Abstract
An analysis of published data appertaining to the cytokine storms of COVID-19, H1N1 influenza, cytokine release syndrome (CRS), and macrophage activation syndrome (MAS) reveals many common immunological and biochemical abnormalities. These include evidence of a hyperactive coagulation system with elevated D-dimer and ferritin levels, disseminated intravascular coagulopathy (DIC) and microthrombi coupled with an activated and highly permeable vascular endothelium. Common immune abnormalities include progressive hypercytokinemia with elevated levels of TNF-α, interleukin (IL)-6, and IL-1β, proinflammatory chemokines, activated macrophages and increased levels of nuclear factor kappa beta (NFκB). Inflammasome activation and release of damage associated molecular patterns (DAMPs) is common to COVID-19, H1N1, and MAS but does not appear to be a feature of CRS. Elevated levels of IL-18 are detected in patients with COVID-19 and MAS but have not been reported in patients with H1N1 influenza and CRS. Elevated interferon-γ is common to H1N1, MAS, and CRS but levels of this molecule appear to be depressed in patients with COVID-19. CD4+ T, CD8+ and NK lymphocytes are involved in the pathophysiology of CRS, MAS, and possibly H1N1 but are reduced in number and dysfunctional in COVID-19. Additional elements underpinning the pathophysiology of cytokine storms include Inflammasome activity and DAMPs. Treatment with anakinra may theoretically offer an avenue to positively manipulate the range of biochemical and immune abnormalities reported in COVID-19 and thought to underpin the pathophysiology of cytokine storms beyond those manipulated via the use of, canakinumab, Jak inhibitors or tocilizumab. Thus, despite the relative success of tocilizumab in reducing mortality in COVID-19 patients already on dexamethasone and promising results with Baricitinib, the combination of anakinra in combination with dexamethasone offers the theoretical prospect of further improvements in patient survival. However, there is currently an absence of trial of evidence in favour or contravening this proposition. Accordingly, a large well powered blinded prospective randomised controlled trial (RCT) to test this hypothesis is recommended.
Keywords: COVID-19, Cytokine storm, Inflammation, Immune, Interleukin-1, Interleukin-6
1. Introduction
COVID-19 is a biphasic illness where viral driven fever predominates for between 7 and 10 days on average followed by a host mediated immune-inflammatory response of variable intensity [1], [2]. In aggregate, the patterns and levels of inflammation and immune dysregulation observed in the periphery and lungs of COVID-19 patients are characteristic of “cytokine storm”, and this phenomenon is deemed to be the main cause of mortality in patients suffering from this illness [3], [4], [5], [6], [7], [8].
Cytokine storms are also considered to be the cause of high levels of mortality and morbidity in cytokine release syndrome (CRS) [9], macrophage activation syndrome (MAS) [10] and in H1N1 influenza virus-mediated pneumonia [11]. This raises the question of whether the processes which generate cytokine storms are the same in all these conditions or whether they differ. If they are similar, or even identical, then that would suggest that agents such as tocilizumab and anakinra, which have shown success in the treatment of CRS [12] and MAS [13], respectively, could be repurposed for the treatment of COVID-19. However, if these processes are significantly different, such treatments may be ineffective or even harmful as far as COVID-19 patients are concerned. Hence, this paper aims to compare the mechanisms involved in the generation of cytokine storms in each of the conditions above, with a view to proposing answers to these questions.
2. The cytokine storm of COVID-19
2.1. Immune abnormalities reported in patients with COVID-19
There is evidence of severe immune dysregulation, inflammation and hypercytokinemia in the periphery and lungs of patients with severe COVID-19. This is characterised by increased levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-18, IL-10, monocyte chemoattractant protein-1 (MCP-1) and interferon (IFN)-γ-induced protein (IP-10 or CXCL10) [3], [6], [14], [15], [16], [17]. Importantly, this pattern of immune dysregulation is progressive and levels of proinflammatory cytokines and chemokines increase dramatically with illness severity and may be some ten-fold higher in ventilated individuals compared to hospitalised patients with milder disease [18]. In contrast, the weight of evidence suggests that interferon (IFN)-γ levels are reduced in patients with COVID-19 who have not progressed to acute respiratory distress syndrome (ARDS) although in the latter scenario secondary elevation of this molecule may be a significant source of irreversible tissue destruction [19], [20], [21].
Other reported abnormalities commonly associated with excessive systemic inflammation seen in COVID-19 patients include lymphopenia and lymphocyte exhaustion, as evidenced by drastically depleted CD4+ T cells, CD8+ T cells, B cells, and natural killer cell (NK) numbers [6], [15], [22], [23], [24]. This is combined with elevated expression of lymphocyte exhaustion markers including programmed cell death protein 1 (PD-1), natural killer cell receptor NKG2A, and T cell immunoglobulin mucin 3 (TIM-3) [22], [25], [26], [27]. Finally an accepted marker of the intensity of systemic inflammation namely the plasma neutrophil: lymphocyte ratio is predictive of both disease severity [28]and mortality [29]. A summary of the COVID-19 cytokine storm is presented on Fig. 1 .
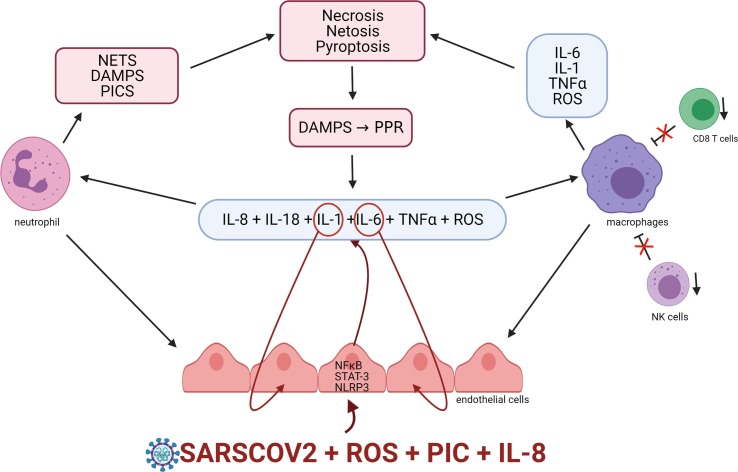
Fig. 1.
The potential origin of the COVID-19 cytokine storm A proposed early event in the development of the COVID-19 cytokine storm is the activation of endothelial cells by SARSCOV2 and or the presence of high levels of proinflammatory cytokines chemokines and ROS species. Such activation is associated with increased STAT-3, NFκB and activation of the NLRP3 inflammazome. The net result is the release of proinflammatory cytokines IL-1, TNF alpha, IL-6, IL-8 and reactive oxygen species (ROS) and the subsequent recruitment of macrophages and neutrophils which become an additional source of IL-1, IL-6, TNF alpha, ROS and several damage associated molecular patterns (DAMPS). IL-1 and IL-6 may engage in autoinflammatory signalling via targeting their receptors on endothelial cells leading to a self-amplifying cascade of inflammation and oxidative stress. Increasing levels of PICs and ROS result in loss of NK and CD8 T cell numbers and function increasing macrophage survival further amplifying PIC and ROS production and compromising macrophage efferocytosis. The resulting increase in survival of NET producing neutrophils further adds to pathological levels of PICs and ROS ultimately resulting in the pyroptosis and necrosis of immune cells and widespread tissue damage The resulting release of DAMPs may trigger further activation of immune and endothelial cells which further sustains and amplifies the inflammatory cascade.
2.2. Biochemical abnormalities reported in patients with COVID-19
Another accepted index of severe inflammation seen in COVID-19 patients is hyperactivation of the coagulant cascade and a relatively exhausted anticoagulant and fibrinolytic system with increased levels of D-dimer; evidence of microthrombi in large and small blood vessels; and in many cases, disseminated intravascular coagulation (DIC) [30], [31], [32], [33], [34]. In addition, the excessive levels of inflammation seen in severe COVID-19 makes the presence of hyperferritinemia predictable [35], [36].
2.3. Elements involved in the pathophysiology of the COVID-19 cytokine storm
The weight of evidence also suggests significant levels of inflammasome activity in the early and latter stages of the disease [37], [38], [39], [40]. Authors have also reported extensive necrosis and pyroptosis of lymphocytes, leucocytes organ-specific cells, lymph nodes and the spleen, and vascular endothelial cells, using a range of histological techniques which is consistent with the inflammasome activation discussed above [41], [42], [15]. Increased IL-1 is also prognostic marker of disease severity and death and a major source of tissue damage in patients severely affected with this disease [43], [44], [45], [46]. Unsurprisingly, several research teams have also reported escalating levels of IL-18 [47], [48], [49], which are approximately 4 times higher in patients with severe disease compared to those mildly affected [48]. In the light of these findings, it is interesting to note that SARSCOV2 infection of epithelial and endothelial cells and resultant endoplasmic reticulum (ER) stress is an acknowledged cause of NLP3 inflammasome activation and pyroptosis suggesting that direct viral infection of these cells is a source of inflammasome activation at least in the early stages of the disease [50], [51], [52], [53], [54], [55], [56].
Furthermore, while inflammasome activation would appear to be a significant source of inflammatory damage, the weight of evidence suggests that infiltrating TNF-α, IL-1 and IL-6-secreting monocytes and macrophages are also major drivers of tissue damage in the periphery and the lung [42], [57], [58]as reviewed by [41]. Additional evidence of macrophage involvement in the pathophysiology has been supplied by the results of a recent study revealing the presence of high serum levels of soluble haemoglobin Scavenger Receptor sCD163 and sCD164 in hospitalised patients [59]. In addition, several authors have reported increased levels neutrophils secreting histones, HMBG1, mitochondrial DNA (mtDNA),[50], [56]review [6], which act as damage associated molecular patterns (DAMPS) which are a known source of extensive inflammation and tissue damage [60], [61]. There is also accumulating evidence of increased activity of nuclear factor kappa beta (NFκB) in patients with severe COVID-19 [62], [63], [64].
