Abstract
BACKROUND AND PURPOSE: Cerebral vasospasm secondary to subarachnoid hemorrhage (SAH) has been a serious clinical problem. The aim of the present study is to evaluate the efficacy of selective intraarterial (IA) nimodipine treatment in a rabbit model of chronic cerebral vasospasm.
METHODS: Twenty-two adult New-Zealand rabbits of either sex, weighing 2500–3800 g were used for this study. Following a control angiography, all animals received 1 mL of fresh unheparinized autologuous arterial blood into the cisterna magna. Three days later, the presence of vasospasm was demonstrated angiographically by selective vertebral artery injection. The experimental design was as follows: separate groups of animals (n=5, in each group) received nimodipine (0.05 mg/kg), papaverine (6 mg/kg), or vehicle intraarterially, after placement of a microcatheter into the vertebral artery. Another group (n=5) received nimodipine (0.05 mg/kg) directly into the cisterna magna, and vehicle injection was made into cisterna magna in two other animals. Thirty minutes after treatment, angiographies were repeated and changes in arterial diameter were expressed as percentages of control.
RESULTS: IA nimodipine and IA papaverine were effective in relieving veretebral and basilary vasospasm (P < .05). IA nimodipine was more effective than IA papaverine (P < .05). IA nimodipine was not more effective than intrathecal (IT) nimodipine in relieving vertebral artery vasospasm, although it was more effective than IT nimodipine in basilar artery. Vehicle injections (IA or IT) failed to reverse the vasospasm induced by autologuous blood injection.
CONCLUSION: This study showed that selective IA nimodipine treatment may be considered as an alternative in the treatment of chronic vasospasm following SAH.
Cerebral vasospasm is one of the most important complications of subarachnoid hemorrhages (SAHs), causing high rates of morbidity and mortality. Despite extensive experimental and clinical studies, the level of clinical success has not been satisfactory (1–8). Hypervolemia, hypertension, hemodilution, and nimodipine (oral or intravenous) treatments are used in clinical practice for the medical treatment of cerebral vasospasm (9–11). If the medical treatment is not successful, endovascular treatment has been proposed as an alternative technique.
Endovascular treatment consists of balloon angioplasty and intraarterial (IA) papaverine infusion. Although balloon angioplasty could be used only in the proximal circulation, IA papaverine infusion could be used in the vasospasm of proximal and distal cerebral arteries. These treatment modalities have limited value because of the risk of vascular rupture, the unavailability of balloon angioplasty in the distal arteries, and side effects and reversibility of vasodilatory effect of IA papaverine infusion (3, 5, 7, 12–14). Intrathecal (IT) drug infusions, such as sodium nitroprusside and nimodipine, were also used in the treatment of cerebral vasospasm, but clinical results were not satisfactory (1, 2, 6, 15). As a result, new treatment modalities were needed.
Nimodipine, a calcium channel blocker, is used orally and intravenously in vasospasm therapy (1, 2, 4, 9, 11, 16, 17). There are two clinical reports in which selective IA nimodipine infusion was tried on a limited number of patients for the treatment of cerebral vasospasm (18, 19). There is no experimental or clinical study investigating the effects of selective IA nimodipine infusion or the comparison of this treatment with the routine treatment modalities.
The purpose of the present study is to investigate the effects of selective IA nimodipine infusion and to compare this technique with IT nimodipine and IA papaverine infusions in the treatment of experimentally induced cerebral vasospasm in a rabbit model.
Methods
All protocols were approved by the Hacettepe University Laboratory Animals Ethics Committee. We preferred a rabbit model for this study, because a selective IA catheterization of the vertebral artery is relatively straightforward and the angiographic demonstration of vasospasm is possible in these species.
Experiments were carried out in 22 adult rabbits (New Zealand albino) of either sex weighting 2500–3800 g. All animals were starved for 6 hours before the experiment and anesthetized with a mixture of ketamine (35 mg/kg) and xylazine (5 mg/kg) administered intramuscularly. Additional doses of the anesthetic mixture were given at 20–30 minutes intervals whenever deemed necessary.
