To the Editor
At least 20% of coronavirus disease 2019 (COVID-19) patients develop acute hypoxemic respiratory failure requiring admission to intensive care unit (ICU) in 5–32% of the cases [1]. Hyper-inflammatory activation characterized by immune cell infiltration and elevated levels of cytokines as interleukin (IL)-6, IL-8, IL-10, IP-10 and PTX3 was reported as the main mechanism leading to critical illness and severe acute respiratory distress syndrome (ARDS) [2,3]. Thus, reducing pro-inflammatory cytokines may represent a promising therapeutic strategy.
A treatment able to reduce circulating cytokines in critically ill patients is CytoSorb® (Cytosorbent™, USA), a device containing adsorbent polymer beads designed to irreversibly remove cytokines currently used for septic shock and other conditions where elevated levels of cytokines are present. Along with the beneficial effect on systemic inflammation, CytoSorb® can be easily integrated with all extracorporeal circulation systems, as Continuous Renal Replacement Therapy (CRRT) or Extra Corporeal Membrane Oxygenation (ECMO). Despite the lack of published results, based on bench performance testing and reported clinical experience, CytoSorb® was granted FDA emergency approval for critically ill SARS-CoV-2 patients on April 10, 2020.
In this report we present the laboratory and clinical outcomes of COVID-19 patients treated with CytoSorb® during the March 2020 pandemic crisis at Papa Giovanni XXIII Hospital, Bergamo, Italy.
From March 7th to March 24th, 2020, 11 consecutive patients with microbiological confirmed SARS-CoV-2 infection and severe disease requiring invasive mechanical ventilation (IMV) were admitted in ICU and treated with CytoSorb® to remove the excess of cytokine. Detailed description of the study is presented in the Supplementary Material and Methods. All patients were male, overweight and only 3 (27%) were over 70 years old. Specifically, median age was 62 years (range 35–75) and median body mass index was 28 (range 22–37). History of hypertension was present in 64% of the patients while 27% were affected by diabetes. Despite having no severe comorbidity, all patients had rapidly evolving aggressive disease characterized by the need of non-invasive ventilation, followed by IMV, after a median of 3 days (range 0–4 days) from hospital admission. Median SOFA score at ICU admission was 6 (range 4.5–6.5). A complete summary of clinical and laboratory characteristics of the patients are presented in Supplementary Table 1. Best supportive care was provided according to hospital guidelines of that moment and included antibiotic therapy for community-acquired pneumonia (azithromycin), antiretroviral therapy (lopinavir/ritonavir 200/50 mg two tablets twice daily, darunavir/cobicistat 800/150 mg one tablet once daily) and hydroxychloroquine 200 mg twice daily. All patients received a protective ventilation (Tidal Volume 6–8 ml/kg of predicted body weight, Driving Pressure ≤14 cmH2O, Plateau Pressure ≤28 cmH2O). If needed, after an adequate fluid resuscitation based on goal-directed therapy, they received a catecholamine support to obtain a mean arterial pressure > 65 mmHg and/or a diuretic therapy if urine output was < 0.5 ml/kg/min for 6 hours. Catecholamine support, concomitant use of steroid and antimicrobial therapy at enrolment and during study are described in Supplementary Table 2.
A double lumen venous catheter was inserted and CytoSorb® cartridge was used with continuous veno-venous hemodiafiltration (CVVHDF) circuit with Regional Citrate Anticoagulation (RCA), to allow a longer duration of the adsorber. Ultraflux AV1000S (Fresenius) filter was used. The CVVHDF was applied at the dose of 35 ml/kg/h with interquartile range of 32–39 and blood flow rate was 100–150 ml/min. Cytosorb® adsorber was changed every 24 hours. Two patients were treated with one 24-hour cycle, while the remaining 9 were treated for 48 hours.
No unexpected adverse event was observed with CytoSorb® use. After a median follow-up of 16 days (range 6–30 days), 2 patients died (18%) after 6 and 16 days from enrolment, and 9 survived. In 3 patients weaning was completed with extubation at day 14, 15 and 27 from enrollment. Six patients required percutaneous tracheostomy to achieve respiratory weaning. All 9 patients were discharged from ICU after a median of 25 days (range 11–52). Additional details are described in Supplementary Table 3.
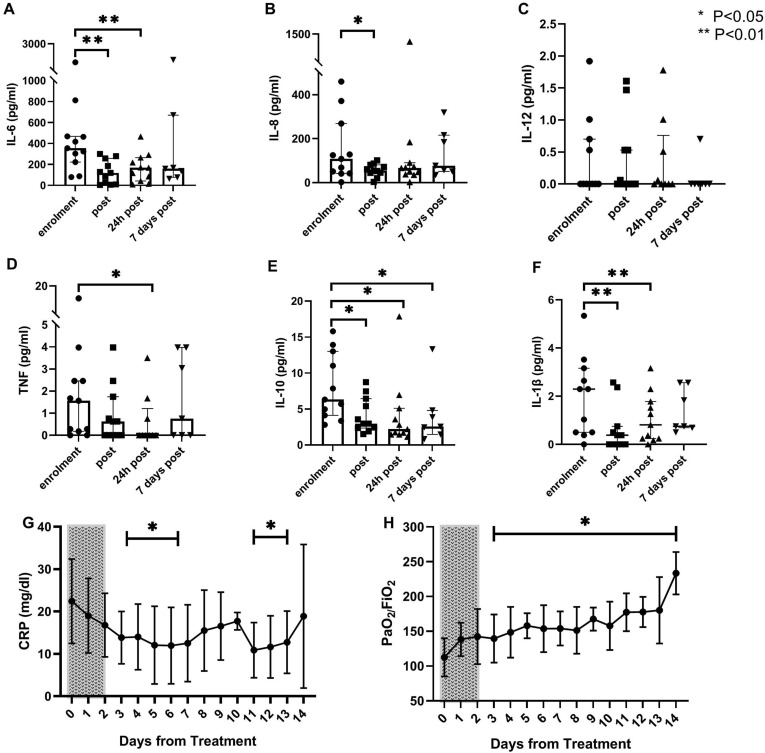
Cytokines levels were evaluated before and immediately after treatment. In addition, samples were collected after 24 hours and 7 days from treatment end. With the exception of IL-12, all the cytokines were elevated before treatment (Fig. 1 ). In particular, markedly elevated levels of IL-6 and IL-8 were observed. At treatment end and 24 hours after, a general reduction of cytokines concentration was observed, with a significant reduction of IL-6, IL-8, IL-10 and IL-1β (P < 0.05). After 7 days from treatment end, samples were available in 7 patients (64%); median cytokines levels were stable compared to the previous assessment.