Severe COVID-19 is also associated with the presence of systemic endotheliopathy characterised by a severely damaged permeable vascular endothelial barrier and activated endothelial cells in patients with severe disease [65], [66], [67]. This may be relevant from the perspective of COVID-19 pathophysiology as there is extensive evidence establishing a causative association between endothelial cell activation, inflammation, and damage with the development of self-amplifying inflammation, and coagulation [68], [69]. Moreover, there is accumulating evidence establishing endothelial activation and damage with the genesis and exacerbation of the cytokine storms associated with severe cytokine release syndrome [70], [71], [72]and severe H1N1 influenza [73], [74], [75], [76]. In addition, endothelial activation by IL-6 [77]could explain, at least in part, the a correlation between levels of this cytokine present in the circulation with illness severity and mortality [18], [78], [4], [79].
Finally, the importance of hypercoagulation in the pathogenesis of severe COVID-19 should not be underestimated as findings from the original outbreak in Wuhan suggest that over 70% of people who died following SARSCOV2 infection satisfied the criteria of DIC compared to only 1% of individuals that survived [80]. This is perhaps unsurprising given the context of data suggesting that the pathology associated with a range of self- amplifying inflammatory syndromes are underpinned by a complex interplay between a hyperactivated immune response and a hyperactivated coagulation cascade often described as immunothrombosis.
3. The cytokine storm of H1N1 (“Pandemic strain”) influenza
3.1. Immune abnormalities associated with severe H1N1 influenza
Patients suffering from H1N1-induced pneumonia and consequent ARDS have excessively elevated levels of serum interferons, cytokines, and chemokines characteristic of a cytokine storm [81], [82], [74], [11]. The extent and pattern of immune dysregulation seen in these individuals varies with the illness severity; however, elevated IFN-γ, IL-6, IL-1α, IL-1β, TNF-α, IL-15, IL-12p70, IL-17, IL-10, MCP-1, MIP-1β, IL-8, MIG, IP-10, MIP-1α, GM-CSF, and RANTES are the most commonly reported findings [83], [84], [85], [86], [87], [75].
In addition, several authors have reported a positive association between levels of IL-6 and disease severity [83], [88], [89], [90], [91]. The same appears to be true of a high neutrophil to lymphocyte ratio [92]. Unsurprisingly, the cytokine storm of H1N1 is associated with the development of severe multiorgan tissue damage and dysfunction [81], [82], [74], [11].
3.2. Biochemical abnormalities in patients with severe H1N1 influenza
Patients with severe H1N1 influenza-induced pneumonia also present with hypercoagulation, as evidenced by vascular leak activated platelets and endothelial cells, coupled with the presence of microemboli and, in many instances, DIC. Expectedly, there is extensive evidence of elevated D-dimer levels in these patients, often accompanied by significant upregulated production of lactate dehydrogenase (LDH) [93], [94], [95]. Importantly, the extent of elevation is predictive of disease severity and a poor outcome [93], [94], [95]. The overactivation of the coagulation cascade seen in such patients is associated with increased risk of deep venous thrombosis and acute cardiac injury [96]. Common manifestations include acute myocardial infarction [97], [98], acute coronary syndrome [99], [100]and several other cardiovascular diseases [99]. In addition, accumulating evidence suggests that an overactivated coagulation cascade and compromised fibrinolysis make an independent contribution to the development of the hyperinflammatory state and the cytokine storm seen in severely or critically ill patients [101], [102], [103] (reviewed in [104].
3.3. Elements involved in the pathophysiology of the H1N1 influenza cytokine storm
Abundant evidence suggests that an indispensable and early event in the generation of a hypercoagulable state in patients with severe H1N1 pneumonia is the activation of endothelial cells [105], [102], [106], [103]. Some strains of influenza infect and activate endothelial cells leading to upregulation of platelet tissue factor (TF) and downregulation of thrombomodulin via Toll-Like receptor (TLR)-3 or TLR-4 activation [107], [108], [109]. However, unlike the case for SARSCOV2 where direct infection of endothelial cells has been reported [110], there is no evidence that H1N1 directly infects endothelial cells or targets membrane TLR-4 receptors [111], [112]and the endothelial cell activation appears to result from the release of TNF-α, HMBG1, oxidized phospholipids and S100 proteins by already activated and damaged epithelial cells [107], [113], [112], [76], [109].
The source of HMBG1 and S100 proteins is not fully understood. However, there is evidence to suggest that H1N1 activates the NLRP3 inflammasome [114], which results in the secretion of HMBG1 from a wide range of immune cells [115], and damaged or stressed cells are a well-documented source of S100 proteins as well a range of other DAMPs as reviewed by [116].
Furthermore, accumulating evidence suggests that endothelial cells orchestrate the H1N1 cytokine storm via the upregulation of sphingosine-1-phosphate (S1P) receptor 1 expression [73], [74], [75], [76]. This conclusion stems from evidence produced by several research teams investigating the use of the S1P receptor agonists fingolimod or CYM5442 in animal models of the H1N1 cytokine storm which were invariably associated with the termination of the hyperinflammatory state accompanied by significant and large reductions in mortality [75], [76], [117], [118]. Importantly, there is evidence to suggest that the absence of endothelial S1P receptor may aggravate immune-mediated pulmonary injury following H1N1 infection [119] (Reviewed [120].
Unlike the situation with COVID-19 there is considerable evidence regarding the influence of individual cytokines in the pathogenesis and pathophysiology of the H1N1 cytokine storm and hence we will consider this area in the hope that these findings might be extrapolated to inform the potential role of such cytokines in the pathogenesis and pathophysiology of COVID-19.
3.4. The potential role of individual cytokines in the genesis of the H1N1 cytokine storm
TNF-α is considered to be the prototypical proinflammatory cytokine at the “centre of the influenza cytokine storm”, escalating the severity of disease in humans with highly pathogenic and pathological influenza infections [121], [122], [123]. This is foreseeable given the acknowledged role of this cytokine as the orchestrator and master regulator of the innate immune response [124], [125], [126], [127]. TNF-α exercises autocrine roles via increasing NFκB activity and paracrine effects on neighbouring cells and tissue, as well as inflammation at the level of organs [128], [127]. TNF-α also plays a major role in activating endothelial cells [129], [130], [131]and is the cytokine responsible for upregulating the production of IL-1 and IL-6 following pathogen invasion [74], [11].
TNF-α blockade results in significant reductions in pulmonary levels of proinflammatory cytokines and chemokines in animal models of severe influenza [132]. More recently, a significant reduction in mortality in mice infected with H1N1 following the administration of etanercept was reported [133]. However, an early TNF-α response following a viral infection is transitory and may quickly fall below levels of detection, hence any opportunity to inhibit TNF-α is likely to be short lived [134], [135], [136].
The role of IL-1 in the genesis and/or exacerbation of an H1N1 cytokine storm is uncertain with most evidence suggesting that the early upregulation of this cytokine has antiviral and overall cytoprotective effects despite its role in inducing pulmonary inflammation [137], [138], [11]. There may be several mechanisms underpinning the net beneficial effects of IL-1 upregulation, which might include upregulated IL-1R receptor activity that is responsible for the recruitment of CD8+ T cells [139]. It is also noteworthy that genetic deletion of IL-1R is associated with increased mortality in H1N1 infected rodents [74]. However, there is accumulating evidence that IL-1 may make a significant contribution to the development and persistence of “secondary” cytokine storms, which may arise as a result of the activity of lymphocytes and leukocytes and inflammasome activation [114], [140]. The sources of elevated levels of IL-1 seen in these patients is debated but one possible mechanism is the activation of the NLRP3 inflammazome which has been observed in mouse models and may play a vital role in the initial innate and latter humoral responses following H1N1 infection [114], [140].
3.4.1. IL-6
Similar to IL-1, the role of IL-6 in the genesis and/or exacerbation of the cytokine storm of H1N1 influenza is a matter of debate and currently the weight of evidence suggests that the early upregulation of this cytokine exerts protective rather than cytotoxic effects [141], [142]. The protective effects appear to stem from the role of this cytokine in regulating the influx of neutrophils into the lung and the differentiation and antibody production of B cells [143], [141](reviewed [144]. This is somewhat puzzling in the face of data establishing a positive correlation between levels of IL-6 and disease severity, as discussed above [83], [88], [89], [90], [91]. However, IL-6 is upregulated during the development of chronic inflammation as a result of upregulated IL-1 and TNF-α, which upregulate the transcription of this cytokine indirectly via the upregulation of NFκB or directly via binding to sites in the IL-6 gene promoter region (reviewed in: [145]. In addition, IL-6 is upregulated as part of the antiviral responses and the pleiotropic roles of this cytokine in this arena are detailed in an excellent review by [146]. The protective effects and inflammatory effects of IL-6 are mediated by classical IL-6 and IL-6 trans-signalling mechanisms, respectively, via mechanisms involving the phosphorylation of STAT-3 and Janus kinase (JAK) [147]described in Fig. 2 . Hence, elevated IL-6 levels may be a marker rather than a cause of disease severity and or may be upregulated in response to influenza virus infection.
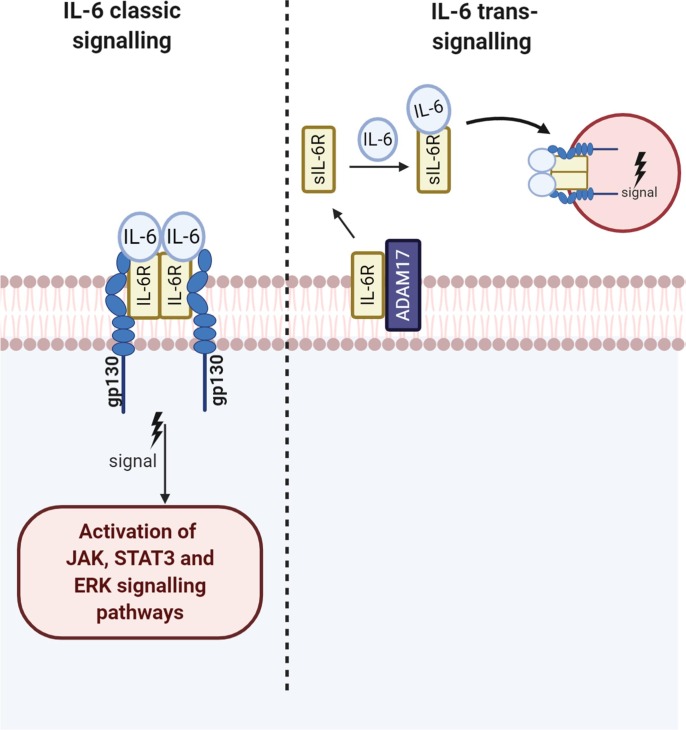
Fig. 2.