Every rabbit underwent an angiography procedure to visualize vertebral and basilar arteries before the formation of SAH. This angiography was used as the baseline measure of the vertebrobasilar circulation. An injection of the autologous blood into the subarachnoid space was then made to induce SAH, and the animals were returned to their cages. Three days later, the formation of vasospasm was verified by a second angiography and, different treatment protocols were applied. A third angiography was performed 30 minutes later to assess the effects of the treatments.
Animals were assigned into four experimental groups according to treatment protocols. Each group consisted of five rabbits receiving one of the following treatments: IA injection of nimodipine (Nimotop; Bayer, Pharmaceuticals Division, West Haven, CT) 0.05 mg/kg; papaverine (Frosst, Kiriland, Quebec, Canada) 6 mg/kg; nimodipine-vehicle and; IT injection of nimodipine 0.05 mg/kg. In addition, nimodipine-vehicle was applied intrathecally in two animals.
Each animal received only one drug injection throughout the study. At the end of the experimental protocol, the animals were killed by an intracardiac injection of 15 mL of alcohol under general anesthesia.
Arterial Access
Femoral arteries were used for access to the vertebral artery. The femoral artery was palpated and the inguinal artery was locally shaved, a 3-cm incision along the course of femoral artery was made under sterile conditions, and the neurovascular bundle was dissected out. The femoral artery was separated and suspended with 3–0 silk sutures at its proximal and distal ends. The artery was then punctured using a micropuncture set needle at a 45° angle. A 4F introducer sheath was placed into the femoral artery by using the micropuncture set system. At the end of procedure, the femoral artery was sutured proximal to the puncture site and the sheath was pulled back carefully. After local cleaning, the skin was continuously sutured with 3–0 silk. The contralateral femoral artery was used for subsequent angiographies. The same method was used in animals in which IT drug treatment was administered.
Selective Vertebral Angiography
Selective vertebral angiographies were performed by using a Vasco microcatheter (2.3F, Balt Extrusion, Montmorency, France) and a 0.016-inch Terumo microguide wire (Terumo Corporation, Tokyo, Japan). In all animals, the left vertebral artery was catheterized at its origin. Extreme care was taken when advancing the tip of the microcatheter to prevent catheter-induced vasospasm.
One mililiter of contrast agent (Omnipaque 300, Nycomed, Cork, Ireland) was injected through the microcatheter, and digital subtraction angiographies (Philips SP4, Best, the Netherlands) were performed on anteroposterior projection. During angiographies exposure factors were kept constant and a metered radiopaque ruler was used to measure a posteriori the diameters of the arteries.
Formation of SAH
Dorsal parts of the head and neck were shaved. Under sterile conditions, the atlanto-occipital membrane was punctured with the head stabilized on hyperflexion to enter the subarachnoid space by using a 27-gauge needle positioned at 90° angle. CSF (1 mL) was aspirated under negative pressure, and an equal volume (i.e., 1 mL) of unheparinized fresh autologous blood, drawn from femoral artery, was injected into the subarachnoid space in 2 minutes. The rabbit was then placed head down for 3 minutes to ease the diffusion of the blood into the subarachnoid space.
Drug Treatments
In IA injection groups, drugs and vehicle were diluted in a final volume of 5 mL and injected slowly through microcatheters over a 5-minute duration. In IT treatment groups, drugs and solvents were injected into the subarachnoid space without dilution. Angiographies were performed 30 minutes later, following the treatments.
A mixture of ethanol (96%, 1.45 mL), polyethylene glycol (1.13 mL), sodium citrate (00118 g), citric oxide-1-H2O (0.002 g), and sterile water (3.68 mL) was used as a nimodipine vehicle. The vehicle injection group served as control when comparing the effectiveness of the treatments.
