Abstract
Background
Polypharmacy, the concomitant use of 5 or more medications, is highly prevalent among older adults and individuals with multimorbid conditions and has been linked to suboptimal clinical outcomes in various diseases. However, little is known about the impact of polypharmacy on clinical outcomes among coronavirus disease 2019 (COVID-19) patients.
Objective
This systematic review summarizes the available literature on the association between polypharmacy and specific drug classes, and clinical outcomes among COVID-19 patients.
Methods
We conducted an electronic database search on Embase, Medline, Cochrane, Scopus, Google Scholar, clinicaltrials.gov, LITCOVID, PubMed, PubMed Central (PMC), and China national knowledge infrastructure for studies on Polypharmacy among COVID-19 patients using relevant combinations of the keywords. Only studies published between November 2019 to September 2020 were included. Seven articles out of 1502 unique articles met the inclusion criteria and were used for the current study. We adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline in conducting and reporting this systematic review.
Results
The total sample size of all studies was 474,342, out of which 10,519 patients were COVID-19 positive, and 4818 COVID-19 positive patients experienced polypharmacy. Five out of the 7 included studies found associations between polypharmacy and negative clinical outcomes among COVID-19 patients. Polypharmacy was associated with increase in the relative risk of a positive COVID-19 test result (P < 0.01), death among male COVID-19 patients (P < 0.001), increase in the rate of acute kidney injury (P = 0.003), and adverse drug reactions (P < 0.001). Antipsychotic drugs were associated with severe COVID-19 morbidity (OR = 2.79; 95% CI 2.23–3.49) and increased risk of death among COVID-19 infected men (OR = 1.71; 95% CI 1.18–2.48) and women (OR = 1.96; 95% CI 1.41–2.73).
Conclusion
Polypharmacy and selected drug classes are associated with increased risk of adverse clinical outcomes among COVID-19 patients. Understanding these relationships can enhance risk stratification and evidence-based decision-making that may improve care and clinical outcomes of COVID-19 patients.
Key Points.
Background
-
•
Little is known about the impact of polypharmacy on coronavirus disease 2019 (COVID-19) clinical outcomes.
-
•
We summarized the available literature on the association between polypharmacy and specific drug classes, and clinical outcomes among COVID-19 patients.
Findings
-
•
Polypharmacy is associated with increased risk of adverse clinical outcomes among COVID-19 patients.
-
•
Antipsychotics, non-tricyclic antidepressants, opioid analgesics and drugs for peptic ulcer and gastroesophageal reflux disease were among drug classes associated with adverse clinical outcomes among COVID-19 patients.
Since coronavirus disease 2019 (COVID-19) was first discovered in Wuhan, China, in November 2019, it has infected nearly 132 million persons and caused over 2.9 million deaths.1 Older adults and individuals with pre-existing multimorbidities, the presence of 2 or more chronic diseases, remain by far the most vulnerable to severe COVID-19 infection, which can result in hospitalization, admission to the intensive care units, or death.2 , 3 For instance, of the 1.7 billion people with underlying conditions who are estimated to be at an elevated risk of severe COVID-19 infection globally, 66% were adults aged 70 years and above.4 In addition to being vulnerable to severe COVID-19 infection, older adults and individuals with pre-existing multimorbidities are also predisposed to or experiencing polypharmacy. Although some have argued that polypharmacy should be determined by the context of the clinical appropriateness, polypharmacy is most commonly defined as the concomitant use of 5 or more medications.5 The true global magnitude of polypharmacy is hard to estimate. Evidence shows that it is highly prevalent globally and will keep increasing as the population ages.6 The high prevalence of polypharmacy in long-term-care (LTC) facilities was noted in a systematic review that reported a polypharmacy prevalence of up to 91.2%.7 In addition, vulnerable groups, especially older persons, are disproportionately affected by severe COVID-19 infection, as evidenced in the ravaging effect of the disease in LTC facilities and among community-dwelling of older adults worldwide.2 , 3
The dual threat of COVID-19 infection and polypharmacy to the same vulnerable group—older adults and individuals with pre-existing multimorbidities—is particularly problematic because polypharmacy has been shown to lead to suboptimal treatment outcomes in various diseases.8 During the influenza epidemic of 1996-1997, polypharmacy was found to be an independent prognostic risk factor for influenza-associated hospitalization and death.9 In addition, evidence suggests that among patients undergoing polypharmacy, certain drug classes can explain the variability in the occurrence of adverse outcomes.10 For instance, whereas cardiovascular polypharmacy was not associated with unplanned noncardiovascular hospitalization,11 antipsychotics were associated with severe clinical outcomes among older patients with pneumonia.12 The overwhelming evidence on the high prevalence of polypharmacy among groups vulnerable to severe COVID-19 infection and its independent negative impact on other respiratory diseases such as influenza9 necessitates an investigation into the role of polypharmacy in COVID-19 clinical outcomes. Currently, little is known in this area because of the novel nature of the disease and hence a paucity of research on polypharmacy among COVID-19 patients.
Understanding the impact of polypharmacy on COVID-19 clinical outcomes can help to inform better pharmacotherapeutic management for the most vulnerable to severe COVID-19 infection and lead to improvement in their health outcomes. This systematic review explores the state of science on polypharmacy among patients with COVID-19. We aimed to assess the association between polypharmacy and specific drug classes on clinical outcomes among patients with COVID-19.
Methods
Search strategy and data sources
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses was followed in conducting and reporting this systematic review.13 Medical subject headings and different combinations of key words relevant to COVID-19 and polypharmacy were used to carry out an electronic search on Embase, Medline, Cochrane, Scopus, Google Scholar, clinicaltrials.gov, LITCOVID, PubMed, PubMed Central, and China national knowledge infrastructure from November 2019 to September 2020 (Appendix 1). In addition, the references of the studies that met the inclusion criteria were searched to identify additional articles for inclusion.
Study selection criteria and process
Only primary research articles involving individuals of any age who tested positive for COVID-19 and had data on polypharmacy (5 or more medications dispensed concurrently) and published between November 2019 and September 2020 were included in this systematic review. No language restriction was applied in the selection of articles. However, all the studies that met eligibility criteria for inclusion were originally published in English language. Studies with insufficient information on the number of medications or incomplete data were excluded. A protocol was developed in collaboration with all authors and registered prospectively on PROSPERO systematic review database (CRD42020205380).
