Abstract
Objectives
We evaluated the clinical, virological and safety outcomes of lopinavir/ritonavir, lopinavir/ritonavir–interferon (IFN)-β-1a, hydroxychloroquine or remdesivir in comparison to standard of care (control) in coronavirus 2019 disease (COVID-19) inpatients requiring oxygen and/or ventilatory support.
Methods
We conducted a phase III multicentre, open-label, randomized 1:1:1:1:1, adaptive, controlled trial (DisCoVeRy), an add-on to the Solidarity trial (NCT04315948, EudraCT2020-000936-23). The primary outcome was the clinical status at day 15, measured by the WHO seven-point ordinal scale. Secondary outcomes included quantification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in respiratory specimens and pharmacokinetic and safety analyses. We report the results for the lopinavir/ritonavir-containing arms and for the hydroxychloroquine arm, trials of which were stopped prematurely.
Results
The intention-to-treat population included 583 participants—lopinavir/ritonavir (n = 145), lopinavir/ritonavir–IFN–β-1a (n = 145), hydroxychloroquine (n = 145), control (n = 148)—among whom 418 (71.7%) were male, the median age was 63 years (IQR 54–71), and 211 (36.2%) had a severe disease. The day-15 clinical status was not improved with the investigational treatments: lopinavir/ritonavir versus control, adjusted odds ratio (aOR) 0.83, (95% confidence interval (CI) 0.55–1.26, p 0.39), lopinavir/ritonavir–IFN–β-1a versus control, aOR 0.69 (95%CI 0.45–1.04, p 0.08), and hydroxychloroquine versus control, aOR 0.93 (95%CI 0.62–1.41, p 0.75). No significant effect of investigational treatment was observed on SARS-CoV-2 clearance. Trough plasma concentrations of lopinavir and ritonavir were higher than those expected, while those of hydroxychloroquine were those expected with the dosing regimen. The occurrence of serious adverse events was significantly higher in participants allocated to the lopinavir/ritonavir-containing arms.
Conclusion
In adults hospitalized for COVID-19, lopinavir/ritonavir, lopinavir/ritonavir–IFN–β-1a and hydroxychloroquine improved neither the clinical status at day 15 nor SARS-CoV-2 clearance in respiratory tract specimens.
Keywords: COVID-19, Hydroxychloroquine, Interferon β-1a, Lopinavir/ritonavir, Randomized controlled trial, SARS-CoV-2
Introduction
Worldwide research efforts against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) initially focused on repurposed drugs that showed broad-spectrum antiviral activity against coronaviruses [1,2]. Lopinavir/ritonavir [3,4], type I interferon (IFN) [[5], [6], [7]], hydroxychloroquine [[8], [9], [10]], and remdesivir [11] were among the first investigational treatments to be tested on the basis of their in vitro activity against SARS-CoV-2.
The DisCoVeRy trial is a European randomized controlled trial evaluating the clinical and virological efficacy and safety of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a, hydroxychloroquine, and remdesivir as compared with standard of care in adults hospitalized for coronavirus 2019 (COVID-19) [12]. As an add-on trial to the international Solidarity trial sponsored by the World Health Organization (WHO), it has contributed to data acquisition on in-hospital mortality, need for mechanical ventilation, and time to hospital discharge. Interim analyses of these variables concluded in futility, leading to discontinuation of three treatment arms, while inclusions continued in the remdesivir arm [13]. The DisCoVeRy trial was designed to further document clinical outcomes, virological kinetics, treatment pharmacokinetics and related safety data. We report here the results for the lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine arms.
Methods
Trial design and oversight
DisCoVeRy is a phase III open-label, adaptive, multicentre, randomized, superiority-controlled trial evaluating the efficacy and safety of repurposed drugs in adults hospitalized for COVID-19. Sponsored by the Institut National de la Santé et de la Recherche Médicale (INSERM, France), the trial was approved by the Ethics Committee (CPP Ile-de-France-III, approval #20.03.06.51 744). Written informed consent was obtained from all included participants or from their legal representatives for those unable to consent. The trial was conducted in accordance with the Declaration of Helsinki and national laws and regulations, and declared on the clinicaltrials.gov registry (NCT 04315948) and on the European Clinical Trials Database (2020-000936-23).
Study population
Eligible participants were adults (≥18 years old) hospitalized with a PCR-proven (<72 h) SARS-CoV-2 infection and pulmonary rales or crackles with a peripheral oxygen saturation ≤94% or requiring supplemental oxygen. Inclusion and exclusion criteria are presented in the Supplementary Material Appendix.
Interventions and randomization
Participants were randomly assigned to treatment arms in a 1:1:1:1 ratio through computer-generated blocks of various sizes and stratification by administrative region and severity of disease at enrolment (moderate: hospitalized participants not requiring oxygen or receiving low-flow supplemental oxygen; severe: hospitalized participants requiring non-invasive ventilation or high-flow oxygen devices, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO)). Randomization was implemented in the electronic Case Report Form to ensure appropriate allocation concealment. Investigational arms were standard of care (SoC, control), SoC plus lopinavir/ritonavir (400 mg lopinavir and 100 mg ritonavir orally twice a day for 14 days [3,14]), SoC plus lopinavir/ritonavir plus IFN-β-1a (44 μg subcutaneous IFN-β-1a on days 1, 3, and 6), SoC plus hydroxychloroquine (400 mg orally, twice on day 1 as a loading dose followed by 400 mg once daily for 9 days) [15]. Supportive treatments—corticosteroids, anticoagulants or immunomodulatory agents—were allowed, but not antivirals. Enrolment in another investigative trial was not allowed.
Clinical and laboratory monitoring
Participants were assessed at days 3, 5, 8, 11, 15 ± 2 and 29 ± 3 while hospitalized. If discharge occurred before day 15, face-to-face visits were set up for days 15 ± 2 and 29 ± 3 for efficacy and safety evaluations. Clinical data, concomitant medications, adverse events (AEs) and measurements for safety biological data (blood cell counts, serum creatinine and liver aminotransferases) were collected. Nasopharyngeal swab and lower respiratory tract specimens were collected for SARS-CoV-2 RNA quantification. For lopinavir and ritonavir, trough plasma concentrations were obtained at days 1 and 3, 12 h (±2 h) after the last administration and for hydroxychloroquine at day 1, 12 h (±2 h) and at day 3, 24 h (±4 h) after the last administration.