Measurements of the Arterial Diameters
Measurements of the artery diameters were made independently by two radiologists in a single-blind fashion. On every angiogram, vertebral and basilar artery diameters were measured at two and three different levels, respectively (for measurement points, see Figure 1). To do so, angiographic images were transferred to a computer (Apple Macintosh Cube) by using a 1200-dots-per-inch scanner (Umax Astra) and arterial diameters were measured in pixels by using the image analysis software NIH-Image (version 1.62, available in the public domain [http://rsb.info.nih.gov/nih-image/download.html]). In preliminary analyses, we noticed that by using digital filters or image-processing tools have led to erroneous measurements of the vessel diameters, so all analyses were made without using digital dark room tools. During the measurement procedure, to determine the arterial boundaries accurately, the area was first enlarged by using the “zoom-in” function of the software and outer margins of the vessel were determined and marked visually perpendicular to the vessel axis (Fig 1A–C, insets). In a second step, to rule out overestimation of vessel lumen due to raster image enlargement, the area zoomed out and selected points were reassessed. If margins were considered accurate, the distance between the marks was taken as the vessel diameter. The data (measured in pixels) were then converted into metric values by using the radiopaque ruler as a reference. To evaluate the reliability of the measurements, data compiled by each observer were pooled separately, and confidence intervals were calculated. Each group’s confidence intervals were then compared with assessed interobserver variability. Subsequently, for each determination of vessel diameter, two observers’ measurements were pooled, provided that the corresponding confidence intervals of the group data overlap with each other. The mean ± SEM obtained from each artery was used as the final value for a particular segment in an angiogram.
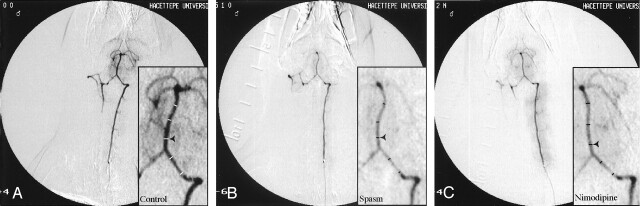
Fig 1.
Rabbit angiograms.
A, Baseline measurements. B, During vasospasm. C, Following IA nimodipine treatment. Insets show enlarged portions of the corresponding angiograms. Measured diameters are given as transecting lines.
Statistical Analysis
Paired Student t test was used to compare the vessel diameter changes after SAH. To compare the effectiveness of the drug therapies, baseline (B), vasospasm (V), and post-treatment (T) vessel diameter values for each animal were plotted on a trigonometric plane, and the angle ∠BVT was expressed in radians. This angle was considered to represent the overall outcome of the therapy for each animal, and values were pooled for different treatment groups. Comparison was initially made with two-way ANOVA and unpaired Student t test was used as post hoc test to compare two different groups. Probability values <0.05 were considered significant throughout the study.
Results
The confidence intervals of the mean values derived from the measurements of two different observers overlapped, and the differences between the measurements were not statistically significant.
Vasospasm Formation
Figure 1A illustrates a typical angiogram of the rabbit vertebrobasilar system. Following the injection of radiopaque material, vertebral arteries, both ipsilateral and contralateral to the injection side and basilar artery, were clearly visualized. Intracranial branches of the basilar artery were also filled. Measurement sites for the artery diameters were shown with horizontal lines (Fig 1A). Same sites were chosen on all subsequent angiograms. The average diameters found were 0.59 ± 0.02 and 0.69 ± 0.01 mm, for vertebral and basilar arteries, respectively (n=22). On the 3rd day following SAH, angiographies showing lack of retrograde filling in the contralateral vertebral artery and intracranial branches of the basilar artery were removed (Fig 1B). In addition, in most of the experiments, retention of the radiopaque material by muscle branches of the ipsilateral vertebral artery was enhanced because of the increase in vertebrobasilar system resistance. The average diameters of vertebral and basilar arteries were 0.39 ± 0.02 and 0.45 ± 0.02 mm, respectively (n=22). The overall decrease in the artery diameters was 34% in vertebral artery and 35% in basilar artery. This decrease was statistically significant (P < .05).
