Abstract
BACKGROUND AND PURPOSE: MR imaging characteristics of optic neuropathy caused by cat scratch disease have not yet been described; this lack of information may result in incorrect diagnosis and may contribute to initiation of inappropriate therapy. Our study was based on the hypothesis that cat scratch disease–related optic neuropathy has distinct MR imaging features compared with those of other types of optic neuropathies.
METHODS: Eighty-two patients with various causes of optic neuropathy and available MR imaging examinations were included in this study. Two readers blinded to the diagnosis reviewed the MR images independently in regard to presence, location, and extent of optic nerve enhancement. The MR imaging findings were correlated with the final diagnosis.
RESULTS: Eleven percent (9/82) of the patients received a final diagnosis of cat scratch disease. Optic nerve enhancement in patients with cat scratch disease (5/37) was localized to a 3- to 4-mm segment at the optic nerve–globe junction. All other patients with optic neuropathy (31/37) with one exception showed enhancement away from the optic nerve–globe junction or a long-segment enhancement when the optic nerve–globe junction was also involved. Four patients with cat scratch disease did not show any optic nerve MR abnormalities.
CONCLUSION: Unilateral, short-segment enhancement localized to the optic nerve–globe junction is highly specific for cat scratch disease as the underlying cause of optic neuropathy and may help in establishing the diagnosis of this condition.
Optic neuropathy refers to a clinical disorder characterized by sudden-to-chronic loss of vision in one or both eyes due to optic nerve dysfunction that might be idiopathic, ischemic, primary demyelinating, infectious, or inflammatory in etiology. Clinically, the various types of optic neuropathies share many signs and symptoms (1). The course of visual function recovery can be used clinically as a differentiating factor among some of these entities; however, using visual function recovery requires time, resulting in a delay in diagnosis and subsequent treatment (2, 3). Other ophthalmological findings such as cup-to-disc ratio or presence of intraocular inflammation may favor a particular diagnosis, but the specificity and sensitivity are low (3–8). MR imaging is currently used as the radiological study of choice for the evaluation of patients with optic neuropathy. The imaging findings of optic neuropathy related to primary demyelinating disease such as multiple sclerosis have been well reported in the radiological literature (9–13). Only limited information is available for ischemic optic neuropathy and its imaging distinction from primary demyelinating disease (3, 14, 15).
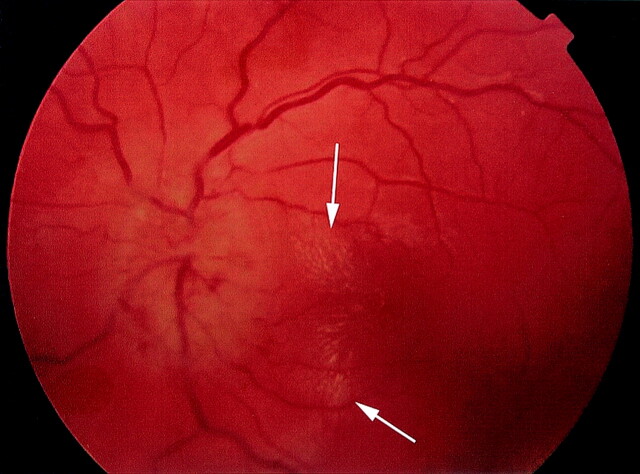
Optic neuropathy due to cat scratch disease is a relatively infrequent occurrence and has been associated with macular star formation on ophthalmological examinations (Fig 1) in some patients on presentation and delayed development in others (16, 17). If cat scratch disease–related optic neuropathy is clinically suspected, confirmatory tests with serological testing for Bartonella henselae, the causative organism of cat scratch disease, or a polymerase chain reaction in patients with inconclusive serology could be obtained (18). The polymerase chain reaction requires a small aspirate from axillary lymphadenopathy. Axillary or any level lymphadenopathy might not be present in patients with cat scratch disease–related optic neuropathy, precluding polymerase chain reaction testing. Direct aspiration of the involved optic nerve is currently not possible.
Fig 1.
The fundoscopic photograph shows the characteristic macular star formation composed of very small bright spots (arrows) to the right of optic nerve disk and aligned in a stellar configuration.
MR imaging is certainly not the study of first choice in suspected cat scratch disease–related optic neuropathy; however, a significant number of patients present with nonspecific clinical findings and a lack of cat scratches on examination, requiring MR imaging for further workup of optic neuropathy. To our knowledge, MR imaging features of optic neuropathy due to cat scratch disease have not yet been described in the literature. We hypothesize that cat scratch disease–related optic neuropathy has distinct MR imaging features in comparison with other types of optic neuropathies.