Fig. 1.
(A–F) Individual level (dots), median level (bars) and interquartile range of serum cytokines before (enrolment) and after (post, after 24 hours and after 7 days) treatment with CytoSorb®. Statistical significance is referred to baseline level, comparison between the timepoints“enrolment” and “after 7 days” was done only for the 7 patients with available samples. (G–H) Daily median serum level and interquartile ranges of CRP and PaO 2/FiO 2 ratio for the first 15 days after enrolment (Day 0–14). Dotted rectangle indicates treatment period. A statistical significance difference from pre-treatment values (Day 0) is present from Day 3 to Day 14 for both parameters. Legend: IL: interleukin; TNF: tumour necrosis factor; CRP: C-reactive protein.
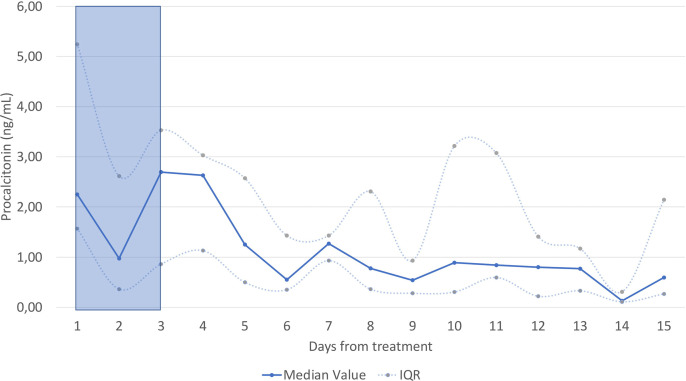
C-reactive protein (CRP) levels and respiratory parameters, including the P/F ratio, were assessed daily. Consistent with the trend observed for IL-6, a significant drop of CRP median levels was observed starting from 48 hours after treatment start (Panel G). Median P/F value at enrolment was 103 (IQR 88–133), consistent with a moderate to severe ARDS (Panel H). The decrease in the inflammatory status was associated with a progressive improvement in the respiratory function, with a significant increase in P/F from the first day after the end of the therapy. A similar trend was observed for procalcitonin (Supplementary Figure 1).
With the limitation of the small sample size, CytoSorb® proved to be safe in COVID-19 patients. A clinical improvement was observed in most of the treated patients despite the severity of the disease. Several treatments have been assessed in COVID-19 as steroids or monoclonal antibodies against interleukins or complement pathway, and some of these have been reported to be beneficial in modulating the inflammatory response [[4], [5], [6]]. However, differently from pharmacological treatments, the use of CytoSorb® does not raise concerns of secondary bacterial infection, as the device is currently used in septic shock. In this study CytoSorb® was used empirically for 24–48 hours based on previous experience in septic shock. The persistence of significant levels of IL-6 and CRP after CytoSorb® treatment may suggest that a prolonged treatment can improve the efficacy in controlling COVID-19 hyperinflammatory status. Further studies are required to assess if CytoSorb® can improve the clinical outcome of COVID-19 patients requiring IMV.
Author's contribution
GG, IR, FF designed the study; MD, LG, GB, FL contributed to data acquisition; MD, GG, IR analyzed data; MD, LG, GG wrote the manuscript; all the authors contributed to data interpretation, critical review and final approval of the manuscript.
Declaration of competing interest
GG reports non-financial support from Gilead Kite, Roche, Takeda and Janssen, and personal fees from Gilead Kite, Autolus, Roche, IQvia, Takeda, Amgen and Italfarmaco, outside the submitted work. IR reports travel support from Aferetica. All other authors declare no competing interests.
Acknowledgments
This work was supported by COVID-2020-12371640 grant “Innate and adaptive immunity in COVID-19: from mechanisms to patients”. Cytosorb® filters were donated by Aferetica srl, Italy. We would like to thank Dr. Andjela Kurevija (Aferetica srl) for the statistical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106477.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Guan W., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetta E., et al. Macropahge expression and prognostic significance of the long pentraxin PTX3 in COVID-19. Nat. Immunol. 2020 doi: 10.1101/2020.06.26.20139923. [DOI] [PubMed] [Google Scholar]
- 4.Gritti G., et al. Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19. Leukemia. 2021 doi: 10.1038/s41375-021-01299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rambaldi A., et al. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020:152001. doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RECOVERY Collaborative Group. Horby P., et al. Dexamethasone in hospitalized patients with covid-19. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021 Feb 25;384(8):693–704. doi: 10.1056/NEJMoa2021436. PMID: 32678530; PMCID: PMC7383595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.