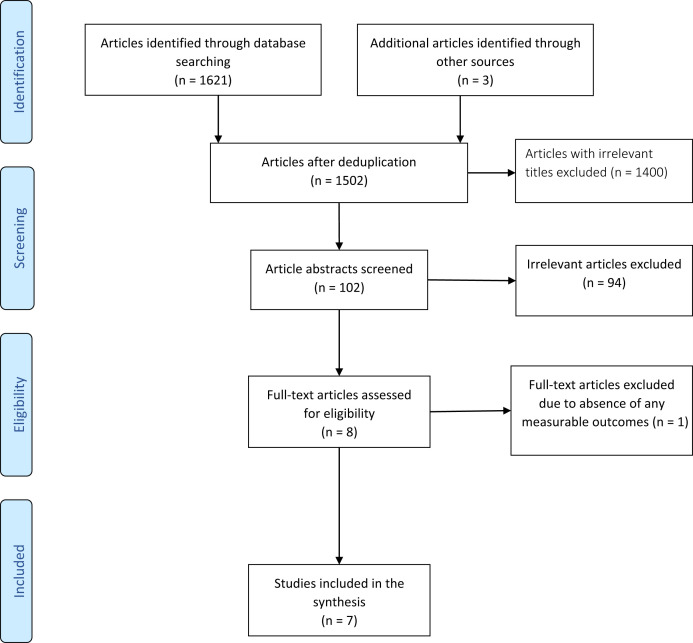
The study selection process and management were conducted using the Rayyan QCRI (Rayyan Systems).14 All identified abstracts and articles were independently screened by 2 of the authors (S.I. and O.M.) for eligibility on the basis of the Rayyan QCRI categorization as “include” (eligible), “exclude” (irrelevant), and “maybe” (unsure). The articles classified as “maybe” were resolved by consensus after a detailed review of the articles by all authors (S.I., O.M., E.J.E.). The initial comprehensive search of the aforementioned databases yielded a total of 1502 unduplicated potentially relevant abstracts and articles. During the preliminary screening of the abstracts, 1400 items were excluded on the basis of the exclusion criteria and relevance. From the remaining 102 items, 94 more items were excluded after reviewing the full articles. After this, 8 articles were found eligible for further evaluation. However, one article was further excluded because of the absence of any measurable clinical outcome. Therefore, only 7 articles met the inclusion criteria and were used for the current study. Figure 1 displays the Preferred Reporting Items for Systematic Review and Meta-Analysis–based flowchart of the studies selection process.
Figure 1.
PRISMA-based flow chart of study selection. Abbreviation used: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study data management and extraction
A predefined data extraction form developed and approved by all authors was used to extract the relevant data from all the articles included. The data items extracted included the publication information, study sample size, number of COVID-19 patients, definition of polypharmacy, number of COVID-19 patients undergoing polypharmacy (taking 5 or more medications), number of medications categorized, class of medications, the associated clinical outcomes (death, adverse drug reactions [ADRs], COVID-19 morbidity, etc.). After the data extraction, all authors reviewed the content of the data extraction form to check for accuracy and determine the suitability of the categorization.
Assessment of the risk of bias
To assess the methodological quality of the studies included, the risk of bias of each article was assessed independently by 2 reviewers (S.I. and O.M.) using the Joanna Briggs Institute (JBI) critical appraisal tool specific for case-control studies, cohort studies, and studies containing prevalence data.15 Generally, studies were evaluated on the basis of clarity of eligibility criteria, sample size, validity of outcome measurement, statistical analysis, and measures to reduce confounding and bias. There were 10 items in the checklist for case-control studies, 12 items in the checklist for cohort studies, and 9 items in the checklist for prevalence studies. Each reviewer independently rated studies by selecting the appropriate response for each item (“Yes,” “No,” “Unclear,” or “Not applicable”). These responses were adapted into numeric scores. All items checked as “Yes” in the checklist were assigned a score of 1, whereas items in the checklist marked as “No,” “Unclear,” or “Not Applicable” were assigned a score of 0 (Appendix 2). The total score for each study was then summed up and calculated as a proportion (%) of the total items to indicate the quality of the study (Table 1 ).
Table 1.
Characteristics of studies included and main clinical findings
| Article author(s), date | Study design | Study country | Study period | Total sample size [N = 474,342] | No. COVID-19 positive patients [n = 10,519] | No. Patients with COVID-19 with polypharmacy [n = 4818] | Mean age (y) | Age range (y) | Drug classification method | Clinical outcome(s) | Methodological quality assessmenta |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Taher et al.,17 July, 2020 |
Retrospective cohort study | Bahrain | April 1–May 31, 2020 | 73 (hospitalized patients with COVID-19) | 73 | 43 | 54 ± 13.5 | N/A | Polypharmacy (increasing No. of medications) for COVID-19 pneumonia was associated with having AKI (n = 24, [82.8%], P < 0.003). Polypharmacy was associated with severe AKI (stage 3) (90%, P < 0.031) |
6/12 (50%) | |
| Poblador-Plou et al.,16 July, 2020 |
Retrospective cohort study | Spain | (Follow-up period = at least 30 d from cohort entry) 4 March 2020 (i.e., date of the first confirmed infection in the region) to 17 April 2020 (enrolment period). The researchers followed patients from the date of inclusion in the cohort to 17 May 2020, or to the date of death | 4412 (All COVID-19 positive patients in the Spanish Region of Aragon) | 4412 | 402 | 67.7 (± 20.7) | Anatomical-Therapeutic-Chemical (ATC) classification system at the third level was used to classify drugs |
|
8/12 (66.7%) | |
| McKeigue et al.,21 July 23, 2020 | Matched case control study | Scotland | Dispensed prescriptions issued in primary care during the last 240 d | 41,220 (all individuals who were tested for COVID-19 in Scotland. 36,948 controls, 4272 cases) | 4272 | 3452 | N/A | 0–75 y ≥ 75y |
Examined noncardiovascular drugs separately from cardiovascular drugs. The Laporte and Healy review was used to classify drug classes postulated to increase the risk of severe COVID-19. The BNF chapter 2 (cardiovascular) and chapter 13 (NSAIDs) was also used to operationalize drug classes |