Methods
Local institutional review board approval was obtained to perform this retrospective study. The institutional review board did not require an informed consent from the subjects.
A list of patients (n = 301) diagnosed with various causes of optic neuropathy by an experienced neuroophthalmologist at our institution between January 1995 and March 2003 was generated. The records of each patient were reviewed for availability of MR imaging examinations performed at the time of initial presentation. Two hundred thirteen patients fulfilled the criteria and were enrolled in this study. All MR imaging examinations were reviewed in regard to imaging parameters and extent of optic nerve and chiasm coverage. Only patients with MR imaging studies consisting of a minimum of coronal T2-weighted and coronal gadolinium-enhanced T1-weighted images covering the entire course of the optic nerves and chiasm with a section thickness ≤4 mm and a section gap of <1 mm were included. One hundred twelve MR imaging studies showed inadequate imaging parameters and/or had incomplete coverage of the optic nerves and/or chiasm. All these patients as well as patients with significantly compromised image quality secondary to motion (n = 6) were excluded from this study. Thirteen additional patients were excluded because of incomplete medical records, leaving 82 eligible patients. Most MR imaging examinations (n = 76) were performed on 1.5-T magnets of different vendors (Vision; Siemens, Erlangen, Germany: LX; General Electric Medical Systems, Milwaukee, WI), and only a few, on an open 0.3-T MR (Open MRI; Siemens, Erlangen, Germany) imaging system (n = 6). All MR images were independently evaluated by a neuroradiologist (IMS) and a neuroophthalmologist (MTB). The readers were aware of the diagnosis of optic neuropathy but were blinded to patients’ identifiers, demographics, medical histories, findings on clinical examinations, laboratory results, final diagnoses, and side of the affected optic nerve.
Image Assessment
All except 2 outside MR imaging examinations were evaluated by the 2 readers independently, using the picture archiving and communication system (Cedara I-Report 5.0.2.1; Cedara, Mississauga, ON, Canada). No limitations were given to the reader in regard to window and level settings and magnification factors. The readers were instructed to use fat-suppressed images whenever available. The type of gadolinium-enhanced T1-weighted sequences used for image assessment (fat-suppressed [n = 74] vs not fat-suppressed [n = 8]) was recorded.
The optic nerves were evaluated individually for the presence, location, and extent of enhancement on the coronal gadolinium-enhanced T1-weighted images. When evaluating the optic nerves in the anteroposterior direction, we defined location as the section number posterior to the optic nerve–globe junction showing the beginning of enhancement. The optic nerve–globe junction was considered to represent section number zero. Extent was recorded as the number of sections showing the optic nerve enhancement. The number of sections used in recording the location and extent of enhancement was later converted into millimeters using the section spacing as the converting factor. Enhancement was assessed visually by comparing the appearance of the optic nerve on the precontrast images in the same plane and at the same levels with that in the postcontrast images. If precontrast images were not available (n = 37), comparison was made from side to side or, if both sides were affected, with portions of optic nerves thought to be normal. If there was significant discrepancy between readers (1 section deviation in extent and location of enhancement), the MR images were reviewed jointly and consensus was reached.
One reader (IMS) also performed additional assessment of available coronal T2-weighted images in regard to presence, location, and extent of optic nerve edema seen as increased signal intensity within the optic nerve. All optic nerves were evaluated independent of the presence or absence of contrast enhancement. The results were recorded in a manner similar to that for enhancement of the optic nerve (see previous description). The diameter of the optic nerve in the craniocaudal extension was also measured approximately 1 cm posterior to the optic nerve–globe junction, using the coronal T2-weighted images. The exact location of the size measurement was dependent on the section thickness and spacing of the T2-weighted images (range, 0.8–1.2 cm posterior to the optic nerve–globe junction).
In all patients, MR images of the brain were also evaluated by this reader in regard to degree of narrowing of the cavernous segment of the internal carotid artery as seen on conventional axial MR images as well as presence and degree of white matter lesions as seen on FLAIR or T2-weighted images obtained with a section thickness of 5 mm and an intersection gap of 0–2.5 mm. In all patients, the degree of white matter disease was classified as none, mild (1–5 lesions), moderate (6–15 lesions), and severe (15 lesions). Enhancement of the white matter lesions as seen on the contrast-enhanced T1-weighted images (section thickness, 5 mm; intersection gap, 0–2.5 mm) was recorded if present. The location, distribution, and size of the white matter lesions were not recorded.
