Introduction
On 21 January 2021, an interim analysis of a phase-3 clinical trial revealed that the investigational, recombinant-vector COVID-19 vaccine developed by Janssen Pharmaceuticals, Janssen Ad26.COV2.S, was both effective and safe in preventing moderate and severe COVID-19 in adults.1 The vaccine was approved in late February 2021 by the Food and Drug Administration, following Pfizer's BNT162b2 and Moderna's messenger RNA-1273 approval in in December 2020. All 3 vaccines are considered safe by clinical trials. Fatigue, myalgias, and headaches have been reported in up to 10% of both of the Pfizer and Moderna vaccine recipients.2 About 1.5% of the patients who received the Moderna vaccine reported hypersensitivity reactions, such as rash and urticaria, and similar morbilliform hypersensitivity rashes have been reported with the Pfizer vaccine.3,4 Johnson and Johnson's vaccine had generally mild side effects in clinical trials, including fever in 9% of volunteers.1
As the number of vaccinated individuals in the general population rises, sporadic, atypical reactions are more likely to be observed and reported. Herein, we report a case of a 74-year old man who experienced a severe cutaneous adverse reaction (SCAR) following administration of the Janssen Ad26.COV2.S vaccine. The differential diagnosis included acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), and AGEP-DRESS overlap.
Case report
A 74-year-old man with panhypopituitarism secondary to craniopharyngioma resection, vision loss of the left eye, neurogenic bladder, and obstructive sleep apnea presented to the emergency room at Virginia Commonwealth University Medical Center with a new-onset rash beginning 3 days (t + 3) after receiving the Janssen Ad26.COV2.S vaccine; he reported ipsilateral arm discomfort, including the axilla, within 24 hours of administration. Sulfa drugs and amoxicillin-clavulanic acid were his only known allergies; no prior vaccination-related reactions were reported. He denied recent illness or medication changes prior to onset. Of note, he took prednisone 5 mg daily for adrenal insufficiency, but his community dermatologist increased this to 20 mg daily for a suspected stasis dermatitis flare when this eruption was mostly on his lower extremities.
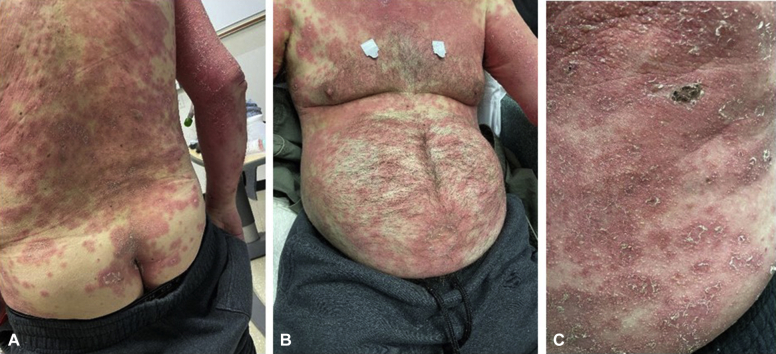
Physical examination revealed a generalized distribution (50% of the body surface area) of erythematous plaques, studded with numerous small, non-follicular pustules (Fig 1). The rash spared the face, genitals, and mucosae. Significant acral swelling was observed in the absence of palpable lymphadenopathy.
Fig 1.
Widespread, erythematous plaques (A and B) with small, non-follicular pustules and scale (C).
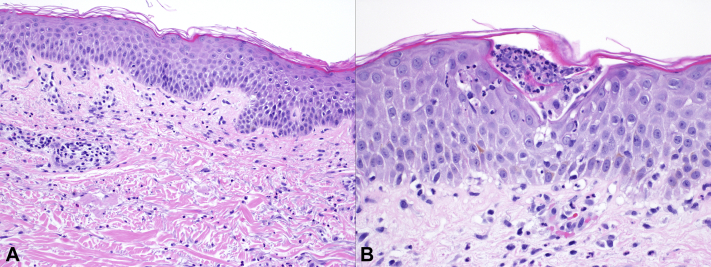
Laboratory test results at presentation (t + 13) were notable for an elevated white blood cell count reflecting absolute neutrophilia and an elevated eosinophil count (Table I). Within 24 hours, his eosinophil count roughly tripled to 1.6 × 109 cells/L. Hepatic function panel results were within the normal limits, and creatinine levels elevated transiently but returned to normal. A biopsy from the right shoulder revealed epidermal spongiosis with focal, occasional, subcorneal neutrophilic pustules and dermal neutrophilic inflammation with eosinophils, consistent with a spongiotic and pustular drug eruption (Fig 2). A direct immunofluorescence study from the same site was negative.
Table I.