Severe COVID-19 is strongly associated with polypharmacy, with increased rate ratio as the number of noncardiovascular drugs increased among those not residing in care homes (P < 0.001). No. meds: rate ratio (95% CI) 4–6: 2.97 (2.47–3.57) 7–9: 4.38 (3.61–5.32) 10–12: 6.5 (5.3–8.0) >12: 10.8 (8.7–13.2) |
8/10 (80%) |
| Gavin et al.,19 July 2020 | Retrospective chart review | United States | March 1–31, 2020 | 140 (hospitalized patients with COVID-19) | 140 | 140 | 60 y | 42–81 y | N/A | There was no statistically significant difference in the mean No. of medications between the different groups of patients with COVID-19 (those who did not receive MV, those who received MV and survived, and those who received MV and died) | 9/9 (100%) |
| De Smet et al.,22 June 2020 | A retrospective, single-center observational study | Belgium | March 12 and April 30, 2020 | 81 (hospitalized patients with COVID-19) | 81 | 52 | 65–97 y | N/A | There was no statistically significant difference in polypharmacy between COVID-19 survivors and nonsurvivors (P = 0.52) | 8/9 (88.9%) | |
| Sun et al.,20 April 2020 | A retrospective study | China | January 17 to February 29, 2020 | 217 (hospitalized patients with COVID-19) | 217 | 217 | 45.7 ± 16.6 y | The WHO-UMC system was used to assess causality for all suspected ADRs | Polypharmacy was associated with having adverse drug reactions (P < 0.001) | 9/9 (100%) | |
| McQueenie et al.,18 August 20, 2020 | Retrospective cohort study | United Kingdom | March 16, 2020–May 18, 2020 | 428,199 (data obtained from United Kingdom biobank) | 1324 | 500 | 48–86 y | N/A | There is a clear dose response relationship in the risk of a COVID-19 positive test result (P < 0.01) as the No. of medications increased no medications: RR (95% CI) 4–6: 1.58 (1.34–1.87) 7–9: 2.24 (1.81–2.77) ≥10: 3.09 (2.37–4.01) |
11/12 (91.7%) |
Abbreviations used: AKI, acute kidney injury; COVID-19, coronavirus disease 2019; GERD, gastroesophageal reflux disease; N/A, not applicable; BNF, British National Formulary; NSAIDs, nonsteroidal anti-inflammatory drugs; RR, relative risk; MV, mechanical ventilation; WHO, World Health Organization; UMC, Uppsala Monitoring Centre; ADRs, adverse drug reactions.
Based on Joanna Briggs Institute critical appraisal tool (Assessment of the Risk of Bias) specific for each respective study design.15
Statistical analysis
The interventions, settings, study designs, and outcome measures of the included studies were heterogenous; hence, a statistical synthesis or meta-analysis was not performed. We conducted a qualitative synthesis of the included studies where possible using descriptive statistics. Mean, standard deviation, and range were used to synthesize and report continuous variables, whereas frequencies and percentages were used to describe categorical variables.
Results
Characteristics of studies
The characteristics of the 7 unique articles from the United States, United Kingdom, China, Spain, and Bahrain, published between April and August 2020, were included in this review (Table 1). All the included studies were originally published in English language. Of the 7 studies included, 3 were retrospective cohort studies,16, 17, 18 2 were retrospective review of health records,19 , 20 1 study was a matched case-control study,21 and the final study was a retrospective observational study.22 The total sample size of all studies was 474,342, with wide variation noted in the sample sizes across studies. The smallest sample size of the included studies was 73,17 and the largest sample size was 428,199.18 The study participants’ age across all studies ranged from 0 to 97 years, with the minimum age in 6 of the 7 studies included being 42 years.16, 17, 18, 19, 20 , 22
In 5 of the 7 included studies, all patients were COVID-19 positive,16 , 17 , 19 , 20 , 22 as were the 4272 cases matched to 36,948 controls in the sixth study,21 and 1324 of the 428,199 patients in the seventh study.18 Across all studies, a total of 10,519 patients were COVID-19 positive. On the basis of the JBI assessment criteria,15 the quality scores for the included studies ranged from 50%-100%.
Description of polypharmacy
Six of the 7 included studies reported on the number of medications used by patients, including those for other comorbid conditions.16 , 18, 19, 20, 21, 22 One of the above 6 studies reported on the number of cardiovascular and noncardiovascular medications separately.21 The final study reported only the number of medications used to treat COVID-19 pneumonia specifically.17 Three of the 7 included studies categorized the number of medications used by COVID-19 patients ranging from 0 to 12 or more,17 , 18 , 21 and 2 studies defined polypharmacy generally as concurrently taking 5 or more medications without giving a breakdown of the exact number of medications (Table 1).16 , 22 The remaining 2 studies reported the mean number of medications among patients with COVID-19 ranging from 5.40 ± 2.10 to 8.57 ± 3.34.19 , 20 Across all studies, 4818 COVID-19 positive patients experienced polypharmacy.