Statistical Analysis
Statistical analysis was performed using the SAS System (SAS, version 9.0, Cary, NC). Descriptive statistics stratified by final diagnosis were calculated. These included age, sex, diameter of the optic nerve, presence of optic nerve edema, presence of optic nerve enhancement, extent of optic nerve enhancement (in millimeters), distance between the optic nerve–globe junction and the beginning of enhancement (in millimeters), and presence of white matter lesions. To test the relationship of extent of enhancement to final diagnosis, we used fixed effects analysis of variance. The dependent variable was “extent of enhancement” and the classification variable was “final diagnosis.” Duncan’s multiple range testing with the experimentwise comparison rate set to 5% was performed to characterize grouping of mean extent of enhancement by final diagnosis. The mean distance between the optic nerve–globe junction and the beginning of enhancement was also tested with an analysis of variance for differences by final diagnosis. Standard test performance metrics were calculated with disease-positive being “optic neuropathy related to cat scratch disease” and test-positive being “optic nerve enhancement confined to the optic nerve–globe junction.” This was performed with the total number of patients (n = 82) and enhancing optic nerves (44 enhancing nerves in 37 patients) as the units of analysis.
Results
Of the 82 patients included in this study, 9 (11%) were diagnosed with optic neuropathy due to cat scratch disease (see Table 1 for summary of the final diagnosis). The diagnosis of cat scratch disease was established by the presence of B henselae–specific IgM and/or elevated IgG with a titer of 1:64; anything above this titer was considered abnormal (Table 2). One patient (case #4) was suspected of having optic neuropathy due to cat scratch disease before review of the MR imaging examination performed in an outside institution. This working diagnosis was primarily based on the presence of a macular exudate (Fig 1) seen on clinical examination. None of the other patients included in this study carried the initial working diagnosis of optic neuropathy due to cat scratch disease (Table 2).
TABLE 1:
Summary of final diagnosis and demographic data of all enrolled patients with optic neuropathy
| Final Diagnosis | No. of Patients (%) | Average Age (yr) | Sex (Female/Male) |
|---|---|---|---|
| Idiopathic optic neuropathy | 42 (51.2) | 44.2 | 28/14 |
| Ischemic optic neuropathy | 18 (22.0) | 59.8 | 12/6 |
| Optic neuropathy related to cat scratch disease | 9 (11) | 31.7 | 5/4 |
| Optic neuropathy related to multiple sclerosis | 9 (11) | 42.0 | 9/0 |
| Others (sarcoidosis, radiation-induced, due to compression by tumor or giant cell arteritis) | 4 (4.9) | 63.7 | 1/3 |
TABLE 2:
Demographic data clinical findings, and laboratory results of patients with optic neuropathy due to cat scratch disease
| Pation No. | Age | Sex | IgM | IgG | Symptom Duration (weeks) | Macular Exudate | MRI | Cats at Home |
|---|---|---|---|---|---|---|---|---|
| 1 | 41 | F | — | 64 | 1 | No (initially) | + | Yes |
| Yes (on follow-up) | ||||||||
| 2 | 28 | M | 128 | 1024 | 2 | No (initially) | + | Unknown |
| Yes (on follow-up) | ||||||||
| 3 | 20 | F | — | 1024 | 2 | No (initially) | + | Unknown |
| Yes (on follow-up) | ||||||||
| 4 | 17 | F | — | 256 | 6 | Yes | + | Yes |
| 5 | 42 | F | — | 64 | 12 | No | + | Unknown |
| 6 | 23 | M | — | 64 | 1 | No (initially) | − | Unknown |
| Yes (on follow-up) | ||||||||
| 7 | 32 | F | 128 | 128 | 0.5 | No | − | Yes |
| 8 | 66 | F | 34 | 1024 | 4 | No | − | Yes |
| 9 | 17 | M | — | 128 | 22 | No | − | Yes |
The analysis of the MR imaging findings showed a trend toward a larger optic nerve in patients with the final diagnosis of idiopathic optic neuropathy or optic neuropathy related to multiple sclerosis (2.54 and 2.36 mm, respectively) when compared with the patients with cat scratch disease. This was not, however, statistically significant (P = .23).