Outcome measures
The primary outcome measure was the clinical status at day 15 as measured on the seven-point ordinal scale of the WHO Master Protocol (v3.0, 3rd March 2020): (1) not hospitalized, no limitation on activities; (2) not hospitalized, limitation on activities; (3) hospitalized, not requiring supplemental oxygen; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, on non-invasive ventilation or high-flow oxygen devices; (6) hospitalized, on invasive mechanical ventilation or ECMO; (7) death.
Secondary efficacy outcome measures were the clinical status at day 29 and the time to an improvement of two categories as measured on the seven-point ordinal scale or hospital discharge until day 29, the time to national early warning score 2 (NEWS2) ≤2 or hospital discharge until day 29, the time to hospital discharge until day 29, oxygenation- and ventilator-free days until day 29, 29-day mortality, and the SARS-CoV-2 detection and quantitative normalized viral loads. Trough plasma concentrations of lopinavir, ritonavir and hydroxychloroquine were measured at days 1 and 3. Secondary safety outcomes included the cumulative incidence of any grade 3 or 4 AE, or of any serious AE (SAE, according to the DAIDS Table for Grading the Severity of Adult and Paediatric Adverse Events, v2.1, July 2017) and the proportion of patients with a premature suspension or discontinuation for any reason of the investigational treatments.
Virological methods
Determination of normalized viral load blinded to treatment arm was performed on nasopharyngeal swab and lower respiratory tract specimens by RNA extraction on the EMAG® platform (bioMerieux, Marcy-l'Étoile, France). The SARS-CoV-2 load was measured by quantitative RT-PCR, according to a scale of calibrated in-house plasmid, using the RT-PCR RdRp-IP4 developed by the Institut Pasteur (Paris, France) [16]. The amplification protocol was developed using QuantStudio 5 rtPCR Systems (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The number of cells in a sample (quality criteria for nasopharyngeal swab and normalization tool for viral load determination) was checked using the CELL Control r-gene® kit (Argene-BioMérieux, Marcy-l'Étoile, France). If cell quantification was <500 cells/reaction, the quality of the sample was considered too low to be measured. We computed a normalized SARS-CoV-2 load by dividing the viral load by the number of cells. All viral loads strictly below 1 log10 RNA copies/10 000 cells were considered under the limit of detection and were reported as negative.
Pharmacological methods
Plasma concentrations of lopinavir, ritonavir and hydroxychloroquine were determined using liquid chromatography coupled with tandem mass spectrometry [17,18]. The limits of quantification were 30 ng/mL for lopinavir and ritonavir, and 10 ng/mL for hydroxychloroquine.
Sample size calculation
The sample size was determined assuming the following scenario under SoC for each item of the ordinal scale at day 15: 1, 42%; 2, 38%; 3, 8%; 4, 7%; 5, 2%; 6, 1%; and 7, 2%. At the time of the trial design there was a significant uncertainty with these assumptions. We powered the study for an odds ratio of 1.5 (an odds ratio >1 indicates superiority of the experimental treatment over the control for each ordinal scale category), with 90% power and using an overall two-sided type I error rate of 0.05. Adjusting for multiplicity of four pairwise comparisons with the control arm in a 5-arm setting, the two-sided false-positive error rate would be 0.0125. We determined that the inclusion of 620 patients in each treatment arm was required.
Statistical and interim analyses
An independent data safety and monitoring board (DSMB) externally reviewed the trial data periodically. Based on interim analyses (see Supplementary Material Appendix), enrolment in the hydroxychloroquine arm was prematurely stopped on 17th June, and enrolment in lopinavir-containing arms was stopped on 29th June 2020.
For the seven-point ordinal scale, data were analysed using a proportional odds model, which assumes a common odds ratio between the seven points of the ordinal scale. All analyses were stratified by severity at randomization, and adjusted effect measures are reported. Full statistical methods are presented in the Supplementary Material Appendix.
Results
Patient characteristics at baseline
Between 22nd March and 29th June, 603 participants were randomized across 30 sites in France and two in Luxembourg; 583 were evaluable for analysis (Supplementary Material Fig. S1): control arm, n = 148; lopinavir/ritonavir arm, n = 145; lopinavir/ritonavir plus IFN-β-1a arm, n = 145; and hydroxychloroquine arm, n = 145. Participants' baseline characteristics are presented in Table 1 . Participants were mostly male (n = 418, 71.7%), median age was 63 years (IQR 54–71). The median time from symptom onset to randomization was 9 days (IQR 7–12). The most frequent underlying conditions were obesity (n = 166, 28.7%), chronic cardiac disease (n = 151, 26.0%) and diabetes mellitus (n = 128, 22.0%). At baseline, severe disease accounted for 211 participants (36.2%). Concomitant treatments are listed in Supplementary Material Table S1.
Table 1.