Polypharmacy and clinical outcomes among COVID-19 patients
The association between polypharmacy and clinical outcomes among patients with COVID-19 extracted from the included studies is summarized in Table 1. Majority (71%) of the studies found statistically significant association between polypharmacy and negative clinical outcomes (Table 1).16, 17, 18 , 20 , 21 In contrast, 2 studies (29%) reported no statistically significant differences in the mean number of medications between comparison groups (COVID-19 survivors and nonsurvivors with or without mechanical ventilation).19 , 22
Morbidity and mortality
A higher mean number of medications was associated with severe COVID-19 morbidity and increased mortality among patients with COVID-19 (Table 1). Poblador-Plou et al.16 found that there was a statistically significant difference in the mean number of drugs between male (P < 0.001) and female (P = 0.006) survivors of COVID-19 compared with their deceased counterparts. Polypharmacy was significantly associated with death among male patients with COVID-19 (n = 242 (59.5%), P < 0.001).16
McKeigue et al.21 reported an association (P < 0.001) between noncardiovascular polypharmacy and severe COVID-19 infection among noncare home residents. This association was strongest in those without any diagnosed comorbidity.21 The study by Taher et al.17 reported that a relationship exists between polypharmacy and acute kidney injury (AKI) among patients with COVID-19. Increase in the number of medications administered for COVID-19 management was strongly associated with increase in the rate of AKI (P = 0.003) and the severity of AKI (P = 0.031).17
ADRs
Patients with COVID-19 who experienced ADR had a higher mean number of medications than patients with COVID-19 with no ADRs (P < 0.001).20 Furthermore, number of medications administered to patients with COVID-19 was found to be an independent risk factor for the occurrence of ADRs, because patients with higher mean number of medications (9 drugs vs. 5 drugs) were 4 times more likely to experience ADRs than patients with an average of 5 (odds ratio [OR] = 3.99, [95% CI 2.23–7.13]).20
Risk of COVID-19 infection
Polypharmacy is associated with increased risk of contracting COVID-19 infection. In the study by McQueenie et al.,18 a clear dose-response relationship between polypharmacy and an increased risk of a positive COVID-19 test result was discovered (P < 0.01). As the degree of polypharmacy increased, the rate ratio of a positive COVID-19 test result increased (P < 0.01) (4-6 medications rate ratio = 1.58, [95% CI 1.34–1.87] and ≥10 medications 3.09 [2.37–4.03]).18 This association remained statistically significant (P < 0.01) even after adjusting for demographic factors, Townsend score, location of assessment centers, smoking status, alcohol intake frequency, body mass index, and physical activity.18
Class of comedications associated with negative clinical outcomes among COVID-19 patients with polypharmacy
The specific class of comedications associated with clinical outcomes among COVID-19 patients is summarized in Table 2 . Four of the 7 included studies reported on the class of medications or specific drugs suspected of or linked to negative clinical outcomes.16 , 17 , 20 , 21 Among these 4 articles, 2 noted anassociation between some class of medications and specific drugs with adverse clinical outcomes among the cohort.16 , 21 A third article documented a list of drugs suspected in adverse reactions among the study sample.20 The fourth article reported no statistically significant association between the selected medications and the adverse outcome investigated.
Table 2.
Association of specific drug classes with COVID-19 clinical outcomes
| Drugs associated with death (ATC classification system code)16 OR (95% CI) | Drugs associated with severe COVID-1921 Adjusted Rate Ratio (95% CI); P value | Drugs suspected in ADRs20 n (%) |
|---|---|---|
| Men vs women: Potassium-sparing agents (C03D): 2.52 (1.27–4.97) vs. 2.86 (1.50–5.44) high-ceiling diuretics (C03C): 1.79 (1.30–2.49) vs. 1.71 (1.29–2.27) Antipsychotics (N05A): 1.71 (1.18–2.48) vs. 1.96 (1.41–2.73) Drugs for peptic ulcer and GERD (A02B): 1.45 (1.13–1.86) vs. 1.32 (1.02–1.71) Among women: Other beta-lactam antibacterials (J01D): 2.17 (1.00–4.71) Antigout preparations (M04A): 2.06 (1.05–4.04) Antiepileptics (N03A): 1.94 (1.31–2.88) Antithrombotic agents (B01A): 1.51 (1.16–1.97) Antidepressants (N06A): 1.33 (1.02–1.73) Among Men: Corticosteroids (D07A): 3.83 (1.43–10.3) Vasodilators for cardiac diseases (C01D): 3.15 (1.55–6.41) Immunosuppressants (L04A): 2.49 (1.08–5.76) Insulins and analogs (A10A): 2.33 (1.21–4.48) Vitamin B12 and folic acid (B03B): 1.88 (1.20–2.97) Other drugs for obstructive airway diseases, inhalants (R03B): 1.83 (1.10–3.05) Beta-blocking agents (C07A): 1.53 (1.11–2.11) Anxiolytics (N05B): 1.52 (1.05–2.22) |
Antipsychotic drugs: 2.79 (2.23–3.49) Opioid analgesics: 1.83 (1.61–2.07) Antidepressant drugs (not Tricyclic antidepressants): 1.76 (1.50–2.07) Proton pump inhibitors: 1.44 (1.31–1.58) Drugs used in nausea and vertigo: 1.43 (1.21–1.69) Gabapentinoids: 1.37 (1.16–1.60) Other drugs for Epilepsy 1.34 (1.06–1.69) Antihistamine: 1.30 (1.12–1.50) Tricyclic and related antidepressant drugs: 1.18 (1.03–1.36) |
Suspected drugs Lopinavir and ritonavir (All ADRs: 60 [63.8]; Severe ADRs: 10 [10.6]) Umifenovir (All ADRs: 17 [18.1]; Severe ADRs: 6 [6.38]) Chloroquine (All ADRs: 5 [5.31]; Severe ADRs: 0) Xuebijing injection (All ADRs: 3 [3.19]; Severe ADRs: 1) Antibacterial drugs (All ADRs: 4 [4.25]; Severe ADRs: 0) Other drugs 5 (All ADRs: [5.31]; Severe ADRs: 0) Causality assessment Lopinavir and ritonavir: (Probable: 35 (37.2); Possible: 25 (26.6)) Umifenovir (Probable: 5 [5.31]; Possible: 12 [12.8]) Chloroquine (Probable: 4 [4.25]; Possible: 1 [1.06]) Xuebijing injection: (Probable: 2 [2.13]; Possible: 1 [1.06]) Antibacterial drugs: (Probable: 2 [2.13]; Possible: 2 [2.13]) Other drugs 5 (Probable: 4 [4.25]; Possible: 1 [1.06]) |
Abbreviations used: COVID-19; coronavirus disease 2019; ATC, anatomical therapeutic chemical; OR, odds ratio; ADRs, adverse drug reactions; GERD, gastroesophageal reflux disease.