Optic nerve enhancement in one or both eyes was observed in 45.1% (37/82 patients). Table 3 summarizes the distribution of optic nerve enhancement alone and optic nerve edema by disease process. Enhancement of both optic nerves was seen in 18.9% (7/37 patients), of the right optic nerve only in 51.4% (19/37), and of the left optic nerve only in 29.7% (11/37), leading to a total of 44 enhancing optic nerves. None of the patients with optic neuropathy due to cat scratch disease had bilateral optic nerve involvement. Most bilateral optic nerve involvement was seen in idiopathic optic neuropathy (5/7 patients). The bilateral optic nerve enhancement in the remaining 2 patients was due to suspected vascular compromise (ischemia and/or venous engorgement) caused by compression of the optic nerve by a recurrent tumor in one and sarcoidosis in the other.
TABLE 3:
Distribution of optic nerve enhancement by final diagnosis
| Final Diagnosis | Optic Nerve Enhancement (%) | Average Extent of Enhancement (mm ± SD) | Optic Nerve Edema and Enhancement (%) | Optic Nerve Edema without Enhancement (%) | White Matter Lesions and Optic Nerve Enhancement (%) |
|---|---|---|---|---|---|
| Idiopathic optic neuropathy | 21/42 | 19.6 ± 9.3 | 21/21 | 5/42 | 12/21 |
| (50.0) | (100) | (11.9) | (57) | ||
| Ischemic optic neuropathy | 2/18 | 21.5 ± 18.5 | 1/2 | 2/18 | 1/2 |
| (11.1) | (50) | (11.1) | (50) | ||
| Optic neuropathy related to cat scratch disease | 5/9 | 3.58 ± 0.48 | 0/5 | 0/9 | 0/5 |
| (55.6) | (0) | (0) | (0) | ||
| Optic neuropathy to multiple sclerosis | 6/9 | 17.95 ± 9.9 | 5/6 | 0/9 | 4/6 |
| (66.7) | (83.3) | (0) | (66.7) | ||
| Others | 3/4 | 10.7 ± 9.8 | 3/3 | 1/4 | 1/4 |
| (75) | (100) | (25) | (25) |
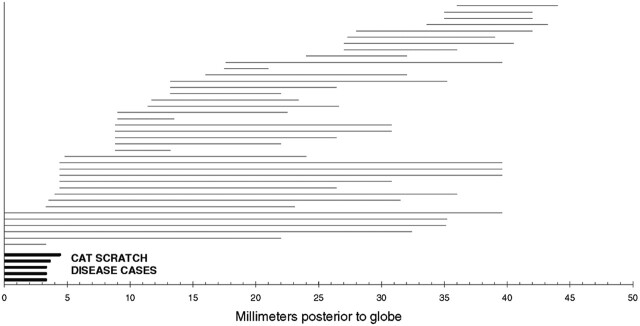
The enhancement of the optic nerve in patients with cat scratch disease (5/37) was localized to a 3- to 4-mm segment at the optic nerve–globe-junction (Figs 2–4). All other patients (31/37) with one exception showed enhancement away from the optic nerve–globe junction or a long-segment enhancement when the optic nerve globe–junction was involved. Figure 5 shows the location and extent of enhancement for each individual enhancing optic nerve. Four patients with the final diagnosis of optic neuropathy due to cat scratch disease had normal-appearing optic nerves on the reviewed MR images (Table 2).
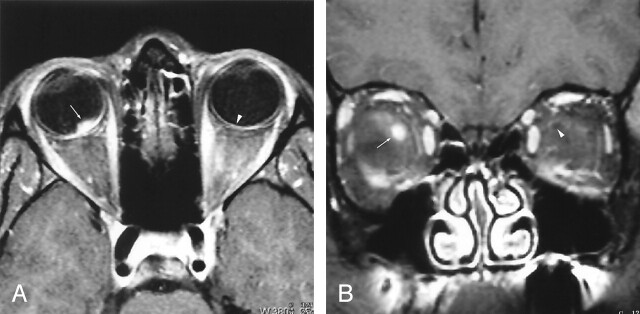
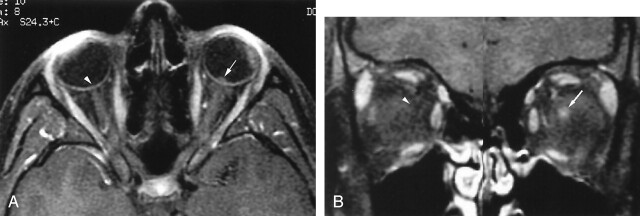
Fig 2.
