Graphical abstract
Keywords: DFT study, Heterocyclic compounds, Spectroscopic analyses, Geometrical calculation, Non-covalent interaction, Molecular docking, SARS-Cov-2
Abstract
Investigation the molecular structure of the system requires a detailed experience in dealing with theoretical computational guides to highlight its important role. Molecular structure of three heterocyclic compounds 8,10-diphenylpyrido[3,2–e][1,2,4]triazolo[4,3–c]pyrimidine-3(2H)-thione (HL), 8-phenyl-10-(p-tolyl)pyrido[3,2–e][1,2,4]triazolo[4,3–c]pyrimidine-3(2H)-thione (CH3L) and10-(4-nitrophenyl)-8-phenylpyrido[3,2–e][1,2,4]triazolo[4,3–c]pyrimidine-3(2H)-thione (NO2L) was studied at DFT/B3LYP/6-31G (d,p) level in ethanol solvent. Spectroscopic properties such Infrared (IR, 1H NMR and 13C NMR) and ultraviolet–visible (UV–VIS) analyses were computed. Some quantum and reactivity parameters (HOMO energy, LUMO energy, energy gap, ionization potential, electron affinity, chemical potential, global softness, lipophelicity) were studied, also molecular electrostatic potential (MEP) was performed to indicate the reactive nucleophilic and electrophilic sites. The effects of H-, CH3– and NO2– substituents on heterocyclic ligands were studied and it was found that the electron donation sites concerned with hydrogen and methyl substituents over nitro substituent. Topological analysis using reduced density gradient (RDG) was discussed in details. To predict the relevant antiviral activity of the reported heterocyclic compounds, molecular docking simulation was applied to the crystal structure of SARS-Cov-2 viral Mpro enzyme with 6WTT code and PLpro with 7JRN code. The enzymatic viral protein gives an image about the binding affinity between the target protein receptor and the heterocyclic ligands entitled. The hydrogen bonding interactions were evaluated from molecular docking with different strength for each ligand compound to discuss the efficiency of heterocyclic ligands toward viral inhibition.
1. Introduction
2-amino-3-cyanopyridines are good starting reagents that have been used to synthesize many heterocyclic compounds such as pyridopyrimidines [1], [2], [1,2,4 ]triazolo and [1,2,3,4] tetrazolo derivatives. Pyridopyrimidines are interesting compounds for its biological activities such as anti-microbial and cytotoxic activities [3]. At this time, studies have been intensified on the causes and forms of different types of cancers and how to find solutions to treat them or limit their occurrence. Ligands of low molecular weight, such as Schiff bases and heterocyclic compounds, can bind to protein receptors by forming a number of non-covalent interactions of different amino acid residues. Interactions between different non-covalent molecules are weak compared to covalent bonds because a variety of non-covalent interactions do not include electron sharing. Types of interactions between ligands and protein target include π–π aromatic stacking [4], [5], hydrogen bonds [6], [7] hydrophobic effects, [8], [9], cation–π interactions, [10], [11] halogen bonds, [12], [13] and salt bridges [14], [15]. π–π stacking is defined as a noncovalent attractive interaction between the aromatic rings, but this type not as widespread as hydrophobic contacts and hydrogen bonds, this type plays a major role in biological molecular recognition and the organization of structural biomolecules. The study of geometrical and energetic properties of these non-covalent interactions is mainly important in various applications of drug discovery [11], [16], [17]. Egli and his co-workers first reported that formation a non-covalent contact between the lone pair of O4′ atom of cytosine deoxyribose and the π cloud of the aromatic system of guanine nucleobase that stabilizes the structural component of the Z-DNA double-helix structure [18]. This type of interaction is called lone pair–π (lp–π) interaction. There are many organic heterocyclic ligands [19], [20], [21] that have been studied according to their applications in pharma, drug and catalysis. To relate heterocyclic ligands with their complexes, complexes with transition metals get a great attention due to extensive applications in fields such as antibacterial, antiviral and flame retardant [22], [23] . Cancer treatment with coordination complexes was considered to be a great success since the discovery of cisplatin. [24].
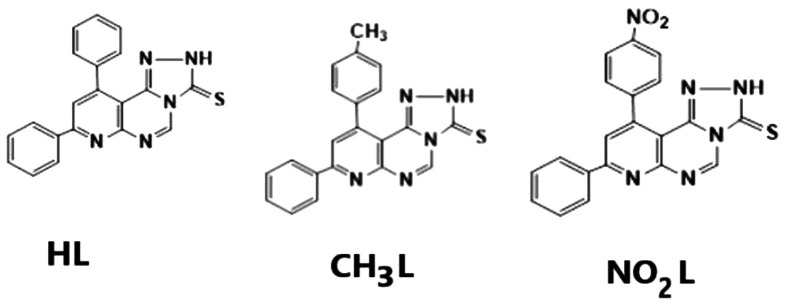
This study aimed to elucidate the structural, electronic and molecular antiviral properties of HL, CH3L and NO2L heterocyclic ligand compounds with DFT computational study. These compounds have been experimentally synthesized with spectral characterization occurred [25]. Scheme 1 shows the structures of heterocyclic ligands afforded in this study. The binding modes of different substituents (H, CH3 and NO2) to the phenyl ring of the parent compound of the hydroxyl group were investigated with complete computational details.
Scheme 1.
The chemical structure of recently synthesized heterocyclic compounds [25].
Spectroscopic features such as electronic circular dichroism is termed as the differential absorption of left and right circularly polarized electromagnetic radiation by a sample, this analysis is also defined as chiroptical counterpart of UV–Vis absorption spectroscopy. Similarly, Raman spectroscopy is the counterpart of infrared (IR). The main advantages of chiroptical spectroscopic, instead of non-chiral identification, are sensitive to the absolute configuration of a sample. This type of chiroptical spectroscopies is sensitive toward the all molecular structure rather than their non-chiral counterpart. ECD spectrum involves a lot of structural information about the molecular structure such conformation and configuration [26] .
2. Method of calculation
2.1. Experimental
The heterocyclic compound class was synthesized as the reported method presented by El-sayed et al [25] Infrared spectra were measured on Perkin-Elmer FT/IR spectrophotometer, 1H NMR and 13C NMR spectra were measured on (300 MHz), in solvent DMSO‑d 6, using the internal reference standard tetramethyl-silane (TMS).
2.2. Computational details
Computational study has been performed to evaluate the structural properties of three heterocyclic compounds HL, CH3L, NO2L. Gaussian 09 software [27] was used for this calculation based on density functional theory (DFT). The selected computational method is using Becke’s three parameter exchange function [28], [29] from the Lee Yang Parr (B3LYP) correlation function with polarized basis set 6-31G (d,p). the calculations were performed in ethanol using the model of conductor-like polarizable contimum (CPCM) with no imaginary frequency [30] . Gauss view software [31] and Chemcraft program [32] were used to create the Gaussian input file and visualize the optimized structure. IR spectra and UV/visible properties of the optimized compounds were performed and calculated at the same level of DFT theory. 1H and 13C NMR spectral analyses were performed after optimization using the gauge-including-atomic-orbital (GIAO) method. UV–VIS study was followed by time-dependent density functional theory (TD-DFT) method [33] to confirm different electronic transitions in the heterocyclic ligands. Some quantum chemical parameters were estimated for the optimized structures to get an information about their stability and reactivity. Topological analysis was considered as it is helpful in the interaction findings between atoms at the structure surface. Molecular electrostatic potential (MEP) was applied to investigate the electrophilic and nucleophilic binding sites. Non covalent interaction analysis using reduced density gradient (NCI-RDG) were analyzed which is helpful in illustrating the type of intramolecular interactions.