Vertebral Artery Spasm
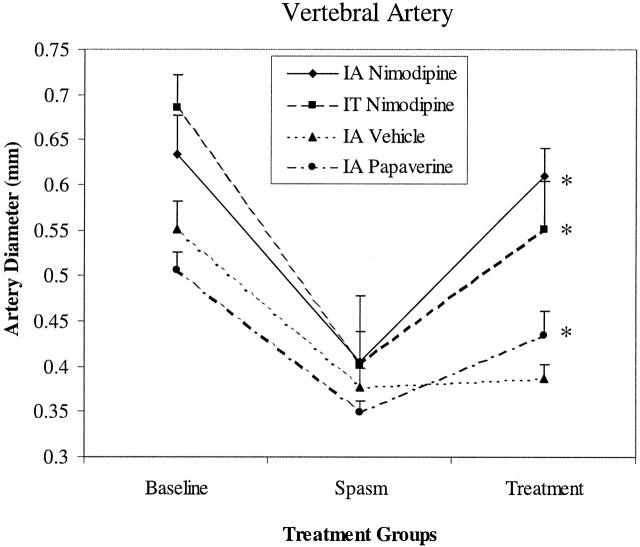
IA nimodipine treatment reversed the vasospasm induced by experimental SAH in vertebral artery. An example was given in Fig 1C and Table 1 shows the arterial diameter measurements in different treatment groups. Mean artery diameter, 30 minutes after IA nimodipine, was found 0.61 ± 0.03 mm (97% of the baseline; n=5). This value was statistically significant from that of during the vasospasm (Fig 2; P < .05). On the other hand, vehicle administration failed to reverse the vasospasm induced by SAH (P > .05; n=5; Fig 2).
TABLE 1:
Changes in vertebral artery diameter
| Treatment groups | Baseline (mm) | Vasospasm (mm) | Treatment (mm) |
|---|---|---|---|
| ia nimodipine | 0.63 ± 0.04 | 0.41 ± 0.03* | 0.61 ± 0.03†§ |
| it nimodipine | 0.69 ± 0.04 | 0.40 ± 0.08* | 0.55 ± 0.55† |
| ia vehicle | 0.55 ± 0.03 | 0.38 ± 0.02* | 0.39 ± 0.02 |
| it vehicle | 0.52 ± 0.04 | 0.40 ± 0.05* | 0.36 ± 0.07 |
| ia papaverine | 0.51 ± 0.02 | 0.35 ± 0.01* | 0.44 ± 0.03† |
All values were derived from n=5 experiments except for IT vehicle group, where n=2 experiments were applied.
Statistically significant from baseline values (P < .05).
Statistically significant from values obtained at vasospasm (P < .05).
Statistically significant from values obtained in IA papaverine treatment.
Fig 2.
Changes in vertebral artery diameters in different treatment groups. Values are obtained from five experiments. Asterisk indicates a value that is statistically significant from spasm values (P < .05).
IT nimodipine reversed the vasospasm induced by experimental SAH to a lesser extent than did the IA treatment, but this reversal was also found statistically significant (P < .05, n=5). The average diameter of vertebral arteries after IT nimodipine was 80% of the baseline value (Fig 2).
Likewise, IA papaverine treatment reversed the vasospasm induced by autologous blood injection into cisterna magna in five rabbits (Fig 2, P < .05). On average, the vessel diameter value was 86% of the baseline.
Two-way ANOVA showed that there were no statistically significant differences between IA and IT nimodipine treatments, but IA papaverine treatment was found to be statistically less effective in reversing the vasospasm in vertebral artery.
Basilar Artery Spasm
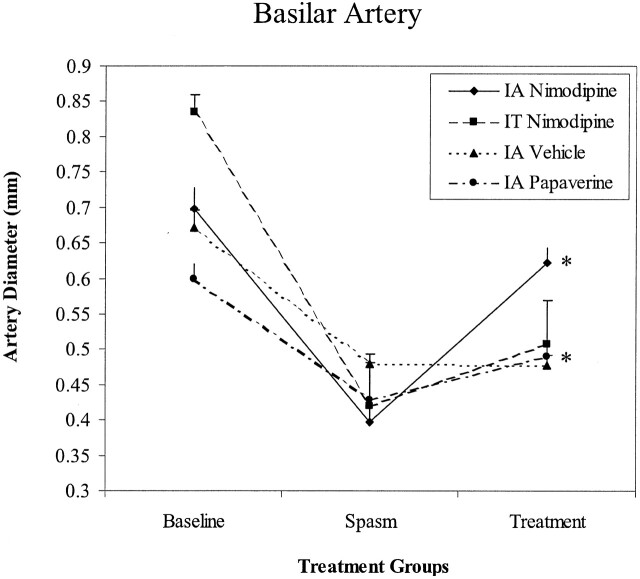
Figure 1C also illustrates the changes obtained after IA nimodipine treatment in the basilar artery. As in the previous section, this treatment technique reversed the vasospasm induced by experimental SAH in basilar artery. Mean basilar artery diameter, 30 minutes after IA nimodipine, was found 0.62 ± 0.02 mm (89% of the baseline; n=5). This value was statistically significant from that of during the vasospasm (Fig 3; P < .05). Vehicle administration failed to reverse the vasospasm induced by SAH (P > .05; n=5; Fig 3).