Baseline characteristics of patients included in the intention-to-treat population of the present analysis of DisCoVeRy trial
| Overall (n = 583) | Control (n = 148) | Lopinavir/ritonavir (L/r) (n = 145) | Lopinavir/ritonavir + interferon β-1a (L/r + IFN) (n = 145) | Hydroxychloroquine (HCQ) (n = 145) | |
|---|---|---|---|---|---|
| Median age: years [IQR] | 63 [54–71] | 62 [52–71] | 63 [55–71] | 64 [53–71] | 65 [55–71] |
| Male sex: n (%) | 418 (71.7%) | 105 (70.9%) | 106 (73.1%) | 103 (71.0%) | 104 (71.7%) |
| Coexisting condition:an (%) | |||||
|
151 (26.0%) | 39 (26.4%) | 35 (24.1%) | 36 (25.2%) | 41 (28.3%) |
|
88 (15.1%) | 31 (20.9%) | 19 (13.1%) | 19 (13.3%) | 19 (13.1%) |
|
24 (4.1%) | 7 (4.7%) | 2 (1.4%) | 5 (3.5%) | 10 (6.9%) |
|
13 (2.2%) | 6 (4.1%) | 3 (2.1%) | 0 (0.0%) | 4 (2.8%) |
|
23 (4.0%) | 6 (4.1%) | 5 (3.4%) | 4 (2.8%) | 8 (5.5%) |
|
35 (6.0%) | 10 (6.8%) | 8 (5.5%) | 6 (4.1%) | 11 (7.6%) |
|
26 (4.5%) | 8 (5.4%) | 4 (2.8%) | 9 (6.3%) | 5 (3.4%) |
|
166 (28.7%) | 46 (31.3%) | 36 (24.8%) | 41 (28.7%) | 43 (30.1%) |
|
128 (22.0%) | 35 (23.6%) | 35 (24.1%) | 27 (18.9%) | 31 (21.4%) |
|
18 (3.3%) | 5 (3.5%) | 4 (2.9%) | 5 (3.6%) | 4 (3.0%) |
| Median time from symptom onset to randomization:a days [IQR] | 9.0 [7.0–12.0] | 10.0 [7.0–12.0] | 10.0 [7.0–13.0] | 10.0 [7.0–12.0] | 8.0 [7.0–11.0] |
| Baseline severity of COVID-19:bn (%) | |||||
|
372 (63.8%) | 94 (63.5%) | 94 (64.8%) | 91 (62.8%) | 93 (64.1%) |
|
211 (36.2%) | 54 (36.5%) | 51 (35.2%) | 54 (37.2%) | 52 (35.9%) |
| Randomization site:an (%) | |||||
|
254 (43.6%) | 64 (43.2%) | 65 (44.8%) | 65 (45.1%) | 60 (41.4%) |
|
328 (56.4%) | 84 (56.8%) | 80 (55.2%) | 79 (54.9%) | 85 (58.6%) |
| Seven-point ordinal scale at baseline: n (%) | |||||
|
27 (4.6%) | 8 (5.4%) | 4 (2.8%) | 9 (6.2%) | 6 (4.1%) |
|
341 (58.5%) | 84 (56.8%) | 88 (60.7%) | 84 (57.9%) | 85 (58.6%) |
|
63 (10.8%) | 21 (14.2%) | 15 (10.3%) | 13 (9.0%) | 14 (9.7%) |
|
152 (26.1%) | 35 (23.6%) | 38 (26.2%) | 39 (26.9%) | 40 (27.6%) |
| Median NEWS-2 at baselinea, median [IQR] | 9.0 [7.0–12.0] | 9.0 [7.0–12.0] | 9.0 [7.0–11.0] | 10.0 [7.0–12.0] | 9.0 [6.0–11.0] |
| Median viral load at baseline, median [IQR] | |||||
|
2.4 [0.7–3.7] n = 349) | 2.5 [1.1–3.9] (n = 87) | 2.4 [0.7–3.6] (n = 88) | 2.5 [0.7–3.8] (n = 79) | 2.0 [0.7–3.4] (n = 95) |
|
4.1 [2.8–4.9] (n = 56) | 3.6 [2.4–4.5] (n = 14) | 4.4 [3.2–4.8] (n = 14) | 3.5 [1.0–4.8] (n = 10) | 4.3 [3.3–5.5] (n = 18) |
| Biological data at baselinea, median [IQR] | |||||
|
0.9 [0.6–1.2] | 0.9 [0.6–1.4] | 0.8 [0.6–1.2] | 0.9 [0.7–1.3] | 0.9 [0.6–1.1] |
|
5.8 [4.0–7.9] | 5.7 [4.1–7.8] | 6.3 [4.3–8.0] | 5.7 [3.9–8.3] | 5.6 [3.8–7.8] |
|
74.0 [62.0–91.0] | 72.5 [60.0–88.0] | 73.5 [62.0–88.0] | 77.0 [65.0–91.0] | 74.0 [62.0–93.0] |
|
49.0 [35.0–72.0] | 53.0 [38.0–74.0] | 47.0 [34.0–64.0] | 47.0 [35.0–70.0] | 53.5 [34.0–81.0] |
|
37.0 [25.0–63.0] | 41.0 [25.0–62.0] | 34.0 [22.5–60.5] | 37.0 [24.0–59.0] | 41.5 [26.0–67.0] |
|
119.5 [72.0–185.0] | 132.0 [86.0–191.0] | 124.0 [75.0–188.0] | 105.0 [57.0–164.0] | 118.0 [72.0–188.0] |
|
1080.0 [649.0–1860.0] | 1170.0 [689.0–2000.0] | 1060.0 [626.0–1987.0] | 956.0 [560.0–1673.0] | 1140.0 [654.0–1820.0] |
|
0.2 [0.1–0.9] | 0.3 [0.1–1.1] | 0.2 [0.1–0.6] | 0.3 [0.1–0.9] | 0.3 [0.1–0.9] |
|
480.5 [2.0–1344.0] | 98.0 [2.0–1041.0] | 608.0 [2.0–1288.0] | 761.0 [3.0–1344.0] | 377.0 [2.0–1610.0] |
NPS, nasopharyngeal swabs; LRT, lower respiratory tract; SGOT, serum glutamic–oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
Denotes variables with missing data. Data on chronic cardiac disease, chronic pulmonary disease, mild liver disease, chronic neurological disorder, active cancer and diabetes mellitus were missing for two patients. Data on chronic kidney disease were missing for three patients. Data on autoinflammatory disease were missing for one patient. Data on obesity were missing for five patients. Data on smoking status were missing for 30 patients. Data on the time from symptoms onset to randomization were missing for eight patients. Data on BMI were missing for 83 patients. Data on randomization site were missing for one patient. Data on viral load from NPS were missing for 234 patients. Data on viral load from lower respiratory tract specimens were missing for 527 patients. Data for lymphocyte count were missing for 90 patients. Data for neutrophil count were missing for 136 patients. Data on creatinine were missing for 15 patients. Data on AST/SGOT were missing for 56 patients. Data on ALT/SGPT were missing for 51 patients. Data on CRP were missing for 137 patients. Data on D-dimers were missing for 299 patients. Data on PCT were missing for 356 patients. Data on ferritin were missing for 421 patients.