Results of the main diagnostic workup during admission. The workup was generally unremarkable, with the exception of neutrophilia and the eosinophil count; the eosinophil count later increased by a factor of 3 (data not shown). The electrocardiogram was within normal limits
| Biochemistry | ||
|---|---|---|
| Component | Value w/units | Normal range |
| Sodium | 124 mmol/L | 135-145 |
| Potassium | 5.5 mmol/L | 3.6-5.1 |
| Chloride | 91 mmol/L | 100-110 |
| Carbon dioxide | 27 mmol/L | 21-33 |
| Anion gap | 6 mmol/L | 0-12 |
| Glucose | 127 mg/dL | 65-100 |
| BUN | 19 mg/dL | |
| Creatinine | 1.08 mg/dL | 0.60-1.20 |
| Calcium | 8.8 mg/dL | 8.9-10.7 |
| AST | 16 units/L | 0-50 |
| ALT | 7 units/L | 0-60 |
| Alkaline Phosphatase | 87 units/L | 0-120 |
| Bilirubin, total | 0.6 mg/dL | 0.0-1.3 |
| Bilirubin, conjugated | 0.3 mg/dL | 0.0-0.4 |
| Complete blood count | ||
|---|---|---|
| Component | Value w/units | Normal range |
| White blood cells | 19.7 109/L | 3.7-9.7 |
| Red blood cells | 4.47 1012/L | 4.54-5.78 |
| Hemoglobin | 14.6 g/dL | 13.3-17.2 |
| Hematocrit | 43% | 38.9-50.9 |
| Platelets | 359 109/L | 179-373 |
| % Neutrophils | 92.10% | |
| % Lymphocytes | 2.30% | |
| % Monocytes | 2.50% | |
| % Eosinophils | 2.80% | |
| % Basophils | 0.30% | |
| Neutrophils | 18.1 109/L | 2.0-6.7 |
| Lymphocytes | 0.5 109/L | 1.1-3.3 |
| Monocytes | 0.5 109/L | 0.2-0.7 |
| Eosinophils | 0.6 109/L | 0.0-0.4 |
| Basophils | 0.1 109/L | 0.0-0.1 |
| % Nucleated red blood cells | 0.00% | 0.0-0.2 |
| Respiratory pathogen panel | ||
|---|---|---|
| Pathogen | Value w/units | Normal range |
| Adenovirus | Not Detected | |
| Coronavirus 229E | Not Detected | |
| Coronavirus HKU1 | Not Detected | |
| Coronavirus NL63 | Not Detected | |
| Coronavirus OC43 | Not Detected | |
| Influenza A | Not Detected | |
| Influenza B | Not Detected | |
| Human metapneumovirus | Not Detected | |
| Parainfluenza 1 | Not Detected | |
| Parainfluenza 2 | Not Detected | |
| Parainfluenza 3 | Not Detected | |
| Parainfluenza 4 | Not Detected | |
| Respiratory syncytial virus | Not Detected | |
| Rhinovirus/enterovirus | Not Detected | |
| Bordetella pertussis | Not Detected | |
| Chlamydophila pneumoniae | Not Detected | |
| Mycoplasma pneumoniae | Not Detected | |
| SARS-CoV-2, NAA | Not Detected | |
| Other tests | ||
|---|---|---|
| Component | Value w/units | Normal range |
| Erythrocyte sedimentation rate | 14 mm/hour | 0-15 |
| C-reactive protein | 8.7 mg/dL | 0-0.5 |
| Antinuclear antibody | Negative | |
| Cytomegalovirus DNA | 0.00 (log) | |
| Epstein-Barr virus DNA | 0.00 (log) | |
| Hepatitis A IgM/IgG | Not detected | |
| Hepatitis C core antibody | Not detected | |
| Hepatitis B core antibody | ||
| IgG | Positive | |
| IgM | Equivocal | |
| Hepatitis B surface antigen | Negative | |
| Hepatitis B surface antibody | Positive | |
| Hepatitis B viral DNA | 0.00 (log) | |
| HIV screening | Negative | |
| Chlamydia NAA | Negative | |
| Neisseria gonorrhea NAA | Negative | |
| Urinalysis | WNL | |
| Blood culture | Negative | |
ALP, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; NAA, nucleic acid amplification; WNL, within normal limits.
Fig 2.
Histopathology of a biopsy from the right shoulder with (A) epidermal spongiosis and dermal neutrophilic inflammation with occasional eosinophils (hematoxylin-eosin staining; original magnification, ×200) and (B) scattered subcorneal neutrophilic pustules. (Hematoxylin-eosin staining; original magnification, ×400.)