The most frequently used class of medications among patients with COVID-19 (men vs. women) in the cohort who died were drugs for peptic ulcer and gastro-esophageal reflux disease (GERD) (49.6% vs. 58.5%), antithrombotic agents (36.9% vs. 39.0%), and other analgesics and antipyretics (31.0% vs. 37.4%).16 Proton pump inhibitors were associated with a higher risk of severe COVID-19 (RR = 1.44; [95% CI 1.31–1.58]).21 Drugs for peptic ulcer and GERD were associated with greater odds of death in men (OR = 1.45, [95% CI 1.13–1.86]) and women (OR = 1.32, [95% CI 1.02–1.71]).16 Among male patients with COVID-19, corticosteroids (OR = 3.83, [95% CI 1.43–10.3]), vasodilators for cardiac diseases (OR = 3.15, [95% CI 1.55–6.41]), and immunosuppressants (OR = 2.49, [95% CI 1.08–5.76]) were strongly associated with death.16 In contrast, among female patients with COVID-19, other beta-lactam antibacterial (OR = 2.17, [95% CI 1.00–4.71]), antigout preparations (OR = 2.06, [95% CI 1.05–4.04]), and antiepileptic medication (OR = 1.94, [95% CI 1.31–2.88) were most strongly associated with death.16
Two studies reported an association between antipsychotics and adverse clinical outcomes among patients with COVID-19.16 , 21 Antipsychotic drugs were associated with nearly 3 times (RR = 2.79, [95% CI 2.23–3.49]) more risk of severe COVID-19 morbidity21 and nearly 2 times the risk of death in men (OR = 1.71, [95% CI 1.18–2.48]) and women (OR = 1.96, [95% CI 1.41–2.73]).16 A statistically significant association was reported between antidepressants (nontricyclic antidepressants: RR = 1.76, [95% CI 1.50–2.07], tricyclic antidepressants RR = 1.76, [95% CI 1.50–2.07]) and severe COVID-19 infection.21 However, one study noted an association between antidepressants and death (OR = 1.33, [95% CI 1.02–1.73]) in women but not in men.16 One study identified several medications suspected to cause ADRs among patients with COVID-19 in the study group.20 In particular, lopinavir and ritonavir were suspected in 63.8% of all the ADRs and 10.6% of severe ADRs among patients with COVID-19 in the study.20
Discussion
The findings from this systematic review of the available evidence suggest that polypharmacy is associated with adverse clinical outcomes among patients with COVID-19. The adverse clinical outcomes reported include ADRs, AKI, increased risk of COVID-19 infection, severe COVID-19, and mortality. This is consistent with the result of other studies that have reported the negative clinical impact of polypharmacy on different viral and respiratory diseases such as pneumonia and influenza.8 , 9 , 23 , 24 The findings from our systematic review provide further evidence for the argument in favor of deprescribing, especially among older patients with COVID-19 for whom drug clearance is altered because of age-associated physiological changes.25 Deprescribing refers to a patient-tailored intervention to prevent inappropriate polypharmacy through medication simplification and optimization.26 Because deprescribing has been shown to prevent medication harm in patients,26 there is a need to incorporate it in the pharmacotherapeutic management of COVID-19 patients undergoing polypharmacy.27 Incorporating this intervention early-on in the management of patients with COVID-19 may prevent further exacerbation of the disease. In addition, because increasing level of polypharmacy has been found to increase the risk of getting infected with COVID-19, prophylactically optimizing medication regimens for older adults and individuals with multimorbid conditions might help to lower their risk of getting infected with COVID-19.28 To achieve this, prescribers and pharmacists must collaborate to review patient medications and ensure that the benefit of the medications outweighs the risk in the context of the specific patient.
Our findings also linked specific drug classes to adverse outcomes in COVID-19 patients. Generally, medications with anticholinergic properties, sedative effect, respiratory depression, and some medications acting on the gastrointestinal tracts were more likely to increase the risk of adverse outcomes among patients with COVID-19. This is understandable because COVID-19 compromises several other organs in the body29 and, hence, may alter the physiological levels of certain drugs, leading to pharmacodynamic and pharmacokinetic interactions often marked by ADRs and other negative clinical outcomes. Evidence from our systematic review revealed that antipsychotics, proton pump inhibitors, antihistamines, and opioid analgesics were among the drug classes with the strongest association with negative clinical outcomes among patients with COVID-19. The aforementioned drug classes have also been implicated in adverse clinical outcomes in other disease states.30, 31, 32 A systematic review of observational studies concluded that exposure to antipsychotics is associated with an increased risk of pneumonia.30 Similarly, proton pump inhibitors and opioid analgesics have also been linked to increased risk of serious infections including pneumonia, endocarditis, and enteric infection.31 , 32 Furthermore, antipsychotics, antihistamine, opioid analgesics, and proton pump inhibitors are often implicated in adverse effects such as infection, falls, hospitalization, emergency department visits, and death,12 , 33, 34, 35 yet these drug classes are commonly used among older patients, especially those dwelling in LTC facilities.36 , 37 For instance, there have been reports of off-label use of antipsychotics in long-stay nursing homes without qualifying diagnoses36 and in older community-dwelling adults with dementia,37 despite modest benefit and high risk of harm. Findings from this systematic review provide additional evidence against such practices as it predisposes older persons to an increased risk of severe COVID-19 infection and mortality.
Lopinavir/ritonavir and chloroquine are among drugs undergoing clinical trial with the aim of repurposing them for COVID-19 treatment.38 However, the study that identified several medications suspected in ADRs among patients with COVID-19 linked lopinavir/ritonavir and chloroquine to a high proportion of ADRs investigated.20 This finding has implications for policy and research, especially because there has been heated scholarly and political debates surrounding the use of these repurposed medications and their analogs in managing patients with COVID-19. Several expert recommendations have advocated for caution in the use of these drugs among patients with COVID-19 because of conflicting and controversial evidence about their benefits and harm.39, 40, 41 Concerns have also been raised as to whether pharmacists should dispense or not dispense prescriptions for these medications for the management of COVID-19 outside of a clinical trial setting, especially for older persons for whom clinical trial data are nonexistent.42 The consensus is that as drug experts, gatekeepers and patient advocates, and pharmacists are encouraged to use their professional judgment in such situations.42 Although a number of COVID-19 vaccines are currently available for immunization against the disease, several countries still struggle with access to COVID-19 vaccines.43 This situation is made complex by the emergence of new variants for which the available COVID-19 vaccines have reduced efficacy44 and the increase in new COVID-19 cases and deaths globally.45 Therefore, clinicians are still faced with situations where they must exercise their clinical judgment in administering certain medications to patients with COVID-19. This systematic review provides evidence for medication optimization for patients with COVID-19 undergoing polypharmacy to improve their health outcomes.