20-year-old woman with most extensive imaging findings of optic neuropathy due to cat scratch fever (patient 3, Table 2).
A and B, Axial (A) and coronal (B) gadolinium-enhanced fat-suppressed T1-weighted images (TR1, TR2/TE, 735, 850/14) show significant bulging of the right optic disk (arrow, A) associated with severe enhancement at the optic nerve–globe junction (arrow, A and B). Note the normal appearance of the optic nerve–globe junction on the left (arrowhead, A and B).
Fig 4.
42-year-old woman with very subtle imaging findings of optic neuropathy due to cat scratch fever (patient 5, Table 2).
A and B, Axial (A) and coronal (B) gadolinium-enhanced fat-suppressed T1-weighted images (TR1, TR2/TE, 735, 882/14) show very minimal bulging of the left optic disk (arrow, A) compared with those in Figures 1 and 2. The associated enhancement at the left optic nerve–globe junction (arrow, A and B) is very subtle, in particular in the coronal plane. Note the normal appearance of the optic nerve–globe junction region on the right (arrowhead, A and B).
Fig 5.
Plot of enhancement in 44 optic nerves (37 patients). Each horizontal line represents the area of enhancement in a single nerve. The distance from zero on the x-axis to the left end of the line represents length (if any) of nonenhancing nerve adjacent to the globe. The length of the line itself represents the extent of enhancement. The 5 enhancing nerves in patients with optic neuropathy due to cat scratch disease are at the bottom (bold lines). The other 39 enhancing nerves with optic neuropathy from other causes are above these (thin lines). The one patient with false-positive findings is marked as a short thin line just above the patients with cat scratch disease.
The analysis of variance revealed a significant difference in both extent of enhancement (P < .018) and distance between the optic nerve–globe junction and the beginning of enhancement (P = .004) between the patients in the cat scratch disease group and those with other types of optic neuropathy. Duncan’s multiple range test on the extent of enhancement revealed that optic neuropathy due to cat scratch disease was significantly shorter than all the other diagnoses when enhancement was present. At the patient level (n = 82), the finding of optic nerve enhancement confined to the optic nerve–globe-junction was 56% sensitive (5/9 disease-positive patients) and 98.6% specific (1/73 disease-negative patients) for optic neuropathy due to cat scratch disease. The one patient with optic nerve enhancement confined to the optic nerve–globe junction (false-positive) was diagnosed with ischemic optic neuropathy and had subtle enhancement of the right optic nerve–globe junction only, similar to that of the patient with cat scratch disease shown in Figure 3. When the 37 enhancing optic nerves were used as the unit of analysis, the finding of enhancement confined to the optic nerve globe–junction was 100% sensitive (5/5 disease-positive patients) and 96.9% specific (1/32 disease-negative patients) for optic neuropathy due to cat scratch disease.
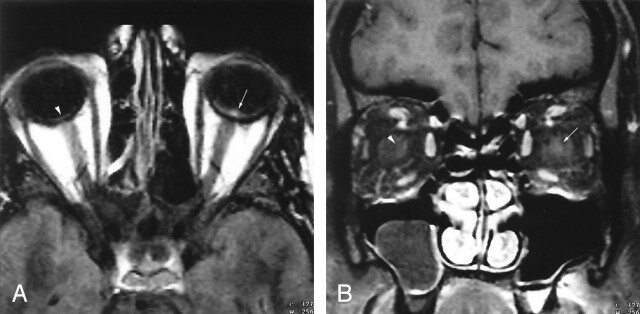
Fig 3.
17-year-old girl with moderate imaging findings of optic neuropathy due to cat scratch fever (patient 4, Table 2).
A and B, Axial (A) and coronal (B) gadolinium-enhanced fat-suppressed T1-weighted images (TR1, TR2/TE, 735, 875/14) show bulging of the left optic disc (arrow, A) that is markedly less pronounced than that on the right (arrowhead, A) as well as that in Figure 1. The associated enhancement at the left optic nerve–globe junction (arrow, A and B) is also markedly less extensive. Note the normal appearance of the optic nerve–globe junction region on the right (arrowhead, A and B).
None of the patients showed narrowing of the cavernous segment of the internal carotid artery on either side. Cerebral white matter lesions were observed in significant numbers of patients; however, none of the patients with cat scratch disease (0/9) showed any white matter lesions (Table 3).