Fig 3.
Changes in basilar artery diameters in different treatment groups. Values are obtained from five experiments. Asterisk indicates a value that is statistically significant from spasm values (P < .05).
IT nimodipine treatment failed to reverse the vasospasm in basilar artery (P > .05, n=5). The average diameter of basilar arteries after IT nimodipine treatment was 61% of the baseline value (Fig 3).
IA papaverine treatment also reversed the vasospasm in basilar artery (Fig 3, P < .05). On the average, the vessel diameter value was 82% of the baseline (Table 2).
TABLE 2:
Changes in basilar artery diameter
| Treatment groups | Baseline (mm) | Vasospasm (mm) | Treatment (mm) |
|---|---|---|---|
| ia nimodipine | 0.70 ± 0.03 | 0.40 ± 0.06* | 0.62 ± 0.02†§ |
| it nimodipine | 0.83 ± 0.03 | 0.42 ± 0.06* | 0.51 ± 0.06† |
| ia vehicle | 0.67 ± 0.02 | 0.48 ± 0.01* | 0.48 ± 0.01 |
| it vehicle | 0.59 ± 0.01 | 0.47 ± 0.01* | 0.41 ± 0.05 |
| ia papaverine | 0.60 ± 0.02 | 0.43 ± 0.03* | 0.49 ± 0.03† |
All values were derived from n=5 experiments except for IT vehicle group where n=2 experiments were applied. Data were expressed as mean ± SEM.
Statistically significant from baseline values (P < .05).
Statistically significant from values obtained during vasospasm (P < .05).
Statistically significant from values obtained in either IT nimodipine or IA papaverine treatments.
Two-way ANOVA showed that IA nimodipine was more effective in reversing the vasospasm in basilar artery than either IT nimodipine or IA papaverine treatment.
IT vehicle treatment was applied only in two rabbits in which vasospasm has been induced. As expected, there were no statistically significant changes in the diameters of either vertebral or basilar arteries following IT vehicle treatment (data not shown).
Discussion
Experimental Model
Several methods have been used to induce experimental chronic cerebral vasospasm in animals. There are two models that are commonly used to establish SAH in rabbits. The first one is the injection of fresh blood into the cisterna magna. Other authors have suggested modifications in this model to maintain the reproducibility of the vasospasm, such as increasing the injection volume, injection of blood rich in oxyhemoglobin, repeated blood injections, and open or closed access to cisterna magna (20–28). These methods have been successful in obtaining a vasospasm that accounts 31–55% of the baseline in basilar artery. The second one is the puncture of basilar artery with a fine needle. Egemen et al (20) observed a 68% rate of vasospasm in basilar artery by puncturing after transclival exploration. The vasospasm ratios that we report in this study are comparable to that obtained in previous studies. We have not relied on histologic measurements to demonstrate the vasospasms, mainly because we intend to compare the effectiveness of different treatment modalities in real-time procedures. Thus, all measurements and comparisons were made radiologically to mimic the clinical setting more appropriately.
In previous studies, vasospasm was angiographically demonstrated by using injections made into arcus aorta or subclavian artery with 4F catheters (20, 24). This is the first study to demonstrate cerebral artery vasospasm in a rabbit model by using selective vertebral artery injection and also to administer IA nimodipine treatment selectively.
We preferred selective vertebral artery catheterization with microcatheters to perform selective IA treatments and to avoid arterial superposition. To prevent catheter-induced vasospasm, the tips of microcatheters and microwires were placed into the proximal vertebral artery. Contrast agent was injected in fixed volume, and repeated injections were thus avoided.