IL-6 signalling Trans IL-6 signalling is initiated via binding with the soluble receptors and IL-6R/sIL-6R and subsequent engagement of the complex with the trans membrane gp130 receptor possessed by a endothelial cells lymphocytes and leucocytes reviewed this engagement results in the activation of STAT-3 which is in turn phosphorylated by JAK kinase resulting in the nuclear translocation of NFKB SOCS proteins are the main inhibitors of IL-6 -STAT-3 signalling in the immune response and plays a major role in preventing a prolonged or over exuberant immune response. In classical signalling both GP130 the IL-6 receptor are membrane bound and the consequences of signal activation are broadly anti-inflammatory in nature.
4. The cytokine storm of cytokine release syndrome (CRS)
4.1. Immunological abnormalities associated with CRS
CRS is a systemic hyperinflammatory syndrome experienced by some patients following chimeric antigen receptor (CAR) T-cell and bi-specific T-cell engagers (BiTEs) therapy for soluble tumours [148]. IL-6, IL-10, IFN-γ, MCP-1 and GM-CSF appear to be elevated in all CRS patients, irrespective of the severity of the condition [149], [150], [151], [152], [153]. Other cytokines, including TNF-α, IL-1, IL-2, IL-2–receptor-α and IL-8 have also been reported in patients with more severe symptoms [154], [155]. Other universal features of CRS include extensive CD4+ T cell and macrophage activation [156], [157].
4.2. Biochemical abnormalities seen in CRS patients
Patients with severe CRS present with hypotension, fever, haemodynamic instability, increased vascular permeability, low levels of fibrinogen, increased levels of D-dimers, and increased angiopoietin-2 and von Willebrand factor as well as chronic activation of the coagulation cascade [71], [155]. Other commonly reported biochemical abnormalities include grossly elevated levels of ferritin and C-reactive protein (CRP) [158], [159], [160].
4.3. Factors involved in the pathogenesis of CRS
While CRS has traditionally been viewed as a T cell-mediated syndrome, more recent research has proposed that the levels of inflammation and tissue damage seen in the condition are largely mediated by TNF-α, IL-6, IL-1, and nitric oxide (NO) secreted by activated macrophages and monocytes [156], [157]. These findings are supported by the work of other authors who reported that the levels of these molecules produced by macrophages and monocytes in patients with this condition are far higher than the levels produced by T cells [158], [71], [161] (reviewed [162]). In addition, the high levels of IL-8, IL-10, IL-12, TNF-α, IL-6 IFN-α, MCP-1 and MIP-1α seen in CRS patients is a characteristic profile of activated macrophages and monocytes [149], [150], [151], [152], [153].
Multiple lines of evidence suggest that endothelial activation and/or dysfunction plays a crucial role in the development, maintenance and exacerbation of the syndrome [70], [71], [72]. Other biomarkers of endothelial cell activation, such as angiopoietin-2 and von Willebrand factor, predict CRS severity, before CAR T-cell infusion and during CRS [71]. For example, as previously mentioned, grossly elevated levels of von Willebrand Factor and angiopoietin-2 have been reported in patients with severe CRS, which may explain the vascular instability, capillary leakage and hypercoagulability seen in the condition [71], [72]. Moreover, patients with pre-existing endothelial inflammation and/or activation are at a higher risk of developing CRS and developing more severe symptoms than patients whose endothelium is in a quiescent state [163], [164], [165], [166], [167], [157].
4.4. Role of individual cytokines IL-6
Extremely high levels of IL-6 are seen in CRS patients with severe symptoms compared to those with a milder illness profile and healthy controls [158], [161], [9], [168]. In addition, much research suggest that this cytokine plays a key role in the pathophysiology of the condition [169], [170], [157]. This notion is supported by the results of numerous studies reporting the rapid resolution of the condition by the use of the IL-6 receptor antagonist tocilizumab either alone or in combination with the anti-IL-6 chimeric monoclonal antibody siltuximab [12], [163], [171], [172]. Indeed, IL-6 appears to play a major role in the development of many of the characteristic features of CRS such as vascular leakage and activation of the coagulation and complement cascade, which in severe cases may induce DIC [173], [170]. Interestingly, while macrophages and monocytes are significant sources of IL-6 levels in CRS, blood-vessel endothelial cells also appear to make a major contribution to the elevated levels of IL-6 typical of this syndrome [72].
However, the primacy of IL-6 as the central cytokine underpinning the pathophysiology of CRS is under challenge, especially in the early stages of development. For example, there is evidence to suggest that IFN-γ and TNF-α may play a role in the initiation of the CRS cytokine storm and the upregulation of IL-6 is a downstream occurrence [174], [155]. Other authors have cited the importance of IL-1 and the importance of IL-1 blockade in the resolution of the condition [156], [9], [154].
4.4.1. IFN-γ, IL-1 and TNF-α
Overall, most evidence suggests that high concentrations of IFN-γ and TNF-α released by the hyperactivation of CAR T-cells and non-CAR T-cells may trigger macrophage activation, leading to secretion of host cytokines such as IL-6, TNF-α, IL-1 and IL-10 [174], [175], [155]. IL-6, TNF-α and IL-1 in turn activate endothelial cells leading to the production of IL-6 and TNF-α [176]. IL-6 and TNF-α may engage in autocrine signalling with STAT-3 and NFκB, respectively, resulting in self-amplifying and self-sustaining inflammation as explained in Fig. 1 [176], [177], [178], ultimately resulting in a cytokine storm, as reviewed by [179], [180]. The mechanisms involved in the genesis of the cytokine release syndrome are described and summarised in Fig. 3 .
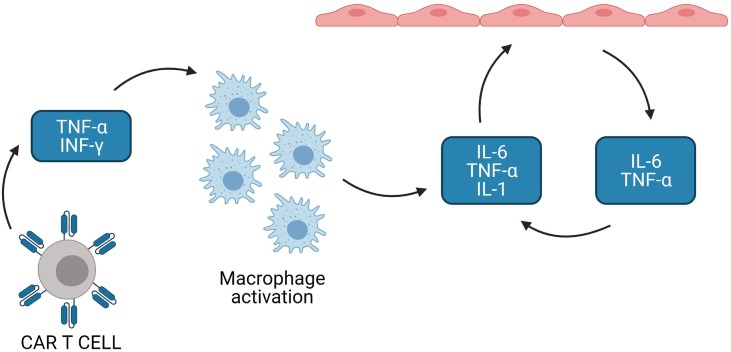
Fig. 3.
Mechanisms underpinning CRS Massive production of TNF alpha and INF gamma by activated T cells activates monocytes and macrophages which in turn produce large amounts of TNF alpha IL-1 and IL-6. These cytokines activate endothelial cells which then increase the production of IL-1 TNF alpha and IL-6 resulting in the production of an autoinflammatory cascade and escalating activation of the coagulation cascade.
5. The cytokine storm of Macrophage activation syndrome
5.1. Immune abnormalities associated with MAS
The term Macrophage Activation Syndrome (MAS) describes an often fatal complication of rheumatic diseases reviewed in [181].
MAS is currently regarded as a form of acquired or secondary hemophagocytic lymphohistiocytosis (sHLH) that is most commonly seen in children with systemic juvenile idiopathic arthritis (SJIA) and, albeit to a lesser degree, in individuals suffering from adult onset Still disease (AOSD), and more rarely in adults with systemic lupus erythematosus (SLE) [182]. Treatment options are limited, and many specialist centres utilise etoposide as a first line treatment for adult and juvenile patients. Readers interested in a consideration of the evidence supporting the use of this drug are invited to consult the work of [183], [184]. This condition is characterised by massive and unregulated expansion of IFN-γ-secreting CD8+ T cells and CD163+ expressing hemophagocytic macrophages [185], and increased NK activity [186]. The most commonly reported peripheral cytokine and chemokine abnormalities include elevated levels of IFN-γ, IL-1β, IL-6, IL-18, TNF-α, sIL-2Rα, CXCL9, CXCL10 and CXCL11 [187], [188], [189], [190], [191](reviewed [192]. Many patients suffering from this condition also possess activated but dysfunctional NK cells with evidence of impaired cytolytic capacity [193], [194], [195] (reviewed [196]). Other commonly observed abnormalities include high levels of soluble sCD163 [197], [198], sTNFR and sIL-1Ra [199] and sIL-2R-α chain (sCD25) [200]. Increased levels of sCD163 would appear to be of particular significance as this molecule, a specific scavenger receptor for haemoglobin-haptoglobin complexes, is associated with disease severity [197], [198], [201]. In addition, the appearance of sCD163 in the serum of SJIA patients is predictive of the development of MAS [202].
5.2. Biochemical abnormalities associated with MAS
MAS patients commonly present with a decreased erythrocyte sedimentation rate (ESR) together with increased levels of D-dimers and fibrinogen [203]. Unsurprisingly, the latter may result in the development of severe coagulopathy and DIC, causing multi-organ failure and death in approximately 20% of patients with severe symptoms [181], [204]. MAS is also characterised by macrophage-mediated hemophagocytosis in the majority of patients, leading to a decrease in the number of platelets, red blood cells, and neutrophils, which may result in thrombocytopaenia, anaemia and leukopenia [200], [205]. Other biochemical abnormalities with diagnostic and pathophysiological significance include grossly elevated levels of CRP, the hepatic transaminases AST and ALT, and LDH, bilirubin, triglycerides and ferritin, together with the presence of hypoalbuminemia and hyponatraemia [206] reviewed [181].