2.3. Molecular docking simulation
The heterocyclic ligands, HL, CH3L, NO2L, are withdrawn to docking simulation with the crystal structure of Mpro and PLpro targets with 6WTT and 7JRN codes were downloaded from Protein Data Bank (PDB) (https://www.rcsb.org/structure). iGemdock 2.1 software [34] was used for docking calculations and Chimera 1.13.1 [35] was used for ligand-target interaction visualization. The docking technique default setting is based on X-ray diffraction with resolution of 2.30 Å and R-factor of 0.193 with total residue count of 207. The docking accuracy settings (GA parameters) was adjusted as a standard docking of population size 200, generations 70 and number of solutions 2. The receptor was prepared by removing any ions, small ligands and water molecules and addition of polar hydrogens.
3. Results and discussion
3.1. Geometry optimization
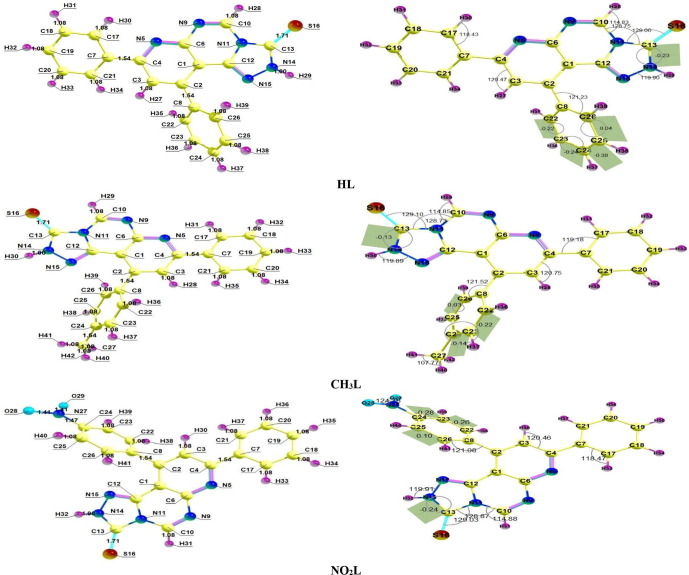
The optimized heterocyclic structures related to ground state were obtained in ethanol at B3LYPl6-31G(d,p) are shown in Fig. 1 with labeling atoms geometrical parameters data (bond length, bond angle and some dihedral values). Most of geometrical parameters are similar for the structure derivatives, while the difference appears in the geometry of the substituent on the phenyl ring branched from C2 atom. The dihedral value of S-C-N-H differs for CH3L (-0.13) that may be attributed to the methyl group on the phenyl ring make it appear far from the triazole ring, where the geometry of triazole moiety became more planar than the other of the two heterocyclic ligands. The optimized structures show a close contact between the phenyl group carry the substituent and the triazole ring, that slightly changes the plane position of triazole ring and the dihedral values also change (for HL is −0.23 and for NO2L is −0.24). Table 1 shows Mulliken charges calculated on each atom where most of charges in the three ligands are the same except the charges of newly added substituent atoms in each ligand which makes the surrounded electronic environment differ from one ligand to another. In the three derived ligands, N5 and N11 have approximately similar high charges (-0.536 and −0.563), but the charge on N14 (-0.350) and N15 (-0.383) have small charges. In the case of Sulphur, It has a small charge (-0.258 for HL, −0.260 for CH3L and −0.245 for NO2L) compared with other nitrogen atoms.
Fig. 1.
Labelled optimized heterocyclic compounds with bond lengths, some bond angles and dihedral values.
Table 1.
Mullikan atomic charge distribution of HL, CH3L and NO2L heterocyclic compounds.
| HL | CH3L | NO2L | |||
|---|---|---|---|---|---|
| C1 | 0.096 | C1 | 0.096 | C1 | 0.101 |
| C2 | 0.032 | C2 | 0.035 | C2 | 0.026 |
| C3 | −0.141 | C3 | −0.138 | C3 | −0.138 |
| C4 | 0.265 | C4 | 0.274 | C4 | 0.267 |
| N5 | −0.536 | N5 | −0.544 | N5 | −0.533 |
| C6 | 0.355 | C6 | 0.355 | C6 | 0.358 |
| C7 | 0.068 | C7 | 0.065 | C7 | 0.068 |
| C8 | 0.043 | C8 | 0.040 | C8 | 0.059 |
| N9 | −0.462 | N9 | −0.462 | N9 | −0.461 |
| C10 | 0.320 | C10 | 0.320 | C10 | 0.322 |
| N11 | −0.563 | N11 | −0.564 | N11 | −0.564 |
| C12 | 0.531 | C12 | 0.531 | C12 | 0.534 |
| C13 | 0.354 | C13 | 0.354 | C13 | 0.353 |
| N14 | −0.350 | N14 | −0.349 | N14 | −0.350 |
| N15 | −0.383 | N15 | −0.384 | N15 | −0.389 |
| S16 | −0.258 | S16 | −0.260 | S16 | −0.245 |
| C17 | −0.096 | C17 | −0.097 | C17 | −0.095 |
| C18 | −0.092 | C18 | −0.092 | C18 | −0.092 |
| C19 | −0.076 | C19 | −0.075 | C19 | −0.074 |
| C20 | −0.096 | C20 | −0.097 | C20 | −0.096 |
| C21 | −0.118 | C21 | −0.120 | C21 | −0.118 |
| C22 | −0.101 | C22 | −0.101 | C22 | −0.114 |
| C23 | −0.089 | C23 | −0.128 | C23 | −0.092 |
| C24 | −0.078 | C24 | 0.131 | C24 | 0.251 |
| C25 | −0.092 | C25 | −0.125 | C25 | −0.094 |
| C26 | −0.065 | C26 | −0.064 | C26 | −0.077 |
| H27 | 0.108 | C27 | −0.383 | N27 | 0.387 |
| H28 | 0.171 | H28 | 0.105 | O28 | −0.393 |
| H29 | 0.307 | H29 | 0.171 | O29 | −0.394 |
| H30 | 0.124 | H30 | 0.306 | H30 | 0.111 |
| H31 | 0.094 | H31 | 0.127 | H31 | 0.174 |
| H32 | 0.091 | H32 | 0.093 | H32 | 0.310 |
| H33 | 0.090 | H33 | 0.091 | H33 | 0.126 |
| H34 | 0.084 | H34 | 0.090 | H34 | 0.097 |
| H35 | 0.092 | H35 | 0.084 | H35 | 0.094 |
| H36 | 0.092 | H36 | 0.092 | H36 | 0.093 |
| H37 | 0.092 | H37 | 0.084 | H37 | 0.084 |
| H38 | 0.092 | H38 | 0.083 | H38 | 0.110 |
| H39 | 0.093 | H39 | 0.092 | H39 | 0.143 |
| – | – | H40 | 0.113 | H40 | 0.142 |
| – | – | H41 | 0.127 | H41 | 0.110 |
| – | – | H42 | 0.126 | – | – |
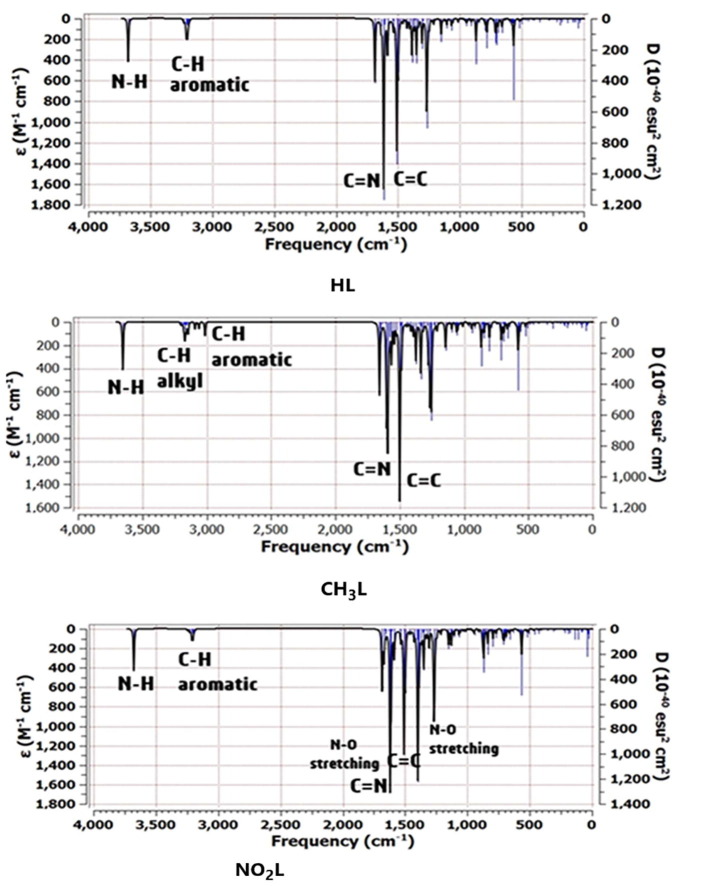
3.2. IR spectral analysis
Vibrational spectra of the studied ligands were calculated to ensure their molecular structures. There are some characteristic bands for the three ligands shown in Fig. 2 . There is a sharp band appeared at 3679 cm−1, 3651 cm−1 and 3679 cm−1 for HL, CH3L and NO2L, respectively that attributed to N-H of triazole ring stretching. The experimental data revealed that presence of a band at 3377 cm−1, 3356 cm−1 and 3332 cm−1 which were corresponding to NH group of HL, CH3L and NO2L, respectively. The difference in theoretical and experimental data matching can be attributed to the solvent environment in the preparation method, also computational study performed calculations in pure ethanol, but not in solid state. Another band appears at 3205 cm−1 for HL, 3169 cm−1 CH3L and 3201 cm−1 NO2L that attributed to C-H aromatic stretching, respectively [36]. There is a strong and sharp band at 1631 cm−1 for HL, 1593 cm−1 for CH3L and 1620 cm-1 for NO2L where these data corresponding to C = N group positions that experimentally appears at the same positions (1624 cm−1 for HL, 1617 cm−1 for CH3L and 1605 cm−1 for NO2L).