Discussion
Optic neuropathy can be due to various causes that show significant overlap in clinical presentation and clinical examination (1). The Optic Neuritis Treatment Trial has shown that intravenous administration of high-dose steroids decreases the risk of progression of optic neuropathy to multiple sclerosis within 2 years of follow-up (2, 19, 20). Therefore, the diagnosis of the underlying cause of optic neuropathy should be established as soon as possible. MR imaging is the study of first choice in evaluation of patients with optic neuropathy. The imaging findings of idiopathic and multiple-sclerosis–related optic neuropathy are well known and have been described in the literature (3, 9–13). Most authors reported long-segment enhancement in patients with idiopathic or multiple sclerosis–related optic neuropathy, typically involving the entire intraorbital portion of the optic nerve (3, 9–11, 21). In addition, Rizzo et al (3) observed that the extent of enhancement overlapped the extent of optic nerve edema. This is congruent with our results.
To our knowledge, the imaging features of optic neuropathy due to cat scratch disease have not yet been reported in the literature and significantly differ from the established MR imaging findings described for idiopathic or multiple sclerosis–related optic neuropathy (3, 9–11). As shown in our study, optic neuropathy due to cat scratch disease is characterized by short-segment enhancement of the optic nerve localized to the optic nerve–globe junction alone (Figs 2–5). None of our patients with idiopathic or multiple sclerosis–related optic neuropathy showed localized enhancement to the optic nerve–globe junction (Fig 5). Only 3 patients with idiopathic or multiple sclerosis–related optic neuropathy in our study had short-segment enhancement of the optic nerve; however, the enhancement was remote from the optic nerve–globe junction (Fig 5). In addition, none of our patients with cat scratch disease showed optic nerve edema in the region of enhancement or in other locations in contrast to the other types of optic neuropathy (Table 3). All our patients with optic neuropathy due to cat scratch disease manifested unilateral findings clinically and radiographically, whereas bilateral involvement was seen in 19% of patients with idiopathic or multiple sclerosis–related optic neuropathy. Similar findings were observed for the presence of white matter disease because none of the patients with cat scratch disease showed white matter lesions in contrast to the other disease entities (Table 3).
The second most common cause of optic neuropathy in our patient population (after idiopathic and multiple sclerosis–related optic neuropathy combined) was ischemia. None of the patients with optic neuropathy related to ischemia had findings or history of vasculitis (e.g., related autoimmune disorders such as systemic lupus erythematosus). Few publications have described the imaging features of ischemic optic neuropathy to date (3, 12). Localized edema and only occasional enhancement of the optic nerve have been associated with ischemic optic neuropathy, which is consistent with our results (3, 12). Interestingly, one of our patients with ischemic optic neuropathy (a 46-year-old woman) showed faint localized enhancement at the optic nerve–globe junction similar to the mildest changes observed in optic neuropathy due to cat scratch disease (Figs 4 and 5). To our knowledge, no explanation in regard to the occasional enhancement of the optic nerve in ischemic optic neuropathy has been reported in the literature. Potentially, this may be related to “luxury perfusion” in the subacute stage of ischemia because it has been well described for cerebral infarctions (22, 23). The only feature differentiating the patient with ischemic optic neuropathy from the patient with cat scratch disease was the presence of mild white matter disease, which has not been observed in any of the patients with cat scratch disease. Certainly, the significantly lower incidence of white matter lesions in patients with cat scratch disease might be primarily due to their overall younger age (mean, 31.78 years) compared with the patients without cat scratch disease combined (mean, 48.59 years). Nevertheless, age should not be considered helpful in distinguishing optic neuropathy due to cat scratch disease from the other types of optic neuropathy because the age in our patients with cat scratch disease ranged from 17 to 66 years (Table 2).
Because cat scratch disease is infectious, one might expect to see similar MR imaging findings in optic neuropathy due to other types of infections such as toxoplasmosis, cytomegalovirus, herpes zoster virus, Lyme disease, syphilis, or others. There are only scattered clinical case reports of isolated infectious optic neuropathy. Only a few of the reports have described the imaging findings associated with these entities (12, 24). Gass et al (12) reported enhancement associated with 15-mm optic nerve edema, first in one optic nerve and 2 weeks later in the other in a patient with toxoplasmosis-related optic neuropathy. Although the exact location of the enhancement was not described, the presence of optic nerve edema and eventual bilateral optic nerve involvement differs from the findings we observed in our patients with cat scratch disease. Choi et al (24) described a retrobulbar mass causing optic neuropathy related to aspergillosis. None of our patients with cat scratch disease had a retrobulbar mass.