5.3. Factors involved in the pathophysiology of MAS
MAS is associated with heterozygous defects in genes such as perforin 1 (PRF1) and UNC homolog D (UNC13D) involved in enabling and regulating CD8 and NK cytolytic activity [207], [208], [209]. Many patients suffering from this condition also possesses heterozygous gain of function mutations in NLR-family CARD domain-containing protein 4 (NLRC4) inflammasome [210], [211]. Other mutations in genes associated with the development of MAS may be broadly categorised as those involved in antiviral defences, the regulation of inflammasome activity, other immune response pathways and overall regulation of metabolism [212].
This is unsurprising given that bi-allelic mutations in syntaxin binding protein 2 (STXBP2), syntaxin11 (STX11), PRF1, UNC13D and other genes regulating and enabling the clearance of peripheral blood mononuclear cells (PBMCs) by CD8+ T lymphocytes and NK cells are the cause of familial hemophagocytic lymphohistiocytosis (FHL) [213], [214]. In this illness, the impaired capacity to lyse antigen presenting cells (APCs) and T cells compromises the clearance of pathogen-infected cells and prolonged antigen stimulation of T cells leads to chronic macrophage activation and massive release of proinflammatory chemokines and cytokines [215], [216], [189], [191], [217].
However, MAS is not a purely genetic syndrome and is commonly triggered by viral infections, most notably influenza and herpesviruses such as Epstein-Barr virus (EBV) [218]– (reviewed [219]). Interestingly, a high rate of heterozygous mutations in genes associated with the development of FHL genes has been detected in patients who died as a result of H1N1 influenza infections, suggesting that impaired cytolytic killing may be involved in influencing mortality or recovery following infection with this virus [181], [220]. MAS is also typically characterised by increased expression of TLR-2, TLR-4 and IL-1R on PBMCs, which suggests the presence of persistent antigens and high levels of IL-1α and/or IL-1β [221], [222].
These findings have prompted several authors to propose a threshold model whereby high levels of systemic inflammation and genetic defects in CD8+ and NK cytolytic function predispose to the development of MAS, which is then triggered by an infection or a disease flare, resulting in increased CD8+ T cell activation and subsequent chronic macrophage activation [200], [223]. The current consensus regarding the mechanisms involved following an acute exacerbation of disease and viral infection are discussed in more detail below.
Increased inflammation during a disease leads to upregulation of CD8+ T cell and NK activity and the resultant increase in secretion of TNF-α and IFN-γ in turn results in increased activation and proliferation of macrophages [200]. Reduced clearance of these macrophages due mainly to genetic defects in the cytolytic function of CD8+ lymphocytes and NK cells allows chronic macrophage activation [224]. Finally, autocrine engagement between the latter and the former allows the development of self-amplifying inflammation, resulting in a cytokine storm.
In the case of a viral infection, the activation and proliferation of TNF-α- and IFN-γ-secreting CD8+ T and NK cells result in massive activation and proliferation of macrophages [225]. In this scenario, there is accumulating evidence that impaired cytolytic activity of CD8+ T and NK cells leads to impaired clearance of infected APCs, resulting in a prolonged immunological synapse and persistent antigenic stimulation [196], [226]. This ongoing stimulation enhances proliferation of T cells and production of proinflammatory cytokines, resulting in further increases in macrophage activation together with the development of haematophagocytosis [196], [226]. The failure to clear these macrophages leads to an autoinflammatory route, whereby increased macrophage activation leads to increased CD8+ T and NK cell proliferation and cytokine production, further increasing macrophage activity and proliferation, ultimately culminating in an often lethal cytokine storm [196], [226], [188]. The mechanisms thought to be involved in the aetiology of MAS are depicted and described in Fig. 4 .
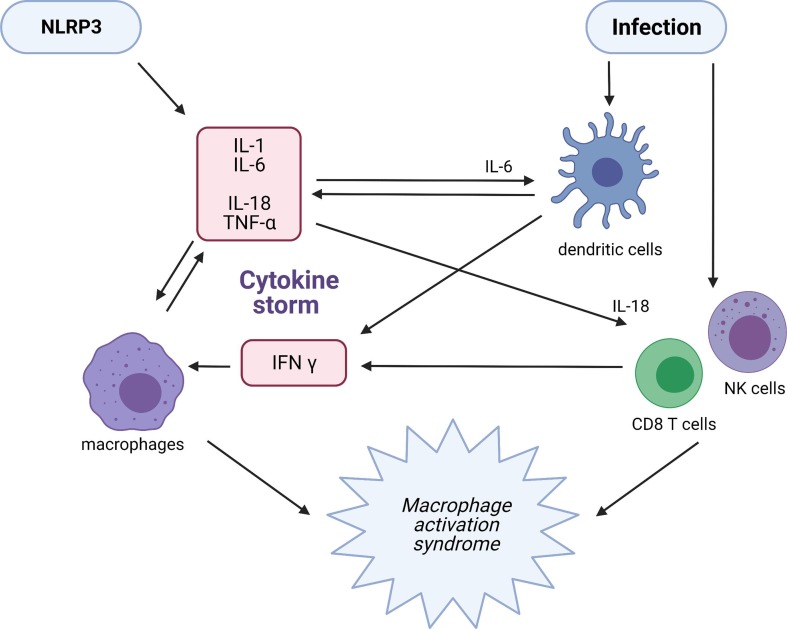
Fig. 4.
Mechanisms underpinning the development of MAS Increased production of IL-1, IL-18 and secondary IL-6 and TNF alpha inflammation in the context of a viral infection or inflammazome activation upregulates CD8+ T cell and NK activity and proliferation of macrophages. Impaired clearance of the latter in the context of defects in the cytolytic function of CD8+ lymphocytes and NK cells predisposes to chronic macrophage activation resulting in prolonged and excessive production of TNF alpha and IL-1. The latter inducing further increases in the activity of CD8 T cells and NK cells and the development of self-amplifying inflammation, resulting in a cytokine storm.
5.4. Role of individual cytokines Ifn-γ
High levels of IFN-γ are found in the serum and tissue of children and adults with MAS [187], [227], [188]. Moreover, these levels may predict increased mortality [228]. There is no evidence of increased serum IFN-γ in patients with SJIA, either during active disease or during remission, suggesting that IFN-γ may be the dominant cytokine driving MAS [229], [230]. Furthermore, several research teams have failed to detect the presence of any increase in the expression of IFN-γ-induced genes in the PBMCs of SJIA patients not displaying any signs or symptoms of MAS [221], [231], [222]. In addition, increased levels of neopterin, a surrogate marker of IFN-γ-driven macrophage activation [232], is commonly found in children with MAS [233], [234], and levels of this cytokine have the capacity to distinguish MAS from active SJIA [235]. Moreover, MAS episodes are frequently triggered by viral infections as discussed above, which are known activators of IFN-γ-induced pathways [236]. In addition, several lines of evidence suggest that elevated IFN-γ is central to the pathogenesis of FLH. For example, IFN-γ expression is highly elevated in these patients, out of proportion to other proinflammatory cytokines, such as TNF-α and IL-6, and rapidly returns to normal upon effective treatment [237], [238]. Further support for the view that MAS is an IFN-γ-mediated condition is supplied by evidence from several RCTs showing significant benefits from the use of emapalumab (an anti-interferon-gamma antibody medication used for the treatment of hemophagocytic lymphohistiocytosis) as a treatment for MAS [239], [240]. Finally, IFN-γ potentiates the release of IL-1 from macrophages and monocytes, implying the possibility that the elevated levels of IL-1 seen in MAS patients, as discussed below, may be a consequence of increased IFN-y production [241] (reviewed [242]).
5.4.1. IL-1
The bulk of the extant data suggests that serum IL-1 levels are significantly increased in MAS [221], [243], [244], [202], [235]. However, this cytokine is also upregulated in patients with SJIA and Still disease and the increased levels seen in MAS patients are relatively modest when compared to levels seen in patients with these conditions [244], [245]. Hence despite convincing data suggesting that IL-1 plays a central role in the pathophysiology of SJIA and AOSD [245], it has been suggested that this cytokine may not be a major player in in the pathogenesis of MAS [244]. This contention is supported by several lines of evidence. For example, the haematological abnormalities seen in MAS are not typical of other IL-1-mediated diseases such as SJIA and AOSD [246]. In addition, IL-1-mediated illnesses tend to be characterised by neutrophilia and high platelet counts while neutropenia and thrombocytopaenia are the common findings in MAS [247], [244]. Finally, patients with FHL do not present with increased serum levels of IL-1 [248].
However, the success of anakinra in the treatment of MAS either alone, or more commonly with high dose corticosteroids and IVIG [249], [250], [251], makes it difficult to conclude that IL-1 does not play a significant role in the development and/or maintenance of the condition, although the precise role is difficult to ascertain. However, there are potential routes that appear to be worthy of consideration.
First, there is evidence to suggest that IL-1α and IL-1β enhance survival, proliferation, differentiation and migration of antigen-primed CD4+ and CD8+ T cells [252], [253]. Second, IL-1 plays a major role in the polarisation of activated macrophages towards an M1 phenotype [254]. Hence, by promoting CD8+ T cell survival and macrophage cytokine production, IL-1 could make a significant contribution towards the exacerbation of pathology. Finally, the direct cytotoxic properties of IL-1 should not be underestimated, as protracted elevations of this cytokine are associated with severe tissue damage [255]. It should, however, be emphasised that these propositions are speculative and the role of IL-1 in the pathogenesis and pathophysiology of MAS, if any, is unknown.