Moderate disease: hospitalized participants receiving low-flow supplemental oxygen or not requiring oxygen; severe disease: hospitalized participants requiring non-invasive ventilation or high-flow oxygen devices, invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
Primary endpoint
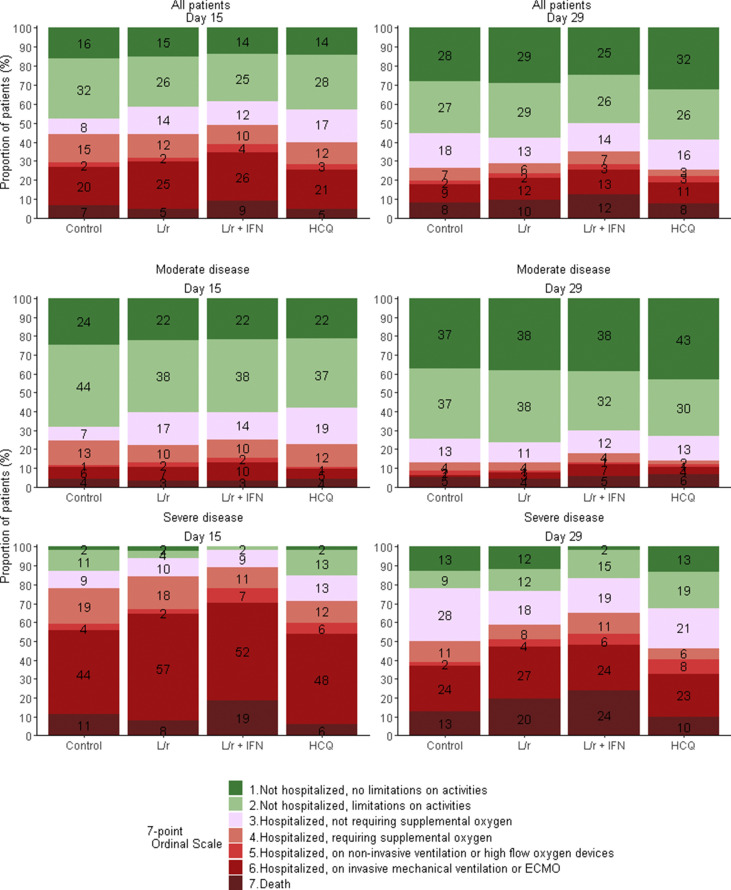
The distribution of the seven-point ordinal scale at day 15 is presented in Fig. 1 and Table 2 . Adjusted OR for clinical improvement (aOR) were not in favour of investigational treatments (<1): lopinavir/ritonavir versus control, aOR 0.83 (95%CI 0.55–1.26, p 0.39); lopinavir/ritonavir plus IFN-β-1a versus control, aOR 0.69 (95%CI 0.45–1.04, p 0.08); hydroxychloroquine versus control, aOR 0.93 (95%CI 0.62–1.41, p 0.75).
Fig. 1.
Clinical status, as measured by the seven-point ordinal scale, at day 15 and day 29 of patients from the intention-to-treat population of the DisCoVeRy trial, according to treatment arm and disease severity at baseline. Reported numbers refer to the proportion of patients with the corresponding level in each group. L/r, lopinavir/ritonavir; L/r + IFN, lopinavir/ritonavir + interferon β-1a; HCQ, hydroxychloroquine.
Table 2.
Primary and secondary outcomes for patients included in the present analysis DisCoVeRy trial, according to disease severity at baselinea
| Overall (n = 583) |
Control (n = 148) |
Lopinavir/ritonavir (L/r) (n = 145) |
Lopinavir/ritonavir + interferon β-1a (L/r + IFN) (n = 145) |
Hydroxychloroquine (HCQ) (n = 145) |
L/r versus control effect measure (95%CI) | L/r + IFN versus control effect measure (95%CI) | HCQ versus control effect measure (95%CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate (n = 372) | Severe (n = 211) | Moderate (n = 94) | Severe (n = 54) | Moderate (n = 94) | Severe (n = 51) | Moderate (n = 91) | Severe (n = 54) | Moderate (n = 93) | Severe (n = 52) | ||||
| Seven-point ordinal scale at day 15,n (%) | |||||||||||||
| 1. Not hospitalized, no limitations on activities | 84 (22.6%) | 3 (1.4%) | 23 (24.5%) | 1 (1.9%) | 21 (22.3%) | 1 (2.0%) | 20 (22.0%) | 0 (0.0%) | 20 (21.5%) | 1 (1.9%) | OR = 0.83 (0.55–1.26) (p = 0.39) | OR = 0.69 (0.45–1.04) (p = 0.08) | OR = 0.93 (0.62–1.41) (p = 0.75) |
| 2. Not hospitalized, limitation on activities | 146 (39.2%) | 16 (7.6%) | 41 (43.6%) | 6 (11.1%) | 36 (38.3%) | 2 (3.9%) | 35 (38.5%) | 1 (1.9%) | 34 (36.6%) | 7 (13.5%) | |||
| 3. Hospitalized, not requiring supplemental oxygen | 54 (14.5%) | 22 (10.4%) | 7 (7.4%) | 5 (9.3%) | 16 (17.0%) | 5 (9.8%) | 13 (14.3%) | 5 (9.3%) | 18 (19.4%) | 7 (13.5%) | |||
| 4. Hospitalized, requiring supplemental oxygen | 41 (11.0%) | 31 (14.7%) | 12 (12.8%) | 10 (18.5%) | 9 (9.6%) | 9 (17.6%) | 9 (9.9%) | 6 (11.1%) | 11 (11.8%) | 6 (11.5%) | |||
| 5. Hospitalized, on non-invasive ventilation or high flow oxygen devices | 6 (1.6%) | 10 (4.7%) | 1 (1.1%) | 2 (3.7%) | 2 (2.1%) | 1 (2.0%) | 2 (2.2%) | 4 (7.4%) | 1 (1.1%) | 3 (5.8%) | |||
| 6. Hospitalized, on invasive mechanical ventilation or ECMO | 27 (7.3%) | 106 (50.2%) | 6 (6.4%) | 24 (44.4%) | 7 (7.4%) | 29 (56.9%) | 9 (9.9%) | 28 (51.9%) | 5 (5.4%) | 25 (48.1%) | |||
| 7. Death | 14 (3.8%) | 23 (10.9%) | 4 (4.3%) | 6 (11.1%) | 3 (3.2%) | 4 (7.8%) | 3 (3.3%) | 10 (18.5%) | 4 (4.3%) | 3 (5.8%) | |||
| 7-point ordinal scale at day 29, n (%) | |||||||||||||
| 1. Not hospitalized, no limitations on activities | 146 (39.2%) | 21 (10.0%) | 35 (37.2%) | 7 (13.0%) | 36 (38.3%) | 6 (11.8%) | 35 (38.5%) | 1 (1.9%) | 40 (43.0%) | 7 (13.5%) | OR = 0.93 (0.62–1.41) (p = 0.74) | OR = 0.76 (0.50–1.15) (p = 0.19) | OR = 1.16 (0.77–1.75) (p = 0.49) |
| 2. Not hospitalized, limitation on activities | 128 (34.4%) | 29 (13.7%) | 35 (37.2%) | 5 (9.3%) | 36 (38.3%) | 6 (11.8%) | 29 (31.9%) | 8 (14.8%) | 28 (30.1%) | 10 (19.2%) | |||