Fig. 2.
Calculated IR spectra of heterocyclic compounds at B3LYP/6-31G(d,p) level.
Bands at 1520 cm−1 and 1263 cm−1 are corresponding to C = C and C = S groups of HL, respectively. Bands appearance at 1510 cm−1 and 1253 cm−1 are corresponding to C = C and C = S groups of CH3L, respectively. Bands appearance at 1524 cm−1 and 1263 cm−1 are corresponding to C = C and C = S groups of NO2L, respectively. Backing to the experimental results, it was found two bands 1596 cm−1, 1589 cm−1, 1574 cm-1which were corresponded to C = C group of HL, CH3L and NO2L, respectively. where the results are slightly closer to the experimental result. Experimental IR C = S band appears at 1274 cm−1, 1263 cm−1 and 1282 cm−1 that give similar calculated results. Also, there is a strong and sharp band at 1267 cm−1 which is corresponding to N-H bending mode in the case of CH3L, a small band is appeared at 2925 cm−1 which is corresponding to C-H of alkyl stretching. In case of NO2L, there are two sharp stretching bands at 1669 cm−1 and 1383 cm−1 due to presence of NO2 group where one of its band is interfered with the position of C = N group of aromatic ring.
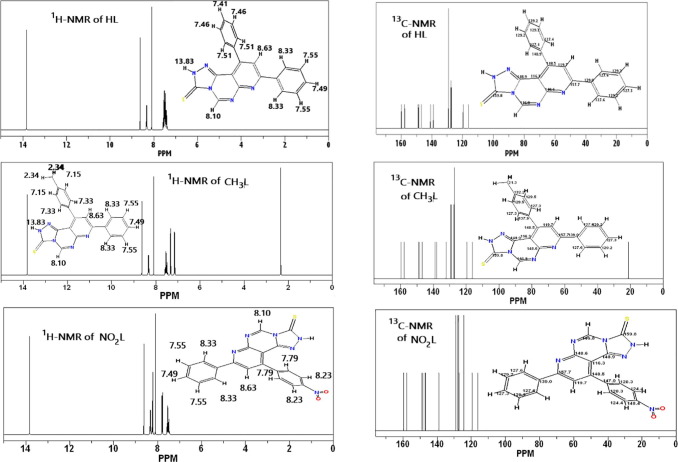
3.3. 1H and 13C NMR spectral investigation
To make a comparison between experimental and theoretical NMR spectral analysis, GIAO method was applied of the B3LYP/ 6-31G (d,p) optimized compounds. Fig. 3 shows 1H and 13C NMR spectral analysis of the three studied heterocyclic compounds. In case of 1H NMR of HL, position of Ar-H peak at δ = 7.41 ppm − 8.33 ppm, the range is similar to the experimental values of δ 7.13–8.17 (m, 10H, Ar-H). but the chemical shift of N-H appears at 13.83 ppm where experimentally differs which is 8.49 ppm (s, 1H, NH, D2O exchangeable); that may be due to the experimental interaction mode of the proton with other surrounded atoms. The peak of H-pyridinic proton appears at 8.10 PPM and experimentally occurs at 8.25 (s, H-5 pyridinic proton). For Ar-H of pyrimidine, it located at 8.63 ppm and that coincides with the experimental result which showed 8.34 (s, 1H, Ar-H of pyrimidine). In case of 13C NMR of HL, C3 of pyridine ring locates at 119.7 ppm and experimentally occurred at 129.3. C4 of pyridine ring locates at 148.5 ppm and experimentally occurred at 149.35 ppm. C2 of pyridine ring present at 157.7 ppm and experimentally present at 155.9 ppm. The peak of C = S appears at 139.8 ppm which differ from the experimental value (182.47 ppm). In case of CH3L, 1H NMR of CH3 group protons present at 2.34 ppm, while experimentally present at 2.83 ppm (s, 3H, CH3). Aryl protons location range from 7.15 ppm to 8.33 ppm, experimentally the range is 6.96 ppm −8.13 ppm (m, 9H, Ar-H). H-5 pyridinic proton shifts 8.63 ppm while it experimentally appears as strong peak at 8.31 ppm. Ar-H of pyrimidine present at 8.10 ppm while experimentally at 8.42 ppm.
Fig. 3.
1H NMR and 13C NMR spectra of HL, CH3L and NO2L using GIAO method on the optimized heterocyclic structures.
In case of NO2L, 1H NMR Ar-H peaks range from 7.49 ppm to 8.33 ppm and experimentally a medium peaks appeared in the range from 7.11 ppm −8.28 ppm. NMR peak of H-5 pyridinic proton locates at 8.63 ppm, experimentally a strong peak is shifted at 8.33 ppm. Ar-H of pyrimidine peak appears at 8.10 ppm, while experimentally was found at higher chemical shift 8.48 ppm. Concluding these results, there is a good matching between experimental and theoretical characterization in the most cases.