The heterogeneity of the included studies is worth mentioning because it impacts the strength of the evidence presented. There were variations in the study design, population characteristics, sample size, definition of polypharmacy, and outcome measurements across the included studies. The study sample of most16 , 17 , 19 , 20 , 22 of the included articles consisted entirely of patients with COVID-19 with differing disease severity from asymptomatic to critical, and 2 of the included studies18 , 21 whose combined sample size made up 98% of the total sample size consisted of both COVID-19 negative and positive patients. Importantly, individual studies differed in whether and how they controlled for confounding factors as it relates to polypharmacy. The presence of comorbidities is one of the strongest confounders for the observed effect of polypharmacy in various disease states, hence the necessity to control for it to improve the strength of evidence.46 We noted that only 1 of the included studies controlled for the presence of comorbidities as it pertains to the effect of polypharmacy on the clinical outcomes in patients with COVID-19.21 The study by McKeigue et al.21 found a statistically significant association between noncardiovascular polypharmacy and severe COVID-19 which remained after adjusting for potential confounders including presence of other comorbidities. This may serve as evidence that polypharmacy presents an independent risk for adverse COVID-19 outcomes, which cannot be fully explained by presence of comorbidities. Similar studies have also noted the independent association between polypharmacy and adverse clinical outcomes in various disease states such as influenza,9HIV,47 and dementia.48 Another interesting point to note is that despite robust evidence that cardiovascular diseases worsen COVID-19 severity,49 , 50 the study by McKeigue et al.21 found no statistically significant association between cardiovascular polypharmacy and severe COVID-19.21 This further suggests that the COVID-19 related risk conferred by polypharmacy may be independent of the risk from comorbidities. A previous study also noted no association between cardiovascular polypharmacy and worsening severity of noncardiovascular conditions.11 More rigorous studies are needed to better understand this phenomenon in the context of COVID-19.
In view of the negative impact of polypharmacy and specific drug classes on COVID-19 clinical outcomes, it suffices to say that pharmaceutical care is critical now more than ever before. Pharmacists, as highly trained drug experts and the most accessible health care providers,51 are therefore strategically placed to improve COVID-19 clinical outcomes of patients through optimized medication management, virtual patient counseling, and medication stewardship, among other services.52 , 53 Recognizing their strategic position in the fight against COVID-19 infection, several countries have extended and expanded the legal role of pharmacists to fully explore their potential during this pandemic.42 The role of pharmacist in other epidemics54 and chronic disease care is well documented;51 , 55 hence, it is pertinent to ensure that they are fully incorporated in COVID-19 infection management. Furthermore, pharmacists are drug information experts and hence play a vital role in evaluating and interpreting the rapidly evolving literature on COVID-19 pharmacotherapy to support other clinicians’ decision-making and inform population-level policy-making. In addition, they also serve as drug information resource for the public who might be confused about inaccurate claims about COVID-19 vaccine or the effects of drugs being repurposed for COVID-19 treatment. To that end, pharmacists must continue to collaborate with other members of the health care team to ensure that patients with COVID-19 and vulnerable groups get the best quality pharmaceutical care to improve their health outcomes.
Study strengths and limitations
Our systematic review has limitations that deserves to be noted. First, all the studies reviewed were observational studies, which may have significant risk of bias due to lack of randomization and a negative impact on the overall quality of the evidence documented in our systematic review.56 In addition, presence of comorbidities is a potential confounder for the observed adverse effects associated with polypharmacy, because patients included in the studies may be experiencing other baseline comorbidities that can independently worsen their COVID-19 outcomes. Consequently, direct causality cannot be inferred from these studies. Second, because of the paucity of literature on the subject matter, one of the studies we included was a preprint article that had not been peer-reviewed at the time of data extraction.57 However, we decided to include it because the valuable information contained in the article outweighed the risk of excluding it. This article has since been published in a peer-reviewed journal, and the information extracted at the time of the review remained unchanged.21 Third, it was not possible to conduct a meta-analysis because the included studies did not meet the criteria for a quantitative/statistical synthesis owing to their heterogenous nature related to the interventions, settings, study designs, and outcome measures. Finally, the potential effect of polypharmacy on patients with COVID-19 is still not fully understood because this requires more rigorous studies and, perhaps, a longer follow-up period. However, because of the novel and rapidly spreading nature of the pandemic, it was necessary to synthesize the available evidence to aid clinical decision-making for vulnerable patients with COVID-19. On the basis of the above limitations, we recommend that the findings of this systematic review be interpreted with utmost caution.
Notwithstanding its limitations, our study is valuable in assessing and presenting the current evidence on polypharmacy among patients with COVID-19. The information synthesized in this study can help lay a strong foundation for further studies in this area and provide clinicians and policymakers with the most relevant and recent information to make evidence-based and informed decisions that will improve care and health outcomes of patients with COVID-19. In addition, we highlighted the knowledge gaps present on the subject matter and provided a resounding case on the strong need for further research on polypharmacy and COVID-19 infection.
Conclusion
Polypharmacy is associated with increased risk of adverse health outcomes among patients with COVID-19. Some drug classes frequently used in older adults are associated with elevated susceptibility to adverse COVID-19 clinical outcomes. In view of this, pharmacists and health care providers need to collaborate to optimize medication management to reduce harm among vulnerable groups, especially the older adults who are more prone to multimorbidity and polypharmacy. The findings from this systematic review can aid informed COVID-19 related decision-making to improve the clinical outcomes in patients with COVID-19. However, further research is needed in this area to provide more evidence on the association between polypharmacy and COVID-19 infection and modes of action across different classes of medication, especially for patients at higher risk of adverse clinical outcomes.
Acknowledgments
The authors would like to thank the authors of the articles used for this systematic review for their scientific contributions, which lay the foundation for understanding the roles of polypharmacy and drug classes on clinical outcomes among patients with COVID-19.
Biographies
SorochiIloanusi, BSPharm, Doctoral Candidate, Department of Pharmaceutical Health Outcomes and Policy, College of Pharmacy, University of Houston, Houston, TX
Osaro Mgbere, PhD, MS, MPH, Adjunct Professor, Department of Pharmaceutical Health Outcomes and Policy; and Research Scientist, Institute of Community Health, College of Pharmacy, University of Houston, Houston, TX
Ekere J. Essien, MD, DrPH, Professor, Department of Pharmaceutical Health Outcomes and Policy; and Director, Institute of Community Health, College of Pharmacy, University of Houston, Houston, TX
Footnotes
ORCID Sorochi Iloanusi: https://orcid.org/0000-0003-3571-0996
Osaro Mgbere: https://orcid.org/0000-0002-2863-6284
Ekere J. Essien: https://orcid.org/0000-0001-8157-8290
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Previous Presentation: This research was presented at the ISPOR (The Professional Society for Health Economics and Outcomes Research) 2021 conference May 19, 2021.