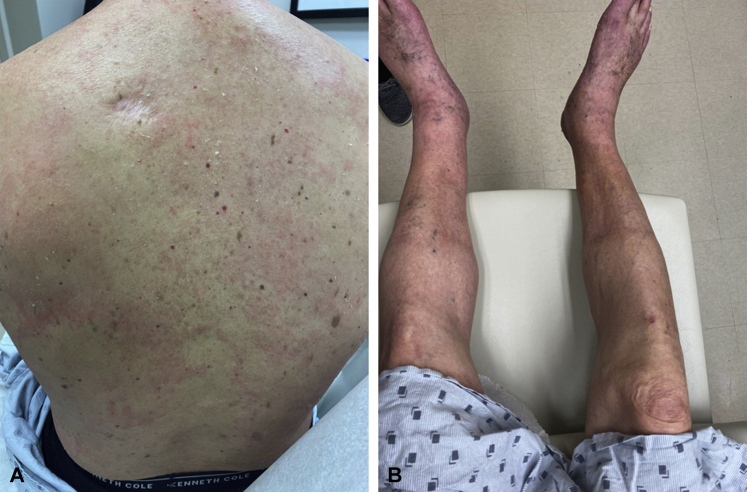
Based on the clinical findings and workup, the differential diagnosis included AGEP, DRESS, and AGEP-DRESS overlap. He responded to oral prednisone 20 mg PO daily and topical steroids. At follow up (t+20), his liver enzymes and creatinine level remained stable and within the normal limits, his absolute eosinophil count was also within the normal range, and his skin had improved (Fig 3). The acral swelling was also reduced.
Fig 3.
Markedly reduced density and decreased intensity of erythematous plaques on the torso and extremities (A and B) compared with the initial presentation (Fig 1). Improving lower extremity edema was also seen (B) but not pictured in Fig 1.
Discussion
SCARs to drugs encompass a broad spectrum of conditions, mainly DRESS syndrome or drug-induced hypersensitivity syndrome, Stevens-Johnson syndrome, toxic epidermal necrolysis, and AGEP.5 SCARs are more commonly associated with certain drug categories, including anticonvulsants, antipsychotics, antibiotics, and allopurinol. Less frequently, cases of systemic and severe cutaneous reactions have been observed after vaccine administration.6 Specifically, Stevens-Johnson syndrome, toxic epidermal necrolysis, and AGEP are serious, rare adverse events associated with a variety of vaccines, including those for pneumonia, influenza, and measles-mumps-rubella.7
Severe systemic and cutaneous responses to vaccinations, although rare, pose a considerable risk for morbidity and mortality for affected patients. The current case demonstrates a SCAR in close temporal association with administration of the Janssen COVID-19 vaccine. Of the various conditions classified as severe cutaneous drug reactions, this case exhibited many features that could suggest AGEP, such as timing of the rash, absolute neutrophilia, lack of visceral involvement, and clinical morphology, although DRESS could not be definitively excluded. Absolute eosinophilia and acral swelling are compatible with DRESS but can be seen in AGEP, and the clinical and histologic morphologies of DRESS and AGEP can overlap.8,9
Although urticarial lesions have been described in patients who have received all 3 of the currently approved COVID-19 vaccines, this case describes a SCAR that was temporally-associated with administration of Johnson and Johnson's Janssen Ad26.COV2.S vaccine. Concerns about possible adverse events may make patients hesitant to receive the vaccine, and physicians should remain up to date on this rapidly-evolving front so as to best advise and educate patients.
Conflicts of interest
None declared.
Footnotes
Funding sources: None.
References
- 1.Livingston E.H., Malani P.N., Creech C.B. The Johnson & Johnson vaccine for COVID-19. JAMA. 2021;325(15):1575. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 2.Wadman M. Public needs to prep for vaccine side effects. Science. 2020;370(6520):1022. doi: 10.1126/science.370.6520.1022. [DOI] [PubMed] [Google Scholar]
- 3.Wei N., Fishman M., Wattenberg D., Gordon M., Lebwohl M. ‘COVID arm’: a reaction to the Moderna vaccine. JAAD Case Rep. 2021;10:92–95. doi: 10.1016/j.jdcr.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jedlowski P.M., Jedlowski M.F. Morbilliform rash after administration of Pfizer-BioNTech COVID-19 mRNA vaccine. Dermatol Online J. 2021;27(1) 13030/qt4xs486zg. [PubMed] [Google Scholar]
- 5.Duong T.A., Valeyrie-Allanore L., Wolkenstein P., Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106):1996–2011. doi: 10.1016/S0140-6736(16)30378-6. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblatt A.E., Stein S.L. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33(3):327–332. doi: 10.1016/j.clindermatol.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Stone C.A., Jr., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85(12):2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D.H., Koh Y.I. Comparison of diagnostic criteria and determination of prognostic factors for drug reaction with eosinophilia and systemic symptoms syndrome. Allergy Asthma Immunol Res. 2014;6(3):216–221. doi: 10.4168/aair.2014.6.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szatkowski J., Schwartz R.A. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73(5):843–848. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]