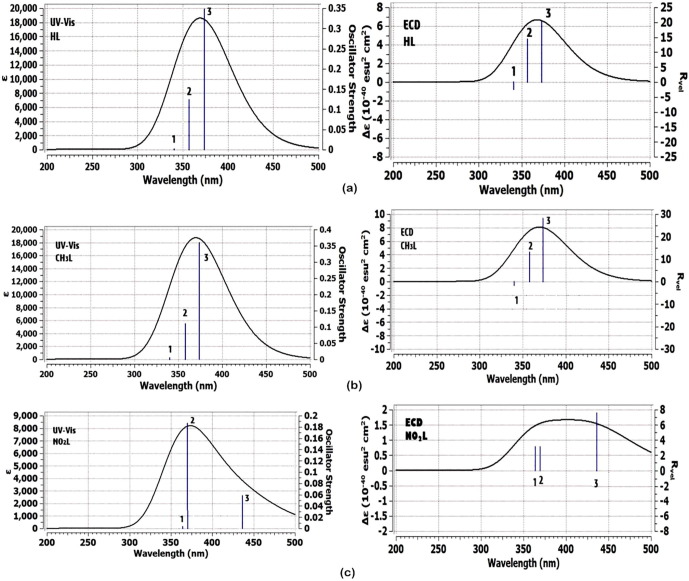
3.4. UV–Vis electronic spectra using TD-DFT method
Describing the electronic behavior of HL, CH3L and NO2L, TD-DFT was applied with CPCM as solvation model. Gaussian 09 default settings were applied for TD-DFT calculations. The program settings were adjusted for Nstate = 6 for analysis of six states. It was shown from Fig. 4 that there are three transition bands present as lines for HL, CH3L and NO2L. From the LOG file of Gaussian calculation, the first transition represents a singlet strong absorption band with orbital contribution of HOMO-LUMO and HOMO-LUMO + 1 for HL and CH3L but only HOMO-LUMO orbital contribution was present for NO2L that corresponding to n-π* transition. LOG outputs estimated the probability of presence this transition as a % with energy of 3.318 eV, 3.317 eV and 2.842 eV at λmax 373.7 nm, 373.8 nm and 436.3 nm for HL, CH3L and NO2L, respectively. Also, the second singlet line represents electronic transition from HOMO-LUMO and HOMO-LUMO + 1 that corresponding to n-π* transition for HL and CH3L.
Fig. 4.
UV–Vis and ECD electronic absorption spectra for (a) HL, (b) CH3L and (c) NO2L heterocyclic ligands.
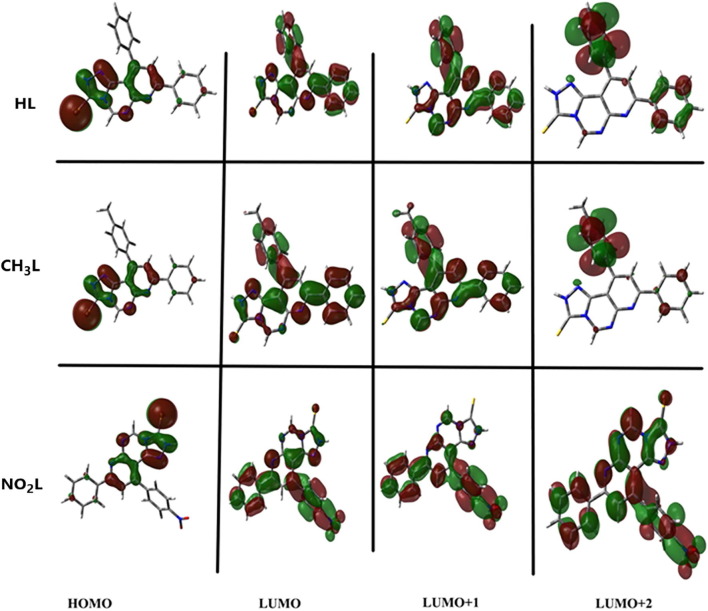
(Fig. 4a,b), while for NO2L, the contribution appeared in HOMO → LUMO + 1 and HOMO → LUMO + 2 with quite smaller energy value (3.354 eV) and higher λmax (369.7 nm). Furthermore, the bands shape of HL and CH3L heterocyclic ligands are quite similar, while in case of NO2L, the band shape and lines position differ as shown in Fig. 4 c. To summarize these calculations, closing the band shape and wavelength values indicate the identical electronic structure of HL and CH3L, but NO2L was attributed as a different electronic behavior system. Fig. 5 it was found that in the third excitation level, the orbital contribution of NO2L electronic structure differ from the other ligands. The orbital contribution character can be estimated from the counter diagrams present for HL, CH3L and NO2L. LUMO + 2 orbital of NO2L compound contributed on nitro group but in the other two heterocyclic compounds, the substituents mostly appear no contribution character. Table 2 shows excitation energies, maximum wavelengths, oscillator strengths and % orbital contribution for HL, CH3L and NO2L compounds.
Fig. 5.
Electronic singlet transition states molecular orbitals of HL, CH3L and NO2L.
Table 2.
Excitation energies, maximum wavelengths, oscillator strengths and % orbital contribution for HL, CH3L and NO2L compounds.
| Compound | Spectral line number | Excitation energy (eV) | λmax (nm) | F | Type of transition | % orbital contribution |
|---|---|---|---|---|---|---|
| HL | 1 | 3.318 | 373.7 | 0.3473 | HOMO → LUMO | 57.54 |
| HOMO → LUMO + 1 | 40.03 | |||||
| 2 | 3.473 | 357.0 | 0.1235 | HOMO → LUMO | 40.00 | |
| HOMO → LUMO + 1 | 57.08 | |||||
| 3 | 3.640 | 340.6 | 0.002 | HOMO-1 → LUMO | 39.49 | |
| HOMO-1 → LUMO + 1 | 55.83 | |||||
| CH3L | 1 | 3.317 | 373.8 | 0.3600 | HOMO → LUMO | 56.59 |
| HOMO → LUMO + 1 | 41.26 | |||||
| 2 | 3.464 | 357.9 | 0.200 | HOMO → LUMO | 41.13 | |
| HOMO → LUMO + 1 | 56.08 | |||||
| 3 | 3.646 | 340.1 | 0.0050 | HOMO-1 → LUMO | 0.3548 | |
| HOMO-1 → LUMO + 1 | 51.10 | |||||
| NO2L | 1 | 2.842 | 436.3 | 0.0580 | HOMO → LUMO | 70.43 |
| 2 | 3.3537 | 369.7 | 0.1870 | HOMO → LUMO + 1 | 36.18 | |
| HOMO → LUMO + 2 | 59.71 | |||||
| 3 | 3.408 | 363.9 | 0.0030 | HOMO-1 → LUMO | 68.71 | |
| HOMO-1 → LUMO + 1 | 13.34 |
Also, Fig. 4 illustrates the different features of ECD plot for each studied ligand. Similar ECD peaks are obtained for HL and CH3L but the peak of NO2L differently occur. In case of HL and CH3L, the first singlet peak, appeared as (-) camphor, while the other two singlet peaks appeared as a (+) camphor. This indicated that the two ligands are identical in UV–Vis spectra and also in conformation and configuration of atoms in the molecular system. In case of the NO2L, all the singlet peaks appeared in a (+) camphor, that confirmed the completely different configuration structure with respect to the other two heterocyclic ligands.
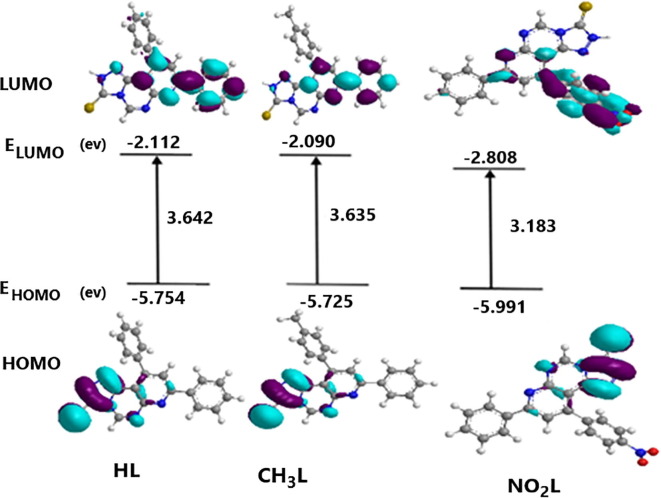
3.5. Molecular orbital energies and reactivity
The energies of molecular orbitals HOMO and LUMO were evaluated for the three tested optimized ligands in gas phase to calculate the energy gap of these ligands. The results can give an indication about the reactivity of molecules associated with chemical reactions [37] . The most of orbital contribution on HOMO for the three ligands include triazole ring with sulfur atom, but the orbital contribution on LUMO for HL and CH3L include the pyrimidine ring with one substituted phenyl group but for NO2L, most of orbital contribution is attributed to nitro-phenyl group. Energy gap of HL (EGAP = 3.642 eV) and of CH3L (EGAP = 3.635 eV) appear to be closer in values, so, their reactivity is the same but energy gap of NO2L (EGAP = 3.183 eV) is slightly with smaller value, so it is reactive towards an electrophile through a nucleophilic part occurred in the molecule. Fig. 6 shows a description of molecular orbitals with transition for the three ligands with energy values of HOMO, LUMO and HOMO/LUMO energy gap. Table 3 shows some important calculated reactivity parameters elucidated from the optimized structures that can explain the electronic behavior of the titled compounds. Such parameters are, total energy of the molecule, ionization potential, electron affinity, chemical potential, chemical hardness, softness and energy fraction. The studied parameters such dipole moment, chemical hardness and energy fraction are with lower values in case of NO2L compared with other ligands. Other parameters such ionization potential, electron affinity, chemical potential and softness are higher values in case of NO2L compared with the other ligands. We can say from this part that NO2L acts as a nucleophile through nitro group slightly stronger than the other ligands.