Morales et al (25) reported the clinical and imaging characteristics of a postviral type of optic neuritis in children. Two-thirds of their patients had a preceding febrile illness within 2 weeks of presentation, similar to acute disseminated encephalomyelitis of the brain. They observed enlargement and enhancement of the affected optic nerve in 63% and associated focal demyelinating white matter lesions of the brain in 33%. None of our patients had a preceding febrile illness. Morales et al also reported that 26% of their patients progressed to develop multiple sclerosis, suggesting that the underlying optic neuritis in their patient population was due to underlying demyelinating disease process triggered by a preceding viral infection rather than by the virus itself.
In our study, 5 of 9 patients having the diagnosis of optic neuropathy due to cat scratch disease showed unilateral short-segment enhancement of the optic nerve localized to the optic nerve–globe junction. Why the enhancement remains so localized to the optic nerve–globe junction is unknown. No pathological correlatives were provided in the literature or in our institution. One might speculate that this localized enhancement is caused by an inflammatory response to the macular exudate (Fig 1); however, only one of our patients had a macular exudate at the time of MR imaging examination. Therefore, this might not be the logical explanation.
Four of the 9 patients with optic neuropathy–related cat scratch disease had normal-appearing optic nerves on the available MR imaging examinations. The absence of optic nerve enhancement in these patients might be due to the timing of the MR scanning performed in relation to the onset of symptoms and/or exposure to cats. As shown in Table 1, only one patient had a macular star formation at the initial clinical examination, whereas others developed it later or not at all. Because no follow-up MR imaging examinations were available, we are not able to document if any of these patients eventually developed these imaging features. There was no significant difference in age, sex, IgG and IgM titers, imaging parameters, quality of the imaging studies, and duration of symptoms between the MR-positive and MR-negative patients with cat scratch disease. Why some patients developed enhancement while others did not is therefore unclear. Potentially, evaluation of a larger number of patients with optic neuropathy due to cat scratch disease might bring some insight in this regard. Consequently, a normal finding on an MR imaging examination does not exclude cat scratch disease as the cause of optic neuropathy; however, short-segment enhancement localized to the optic nerve globe–junction is highly suggestive of underlying cat scratch disease and confirmation with serological testing for B henselae, the causative organism of cat scratch disease, should be considered.
Since the closure of this retrospective study, we have seen 2 additional patients presenting to our institution with optic neuropathy due to cat scratch disease. Both patients underwent an MR imaging examination showing the characteristic short-segment enhancement at the optic nerve–globe junction (1 severe and 1 subtle case). The patient with severe enhancement (similar to that in Figure 2) had been symptomatic with fever and upper respiratory symptoms for approximately 1 month before presentation and presented to 2 different outside hospitals with progressive vision loss in 1 eye. The MR examination was prospectively reported as strongly suggestive of optic neuropathy due to cat scratch disease. The imaging diagnosis was confirmed by elevated antibody titers against B henselae (IgM = 1:32; IgG1:1024). In addition, the patient showed numerous cat scratches on the skin. The patient with subtle enhancement at the optic nerve globe–junction similar to that in the patient with cat scratch disease shown in Figure 4 had been symptomatic for a few days before presentation, and the serological testing was positive for cat scratch disease (IgM = negative; IgG1:1024).
In conclusion, a normal MR imaging appearance of the optic nerve does not exclude cat scratch disease as the underlying cause of optic neuropathy. The presence of short-segment enhancement at the optic nerve–globe junction is suggestive of cat scratch disease infection; therefore, confirmation with serological testing for B henselae antibodies should be obtained.