Appendix
Appendix 1 Search strategy
| A Combination of key words and MeSH terms was used on different electronic databases (Embase, Medline, Cochrane, Scopus, Google Scholar, clinicaltrials.gov, LITCOVID, PubMed, PubMed Central [PMC], and China national Knowledge infrastructure [CNKI]) with necessary adaptations to each database. |
|
|
|
|
|
|
|
|
Appendix 2 Methodological quality of studies included
| Joanna Briggs Institute Checklist | McKeigue et al.421 | Taher et al.117 | Poblador-Plou et al.216 | McQueenie et al. 202018 | Gavin et al 202019 | De Smet et al.322 | Sun et al. 202020 |
|---|---|---|---|---|---|---|---|
| Case Control Studies£ | |||||||
|
Yes | ||||||
|
Yes | ||||||
|
No | ||||||
|
Yes | ||||||
|
Yes | ||||||
|
Unclear | ||||||
|
Yes | ||||||
|
Yes | ||||||
|
Yes | ||||||
|
Yes | ||||||
| Total Score | 8 | ||||||
| Cohort Studies# | |||||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
No | No | Yes | ||||
|
Yes | Yes | Yes | ||||
|
No | No | Yes | ||||
|
No | No | Yes | ||||
|
No | No | No | ||||
|
Yes | Yes | Yes | ||||
|
No | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
No | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
| Total Score | 6 | 8 | 11 | ||||
| Prevalence Dataπ | |||||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
Yes | No | Yes | ||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
|
Yes | Yes | Yes | ||||
| Total Score | 9 | 8 | 9 |
Maximum Attainable Score for Case Control Studies is 10.
Maximum Attainable Score for cohort Studies is 12.
Maximum Attainable Score for prevalence Studies is 9.
References
- 1.World Health Organization (WHO) Coronavirus disease (COVID-19) sashboad. https://covid19.who.int/ Available at:
- 2.Atkins J.L., Masoli J.A.H., Delgado J., et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iaccarino G., Grassi G., Borghi C., et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 4.Clark A., Jit M., Warren-Gash C., et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khezrian M., McNeil C.J., Murray A.D., Myint P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther Adv Drug Saf. 2020;11 doi: 10.1177/2042098620933741. 2042098620933741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jokanovic N., Tan E.C., Dooley M.J., Kirkpatrick C.M., Bell J.S. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(6) doi: 10.1016/j.jamda.2015.03.003. 535.e1–12. [DOI] [PubMed] [Google Scholar]
- 8.Fried T.R., O’Leary J., Towle V., Goldstein M.K., Trentalange M., Martin D.K. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. doi: 10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hak E., Verheij T.J., van Essen G.A., Lafeber A.B., Grobbee D.E., Hoes A.W. Prognostic factors for influenza-associated hospitalization and death during an epidemic. Epidemiol Infect. 2001;126(2):261–268. doi: 10.1017/s0950268801005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E.A., Brettler J.W., Kanter M.H., et al. Refining the definition of polypharmacy and its link to disability in older adults: conceptualizing necessary polypharmacy, unnecessary polypharmacy, and polypharmacy of unclear benefit. Perm. J. 2020;24:18–212. doi: 10.7812/TPP/18.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appleton S.C., Abel G.A., Payne R.A. Cardiovascular polypharmacy is not associated with unplanned hospitalisation: evidence from a retrospective cohort study. BMC Fam Pract. 2014;15:58. doi: 10.1186/1471-2296-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knol W., van Marum R.J., Jansen P.A., Souverein P.C., Schobben A.F., Egberts A.C. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56(4):661–666. doi: 10.1111/j.1532-5415.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 13.Shamseer L., Moher D., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation [published correction appears in BMJ. 2016;354:i4086] BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 14.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.University of Adelaide JBI: critical appraisal tools. https://joannabriggs.org/critical-appraisal-tools Available at:
- 16.Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., et al. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int J Environ Res Public Health. 2020;17(14):5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taher A., Alalwan A.A., Naser N., Alsegai O., Alaradi A. Acute kidney injury in COVID-19 pneumonia: a single-center experience in Bahrain. Cureus. 2020;12(8) doi: 10.7759/cureus.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQueenie R., Foster H.M.E., Jani B.D., et al. Multimorbidity, Polypharmacy, and COVID-19 infection within the UK Biobank cohort. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavin W., Campbell E., Zaidi S.A., et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. 2021;49(2):158–165. doi: 10.1016/j.ajic.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J., Deng X., Chen X., et al. Incidence of adverse drug reactions in COVID-19 patients in China: an active monitoring study by hospital pharmacovigilance System. Clin Pharmacol Ther. 2020;108(4):791–797. doi: 10.1002/cpt.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeigue P.M., Kennedy S., Weir A., et al. Relation of severe COVID-19 to polypharmacy and prescribing of psychotropic drugs: the REACT-SCOT case-control study. BMC Med. 2021;19(1):51. doi: 10.1186/s12916-021-01907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Smet R., Mellaerts B., Vandewinckele H., et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21(7):928–932.e1. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakozaki T., Hosomi Y., Shimizu A., Kitadai R., Mirokuji K., Okuma Y. Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J Cancer Res Clin Oncol. 2020;146(10):2659–2668. doi: 10.1007/s00432-020-03252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene M., Steinman M.A., McNicholl I.R., Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 2014;62(3):447–453. doi: 10.1111/jgs.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross S.B., Wilson M.G., Papillon-Ferland L., et al. COVID-SAFER: deprescribing guidance for hydroxychloroquine drug interactions in older adults. J Am Geriatr Soc. 2020;68(8):1636–1646. doi: 10.1111/jgs.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott I.A., Hilmer S.N., Reeve E., et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. doi: 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- 27.IUPHAR SamerC., Webb D., et al. International union for basic and clinical pharmacology (IUPHAR) clinical division considerations in the context of COVID-19 pandemics. https://iuphar.org/wp-content/uploads/2020/05/IUPHAR-Clinical-Division-Considerations-in-the-Context-of-COVID-19-Pandemics.pdf Available at:
- 28.Brandt N., Steinman M.A. Optimizing medication management during the COVID-19 pandemic: an implementation guide for post-acute and long-term care. J Am Geriatr Soc. 2020;68(7):1362–1365. doi: 10.1111/jgs.16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosè M., Recla E., Trifirò G., Barbui C. Antipsychotic drug exposure and risk of pneumonia: a systematic review and meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2015;24(8):812–820. doi: 10.1002/pds.3804. [DOI] [PubMed] [Google Scholar]
- 31.Wiese A.D., Griffin M.R., Schaffner W., et al. Long-acting opioid use and the risk of serious infections: a retrospective cohort study. Clin Infect Dis. 2019;68(11):1862–1869. doi: 10.1093/cid/ciy809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshpande A., Pant C., Pasupuleti V., et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(3):225–233. doi: 10.1016/j.cgh.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez C.A., Mortensen E.M., Makris U.E., et al. Association of skeletal muscle relaxers and antihistamines on mortality, hospitalizations, and emergency department visits in elderly patients: a nationwide retrospective cohort study. BMC Geriatr. 2015;15:2. doi: 10.1186/1471-2318-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado-Duque M.E., Castaño-Montoya J.P., Medina-Morales D.A., Castro-Rodríguez A., González-Montoya A., Machado-Alba J.E. Drugs with anticholinergic potential and risk of falls with hip fracture in the elderly patients: a case–control study. J Geriatr Psychiatry Neurol. 2018;31(2):63–69. doi: 10.1177/0891988718757370. [DOI] [PubMed] [Google Scholar]
- 35.Rolita L., Spegman A., Tang X., Cronstein B.N. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013;61(3):335–340. doi: 10.1111/jgs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips L.J., Birtley N.M., Petroski G.F., Siem C., Rantz M. An observational study of antipsychotic medication use among long-stay nursing home residents without qualifying diagnoses. J Psychiatr Ment Health Nurs. 2018;25(8):463–474. doi: 10.1111/jpm.12488. [DOI] [PubMed] [Google Scholar]
- 37.Bain K.T., Schwartz E.J., Chan-Ting R. Reducing off-label antipsychotic use in older community-dwelling adults with dementia: a narrative review. J Am Osteopath Assoc. 2017;117(7):441–450. doi: 10.7556/jaoa.2017.090. [DOI] [PubMed] [Google Scholar]
- 38.Giovane R.A., Rezai S., Cleland E., Henderson C.E. Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID-19) and the rationale for their utilization: a review. Rev Med Virol. 2020;30(5):e2136. doi: 10.1002/rmv.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laporte J.-R., Healy D. Medications compromising Covid infections: in the midst of the SARS-CoV-2 pandemia, caution is needed with commonly used drugs that increase the risk of pneumonia. https://rxisk.org/medications-compromising-covid-infections/ Available at:
- 40.Ailabouni N.J., Hilmer S.N., Kalisch L., Braund R., Reeve E. COVID-19 pandemic: considerations for safe medication use in older adults with multimorbidity and polypharmacy. J Gerontol A Biol Sci Med Sci. 2021;76(6):1068–1073. doi: 10.1093/gerona/glaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemaitre F., Solas C., Grégoire M., et al. Potential drug-drug interactions associated with drugs currently proposed for COVID-19 treatment in patients receiving other treatments. Fundam Clin Pharmacol. 2020;34(5):530–547. doi: 10.1111/fcp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox S. To dispense or not to dispense: lessons to be learnt from ethical challenges faced by pharmacists in the COVID-19 pandemic. Dev World Bioeth. 2020;00:1–8. doi: 10.1111/dewb.12284. Accessed June 7, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Tenchov R., Smoot J., Liu C., Watkins S., Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021;7(4):512–533. doi: 10.1021/acscentsci.1c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahase E. Covid-19: where are we on vaccines and variants? BMJ. 2021;372:n597. doi: 10.1136/bmj.n597. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO) Weekly epidemiological update on COVID-19- 6 April 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-april-2021 Available at:
- 46.Schöttker B., Saum K.U., Muhlack D.C., Hoppe L.K., Holleczek B., Brenner H. Polypharmacy and mortality: new insights from a large cohort of older adults by detection of effect modification by multi-morbidity and comprehensive correction of confounding by indication. Eur J Clin Pharmacol. 2017;73(8):1041–1048. doi: 10.1007/s00228-017-2266-7. [DOI] [PubMed] [Google Scholar]
- 47.Justice A.C., Gordon K.S., Skanderson M., et al. Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS. 2018;32(6):739–749. doi: 10.1097/QAD.0000000000001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hein C., Forgues A., Piau A., Sommet A., Vellas B., Nourhashémi F. Impact of polypharmacy on occurrence of delirium in elderly emergency patients. J Am Med Dir Assoc. 2014;15(11):850.e11–850.e15. doi: 10.1016/j.jamda.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29(8):104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsushita K., Ding N., Kou M., et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Glob Heart. 2020;15(1):64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohiuddin A.K. Universal-Publishers; Irvine, CA: 2020. The Role of the Pharmacist in Patient Care: Achieving High Quality, Cost-Effective and Accessible Healthcare Through a Team-Based, Patient-Centered Approach. [Google Scholar]
- 52.Gross A.E., MacDougall C. Roles of the clinical pharmacist during the COVID-19 pandemic. J Am Coll Clin Pharm. 2020;3(3):564–566. doi: 10.1002/jac5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhat S., Kehasse A. Additional clinical pharmacists roles during COVID-19. J Am Coll Clin Pharm. 2020;3(4) doi: 10.1002/jac5.1243. 825–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isenor J.E., Edwards N.T., Alia T.A., et al. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine. 2016;34(47):5708–5723. doi: 10.1016/j.vaccine.2016.08.085. [DOI] [PubMed] [Google Scholar]
- 55.Swaden L. The role of the HIV pharmacist in managing complex polypharmacy has never been more important. Clin Pharm. 2011;3(7):218–219. [Google Scholar]
- 56.Colditz G.A. Overview of the epidemiology methods and applications: strengths and limitations of observational study designs. Crit Rev Food Sci Nutr. 2010;50 Suppl 1(s1):10–12. doi: 10.1080/10408398.2010.526838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKeigue P.M., Kennedy S., Weir A., et al. Associations of severe COVID-19 with polypharmacy in the REACT-SCOT case-control study. https://www.medrxiv.org/content/10.1101/2020.07.23.201607471v Available at: [DOI] [PMC free article] [PubMed]