5.4.2. IL-18
Extremely high Levels of free IL-18 occur in patients with MAS compared to patients with SJIA and AOSD [256], [257], [235], [190], [258]. Moreover, levels of this cytokine strongly correlate with several measures of disease activity, including CRP, ferritin, lactate dehydrogenase, CXCL9 and S100 [257], [235], [190], [258]. In addition, levels of IL-18 in SJIA patients are predictive of developing MAS and are higher in patients with a history of MAS [259], [235], [258]. Furthermore, the increase in IL-18 seen in MAS patients is accompanied by a relative decrease in the levels of IL-18 binding protein [257], [235], [190], which is a phenomenon not seen in other inflammatory diseases such as sepsis, rheumatoid arthritis (RA) and SLE [257], [260], [261]. This may be of pathophysiological significance, as a balance between levels of IL-18 binding protein and IL-18 is needed to restrain the inflammatory effect of the latter under physiological conditions [262]. Unsurprisingly, an imbalance between IL-18 and its binding protein is associated with increased severity of symptoms in several inflammatory diseases [257], [260], [261]. Moreover, there is in vivo evidence to suggest that elevated levels of this cytokine also results in the activation and proliferation of IFN-γ-producing CD8+ T cells [263], [264], [265]. Overall, the evidence suggests that IL-18 enhances the proliferation, survival and renewal of CD8+ cells [266], [267], [268], [269]. Hence, it is tempting to speculate that increased levels of IL-18 may be one potential source of the massive CD8+ T cell expansion seen in MAS, which in many instances is not triggered by a viral infection, but seems to coincide with disease exacerbation and increased inflammation [200], [245].
The source of IL-18 in MAS patients continues to be a matter of debate and there is some evidence to suggest that epithelial cells are one source of this cytokine and that increased activity of the NLP3 inflammasome may be involved [270]. Cases of MAS following SJIA are strongly suggestive of NLRC4 activity [271], although macrophages, monocytes and dendritic cells likely also make a contribution [272]. In the case of AOSD, there is considerable evidence to suggest that the source of this cytokine is upregulated by NLRP3 activity. For example, increased NLRP3 activity is a major driver of macrophage activation [273]and correlates with disease activity [274].
NLRP3 activation in macrophages, monocytes and neutrophils plays a major role in the immune response to RNA viruses, hence, IL-18 may dramatically increase following influenza or EBV infection [275], [276], [277]. In addition, inflammasome activity may be enhanced in an environment of increasing inflammation and oxidative stress characteristic of disease flares in SJIA and AOSD, leading to elevated levels of IL-18 [278], [277]. Therefore, increased IL-18 would be expected to occur either following a virus infection or a disease flare-up if largely produced by inflammasome activity. Thus, the involvement of inflammasomes would explain how disease flares and viral infections may be involved in the precipitation of MAS in the context of genetic vulnerability towards impaired macrophage or other PBMC clearance. Hence, it is tempting to speculate that increased levels of IL-18 may be one potential source of massive CD8+ T cell expansion and IFN-y production seen in MAS, which in many instances is not triggered by a viral infection but seems to coincide with disease exacerbation and increased inflammation [200], [245].
5.4.3. IL-6
Levels of IL-6 are elevated in patients with SJIA and the magnitude of elevation correlates with disease activity [279], [280]. In addition, IL-6 levels are elevated in individuals with MAS, although the extent of such elevation is extremely modest in comparison with other inflammatory syndromes, most notably sepsis [215], [191]. There continues to be debate regarding the potential sources of elevated IL-6 seen in the condition but evidence suggests that activated macrophages may make a major contribution [281]. IL-1 and IL-18 are potent inducers of IL-6 [282], [283].
The role of IL-6 in the pathophysiology of MAS appears to be equally uncertain. Although high levels of tocilizumab may be highly effective in the treatment of SJIA [284], these treated patients remain at high risk of developing MAS [285], suggesting that IL-6 does not play a dominant role in the pathophysiology of the condition. However, it should be noted that elevated IL-6 is an acknowledged cause of impaired NK cytolytic performance [193], [194], [195]and increased levels of this cytokine prolongs virus-specific CD8+ T cell survival [146]. These data suggest that high IL-6 levels may enhance the defects in NK cell and CD8+ T cell function implicated in the pathogenesis of MAS.
5.4.4. TNF-α
Serum levels of TNF-α are increased in patients with SJIA, although of the same order as seen in other illnesses involving systemic inflammation, such as Kawasaki disease [235]. In addition, results from trials involving the TNF-blocking agent etanercept have been inconsistent, with positive [286], [287], negative [285]and harmful results [288], [289], [290], [291]reported. Furthermore, levels of TNF-α are only slightly elevated in MAS compared to those reported in children with active SJIA [228], [235]. This is somewhat difficult to comprehend in the context of massive CD8+ T cell expansion characteristic of MAS, which would be expected to result in extremely high levels of the cytokines. However, there is evidence to suggest that TNF-α expression by CD8+ T cell activated macrophages is muted in the context of high levels of IL-10, which is seen in many MAS patients [292]. Hence it is possible that the relatively higher levels of the latter cytokine may compensate for the increased TNF-α produced by increasing CD8+ T cell activity in MAS so that overall TNF-α levels may only be moderately increased (reviewed [292]).
The similarities and differences in the immune and biochemical abnormalities seen in the cytokine’s storms of H1N1 COVID-19 CRS and MAS cytokine storms are presented in Table 1 . We will now proceed to a consideration of therapeutic targets based on the abnormalities identified beginning with NFκB for reasons which will become apparent upon perusal of the section below.
Table 1.
Comparison and contrast of immunological and biochemical abnormalities reported in COVID-19, H1N1 influenza, CRS and MAS.
| Abnormality | COVID-19 | H1N1 | CRS | MAS |
|---|---|---|---|---|
| IL-6 | +++ | ++ | +++ | NO |
| IL-1 | +++ | ++ | ++ | ++ |
| High neutrophil to Lymphocyte ratio | ++ | ++ | NO evidence | NO evidence |
| Interferon gamma | + | + | +++ | +++ |
| TNF alpha | + | + | +++ | + |
| High D-Dimer levels | ++ | ++ | ++ | ++ |
| Elevated Ferritin | ++ | ++ | +++ | ++ |
| Hypercoagulative state | +++ | ++ | +++ | ++ |
| Activated macrophages | +++ | + | +++ | ++++ |
| Endotheliopathy | +++ | +++ | +++ | NO evidence |
| Elevated IL-18 | +++ | + | NO evidence | ++++ |
| NLRP3 inflammazome activation | +++ | ++ | NO evidence | +++ |
| DIC | ++ | ++ | NO evidence | ++ |
| DAMP production | ++ | ++ | NO evidence | NO evidence |
| CD4 T cell apoptosis and or dysfunction | +++ | NO evidence | +++ | +++ |
| CD8 T cell dysfunction and or apoptosis | +++ | NO evidence | NO evidence | +++ |
| Increased TH17/T reg ratio | +++ | NO evidence | NO evidence | NO evidence |
| Impaired NK cell function and or levels | +++ | NO Evidence | NO evidence | +++ |
| Immunothrombosis | ++ | ++ | ++ | ++ |
| Increased Neutrophil activity | +++ | NO evidence | NO evidence | NO evidence |
6. Potential therapeutic targets
6.1. NFκB
As previously discussed, NFκB activation plays an indispensable orchestrating and nurturing role in the initiation and amplification role in the development and progression of severe H1N1 influenza [73], [74], [75], [76]and cytokine release syndrome [70], [71], [72]cytokine storms. This would also seem to be the case in the cytokine storms experienced by patients with severe COVID-19 [62], [63], [64]and less certainly MAS [293], [294], [295]. In addition, endothelial activation and damage mediated and maintained by the elevation of NFκB [296], [297], [298], [299], [300] Chronically increased NFκB activity is also associated with impaired T cell function, increased Th17 differentiation and reduced numbers and immunosuppressant capacity of regulatory T cells [301], [302], [303]. In addition, prolonged hyperactivity of NFκB and subsequent reactive oxygen species (ROS) production also leads to T cell apoptosis [304], [305]. Furthermore, increased NFκB is also an essential element in neutrophil activation [306], [307]and DAMP formation [308], [309] and NLRP3 activity [310], [311]. Finally, Increased NFκB upregulates levels of IL-6 and IL-1 which appear to be involved in the pathophysiology of COVID-19 cytokine storms and the cytokine storms of H1N1 and CRS at least to some degree. These are important findings as, once activated, IL-1 and IL-6 form autoinflammatory circuits which may speak to the origin of the cytokine storms in severe COVID-19 as we discussed below leading to self-perpetuating increases in their levels.
6.2. IL-6 signalling and autoinflammation
This signalling pathway is initiated when IL-6 binds to the cytokine’s membrane bound receptor (mIL-6R) on endothelial cells resulting in homodimerization of gp130 leading to the phosphorylation of Janus Kinase (JAK) family members leading to the activation of the transcription factor STAT-3 [312]. This in turn results in the upregulation of the endothelial membrane receptor S1PR1, and increased activity of the enzymes sphingosine phosphate kinase 1 and 2 (SIPK1 and S1PK 2) [313]. Increased activity of SIPK1 and S1PK2, in turn, increases levels of S1P which then provokes further activation of S1PR1 via a mechanism often described as “inside out “ signalling, resulting in further increases in the activation of JNK, and STAT-3 [314]. S1P also activates NFκB via an intracellular signalling pathways downstream [315], [313], [316], [317], [318]. The resultant upregulation of IL-6 induced via the transcriptional activity of NFκB forms the foundation of self-amplifying endothelial and systemic inflammation and tissue damage characteristic of CRS, H1N1 influenza MAS and severe COVID-19 [315], [313], [311], [316], [317], [318] as discussed above.
Finally, it should be noted that another self-amplifying loop of IL-6 production may be seen in endothelial epithelial and other non-immune cells described as the IL-6 amplifier [180], [319], [320]. In this case the mechanism appears to rely on IL-17 produced by T lymphocytes following stimulation by IL-6 secreting antigen presentation cells which simultaneously activate STAT-3 and NFκB [180], [319], [320]. Readers interested in an in depth consideration of this mechanism are invited to consult an excellent treatment of the subject by [319]. The importance of this mechanism in the development of cytokine storms is currently unknown but there is evidence that the IL-6 amplifier plays a role in the pathophysiology of at least some autoimmune diseases [180].