It has been shown elsewhere that cerebral vasospasm reaches its maximal level in the 3rd day of SAH (23–28). The effect of IA papaverine treatment is at its maximum on the 3rd day and decreases over time (26). Hence, we preferred the 3rd day of SAH for the visualization of vasospasm and for the timing of the treatment. We performed last angiogram 30 minutes after the drug treatments. Although this interval was not enough to assess the long-term effects of these drugs, the time frame represents the average angiography duration for a SAH patient in routine clinical setting. It is, however, senseless to follow the rabbits for longer periods, because neurologic examination in these species lacks specificity for SAH symptoms.
Because we are the first to apply nimodipine intraarterially in an experimental model, we decided to use a single nimodipine dose of 0.05 mg/kg for both IA and IT treatments, to allow comparison of the drug effects independent of the dosage. The dose for papaverine, 6 mg/kg, was derived from the dose that is currently used in clinical practice, and it was in conformity with previous studies (3, 5, 7, 12).
Calcium Antagonists and Nimodipine
Calcium ions play a critical role in almost all stages of cerebral vasospasm formation as well as in the physiopathology of cerebral ischemia. Calcium influx leading to the blockage of nitric oxide release induces the development of the vasospasm and, subsequent release of leucotriens, prostanoids, tromboxanes, lipid peroxides, and free radicals contributes to the process. It has been suggested that calcium channel blockers have cellular neuroprotective and vasodilatory effects by decreasing the calcium influx. Moreover, calcium antagonists also induce the development of collateral circulation (2, 7, 11).
In fact, nimodipine is applied orally and intravenously for cerebral vasospasm treatment in SAH patients. In clinical studies, oral or intravenous nimodipine treatment before the formation of cerebral vasospasm, was found ineffective angiographically. It has then been concluded that oral or intravenous nimodipine treatment could not resolve the angiographic vasospasm, although it could be effective in neuronal protection (2, 7, 11, 16).
Nimodipine Treatment
No experimental study has been conducted previously by using selective IA nimodipine treatment in cerebral vasospasm. Nimodipine was used intraarterially only in two clinical studies. Böker et al (18) reported selective IA nimodipine injection in the treatment of three vasospasm cases following SAH. They concluded that IA nimodipine was angiographically effective. Grotenhius et al (19) have treated six patients with IA nimodipine who had vasospasm after SAH. In their study, IA nimodipine (0.068–1 mg) was found ineffective when administered after the onset of the vasospasm. They concluded that cerebral vasospasm was probably resulted from the structural changes in the vascular wall. Recent studies, however, have shown that structural changes occurred in the late stages of vasospasm and were considered nonspecific (7).
In the present study, the average diameters of vertebral and basilar arteries were reduced to 35% and 43% of the baseline, respectively. Statistically significant increase in the diameter of vertebral and basilar artery was noted after IA nimodipine administration (97% of initial diameter of vertebral and 89% of basilar artery). Better response at the vertebral artery may be due to a lower degree of established vasospasm. To evaluate the relation between the degree of vasospasm and the response to IA nimodipine treatment is not the focus of this study.
We applied 0.05 mg/kg of nimodipine, which is higher than the dosage used in both studies in the literature. No acute side effect was observed due to application of IA nimodipine. Our results are challenging with the results of Grotenhius et al (19); however, methodological differences including the dose and timing may account this discrepancy.
IT nimodipine treatment has been used experimentally and clinically to resolve the vasospasm and reported to be an effective technique (1, 2, 6, 8, 15, 29). Direct application of nimodipine prevents dose-dependent systemic side effects (8). Auer et al (15) used IT nimodipine during aneurysm surgery and observed improvement in vasospasm in all patients. Gioia et al (29) applied nimodipine sublingually, intravenously, and intrathecally and observed improvement only in the IT treatment group. Zabramski et al (8) used IT nimodipine in a dog vasospasm model and reported an increase in the diameter of the vasospastic arteries. In topical usage, however, clot formation may prevent the drug from reaching its site of action (3). This is in conformity with our results that IT nimodipine improved the vasospasm in vertebral artery but not in basilar artery.