| 3. Hospitalized, not requiring supplemental oxygen | 45 (12.1%) | 45 (21.3%) | 12 (12.8%) | 15 (27.8%) | 10 (10.6%) | 9 (17.6%) | 11 (12.1%) | 10 (18.5%) | 12 (12.9%) | 11 (21.2%) | |||
| 4. Hospitalized, requiring supplemental oxygen | 14 (3.8%) | 19 (9.0%) | 4 (4.3%) | 6 (11.1%) | 4 (4.3%) | 4 (7.8%) | 4 (4.4%) | 6 (11.1%) | 2 (2.2%) | 3 (5.8%) | |||
| 5. Hospitalized, on non-invasive ventilation or high flow oxygen devices | 5 (1.3%) | 10 (4.7%) | 2 (2.1%) | 1 (1.9%) | 1 (1.1%) | 2 (3.9%) | 1 (1.1%) | 3 (5.6%) | 1 (1.1%) | 4 (7.7%) | |||
| 6. Hospitalized, on invasive mechanical ventilation or ECMO | 14 (3.8%) | 52 (24.6%) | 1 (1.1%) | 13 (24.1%) | 3 (3.2%) | 14 (27.5%) | 6 (6.6%) | 13 (24.1%) | 4 (4.3%) | 12 (23.1%) | |||
| 7. Death | 20 (5.4%) | 35 (16.6%) | 5 (5.3%) | 7 (13.0%) | 4 (4.3%) | 10 (19.6%) | 5 (5.5%) | 13 (24.1%) | 6 (6.5%) | 5 (9.6%) | |||
| Time to improvement of 2 categories of the 7-point ordinal scale or hospital discharge within day 29 (days), median [IQR] | 10 [7-16] | 19 [14-29] | 9 [6-14] | 19 [10-29] | 11 [7-17] | 27 [14-29] | 10 [7-19] | 22 [15-29] | 10 [7-17] | 18 [13-29] | HR = 0.71 (0.54–0.93) (p = 0.012) | HR = 0.70 (0.54–0.92) (p = 0.009) | HR = 0.79 (0.61–1.03) (p = 0.08) |
| Time to National Early Warning Score ≤2 or hospital discharge within 29 days (days), median [IQR] | 9 [5-16] | 29 [17-29] | 8 [5-14] | 26 [15-29] | 9 [6-16] | 29 [22-29] | 9 [6-18] | 29 [19-29] | 9 [5-15] | 29 [16-29] | HR = 0.83 (0.63–1.09) (p = 0.17) | HR = 0.75 (0.56–0.99) (p = 0.046) | HR = 0.90 (0.68–1.18) (p = 0.45) |
| Time to hospital discharge within 29 days (days), median [IQR] | 10 [7-20] | 29 [19-29] | 9 [6-16] | 29 [19-29] | 12 [8-21] | 29 [24-29] | 11 [8-26] | 29 [28-29] | 11 [7-20] | 29 [16-29] | HR = 0.77 (0.58–1.02) (p = 0.07) | HR = 0.72 (0.54–0.96) (p = 0.026) | HR = 0.83 (0.62–1.10) (p = 0.20) |
| Oxygenation-free days until day 29 (days), median [IQR] | 22 [15-25] | 0 [0-13] | 22 [15-25] | 4 [0-14] | 22 [15-25] | 0 [0-12] | 22 [13-25] | 0 [0-6] | 22 [16-25] | 3 [0-15] | LSMD = –0.86 (–2.80 to 1.08) (p = 0.39) | LSMD = –1.68 (–3.66 to 0.29) (p = 0.10) | LSMD = 0.17 (–1.84 to 2.17) (p = 0.87) |
| Ventilator-free days until day 29 (days), median [IQR] | 29 [29-29] | 11 [0-20] | 29 [29-29] | 14 [0-22] | 29 [29-29] | 3 [0-19] | 29 [29-29] | 4 [0-16] | 29 [29-29] | 14 [1-22] | LSMD = –0.98 (–2.96 to 1.00) (p = 0.33) | LSMD = –2.01 (–4.03 to 0.00) (p = 0.05) | LSMD = 0.09 (–1.93 to 2.10) (p = 0.93) |
| Death within 28 days, no. (%) | 19 (5.1%) | 35 (16.6%) | 5 (5.3%) | 7 (13.0%) | 4 (4.3%) | 10 (19.6%) | 4 (4.4%) | 13 (24.1%) | 6 (6.5%) | 5 (9.6%) | OR = 1.24 (0.55–2.82) (p = 0.60) | OR = 1.51 (0.69–3.34) (p = 0.30) | OR = 0.93 (0.40–2.20) (p = 0.88) |
NP, nasopharyngeal; LRT, lower respiratory tract; OR, odds ratio; HR, hazard ratio; LSMD, least-square mean difference.
Analyses were stratified on the disease severity at baseline (moderate: seven-point ordinal scale 3 or 4; severe: seven-point ordinal scale 5 or 6), and adjusted effect measures are reported in the table.
Secondary endpoints
There was no significant difference between any of the treatment and control arms on the seven-point ordinal scale at day 29 (Fig. 1 and Table 2). The time to improvement of two categories of the same scale or hospital discharge within day 29 was significantly higher in lopinavir/ritonavir-containing arms than in the control arm: lopinavir/ritonavir versus control, HR 0.71 (95%CI 0.54–0.93, p 0.012 and lopinavir/ritonavir plus IFN-β-1a versus control, HR 0.70 (95%CI 0.54–0.92, p 0.009). The time to NEWS ≤2 or hospital discharge within 29 days was significantly higher in the lopinavir/ritonavir plus IFN-β-1a arm than in the control arm (HR 0.75, 95%CI 0.56–0.99, p 0.046), as was the time to hospital discharge within day 29 (HR 0.72, 95%CI 0.54–0.96, p 0.026).No other significant difference was observed for other secondary outcomes (Table 2 and Supplementary Material Fig. S2–S4).