6.3. IL-1 signalling and autoinflammation
IL-1α or IL-1β binding to IL-1R1 leads to the formation of a heterotrimeric (TIR) complex. These recruits and phosphorylates MyD88 IRAK and TRAF-6 leading to the degradation of IκB and release of NFκB IκB and release of NFκB [321], [322]. IL-1 engagement with ILR1R results in the upregulation of NFκB which, in turn, increases IL-1 transcription creating an autocrine autoinflammatory loop in a similar manner to the case for IL-6 [45], [323], [324] reviewed [325], [326].
6.4. IL-6 versus IL-1
High levels of inflammasome activity in the early and latter stages of the disease is a plausible source of elevated IL-1 seen in severely and mildly affected patients with COVID-19 source of IL-1 [37], [38], [39], [40]. There would seem to be little doubt that the extent IL-1 mediated tissue damage plays a role in determining the severity of the disease [43], [44], [45], [46]. It is also noteworthy that high levels of IL-18 are seen in many patients with COVID-19 [47], [48], [49]also speaking to a role of NLRP3 activation in the pathophysiology of this illness. In addition, there is a wealth of data implicating NLRP3 mediated release of IL-1 from activated macrophages in self amplifying inflammation and tissue damage in a range of autoinflammatory conditions [327], [255], [328].
IL-6 transcription is a downstream target of IL-1 [329], [330], [331], [332], [145]. For example, IL-1 promotes the transcription of IL-6 and the subsequent release of this cytokine from macrophages [333]. Furthermore, IL-1 activates endothelial cells and their resultant production of IL-6 [334], [335], [336]. This at least raises the possibility that increased levels of IL-6 seen in COVID-19 patients are secondary to elevated levels of IL-1. It is also noteworthy that IL-18 may transactivate the expression of IL-6 once again suggesting that NLRP3 activation and the production of IL-1 and IL-18 may precede elevations of IL-6 [337].
7. Therapeutic options
7.1. Tocilizumab
Tocilizumab is a recombinant IgG1 class humanised monoclonal antibody targeting the soluble and membrane bound IL-6 receptors. The product has a history of considerable success in reducing mortality and morbidity in systemic juvenile idiopathic arthritis [338]and CRS provoked by CART [339]. However, tocilizumab does not prevent the transition between SJIA and MAS and may even mask the clinical signs of the syndrome. The data regarding efficacy of tocilizumab monotherapy in preventing mortality or reducing illness severity in COVID-19 patients is also equivocal and thus far prospective blinded RCTs have failed to detect a beneficial effect on patient survival despite a recent large prospective RCT reporting a significant reduction in the composite endpoint of mechanical ventilation and death [340]. In addition, a very recent open label RCT conducted by the REMAP-CAP investigators reported a positive effect on mortality in critically ill ventilated patients when the results were subject to a Bayesian analysis (2021) although a large blinded RCT concluded that Tocilizumab conferred no benefit on patient survival [341]. However there is robust data confirming improved mortality when Tocilizumab is given to patients already on dexamethasone compared to those on dexamethasone alone [342]. Readers interested in an analysis aimed at explaining the apparently contradictory results in these studies are referred to an elegant review by [343]. Readers interested in a review of the studies and conclusions provided by meta-analyses of trial results thus far are invited to consult the work of [344]. Finally, it is noteworthy that prolonged tocilizumab therapy appears to exert no favourable effects on levels of IL-1, IL-18 or activity of NFκB [345], [258].
IL-6 plays a number of essential roles in anti- viral defences including T cells responses including CD8 function and differentiation, specific IgG responses macrophage activation and migration [346], [146]. In this context the presence of data suggesting that monoclonal antibodies targeting IL-6 is associated with reduced B and helper T cell responses, CD8 levels and function and memory T cell production would appear to caution against the use of tocilizumab in the early stages of the disease [347], [143], [348], [349].
7.2. JAK inhibitors
There are several excellent reviews examining the mechanisms involving JAK-STAT activation and the role of this pathway as a major effector of cytokine mediated cellular signalling pathways [350]. Similarly, there are several excellent reviews of specific consequences stemming from the activation of various members of the JAK family of receptors and the mode of actions of various commercial Jak inhibitors. Readers interested in these areas are invited to consult the work of [351], [352], respectively Several small trials have produced evidence suggesting that baricitinib might reduce morbidity in patients with severe COVID-19 [353], [354], [355]. This was also suggested in a larger prospective randomised clinical trial when the drug was combined with remdesivir [356]. However, significant benefits on mortality and morbidity have not been demonstrated in well powered RCTs either with baricitinib alone or in combination with Remdesivir. Indeed, the results of the recent phase 3 trial involving 1400 patients entitled “Study of Baricitinib (LY3009104) in Participants With COVID-19 (COV-BARRIER)” were negative with no significant effect on morbidity or mortality reported. This is also the case for Ruxolitinib where small randomised clinical trials have suggested potential benefit [357], but recent large prospective randomised clinical trial dubbed the RUXCOVID study failed to meet any of its primary or secondary endpoints [358]. Finally, while there is evidence that JAK inhibitors reduce levels of IL-6, they appear to have no effect on levels of IL-1 [359], [360], [361] or IL-18 [362], [363].
Moreover, JAK-STAT pathway activation by interferons is an essential player in antiviral defences cautioning against this use of these agents in patients with high viral loads and or relatively early in the course of COVID19 [364], [365]. In addition, STAT-3 activation plays a major role in defences against a range of viruses [366]and may have an essential role in orchestrating anti-viral defences towards beta Coronaviruses such as SARS and SARSCOV2 as reviewed in [367]. There is also some concern with the use of JAK inhibitors in patients in a hypercoagulative state due to increasing risk of thrombosis as reviewed in [368].
7.3. Canakinumab
Canakinumab is a monoclonal antibody binding and neutralising human IL-1β [369]. The drug is administered subcutaneously resulting in peak plasma levels in approximately 7 days [370]. In addition, canakinumab has an elimination half-life of 26 days and a bioavailability of 70% [370]. This monoclonal antibody is Increasingly considered as a treatment option to prevent the recurrence of cardiovascular events [371]and has an excellent record of safety and efficacy in the treatment of SJIA and AOSD [372], [373]. However, a clinical trial reported no benefit on MAS [285]. In addition, despite the promising results suggesting that canakinumab might reduce the inflammatory status and improve oxygenation in COVID-19 patients [374]the negative results regarding any improvement in morbidity and mortality provided by the “Three Cs” study were extremely disappointing [375].
On a more positive note there appear to be no adverse effects on viral clearance and antibody production [376]and there appears to be little if any increased susceptibility to secondary infections following long term use [377]. In addition, there is evidence that the administration of this monoclonal antibody leads in the reduction of IL-6 levels [378], [379]although as yet there is no evidence of any favourable benefit on levels of IL-18 [285], [380], [378], [379].
7.4. Anakinra
Anakinra is a non-glycosylated form of human IL-1Ra, which acts as a dose related inhibitor of IL-1α and IL-1β binding to IL1-R [381], [382]. This preparation is usually administered subcutaneously resulting in a peak plasma concentration in 3–7 h [382], [383]. In addition, anakinra administered in this fashion has a bioavailability of 95% and an elimination half- life of 4–6 h [382], [383]. This product has been used successfully in the treatment of Rheumatoid Arthritis (RA), SJIA and AOSD and has an excellent record of safety in the treatment of those conditions [384], [385], [386]. Moreover, this drug is increasingly being used as an effective first‐line treatment for MAS and sHLH in children and adults [387], [10], [388]. Small prospective studies have reported significant benefits regarding patient inflammatory oxygenation status and reduced need for ventilation in patients hospitalised with severe COVID-19, but thus far there are no findings suggesting reduced mortality [389], [390], [382], [391].
However, anakinra potentially offers additional benefits to the drugs considered above not least via directly inhibiting of NFKB translocation to the nucleus [392], [324]and the activity of the NLRP3 inflammazome [393], [394], [395]. The latter is an important point as it offers the prospect of decreasing levels of IL-18 which is an independent driver of NFKB activation and directly induces the production of IFN-γ from T lymphocytes and NK cells [327], [255], [322]. In this context it is worth noting that anakinra therapy also results in decreased levels of IFN-γ, IL-17, IL-22, and Th17 and increased T regs [396]. There are also several reports of reduced IL-6 levels following anakinra therapy [397], [380], [334]lending further support to the proposition that IL-6 transcription may depend on elevated levels of IL-1 positive effects on endothelial functions in part by inhibiting the activation of tissue factor [331], [398]. These are all highly pertinent findings as these abnormalities have all been identified in patients with COVID-19, suggesting that anakinra might be a “broad spectrum” approach to treatment.
This latter point is extremely important as, while excessive levels of proinflammtory cytokines are an important driver of pathology in cytokine storms, other factors are involved as discussed above. One such factor is the activation of the coagulation cascade which makes an independent and synergistic contribution to inflammation and tissue damage as well as well as being the origin of DIC microthrombi and end-organ failure. This pathological interplay between a hyperactivated immune system and hyperactivity of the coagulation cascade is increasingly described as immunothrombosis [399], [400], [311]. Readers interested in details of the mechanism driving this mutually self-amplifying cascade are referred to the work of [37]. Another source of cytokine storm initiation and amplification is the activation of Inflammasomes leading to the release of DAMPs such as ILA and HMGB1 which may perpetually activate pattern recognition receptors. The activation of inflammasomes may also result in cell death by necrosis and necroptosis releasing high levels of DAMPS resulting in spiralling levels of proinflammatory cytokine production, immune activation, coagulation, tissue damage and cell death [311].
However, NLRP3 activation and immunothrombosis is dependent on NFκB activation and hence the capacity of anakinra to inhibit both entities offer theoretical advantages over unidimensional cytokine suppression and thus, at least hypothetically is a more rational choice in the treatment of COVID-19 than the other medicines discussed above. In the light of this we would suggest that a randomised clinical trial examining the effects of anakinra in combination with dexamethasone in an effort to improve reductions in mortality already achieved via the use of this drug alone and in combination with tocilizumab [401].