IA Papaverine Treatment
Papaverine produces a transient vasodilatation on spastic vessels (3, 5, 7). Kaku et al (3) demonstrated effectiveness of IA papaverine in 10 vasospastic patients. They postulated that the effect of papaverine was continuous when applied by superselective IA infusion. In this study IA papaverine infusion was found more effective on vertebral artery than on basilar artery.
Conclusion
This is the first study to show that selective IA nimodipine treatment is effective in reversing vasospasm in experimental SAH. IA nimodipine infusion was found more effective than its IT application in basilar artery. This is probably due to the limited diffusion of the drug in IT application. IA and IT applications of nimodipine were equally effective in vertebral artery, which demonstrates the potent vasodilator effect of the drug. IA nimodipine infusion was found more effective than IA papaverine infusion in both vertebral and basilar arteries. Selective IA nimodipine treatment can be considered as an alternative technique for the treatment of vasospasm due to SAH. Further studies are needed to implement this treatment option into clinical practice.
Acknowledgments
We thank Halil Ugurlu, for his valuable technical assistance, and Aysun Kara, for the preparation of the drug vehicle. This study was supported by Hacettepe University Research Fund (grant HUTF 99.02.101.007).
Footnotes
Present affiliation of M.M.F. is Department of Radiology, Faculty of Medicine, Gazi Osman Pasa University, Tokat, Turkey.
References
- 1.Barker FG, 2nd, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg 1996;84:405–414 [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Rinkel GJ, Algra A, et al. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 1998;50:876–883 [DOI] [PubMed] [Google Scholar]
- 3.Kaku Y, Yonekawa Y, Tsukahara T, et al. Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg 1992;77:842–847 [DOI] [PubMed] [Google Scholar]
- 4.Karinen P, Koivukangas P, Ohinmaa A, et al. Cost-effectiveness analysis of nimodipine treatment after aneurysmal subarachnoid hemorrhage and surgery. Neurosurgery 1999;45:780–784; discussion 784–785 [DOI] [PubMed] [Google Scholar]
- 5.Marks MP, Steinberg GK, Lane B. Intraarterial papaverine for the treatment of vasospasm. AJNR Am J Neuroradiol 1993;14:822–826 [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas JE, Rosenwasser RH, Armonda RA, et al. Safety of intrathecal sodium nitroprusside for the treatment and prevention of refractory cerebral vasospasm and ischemia in humans. Stroke 1999;30:1409–1416 [DOI] [PubMed] [Google Scholar]
- 7.Treggiari-Venzi MM, Suter PM, Romand JA. Review of medical prevention of vasospasm after aneurysmal subarachnoid hemorrhage: a problem of neurointensive care. Neurosurgery 2001;48:249–261; discussion 261–2 [DOI] [PubMed] [Google Scholar]
- 8.Zabramski J, Spetzler RF, Bonstelle C. Chronic cerebral vasospasm: effect of calcium antagonists. Neurosurgery 1986;18:129–135 [DOI] [PubMed] [Google Scholar]
- 9.Kassel N, Shaffrey M, Shaffrey C. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. In: Apuzzo M, ed. Brain Surgery, 1st ed. New York: Churchill-Livingstone;1993. :847–856
- 10.Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000;31:383–391 [DOI] [PubMed] [Google Scholar]
- 11.Levy M, Rabb C, Couldwell W, et al. Protection of neuronal pool. In: Apuzzo M, ed. Brain Surgery, 1st ed. New York: Churchill-Livingstone1993;857–889
- 12.Connors J, Wojak J. Endovascular therapy of postsubarachnoid hemorrhage vasospasm. In: Connors J, Wojak J, eds. Interventional Neuroradiology: Strategies and Practical Techniques, 1st ed. Philadelphia: W.B. Saunders Company1999;581–601