Virological endpoints
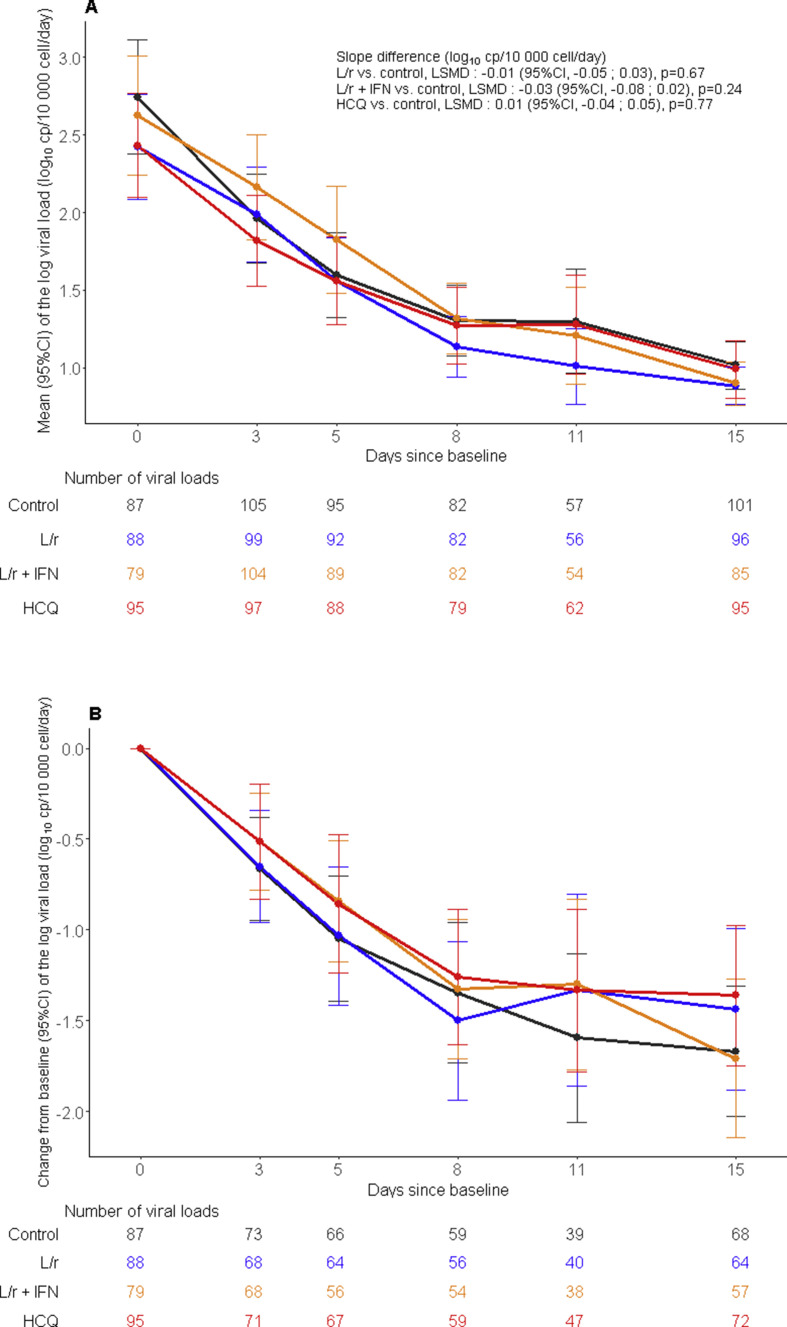
The slope of the decrease of the viral loads in nasopharyngeal swabs over time was not significantly affected by any of the investigational treatments (Fig. 2 ). No significant difference in the proportion of participants with detectable viral loads at each sampling time was observed in the nasopharyngeal swab nor in the lower respiratory tract specimens (Supplementary Material Tables S2 and S3).
Fig. 2.
Evolution of the normalized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in nasopharyngeal swabs between baseline and day 15 in the intention-to-treat population of the DisCoVeRy trial. Means (95%CI) of the log viral loads (panel A), mean changes from baseline (95%CI) of the log viral loads (panel B). L/r, lopinavir/ritonavir (blue line); L/r + IFN, lopinavir/ritonavir + interferon β-1a (yellow line); HCQ, hydroxychloroquine (red line); control (black line). LSMD, least-square mean difference; 95%CI, 95% confidence interval.
Trough concentrations of experimental treatments
At day 3, median trough plasma concentrations of lopinavir were 20 328 ng/mL (IQR 13 033–26 640) and 20 028 ng/mL (15 290–25 718) and of ritonavir were 536 ng/mL (312–1010) and 606 ng/mL (388–1070) in the lopinavir/ritonavir and in the lopinavir/ritonavir plus IFN-β-1a, respectively (Supplementary Material Table S4). Median trough plasma concentrations of hydroxychloroquine were 120 ng/mL (65–271).
Safety
The safety analysis included 579 participants (control, n = 148; lopinavir/ritonavir, n = 144; lopinavir/ritonavir plus IFN-β-1a, n = 144; hydroxychloroquine, n = 143). Safety outcomes are presented in Table 3 . Among 2399 reported AEs, 477 were graded 3 or 4 in 205 patients and reported mostly in lopinavir/ritonavir-containing arms (Table 3).
Table 3.
Summary of adverse events according treatment group in the modified intention-to-treat population
| Overall (n = 579) |
Control (n = 148) |
Lopinavir/ritonavir (L/r) (n = 144) |
Lopinavir/ritonavir + interferon β-1a (L/r + IFN) (n = 144) |
Hydroxychloroquine (HCQ) (n = 143) |
L/r versus control p-value | L/r + IFN versus control p-value | HCQ versus control p-value | |
|---|---|---|---|---|---|---|---|---|
| No. events/no. Patients | No. patients (%) | No. patients (%) | No. patients (%) | No. patients (%) | ||||
| Any adverse events | 2399/450 | 105 (70.9%) | 119 (82.6%) | 117 (81.3%) | 109 (76.2%) | 0.02 | 0.04 | 0.35 |
| Any grade 3 or 4 adverse events | 477/205 | 48 (32.4%) | 56 (38.9%) | 58 (40.3%) | 43 (30.1%) | 0.27 | 0.18 | 0.71 |
| Any serious adverse events | 608/274 | 57 (38.5%) | 76 (52.8%) | 78 (54.2%) | 63 (44.1%) | 0.02 | 0.01 | 0.34 |
| Any serious adverse event related to the experimental treatmenta | — | — | 27 (18.8%) | 45 (31.3%) | 25 (17.5%) | — | — | — |
| Death related to the experimental treatmenta | — | — | 1 (0.1%) | 3 (2.1%) | 0 (0%) | — | — | — |
| Premature suspension or discontinuation of the experimental treatmentb | 77 (13.3%) | — | 17 (11.8%) | 43 (29.9%) | 17 (11.9%) | — | — | — |
| Most relevant serious adverse events: | ||||||||
|
65/65 | 18 (12%) | 19 (13%) | 17 (12%) | 11 (8%) | |||
|
47/46 | 16 (11%) | 7 (5%) | 10 (7%) | 13 (9%) | |||
|
50/50 | 9 (6%) | 16 (11%) | 11 (8%) | 14 (10%) | |||
|
17/17 | 3 (2%) | 3 (2%) | 8 (6%) | 3 (2%) | |||
|
41/35 | 3 (2%) | 8 (6%) | 12 (8%) | 12 (8%) | |||
|
27/27 | 6 (4%) | 10 (7%) | 5 (3%) | 6 (4%) | |||
|
25/25 | 2 (1%) | 5 (3%) | 12 (8%) | 6 (4%) | |||
|
21/21 | 2 (1%) | 6 (4%) | 7 (5%) | 6 (4%) | |||
|
6/6 | 0 (0%) | 2 (1%) | 3 (2%) | 1 (1%) | |||
In the ‘Overall’ column, numbers refer to number of events and number of patients. In other columns, numbers refer to number of patients (%). Some patients had more than a single serious adverse event. Analyses were performed on the modified intention-to-treat population. P-value refer to Fisher exact test.