References
- 1.Rizzo JF III, Lessell S. Optic neuritis and ischemic optic neuropathy: overlapping clinical profiles. Arch Ophthalmol 1991;109:1668–1672 [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis: experience of the Optic Neuritis Treatment Trial. Ophthalmology 1994;101:1771–1778 [DOI] [PubMed] [Google Scholar]
- 3.Rizzo JF III, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2002;109:1679–1684 [DOI] [PubMed] [Google Scholar]
- 4.Doro S, Lessell S. Cup-disc ratio and ischemic optic neuropathy. Arch Ophthalmol 1985;103:1142–1144 [DOI] [PubMed] [Google Scholar]
- 5.Mansour AM, Shoch D, Logani S. Optic disc size in ischemic optic neuropathy. Am J Ophthalmol 1988;106:587–589 [DOI] [PubMed] [Google Scholar]
- 6.Beck RW, Savino PJ, Repka MX, Schatz NJ, Sergott RC. Optic disc structure in anterior ischemic optic neuropathy. Ophthalmology 1984;91:1334–1337 [DOI] [PubMed] [Google Scholar]
- 7.Warner JE, Lessell S, Rizzo JF III, Newman NJ. Does optic disc appearance distinguish ischemic optic neuropathy from optic neuritis? Arch Ophthalmol 1997;115:1408–1410 [DOI] [PubMed] [Google Scholar]
- 8.Armaly MF, Sayegh RE. The cup/disc ratio: the findings of tonometry and tonography in the normal eye. Arch Ophthalmol 1969;82:191–196 [DOI] [PubMed] [Google Scholar]
- 9.Guy J, Mancuso A, Quisling RG, Beck R, Moster M. Gadolinium-DTPA-enhanced magnetic resonance imaging in optic neuropathies. Ophthalmology 1990. :97:592–600 [PubMed] [Google Scholar]
- 10.Guy J, Mao, J, Bidgood WD Jr, et al. Enhancement and demyelination of the intraorbital optic nerve: fat suppression magnetic resonance imaging. Ophthalmology 1992;99:713–719 [DOI] [PubMed] [Google Scholar]
- 11.Merandi SF, Kudryk BT, Murtagh FR, Arrington JA. Contrast-enhanced MR imaging of optic nerve lesions in patients with acute optic neuritis. Am J Neuroradiol AJNR 1991;12:923–926 [PMC free article] [PubMed] [Google Scholar]
- 12.Gass A, Moseley I F. The contribution of magnetic resonance imaging in the differential diagnosis of optic nerve damage. J Neurolog Sciences 2000;172(suppl):S17–S22 [DOI] [PubMed] [Google Scholar]
- 13.Kupersmith MJ, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain 2002;125(Pt 4):812–822 [DOI] [PubMed] [Google Scholar]
- 14.Cornblath WT, Quint DJ. MRI of optic nerve enlargement in optic neuritis. Neurology 1997;48:821–825 [DOI] [PubMed] [Google Scholar]
- 15.Sklar EML, Schatz NJ, Glaser JS, Post MJD, ten Hove M. MR of vasculitis-induced optic neuropathy. Am J Neuroradiol AJNR 1996;17:121–128 [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatti MT, Asif R, Bhatti LB: Macular star in neuroretinitis. Arch Neurol 2001;58:1008–1009 [DOI] [PubMed] [Google Scholar]
- 17.Kodama T, Masuda H, Ohira A. Neuroretinitis associated with cat-scratch disease in Japanese patients. Acta Ophthalmol Scand 2003;81:653–657 [DOI] [PubMed] [Google Scholar]
- 18.Labalette P, Bermond D, Dedes V, Savage C. Car-scratch disease neuroretinitis diagnosed by a polymerase chain reaction approach. Am J Ophthalmol 2001;132:575–576 [DOI] [PubMed] [Google Scholar]
- 19.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis: The Optic Neuritis Study Group. N Engl J Med 1992;326:581–588 [DOI] [PubMed] [Google Scholar]
- 20.Beck RW, Cleary PA, Trobe JD, et al. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. N Engl J Med 1993;329:1764–1769 [DOI] [PubMed] [Google Scholar]
- 21.Youl BD, Turano G, Miller DH, et al. The pathophysiology of optic neuritis. Brain 1991;114:2437–2450 [DOI] [PubMed] [Google Scholar]
- 22.Mountz JM, Deutsch G, Khan SH. Regional cerebral blood flow changes in stroke imaged by Tc-99m HMPAO SPECT with corresponding anatomic image comparison. Clin Nucl Med 1993;18:1067–1082 [DOI] [PubMed] [Google Scholar]
- 23.Baron JC, Delattre JY, Bories J, et al. Comparison study of CT and positron emission tomographic data in recent cerebral infarction. Am J Neuroradiol AJNR 1983;4:536–540 [PMC free article] [PubMed] [Google Scholar]
- 24.Choi MY, Bae IH, Lee JH, Lee SJ. Aspergillosis presenting as an optic neuritis. Korean J Ophthalmol 2002;16:119–123 [DOI] [PubMed] [Google Scholar]
- 25.Morales DS, Siatkowski RM, Howard CW, Warman R. Optic neuritis in children. J Pediatr Ophthalmol Strabismus 2000;37:254–259 [PubMed] [Google Scholar]