Fig. 6.
Description of the molecular orbitals with calculated electronic transition for HL, CH3L and NO2L.
Table 3.
Quantum chemical parameters of the studied compounds in gas phase.
| Parameter | HL | CH3L | NO2L |
|---|---|---|---|
| Total energy (ev) | −39237.20 | −40307.16 | −44801.94 |
| Dipole moment (Debye) | 5.962 | 6.327 | 4.565 |
| EHOMO (eV) | −5.754 | −5.725 | −5.991 |
| ELUMO (eV) | −2.112 | −2.090 | −2.808 |
| EGAP (eV) | 3.642 | 3.635 | 3.183 |
| Ionization potential (eV) | 5.754 | 5.725 | 5.991 |
| Electron affinity (eV) | 2.112 | 2.09 | 2.808 |
| Chemical potential (eV) | 3.933 | 3.908 | 4.399 |
| Chemical hardness (eV) | 1.821 | 1.818 | 1.592 |
| Softness (eV) | 0.549 | 0.550 | 0.628 |
| Energy fraction (unitless) | 2.724 | 2.739 | 2.133 |
3.6. Topological surface study
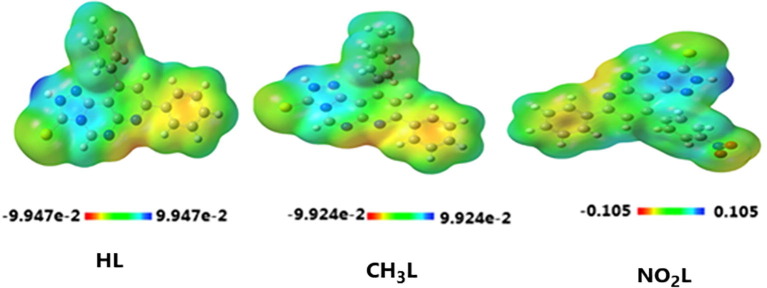
3.6.1. Molecular electrostatic potential (MEP) analysis
Reactivity of the heterocyclic compounds was deduced by analysis of the molecular electrostatic potential (MEP) surface. Detecting the inter and intramolecular interactions in the studied compounds was confirmed by elucidation the electron-rich and electron-poor molecular sited. As shown from Fig. 7 , the electron-rich sites are attributed to one phenyl group carried on pyridine ring and also nitrogen atom of pyridine, this represented as an orange-yellow color on the surface of HL, CH3L and NO2L ligands. Electron-poor sites are attributed to the triazole ring which represented with blue color on the surface of the ligands. For further illustration, the triazole ring is termed as a nucleophilic center and nitrogen of pyridine ring is termed as an electrophilic center. Furthermore, methyl group substituent of CH3L ligand appeared with blue code color, so it is positively charged center and the nitro group substituent of NO2L ligand is colored with yellow code color, that means it is a weekly negatively charged center.
Fig. 7.
MEP surface of the optimized structures of the heterocyclic ligands HL, CH3L and NO2L.
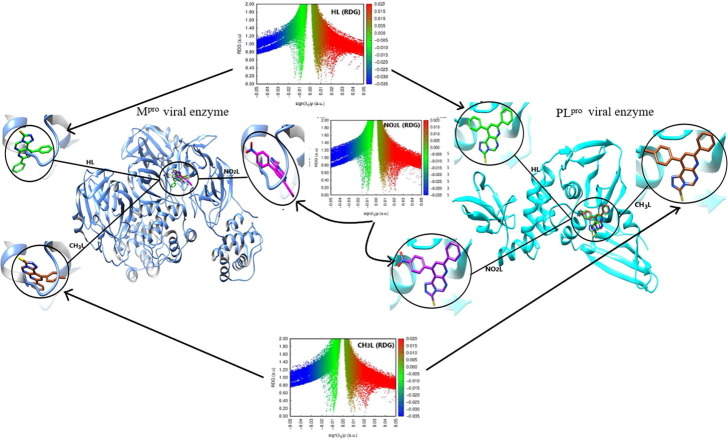
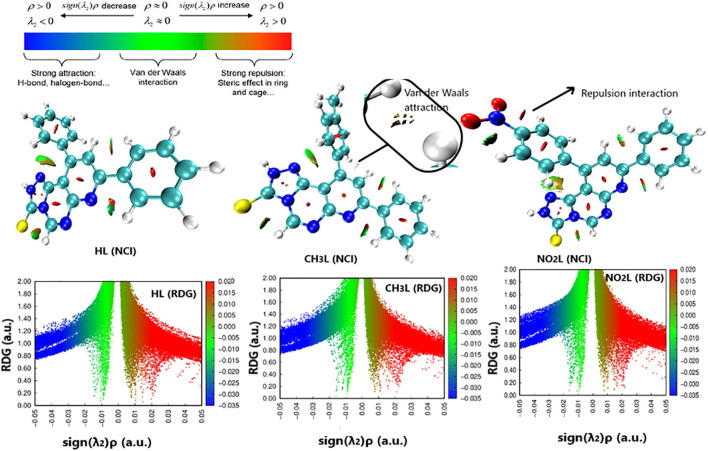
3.6.2. Non covalent interaction analysis (NCI-RDG analysis)
Investigating NCI bonds in the molecular atoms predict, in the future, the behavior of atomic sites for interaction towards other molecular atoms. Reduced density gradient analysis is determined in function of sign sign(λ2)ρ parameter which describes the multiplication of the electron density with the sign of the second Hessian eigenvalue. Diagrams extracted from this analysis are represented in colored codes [38], [39] . As shown from Fig. 8 , HL, CH3L and NO2L ligands were manipulated as plots with a number of weak and strong spikes. To illustrate the type of interactions, it should be understanding the code colors where blue color refers to hydrogen bond formation, green color refers to Van der Waals attraction and red color refers to steric repulsion interaction. In all ligands, there is Van der Waals interaction in between the substituted phenyl ring and triazolo moiety. Also, Van der Waals occur between sulfur atom and pyrimidine ring. Steric repulsion interaction is present inside the aromatic rings for all compounds. There is a slight difference in interaction sites due to exchangeable substituent. In case of CH3L, there is a weak repulsion interaction between the tolyl ring and pyridine ring that may be attributed to the bulkiness of the whole tolyl moiety around the remaining molecular part. In case of NO2L, there is a strong repulsion interaction between nitro group and its phenyl carrier, this feature is not appeared in HL and CH3L ligands, that may be due to the bulkiness of nitro group that take a certain geometry on the structure. From the colored RDG plot of all ligands, spike with negative sign(λ2)ρ sign represents Van der Waals attraction between atoms of each ligand (-0.02 < sign(λ2)ρ < 0). Spike represents highly negative sign(λ2)ρ value (-0.02 > sign(λ2)ρ) attributed to hydrogen bond formation and this feature not present in the studied ligand structure due to the small electron density on the surface leading to inability of hydrogen bond formation. The spike with positive value (sign(λ2) ≥ 0.01) represents strong steric repulsion between atoms of the molecule.