According to the investigator's judgement. Among participants with the occurrence of the serious adverse event related to the experimental treatment, 14 (51.9%) in the lopinavir/ritonavir arm, 32 (71.1%) in the lopinavir/ritonavir plus IFN-β-1a arm and 12 (48.0%) in the hydroxychloroquine arm discontinued the experimental treatment.
Including renal failure in 30 patients, hepatic disorders in 18 patients and electrocardiogram abnormalities in eight patients. IFN treatment was completed in all patients from the lopinavir/ritonavir + interferon β-1a arm.
Excluding acute renal failures defined based on the RIFLE classification.
A total of 608 SAEs were reported in 274 participants; 149 (24.5%) were related to the investigational drug according to the investigator's judgment (lopinavir/ritonavir arm, n = 37; lopinavir/ritonavir plus IFN-β-1a arm, n = 71; hydroxychloroquine arm, n = 41). A significantly higher number of patients experienced at least one SAE in the lopinavir/ritonavir-containing arms than in the control arm (Table 3). The most frequently reported SAEs were acute respiratory failure (n = 65, 11%), acute kidney injury (n = 50, 8.2%), acute respiratory distress syndrome (n = 47, 8%), arrhythmia (n = 41, 7%), pulmonary embolism (n = 27, 5%), and sepsis including those related to super-infections (n = 21, 4%). Thirteen per cent of participants (n = 76) developed at least one kidney-related SAE. Among these, 12 had acute renal failure upon admission, and 66 were critically ill ventilated patients with acute kidney injury. Among 57 fatal SAEs, 23 had a pulmonary origin, and 34 had a non-pulmonary origin. Four non-pulmonary-related deaths were linked to investigational treatments by investigators (lopinavir/ritonavir arm, n = 1; lopinavir/ritonavir plus IFN-β-1a arm, n = 3).
Discussion
We report here the results of the DisCoVeRy clinical trial evaluating lopinavir/ritonavir with or without IFN-β-1a, or hydroxychloroquine in comparison with control, for the treatment of inpatients with COVID-19. Participants had mostly moderate disease (63.4%) covering a large spectrum of clinical presentations. Inclusions were prematurely stopped for futility, so that the number of included patients is lower than the estimated sample size. Consistently with the results of the Solidarity trial, investigational treatments failed to improve the clinical course of COVID-19. No effect on SARS-CoV-2 clearance was observed, using a reproducible normalized method. Furthermore, significantly more SAEs were reported in the lopinavir/ritonavir-containing arms than in the control arm.
Two randomized trials conducted in hospitalized COVID-19 patients found no benefit of lopinavir/ritonavir in terms of 28-day mortality or of progression to mechanical ventilation or death [9,19]. No added benefit was observed using IFN-β-1a, as the median time to randomization of 9 days may have been too long to allow an immune-mediated boosting effect on viral clearance. We observed plasma overexposure of lopinavir relative to target concentrations obtained in HIV-infected patients, which was possibly responsible for the higher rate of SAEs and more acute kidney injury than in controls. The SARS-CoV-2-induced inflammatory burden may have reduced cytochrome P450 activity and modified plasma α-1-acid glycoprotein levels, an acute-phase protein which binds protease inhibitors [20,21]. Reported in vitro half-maximal inhibitory concentration (EC50) for SARS-CoV-2 is 16 400 ng/mL [22] (while the EC50 for HIV is 70 ng/mL [23]), a >200-fold difference, suggesting that significantly higher concentrations of lopinavir are needed to enhance SARS-CoV-2 clearance. A recent physiologically based pharmacokinetic model suggested that standard regimens of lopinavir/ritonavir are not sufficient to achieve efficacy through unbound lung concentrations [24]. In our study, trough lopinavir plasma concentrations at day 3 were more than two-fold higher than expected with the standard dose [25], but were below the EC50 of SARS-CoV-2 in 25% of participants.
Several larger-scale randomized controlled trials conducted in hospitalized COVID-19 patients failed to demonstrate the clinical efficacy of hydroxychloroquine [26,27]. Our results are in line with these conclusions. We report that hydroxychloroquine does not accelerate SARS-CoV-2 clearance, a finding consistent with preclinical data [28]. Based on in vitro EC50 against SARS-CoV-2 (242 ng/mL), the target plasma concentration was reached in only 25% of participants at day 3, and optimal intrapulmonary exposure might have been achieved only at day 10 [10,15]. It could be argued that the dosing regimen administered in the DisCoVeRy trial was insufficient to rapidly reach target concentrations. However, the Solidarity and Recovery trials, which both used a doubled hydroxychloroquine dosing regimen, did not bring evidence of clinical benefit either [13,27].
The trial has limitations: the complexity of blinding treatments with different routes of administration and the need to initiate the trial very rapidly led to the choice of an open-labelled design. The trial did not target patients at the early phase of the disease, nor did it include arms testing anti-inflammatory agents that could be used as part of the SoC in any arm. In addition, the trial was performed in the early phase of the COVID-19 pandemic and the SoC underwent substantial changes over time, adapting to knowledge acquisition, especially regarding the use of corticosteroids in COVID-19.
Conclusion
In patients admitted to hospital with COVID-19, lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine were not associated with clinical improvement at day 15 and day 29, nor in a reduction in viral shedding, and generated significantly more SAEs in lopinavir/ritonavir-containing arms. These findings do not support the use of these investigational treatments for patients hospitalized with COVID-19.