For the sake of completeness it should be noted that venous thromboembolism (VTE) is the most common complication in patients with severe COVID-19 [402] and may occur in almost 30% of patients in ITU [403]. In addition, up to 70% of non survivors display evidence of DIC as previously discussed [37]. Hence a brief discussion regarding the evidence for anticoagulant therapy appears to be warranted.
Unsurprisingly, there have been numerous studies of varying design investigating the use of anticoagulants in this patient group aimed at reducing mortality or at least improving clinical parameters such as oxygenation status [404]. However, the evidence for anticoagulant therapy is mixed. For example, a recent meta-analysis of retrospective observational cohort studies concluded that the use of high-dose anticoagulants in critically ill patients was associated with a significant reduction in 30 day mortality [405]. However, another recent meta-analysis concluded that there was no benefit to mortality but that that it was a significant increase in risk of bleeding [406]. The evidence from prospective RCTs is also mixed. A small phase 2 RCT has reported improved oxygen status in critically ill patients [407]. However a more recent prospective RCT examining the use of high dose enoxaparin at a dose of 1 mg per kilogram per day reported no significant improvement in mortality at 30 days [408].
8. Discussion
In this paper, key features of the cytokine storm associated with three conditions, namely H1N1 influenza, CRS, and MAS have been described. These are summarised in Table 1. Certain haematological and vascular abnormalities are common to all three, including hypercoagulation and increased D-dimer levels. In terms of immune abnormalities, increased levels of IFN-γ, IL-6, IL-1, and TNF-α also tend to be common to the three conditions. However, there are also marked differences between the three conditions in respect of biochemical abnormalities and the pathophysiology of the associated cytokine storm, as well as regarding immune abnormalities and haematological and vascular abnormalities. All cytokine storms and their proposed drivers are dependent on NFκB however and an approach targeting this transcription factor may have universal utility. From the perspective of COVID-19, anakinra has the theoretical potential to alleviate many of the immunological and biochemical abnormalities reported in patients suffering from this illness and thought to be involved in the genesis of cytokine storms potentially improving on decreases in mortality achieved by tocilizumab combined with dexamethasone. However, there is currently a lack of trial data in support of or contravening this hypothesis. Accordingly, large blinded RCTs to test this proposition are recommended.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
MB is supported by a NHMRC Senior Principal Research Fellowship (1059660 and 1156072).
Author’s contributions
All the contributing authors have participated in the preparation of the manuscript and approved the final version.
References
- 1.Chalmers J.D., Chotirmall S.H. Rewiring the immune response in COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:784–786. doi: 10.1164/rccm.202007-2934ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020:eabb8925. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 6.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy H., Iqbal M., Chavez J.C., Kharfan-Dabaja M.A. Cytokine release syndrome: current perspectives. Immunotargets Ther. 2019;8:43–52. doi: 10.2147/ITT.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020;2:276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonmez O., Sonmez M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017;2:311–314. doi: 10.1016/j.pbj.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020 2020.02.23.20026690. [Google Scholar]
- 15.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C., Cai J., Chen R., Shi Z., Bian X., Xie J., et al. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Res. Square. 2020 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020 doi: 10.1093/cid/ciaa449. 2020.02.29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadotti A.C., de Castro Deus M., Telles J.P., Wind R., Goes M., Garcia Charello Ossoski R., et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171. doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galani I.-E., Rovina N., Lampropoulou V., Triantafyllia V., Manioudaki M., Pavlos E., et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021;22:32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z.-J., Xu J., Yin J.-M., Li L., Hou W., Zhang L.-L., et al. Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.585647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) medRxiv. 2020 doi: 10.3389/fimmu.2020.00827. 2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon C. Fighting COVID-19 exhausts T cells. Nat. Rev. Immunol. 2020;20:277. doi: 10.1038/s41577-020-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng H.-Y., Zhang M., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang A.-P., Liu J., Tao W., Li H-m. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020;106504 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maes M., Sirivichayakul S., Kanchanatawan B., Vodjani A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox. Res. 2019;36:306–322. doi: 10.1007/s12640-019-00054-6. [DOI] [PubMed] [Google Scholar]
- 33.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thrombosis Haemostasis: JTH. 2020 doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang X., Du R., Wang R., Cao T., Guan L., Yang C., et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020 doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. (Hoboken, NJ) 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., Oeil A., et al. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life Sci. 2020;258:118166. doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paniri A., Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: role of lncRNAs in cytokine storm modulation. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theobald S., Simonis A., Kreer C., Zehner M., Fischer J., Albert M.-C., et al. The SARS-CoV-2 spike protein primes inflammasome-mediated interleukin-1- beta secretion in COVID-19 patient-derived macrophages. Res. Square. 2020 [Google Scholar]
- 40.Toldo S., Bussani R., Nuzzi V., Bonaventura A., Mauro A.G., Cannatà A., et al. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm. Res. 2021;70:7–10. doi: 10.1007/s00011-020-01413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park M.D. Macrophages: a Trojan horse in COVID-19? Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conti P., Caraffa A., Gallenga C.E., Ross R., Kritas S.K., Frydas I., et al. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy. J. Biol. Regul. Homeost. Agents. 2020;34:1971–1975. doi: 10.23812/20-1-E. [DOI] [PubMed] [Google Scholar]
- 44.G. Magro, SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc, Cytokine: X. 2 (2020) 10029.. [DOI] [PMC free article] [PubMed]
- 45.van de Veerdonk F.L., Netea M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit. Care. 2020;24:445. doi: 10.1186/s13054-020-03166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y., Qin L., Zhang P., Li K., Liang L., Sun J., et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gea-Mallorquí E. IL-18-dependent MAIT cell activation in COVID-19. Nat. Rev. Immunol. 2020;20:719. doi: 10.1038/s41577-020-00467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satış H., Özger H.S., Aysert Yıldız P., Hızel K., Gulbahar Ö., Erbaş G., et al. Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine. 2021;137:155302. doi: 10.1016/j.cyto.2020.155302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in covid-19: the first autopsy series from New Orleans. medRxiv. 2020 doi: 10.1016/S2213-2600(20)30243-5. 2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mason R.J. Pathogenesis of COVID-19 from a cell biologic perspective. Eur. Respir. J. 2020;2000607 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thoracic Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN Electronic J. 2020 [Google Scholar]
- 55.Yao X.-H., He Z.-C., Li T.-Y., Zhang H.-R., Wang Y., Mou H., et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020 doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 57.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. MedRxiv. 2020 doi: 10.1038/s41421-020-0168-9. 2020.03.23.20039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. 2020 2020.03.24.20042655. [Google Scholar]
- 59.Gómez-Rial J., Currás-Tuala M.J., Rivero-Calle I., Gómez-Carballa A., Cebey-López M., Rodríguez-Tenreiro C., et al. Increased serum levels of sCD14 and sCD163 indicate a preponderant role for monocytes in COVID-19 immunopathology. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.560381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brostjan C., Oehler R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discovery. 2020;6:26. doi: 10.1038/s41420-020-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng H.-H., Liu Y.-J., Ojcius D.M., Lee C.-M., Chen R.-H., Huang P.-R., et al. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Sci. Rep. 2017;7:16628. doi: 10.1038/s41598-017-16778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2020;1–10 doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kandasamy M. NF-κB signalling as a pharmacological target in COVID-19: potential roles for IKKβ inhibitors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2021;1–7 doi: 10.1007/s00210-020-02035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang J.C. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J. 2019;17:10. doi: 10.1186/s12959-019-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nedeva C., Menassa J., Puthalakath H. Sepsis: inflammation is a necessary evil. Front. Cell Dev. Biol. 2019;7 doi: 10.3389/fcell.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gust J., Hay K.A., Hanafi L.A., Li D., Myerson D., Gonzalez-Cuyar L.F., et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hay K.A., Hanafi L.-A., Li D., Gust J., Liles W.C., Wurfel M.M., et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obstfeld A.E., Frey N.V., Mansfield K., Lacey S.F., June C.H., Porter D.L., et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017;130:2569–2572. doi: 10.1182/blood-2017-08-802413. [DOI] [PubMed] [Google Scholar]
- 73.Gautam M. Endothelial cells as regulators of cytokine storms during influenza infection. Thorax. 2012;67:617. [Google Scholar]
- 74.Liu Q., Zhou Y.-h., Yang Z-q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oldstone M.B.A., Teijaro J.R., Walsh K.B., Rosen H. Dissecting influenza virus pathogenesis uncovers a novel chemical approach to combat the infection. Virology. 2013;435:92–101. doi: 10.1016/j.virol.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F., et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Esteve E., Castro A., López-Bermejo A., Vendrell J., Ricart W., Fernández-Real J.-M. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30:939–945. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- 78.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D'Elia R.V., Harrison K., Oyston P.C., Lukaszewski R.A., Clark G.C. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 2013;20:319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet. Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramírez P., et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Betakova T., Kostrabova A., Lachova V., Turianova L. Cytokines induced during influenza virus infection. Curr. Pharm. Des. 2017;23:2616–2622. doi: 10.2174/1381612823666170316123736. [DOI] [PubMed] [Google Scholar]