Fig. 8.
NCI isosurfaces and RDG scatter mapping diagram of the optimized structures of HL, CH3L and NO2L heterocyclic compounds.
3.7. Investigation of a potent antiviral activity
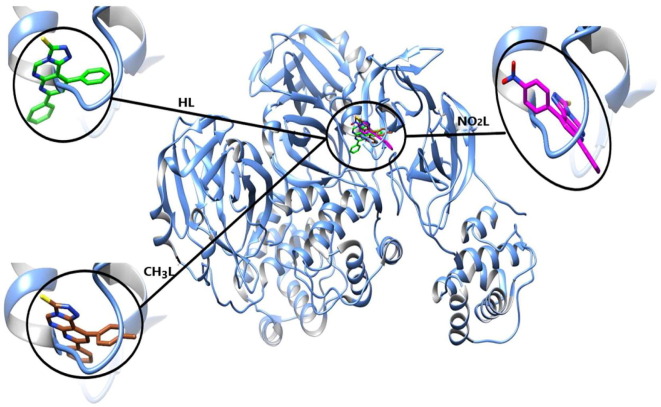
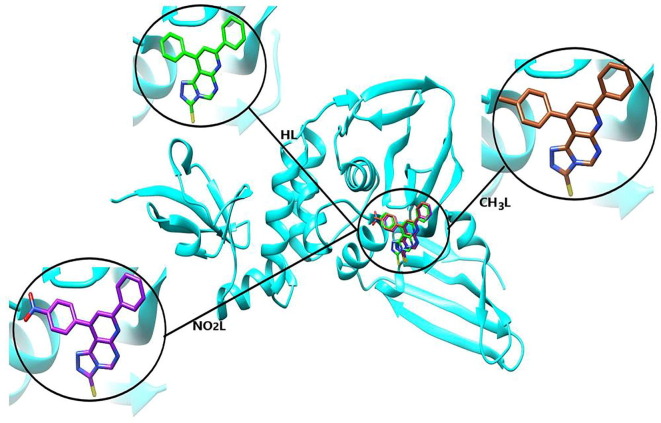
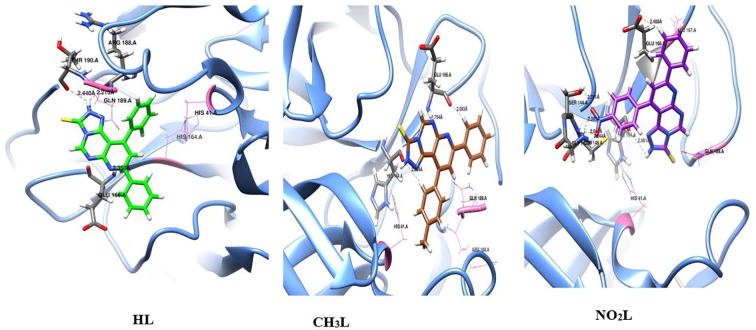
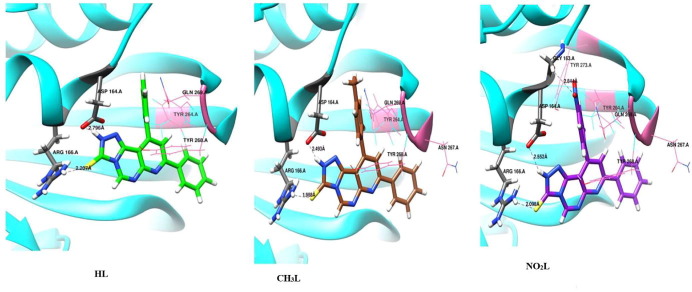
The optimized pyrimidine triazolo compound derivatives were docked in the active sites of Mpro enzyme applying 6WTT target and PLpro enzyme using 7JRN target, as a docking receptor. Fig. 9 shows the strength of interactions of the three tested ligands with different amino acids that surround the docked ligands in Mpro enzyme. Fig. 11 shows the strength of interactions of the three tested ligands with different amino acids that surround the docked ligands in PLpro enzyme.
Fig. 9.
Molecular docking of the studied heterocyclic ligands, HL, CH3L and NO2L with Mpro enzyme (PDB :6WTT).
Fig. 11.
Molecular docking of studied ligands, HL, CH3L and NO2L with PLpro enzyme (PDB :7JRN).
3.7.1. Molecular docking study of triazole derivatives with Mpro of SARS-CoV-2
In-silico docking of three ligands with 6WTT investigate that there are two types of interactions, H-bond and VDW, where HL interact with 6 amino acids, while CH3L interact with 5 amino acids and NO2L interact with 8 amino acids. This investigation reflects that the docked ligand compounds have binding poses ensures a favorable binding modes with Mpro target of PDB 6WTT [39], [40] . Fig. 10 shows the types of amino acids interacted with the tested ligands. The results of optimized HL docking reveals that there are 6 interacting amino acids in chain A of Mpro represented in GLU-166.A, ARG-188.A, THR-190.A, HIS-41, HIS-164 and GLN-189 with docking energy −113.5 kcal/mol. When the optimized CH3L was docked into the target, 5 amino acids were found that interacted with the ligand which are represented in HIS-164.A, GLU-166.A, HIS-41.A , ARG-188.A and GLN-189.A with total docking energy −105.2 kcal/mol. When the optimized NO2L was docked in the protein target, 8 amino acids are interacted with the ligand which are represented in GLY-143.A, SER-144.A, CYS-145.A, HIS-164.A and GLU-166.A, HIS-41.A, LEU-167.A and GLN-189.A with total docked energy −128.6 kcal/mol. Table 4 shows the names of interacted protein residues, bond length and bond energy of the active sites for each ligand. It was found that the NO2L inhibit 4 amino acid active sites with H-bond formation and block other 4 amino acids by VDW interactions but the other studied ligands block from 2 to 3 amino acids by H-bond formation and 3 other active sites by VDW interactions. The higher inhibition power of NO2L is mainly due to presence of nitro group which strongly interacted with GLY-143.A (-3.500 kcal/mol), SER-144.A (-7.995 kcal/mol) and CYS-145.A (-9.500 kcal/mol and −6.210 kcal/mol) with 4H-bond. The three ligand compounds are common interacted with amino acids HIS-41.A and GLN-189.A by VDW interactions. Fig. 10 also shows H-bond and VDW interactions between the surrounded amino acids of Mpro receptor and the tested ligands where the stick grey amino acids represent that interacted with H-bond formation and wire pink amino acids interact with ligands through VDW forces. We can investigate from this virtual screening that the nitro group substituent (polar group) of NO2L is directed to be embedded inside the protein receptor, but the other 2 ligand substituents (non-polar H and CH3 groups) directed outside the protein receptor. This may be an evidence on the polar groups mostly like to be strongly docked within the protein receptor through formation of strong H-bond.
Fig. 10.
H-bond and VDW interactions between the surrounded amino acids of Mpro receptor (PDB :6WTT) and the heterocyclic ligand compounds.
Table 4.