Author contributions
Writing—original draft: FA, NPS, ADi, MBD, CB and FM. Writing—review and editing: JP, DB, MH, MPL, GP, DC and YY. Conceptualization: FA, NPS, JP, MBD, GP, BL, DC, YY and FM. Investigation: FA, NPS, JP, MBD, Adi, NM, FXL, FR, FG, AK, SJ, JR, SN, FD, RCJ, KB, JCN, VT, AC, CDu, JC, SL, JM, RG, BM, EF, VP, SG, OL, KL, JPL, AM, GMB, LB, ÉBN, AGB, OE, LP, FW, JCR, JR, TS, MH, CA, MPL and GP. Methodology: FA, NPS, JP, MBD, DC, CB and FM. Data curation: ADi, ADe, NM, ADu and TA. Formal analysis: DB, ADu, DC, CB and FM. Project administration: FA, CD and FM. Funding acquisition: FA, CDe, JS, DC, YY and FM.
Transparency declaration
FR reports personal fees from Gilead Sciences, personal fees from MSD, personal fees from Pfizer, personal fees from TheraTechnologies, personal fees from ViiV Healthcare, outside the submitted work. FG reports grants from BioMerieux, personal fees and non-financial support from Gilead, non-financial support from Corevio, outside the submitted work. GP reports grants and personal fees from Gilead Sciences, grants and personal fees from Merck, grants and personal fees from ViiV Healthcare, grants and personal fees from TheraTechnologies, outside the submitted work. KL reports personal fees and non-financial support from Gilead, personal fees and non-financial support from Janssen, personal fees and non-financial support from MSD, personal fees and non-financial support from ViiV Healthcare, personal fees and non-financial support from Abbvie, during the conduct of the study. YY has nothing to disclose. He has been a board member receiving consultancy fees from ABBVIE, BMS, Gilead, MSD, J&J, Pfizer, and ViiV Healthcare; however, all these activities have been stopped in the 3 past years. FL reports personal fees from Gilead, personal fees and non-financial support from MSD, non-financial support from Astellas, and non-financial support from Eulmedica, outside the submitted work. AK reports personal fees from Baxter, personal fees from Aspen, and personal fees from Aguettant, outside the submitted work. SN reports personal fees from MSD, personal fees from Pfizer, personal fees from Gilead, personal fees from Biomérieux, and personal fees from BioRad, outside the submitted work. FD reports personal fees from Gilead, outside the submitted work. JN reports non-financial support from MSD France, non-financial support from GILEAD Sciences and personal fees from PASCALEO, outside the submitted work. JM reports non-financial support from GILEAD, outside the submitted work. AM reports personal fees from MSD, personal fees from GILEAD, personal fees from JANSSEN and personal fees from Viiv Healthcare, outside the submitted work. MH reports grants from Fonds Erasme—COVID—Université Libre de Bruxelles, grants from Belgian health Care Knowledge Centre, during the conduct of the study, personal fees from Gilead advisory board on education on invasive fungal infections, personal fees from Pfizer: moderator for session on Isavuconazole, outside the submitted work. DC reports personal fees from Gilead, grants and personal fees from Janssen, outside the submitted work. CB reports personal fees from Da Volterra and personal fees from Mylan Pharmaceuticals, outside the submitted work. FM reports grants from Sanofi and grants and personal fees from Da Volterra, outside the submitted work. All other authors have nothing to disclose. The study was founded by Programme Hospitalier de Recherche Clinique (PHRC-20-0351) (Ministry of Health), from the DIM One Health Île-de-France (R20117HD), and from REACTing, a French multidisciplinary collaborative network working on emerging infectious diseases. The funding sources had no role in the analysis of the data or in the decision of publication.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokugamage K.G., Hage A., de Vries M., Valero-Jimenez A.M., Schindewolf C., Dittmann M., et al. SARS-CoV-2 is sensitive to type I interferon pretreatment. BioRxiv. 2020 doi: 10.1101/2020.03.07.982264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clementi N., Ferrarese R., Criscuolo E., Diotti R.A., Castelli M., Scagnolari C., et al. Interferon-beta-1a inhibition of severe acute respiratory syndrome-coronavirus 2 in vitro when administered after virus infection. J Infect Dis. 2020;222:722–725. doi: 10.1093/infdis/jiaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and Projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ader F., Discovery French Trial Management T. Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Solidarity Trial Consortium Repurposed antiviral drugs for Covid-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S., et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir–ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le M.P., Peiffer-Smadja N., Guedj J., Neant N., Mentre F., Ader F., et al. Rationale of a loading dose initiation for hydroxychloroquine treatment in COVID-19 infection in the DisCoVeRy trial. J Antimicrob Chemother. 2020;75:2376–2380. doi: 10.1093/jac/dkaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etievant S., Bal A., Escuret V., Brengel-Pesce K., Bouscambert M., Cheynet V., et al. Performance assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories. J Clin Med. 2020;9:1871. doi: 10.3390/jcm9061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung B.H., Rezk N.L., Bridges A.S., Corbett A.H., Kashuba A.D.M. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography–tandem mass spectrometry. Biomed Chromatogr. 2007;21:1095–1104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 18.Chhonker Y.S., Sleightholm R.L., Li J., Oupický D., Murry D.J. Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC–ESI–MS/MS: an application for pharmacokinetic studies. J Chromatog B. 2018;1072:320–327. doi: 10.1016/j.jchromb.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzolini C., Stader F., Stoeckle M., Franzeck F., Egli A., Bassetti S., et al. Effect of systemic inflammatory response to SARS-CoV-2 on lopinavir and hydroxychloroquine plasma concentrations. Antimicrob Agents Chemother. 2020;64:e01177–e01220. doi: 10.1128/AAC.01177-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ofotokun I., Lennox J.L., Eaton M.E., Ritchie J.C., Easley K.A., Masalovich S.E., et al. Immune activation mediated change in alpha-1-acid glycoprotein: impact on total and free lopinavir plasma exposure. J Clin Pharmacol. 2011;51:1539–1548. doi: 10.1177/0091270010385118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croxtall J.D., Perry C.M. Lopinavir/Ritonavir: a review of its use in the management of HIV-1 infection. Drugs. 2010;70:1885–1915. doi: 10.2165/11204950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Thakur A., Tan S.P.F., Chan J.C.Y. Physiologically-based pharmacokinetic modeling to predict the clinical efficacy of the coadministration of lopinavir and ritonavir against SARS-CoV-2. Clin Pharmacol Therapeut. 2020;108:1176–1184. doi: 10.1002/cpt.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaletra Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/kaletra-epar-product-information_en.pdf Avalable at:
- 26.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. New Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The RECOVERY Collaborative Group Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.