- 13.Firlik KS, Kaufmann AM, Firlik AD, et al. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol 1999;51:66–74 [DOI] [PubMed] [Google Scholar]
- 14.Oskouian RJ, Jr., Martin NA, Lee JH, et al. Multimodal quantitation of the effects of endovascular therapy for vasospasm on cerebral blood flow, transcranial doppler ultrasonographic velocities, and cerebral artery diameters. Neurosurgery 2002;51:30–41; discussion 41–33 [DOI] [PubMed] [Google Scholar]
- 15.Auer LM, Ito Z, Suzuki A, et al. Prevention of symptomatic vasospasm by topically applied nimodipine. Acta Neurochir (Wien) 1982;63:297–302 [DOI] [PubMed] [Google Scholar]
- 16.Mercier P, Alhayek G, Rizk T, et al. Are the calcium antagonists really useful in cerebral aneurysmal surgery? A retrospective study. Neurosurgery 1994;34:30–36; discussion 36–37 [PubMed] [Google Scholar]
- 17.Paoletti C, Dematons C, Bellec C, et al. Medical manangement of vasospasm and hemodynamic alterations in the neurosurgical ICU. In: Connors J, Wojak J, eds: Interventional Neuroradiology: Strategies and Practical Techniques, 1st ed. Philadelphia: W.B. Saunders Company1999;602–612
- 18.Böker DK, Solymosi L, Wassmann H. Immediate postangiographic intraarterial treatment of cerebral vasospasm after subarachnoid hemorrhage with nimodipine. Report on 3 cases. Neurochirurgia (Stuttg) 1985;28 Suppl 1:118–120 [DOI] [PubMed] [Google Scholar]
- 19.Grotenhuis JA, Bettag W, Fiebach BJ, et al. Intracarotid slow bolus injection of nimodipine during angiography for treatment of cerebral vasospasm after SAH. A preliminary report. J Neurosurg 1984;61:231–240 [DOI] [PubMed] [Google Scholar]
- 20.Egemen N, Sanlidilek U, Zorlutuna A, et al. Transclival approach to rabbit basilar artery for experimental induction of chronic vasospasm. Acta Neurochir (Wien) 1992;115:123–126 [DOI] [PubMed] [Google Scholar]
- 21.Egemen N, Turker RK, Sandlidilek U, et al. The effect of Iloprost on chronic cerebral vasospasm. Gen Pharmacol 1993;24:403–409 [DOI] [PubMed] [Google Scholar]
- 22.Nomura H, Hirashima Y, Endo S, et al. Anticardiolipin antibody aggravates cerebral vasospasm after subarachnoid hemorrhage in rabbits. Stroke 1998;29:1014–1018; discussion 1018–1019 [DOI] [PubMed] [Google Scholar]
- 23.Otsuji T, Endo S, Hirashima Y, et al. An experimental model of symptomatic vasospasm induced by oxyhemoglobin in rabbits. Stroke 1994;25:657–662 [DOI] [PubMed] [Google Scholar]
- 24.Spallone A, Pastore FS. Cerebral vasospasm in a double-injection model in rabbit. Surg Neurol 1989;32:408–417 [DOI] [PubMed] [Google Scholar]
- 25.Vollmer DG, Takayasu M, Dacey RG, Jr. An in vitro comparative study of conducting vessels and penetrating arterioles after experimental subarachnoid hemorrhage in the rabbit. J Neurosurg 1992;77:113–119 [DOI] [PubMed] [Google Scholar]
- 26.Vorkapic P, Bevan RD, Bevan JA. Pharmacologic irreversible narrowing in chronic cerebrovasospasm in rabbits is associated with functional damage. Stroke 1990;21:1478–1484 [DOI] [PubMed] [Google Scholar]
- 27.Vorkapic P, Bevan JA, Bevan RD. Longitudinal in vivo and in vitro time-course study of chronic cerebrovasospasm in the rabbit basilar artery. Neurosurg Rev 1991;14:215–219 [DOI] [PubMed] [Google Scholar]
- 28.Vorkapic P, Bevan JA, Bevan RD. Two indices of functional damage of the artery wall parallel the time course of irreversible narrowing in experimental vasospasm in the rabbit. Blood Vessels 1991;28:179–182 [DOI] [PubMed] [Google Scholar]
- 29.Gioia AE, White RP, Bakhtian B, et al. Evaluation of the efficacy of intrathecal nimodipine in canine models of chronic cerebral vasospasm. J Neurosurg 1985;62:721–728 [DOI] [PubMed] [Google Scholar]