Binding affinity between the optimized ligands and Mpro enzyme of SARS CoV-2 with 7JRN.
| Compound Name | Binding affinity (fitness) |
Protein residue name | Compound residue name | H-Bond length (Å) | Bond energy (kcal/mol) | Protein residue name | VDW Energy (kcal/mol) |
|---|---|---|---|---|---|---|---|
| HL | −113.5 | GLU-166.A | N | 2.350 | −5.242 | HIS-41.A | −6.281 |
| ARG-188.A | O | 2.215 | −7.000 | HIS-164.A | −4.972 | ||
| THR-190.A | O | 2.440 | −2.851 | GLN-189.A | −10.762 | ||
| CH3L | −105.2 | HIS-164.A | HC | 2.839 | −4.255 | HIS-41.A | −6.692 |
| GLU-166.A | HC | 2.093 | −6.692 | ARG-188.A | −12.187 | ||
| N | 1.754 | GLN-189.A | −7.916 | ||||
| NO2L | −128.6 | GLY-143.A | O | 2.244 | −3.500 | HIS-41.A | −4.754 |
| SER-144.A | O | 2.281 | −7.995 | LEU-167.A | −4.271 | ||
| CYS-145.A |
O | 2.046 | −9.500 | GLN-189.A | −8.121 | ||
| O | 2.005 | −6.210 | |||||
| HIS-164.A | HN | 2.981 | −6.474 | ||||
| GLU-166.A | HC | 2.488 | −3.343 |
3.7.2. Molecular docking study of triazole derivatives with PLpro of SARS-CoV-2
In-silico docking of three ligands with 7JRN investigate that there are two types of interactions, H-bond and VDW, where HL interact with 5 amino acids, while CH3L interacts with 6 amino acids and NO2L interact with 8 amino acids. This investigation reflects that the docked ligand compounds have binding poses ensures a favorable binding mode with PLpro target of PDB 7JRN. Fig. 11 shows the types of amino acids interacted with the tested ligands. The results of optimized HL docking reveals that there are 5 interacting amino acids in chain A of PLpro represented in ASP-164.A, ARG-166.A, TYR-264.A, TYR-268.A and GLN-269.A with docking energy −100.3 kcal/mol. When the optimized CH3L was docked into the target, 6 amino acids can interact with the ligand which are represented in ASP-164.A, ARG-166.A, TYR-264.A, TYR-268.A, ASN-267.A and GLN-269.A with total docking energy −102.8 kcal/mol. When the optimized NO2L was docked in the protein target, 8 amino acids can interact with the ligand which are represented in GLY-163.A, ASP-164.A, ARG-166.A, TYR-264.A ASN-267.A, TYR-268.A, GLN-269.A and TYR-273.A with total docked energy −107.6 kcal/mol. Table 5 shows the names of interacted protein residues, bond length and bond energy of the active sites for each ligand. We can investigate that NO2L inhibit 3 amino acid active sites with H-bond formation and block other 6 amino acids by VDW interactions but the other studied ligands block from 2 amino acids by H-bond formation and 3 other active sites by VDW interactions. The higher inhibition power of NO2L is mainly due to presence of nitro group which strongly interacted with GLY-163.A (-3.467 kcal/mol), ASP-164.A −7.718 kcal/mol) and ARG-166.A (-6.533 kcal/mol) with 3H-bond. There are 3 common amino acids interacted with the three ligands (TYR-264.A, TYR-268.A and GLN-269.A) with a strong VDW interaction. Fig. 12 shows H-bond and VDW interactions between the surrounded amino acids of PLpro receptor and the tested ligands where the stick grey amino acids represent that interacted with H-bond formation and wire pink amino acids interact with ligands through VDW forces. We can investigate from this virtual screening that the three ligand substituents are directed in the same direction inside the protein receptor with different mode of interaction. Comparing the total binding energy results of the two proteases enzyme docking, we conclude that Mpro is more effective towards inhibition by triazolo pyrimidine derivatives than PLpro protease. While triazolo compound derivatives give stronger VDW amino acid interactions with receptor in case of PLpro relative to Mpro.
Table 5.
Binding affinity between the optimized ligands and PLpro enzyme of SARS CoV-2 with 7JRN.
| Compound Name | Binding affinity (fitness) |
Protein residue name | Compound residue name | H-Bond length (Å) | Bond energy (kcal/mol) | Protein residue name | VDW Energy (kcal/mol) |
|---|---|---|---|---|---|---|---|
| HL |
−100.3 |
ASP-164.A | HN | 2.796 | −7.336 | TYR-264.A | −13.628 |
| ARG-166.A | S | 2.207 | −4.194 | TYR-268.A | −18.029 | ||
| GLN-269.A | −6.064 | ||||||
| CH3L | −102.8 | ASP-164.A | HN | 2.493 | −6.15 | TYR-264.A | −14.249 |
| ARG-166.A | S | 1.988 | −8.18 | ASN-267.A | −4.6208 | ||
| TYR-268.A | −18.118 | ||||||
| GLN-269.A | −7.142 | ||||||
| NO2L |
−107.6 |
GLY-163.A | O | 2.844 | −3.467 | TYR-264.A | −14.578 |
| ASP-164.A | HN | 2.552 | −7.718 | ASN-267.A | −4.069 | ||
| ARG-166.A | S | 2.098 | −6.533 | TYR-268.A | −16.955 | ||
| GLN-269.A | −7.008 | ||||||
| TYR-273.A | −4.521 |
Fig. 12.
H-bond interactions between the surrounded amino acids of PLpro receptor (PDB :7JRN) and the heterocyclic ligand compounds.
4. Conclusion
A previously synthesized heterocyclic compounds 8,10-diphenylpyrido[3], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [1], [2], [4]triazolo[4], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]pyrimidine-3(2H)-thione derivatives were studied applying DFT and theoretical calculations were performed in this study. In most cases, spectroscopic analyses showed slightly a good matching between theoretical and experimental data reported previously. Electronic properties and reactivity were discussed and it was found that NO2L acts as stronger nucleophile than the other ligands through its nitro group. The studied compounds theoretically proved to be active towards chemical reactions through the small energy gap. NCI-RDG analysis revealed presence of Van der Waals interaction and also strong repulsion interaction inside the aromatic rings. Prediction the biological activity of these compounds was illustrated by performing molecular docking of the optimized molecules with the target of protease enzymes in SARS-CoV-2 viral protein. The docking summarized in studying the interaction with two types of protease enzymes (Mpro with PDB 6WTT and PLpro with PDB 7JRN) and the docking results evaluate the strong inhibition effect of NO2L which contains nitro group that increases the possibility to form a strong H-bonds with the amino acid of viral protein.
Funding
No funding for this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.S.K. El saedany, M. A. Zein, E.M. Abdelrehim, R.M. Keshk, Synthesis, Anti‐Microbial, and Cytotoxic Activities Evaluation of Some New Pyrido[2,3‐d]Pyrimidines, J.Hetrocyclic Chem. 53 (2016) 1534. https://doi.org/10.1002/jhet.2460.
- 2.Abdelrehim E.M., Zein M.A. Synthesis of Some Novel Pyrido[2,3-d]pyrimidine and Pyrido[3,2-e][1,3,4]triazolo and Tetrazolo[1,5-c]pyrimidine Derivatives as Potential Antimicrobial and Anticancer Agents. J. Hetrocyclic Chem. 2018;55:419. doi: 10.1002/jhet.3058. [DOI] [Google Scholar]
- 3.Kurumurthy C., Sambasiva P., Veeraswamy B., Santhoshkumar G., Shanthan P., Narsaiah B., Velatooru L.R., Pamanji R., Venkateswara R. Synthesis of novel alkyltriazole tagged pyrido[2,3-d]pyrimidine derivatives and their anticancer activity. Eur. J. Med. Chem. 2011;46:3462–3468. doi: 10.1016/j.ejmech.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Boehr D.D., Farley A.R., Wright G.D., Cox J.R. Analysis of the π-π Stacking Interactions between the Aminoglycoside Antibiotic Kinase APH(3′)-IIIa and Its Nucleotide Ligands. Chem. Biol. 2002;9:1209. doi: 10.1016/S1074-5521(02)00245-4. [DOI] [PubMed] [Google Scholar]
- 5.Pyrkov T.V., Pyrkova D.V., Balitskaya E.D., Efremov R.G. The role of stacking interactions in complexes of proteins with adenine and guanine fragments of ligands. Acta Nat. 2009;1:124. [PMC free article] [PubMed] [Google Scholar]
- 6.Panigrahi S.K., Desiraju G.R. Strong and weak hydrogen bonds in the protein–ligand interface. Proteins. 2007;67:128. doi: 10.1002/prot.21253. [DOI] [PubMed] [Google Scholar]
- 7.M. A. Williams, J. E. Ladbury, Hydrogen Bonds in Protein– Ligand Complexes (Eds: H.-J.Bohm, G. Schneider), FRG: WileyVCH Verlag GmbH & Co. KGaA, Weinheim (2003) 137-161
- 8.Efremov R.G., Chugunov A.O., Pyrkov T.V., Priestle J.P., Arseniev A.S., Jacoby E. Molecular docking: The role of noncovalent interactions in the formation of protein-nucleotide and protein-peptide complexes. Curr. Med. Chem. 2007;14:393. doi: 10.2174/092986707779941050. [DOI] [PubMed] [Google Scholar]
- 9.Gallina A.M., Bork P., Bordo D. Hydrocarbon binding by proteins: structures of protein binding sites for ≥C10 linear alkanes or long-chain alkyl and alkenyl groups. J. Mol. Recognit. 2014;27:65. doi: 10.1021/jo502488e. [DOI] [PubMed] [Google Scholar]
- 10.Scrutton N.S., Raine A.R. Biochem. Cation-pi bonding and amino-aromatic interactions in the biomolecular recognition of substituted ammonium ligands, J. 1996;1:319. doi: 10.1042/bj3190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu R., McMahon T.B. Investigation of Cation-π Interactions in Biological Systems. J. Am. Chem. Soc. 2008;130:12554. doi: 10.1021/ja802117s. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y., Wang Y., Zhu W. Nonbonding interactions of organic halogens in biological systems: implications for drug discovery and biomolecular design. Phys. Chem. 2010;12:4543. doi: 10.1039/B926326H. [DOI] [PubMed] [Google Scholar]
- 13.Zhou P., Tian F., Zou J., Shang Z. Rediscovery of halogen bonds in protein-ligand complexes. Mini Rev. Med. Chem. 2010;10:309. doi: 10.2174/138955710791331016. [DOI] [PubMed] [Google Scholar]
- 14.Kukic P., Nielsen J.E. Electrostatics in proteins and protein-ligand complexes. Future Med. Chem. 2010;2:647. doi: 10.4155/fmc.10.6. [DOI] [PubMed] [Google Scholar]
- 15.M. T. Neves-Petersen, S. B. Petersen, Biotechnol. Protein electrostatics: A review of the equations and methods used to model electrostatic equations in biomolecules - Applications in biotechnology, Annu. Rev. 9 (2003) 315. DOI: 10.1016/S1387-2656(03)09010-0. [DOI] [PubMed]
- 16.Zhou P., Huang J., Tian F. Specific noncovalent interactions at protein-ligand interface: implications for rational drug design. Curr. Med. Chem. 2012;19:226. doi: 10.2174/092986712803414150. [DOI] [PubMed] [Google Scholar]
- 17.K. Chen, L. Kurgan, Investigation of Atomic Level Patterns in Protein—Small Ligand InteractionsPLoS ONE 4 (2009) 4473. https://doi.org/10.1371/journal.pone.0004473 [DOI] [PMC free article] [PubMed]
- 18.M. Egli, R.V. Gessner, Proc. Natl. Acad. Stereoelectronic effects of deoxyribose O4' on DNA conformation, Sci. U.S.A. 92 (1995) 180–184. DOI: 10.1073/pnas.92.1.180. [DOI] [PMC free article] [PubMed]
- 19.Eynde J.J.V., Fromont D. Bull. Soc. Chim. Belg. 1997;106:393–397. [Google Scholar]
- 20.Sitkowski J., Stefaniak L., Dziembowska T., Grech E., Jagodzinska E., Webb G.A. J. Molecular Structure. 1996;381:177–180. [Google Scholar]
- 21.Tanaka K., Nomura K., Oda H., Yoshida S., Mitsuhashi K. J. Heterocyclic Chem. 1991:907–911. [Google Scholar]
- 22.Kang L., Zhao L., Yao S., Duan C. A new architecture of super-hydrophilic β-SiAlON/graphene oxide ceramic membrane for enhanced anti-fouling and separation of water/oil emulsion. Ceram. Int. 2019;45:16717. [Google Scholar]
- 23.Yang Y., Kang L., Li H. Enhancement of photocatalytic hydrogen production of BiFeO3 by Gd3+ doping. Ceram. Int. 2019;45(6):8017–8022. [Google Scholar]
- 24.Kaya Serpil, Erkan Sultan, Karakaş Duran. Computational investigation of molecular structures, spectroscopic properties and antitumor-antibacterial activities of some Schiff bases, Spectrochim. Acta Part A: Molecular and Biomolecular Spectroscopy. 2021;244 doi: 10.1016/j.saa.2020.118829. [DOI] [PubMed] [Google Scholar]
- 25.Abdelrehim E.M., El-Sayed D.S. A new synthesis of poly heterocyclic compounds containing [1, 2, 4] triazolo and [1, 2, 3, 4] tetrazolo moieties and their DFT study as expected anti-cancer reagents. Curr. Org. Synth. 2020;17:211–223. doi: 10.2174/1570179417666200226092516. [DOI] [PubMed] [Google Scholar]
- 26.Stephens P.J., Harada N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality. 2009;22:229. doi: 10.1002/chir.20733. [DOI] [PubMed] [Google Scholar]
- 27.M. J. Frisch, et al; Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, (2016).
- 28.Becke A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993;98:5648. doi: 10.1063/1.464304. [DOI] [Google Scholar]
- 29.M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, J. Montgomery, T. Vreven, K. Kudin, J. Burant, Inc (2003).
- 30.Güveli Ş., Özdemir N., Bal-Demirci T., Ülküseven B., Dinçer M., Andaç Ö. Quantum chemical, spectroscopic and X-ray diffraction studies on nickel complex of 2-hydroxyacetophenone thiosemicarbazone with triphenylphospine. Polyhedron. 2010;29(12):2393–2403. doi: 10.1016/j.poly.2010.05.004. [DOI] [Google Scholar]
- 31.R. Dennington, T.A. Keith, J.M. Millam, GaussView, Version 6.1, Semichem Inc., Shawnee Mission, KS, 2016.
- 32.G. Zhurko, D. Zhurko, ChemCraft 1.8 http://www.chemcraftprog.com 2005.
- 33.Guillaumont D., Nakamura S. Calculation of the absorption wavelength of dyes using time-dependent density-functional theory (TD-DFT) Dyes Pigments. 2000;46(2):85–92. doi: 10.1016/S0143-7208(00)00030-9. [DOI] [Google Scholar]
- 34.Y.F. Chen, Y.J. Chen, J.M. Yang, GEMDOCK: An Integrated Environment for Computer-aided Drug Design and Its Applications, (2007).
- 35.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004;25:1605. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 36.C.P. Kaushik, Krishan Kumar, S.K. Singh, Dharmendra Singh, Sangita Saini, Synthesis and antimicrobial evaluation of 1,4-disubstituted 1,2,3-triazoles with aromatic ester functionality, Arabian Journal of Chemistry,Volume 9 (6) (2016) 865-871 1878-5352, https://doi.org/10.1016/j.arabjc.2013.09.023.
- 37.Geerlings P., De Proft F. Chemical Reactivity as Described by Quantum Chemical Methods. Int. J. Mol. Sci. 2002;3(4):276–309. doi: 10.3390/i3040276. [DOI] [Google Scholar]
- 38.Venkataramanan N.S., Suvitha A., Kawazoe Y. J. Mol. Liq. 2018;260:18. doi: 10.1016/j.molliq.2018.03.071. [DOI] [Google Scholar]
- 39.Krishna G., Pillai V.S., Veettil M.V. Approaches and advances in the development of potential therapeutic targets and antiviral agents for the management of SARS-CoV-2 infection. Eur. J. Pharmacol. 2020;885 doi: 10.1016/j.ejphar.2020.173450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omolo C.A., Soni N., Fasiku V.O., Mackraj I., Govender T. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur. J. Pharmacol. 2020;883 doi: 10.1016/j.ejphar.2020.173348. [DOI] [PMC free article] [PubMed] [Google Scholar]