- 85.de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J.D., Chau T.N.B., et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin S., Li Y., Pan R., Zou X. Characterizing and controlling the inflammatory network during influenza A virus infection. Sci. Rep. 2014;4:3799. doi: 10.1038/srep03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.La Gruta N.L., Kedzierska K., Stambas J., Doherty P.C. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 88.Coon B.G., Baeyens N., Han J., Budatha M., Ross T.D., Fang J.S., et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagau N., Slavcovici A., Gonganau D.N., Oltean S., Dirzu D.S., Brezoszki E.S., et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit. Care. 2010;14:R203. doi: 10.1186/cc9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oshansky C.M., Gartland A.J., Wong S.-S., Jeevan T., Wang D., Roddam P.L., et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am. J. Respir. Crit. Care Med. 2014;189:449–462. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.To K.K., Hung I.F., Li I.W., Lee K.L., Koo C.K., Yan W.W., et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2010;50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y., Zou P., Gao H., Yang M., Yi P., Gan J., et al. Neutrophil-lymphocyte ratio as an early new marker in AIV-H7N9-infected patients: a retrospective study. Ther. Clin. Risk Manag. 2019;15:911–919. doi: 10.2147/TCRM.S206930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kanchana S., Kanchana S., Vijitsopa T., Thammakumpee K., Yamwong S., Sawanyawisuth K. Clinical factors predictive of pneumonia caused by pandemic 2009 H1N1 influenza virus. Am. J. Trop. Med. Hyg. 2013;88:461–463. doi: 10.4269/ajtmh.12-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiliç H., Kanbay A., Karalezli A., Hasanoğlu H.C., Ateş C. Clinical characteristics of 75 pandemic H1N1 influenza patients from Turkey; risk factors for fatality. Turkish J. Med. Sci. 2015;45:562–567. doi: 10.3906/sag-1401-111. [DOI] [PubMed] [Google Scholar]
- 95.Wang Z.F., Su F., Lin X.J., Dai B., Kong L.F., Zhao H.W., et al. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1) Thromb. Res. 2011;127:198–201. doi: 10.1016/j.thromres.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 96.Ludwig A., Lucero-Obusan C., Schirmer P., Winston C., Holodniy M. Acute cardiac injury events ≤30 days after laboratory-confirmed influenza virus infection among U.S. veterans, 2010–2012. BMC Cardiovasc. Disord. 2015;15:109. doi: 10.1186/s12872-015-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barnes M., Heywood A.E., Mahimbo A., Rahman B., Newall A.T., Macintyre C.R. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart (British Cardiac Society). 2015;101:1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet. Infect. Dis. 2009;9:601–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 99.Kwok C.S., Aslam S., Kontopantelis E., Myint P.K., Zaman M.J.S., Buchan I., et al. Influenza, influenza-like symptoms and their association with cardiovascular risks: a systematic review and meta-analysis of observational studies. Int. J. Clin. Pract. 2015;69:928–937. doi: 10.1111/ijcp.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marsden P.A. Inflammation and coagulation in the cardiovascular system. Circ. Res. 2006;99:1152–1153. doi: 10.1161/01.RES.0000251962.44753.7f. [DOI] [PubMed] [Google Scholar]
- 101.Antoniak S., Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123:2605–2613. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Armstrong S.M., Darwish I., Lee W.L. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence. 2013;4:537–542. doi: 10.4161/viru.25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lê V.B., Schneider J.G., Boergeling Y., Berri F., Ducatez M., Guerin J.-L., et al. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am. J. Respir. Crit. Care Med. 2015;191:804–819. doi: 10.1164/rccm.201406-1031OC. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y., Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell. Mol. Immunol. 2016;13:432–442. doi: 10.1038/cmi.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akiyama R., Komori I., Hiramoto R., Isonishi A., Matsumoto M., Fujimura Y. H1N1 influenza (swine flu)-associated thrombotic microangiopathy with a markedly high plasma ratio of von willebrand factor to ADAMTS13. Intern. Med. 2011;50:643–647. doi: 10.2169/internalmedicine.50.4620. [DOI] [PubMed] [Google Scholar]
- 106.Boilard E., Paré G., Rousseau M., Cloutier N., Dubuc I., Lévesque T., et al. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood. 2014;123:2854–2863. doi: 10.1182/blood-2013-07-515536. [DOI] [PubMed] [Google Scholar]
- 107.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shibamiya A., Hersemeyer K., Schmidt Wöll T., Sedding D., Daniel J.-M., Bauer S., et al. A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood. 2009;113:714–722. doi: 10.1182/blood-2008-02-137901. [DOI] [PubMed] [Google Scholar]
- 109.Tsai S.-Y., Segovia J.A., Chang T.-H., Morris I.R., Berton M.T., Tessier P.A., et al. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003848. e1003848-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y., Chen H., Sun Y., Chen F. Antiviral role of Toll-like receptors and cytokines against the new 2009 H1N1 virus infection. Mol. Biol. Rep. 2012;39:1163–1172. doi: 10.1007/s11033-011-0846-7. [DOI] [PubMed] [Google Scholar]
- 112.Short K.R., Veldhuis Kroeze E.J.B., Reperant L.A., Richard M., Kuiken T. Influenza virus and endothelial cells: a species specific relationship. Front. Microbiol. 2014;5:653. doi: 10.3389/fmicb.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sarvestani S.T., McAuley J.L. The role of the NLRP3 inflammasome in regulation of antiviral responses to influenza A virus infection. Antiviral Res. 2017;148:32–42. doi: 10.1016/j.antiviral.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 114.Ichinohe T., Lee H.K., Ogura Y., Flavell R., Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vande Walle L., Kanneganti T.-D., Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2:162–165. doi: 10.4161/viru.2.2.15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roh J.S., Sohn D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teijaro J.R., Walsh K.B., Rice S., Rosen H., Oldstone M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. U S A. 2014;111:3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Walsh K.B., Teijaro J.R., Rosen H., Oldstone M.B. Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol. Res. 2011;51:15–25. doi: 10.1007/s12026-011-8240-z. [DOI] [PubMed] [Google Scholar]
- 119.Walsh K.B., Teijaro J.R., Brock L.G., Fremgen D.M., Collins P.L., Rosen H., et al. Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J. Virol. 2014;88:6281–6293. doi: 10.1128/JVI.00464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao J., Zhu M., Jiang H., Shen S., Su X., Shi Y. Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: a potential therapy against pathogenic influenza virus. Sci. Rep. 2019;9:5272. doi: 10.1038/s41598-019-41760-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Chan M.C., Cheung C.Y., Chui W.H., Tsao S.W., Nicholls J.M., Chan Y.O., et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung C.Y., Poon L.L., Lau A.S., Luk W., Lau Y.L., Shortridge K.F., et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet (London, England). 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 123.Peiris J.S., Cheung C.Y., Leung C.Y., Nicholls J.M. Innate immune responses to influenza A H5N1: friend or foe? Trends Immunol. 2009;30:574–584. doi: 10.1016/j.it.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arango Duque G., Descoteaux A. Vol. 5. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. (Front. Immunol.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mizgerd J.P., Spieker M.R., Doerschuk C.M. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during <em>Escherichia coli</em> pneumonia in mice. J. Immunol. 2001;166:4042–4048. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- 127.Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caldwell A.B., Cheng Z., Vargas J.D., Birnbaum H.A., Hoffmann A. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 2014;28:2120–2133. doi: 10.1101/gad.244749.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaudhry H., Zhou J., Zhong Y., Ali M.M., McGuire F., Nagarkatti P.S., et al. Role of cytokines as a double-edged sword in sepsis. In Vivo (Athens, Greece). 2013;27:669–684. [PMC free article] [PubMed] [Google Scholar]
- 130.Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J.: Off. Publ. Feder. Am. Soc. Exp. Biol. 1992;6:2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 131.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hussell T., Pennycook A., Openshaw P.J.M. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 133.Shi X., Zhou W., Huang H., Zhu H., Zhou P., Zhu H., et al. Inhibition of the inflammatory cytokine tumor necrosis factor-alpha with etanercept provides protection against lethal H1N1 influenza infection in mice. Crit. Care (London, England). 2013;17:R301. doi: 10.1186/cc13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McClain M.T., Henao R., Williams J., Nicholson B., Veldman T., Hudson L., et al. Differential evolution of peripheral cytokine levels in symptomatic and asymptomatic responses to experimental influenza virus challenge. Clin. Exp. Immunol. 2016;183:441–451. doi: 10.1111/cei.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Falzarano D., de Wit E., Feldmann F., Rasmussen A.L., Okumura A., Peng X., et al. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu X., Bai J., Liu P., Wang X., Jiang P. Suppressor of cytokine signaling 3 plays an important role in porcine circovirus type 2 subclinical infection by downregulating proinflammatory responses. Sci. Rep. 2016;6:32538. doi: 10.1038/srep32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim K.S., Jung H., Shin I.K., Choi B.-R., Kim D.H. Induction of interleukin-1 beta (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J. Med. Virol. 2015;87:1104–1112. doi: 10.1002/jmv.24138. [DOI] [PubMed] [Google Scholar]
- 138.Schmitz N., Kurrer M., Bachmann M.F., Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo X.-Z.J., Thomas P.G. New fronts emerge in the influenza cytokine storm. Semin. Immunopathol. 2017;39:541–550. doi: 10.1007/s00281-017-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tate M.D., Ong J.D.H., Dowling J.K., McAuley J.L., Robertson A.B., Latz E., et al. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci. Rep. 2016;6:27912. doi: 10.1038/srep27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dienz O., Rud J.G., Eaton S.M., Lanthier P.A., Burg E., Drew A., et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lauder S.N., Jones E., Smart K., Bloom A., Williams A.S., Hindley J.P., et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 2013;43:2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dienz O., Eaton S.M., Bond J.P., Neveu W., Moquin D., Noubade R., et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu S., Yan R., Chen B., Pan Q., Chen Y., Hong J., et al. Influenza virus-induced robust expression of SOCS3 contributes to excessive production of IL-6. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reeh H., Rudolph N., Billing U., Christen H., Streif S., Bullinger E., et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: fusing experimental insights and dynamic modelling. Cell Commun. Sig. 2019;17:46. doi: 10.1186/s12964-019-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khadka R.H., Sakemura R., Kenderian S.S., Johnson A.J. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy. 2019;11:851–857. doi: 10.2217/imt-2019-0074. [DOI] [PubMed] [Google Scholar]
- 149.Brentjens R., Yeh R., Bernal Y., Riviere I., Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Hara M.H., Stashwick C., Plesa G., Tanyi J.L. Overcoming barriers of car T-cell therapy in patients with mesothelin-expressing cancers. Immunotherapy. 2017;9:767–780. doi: 10.2217/imt-2017-0026. [DOI] [PubMed] [Google Scholar]
- 151.Tang X., Luo Y.-X., Chen H.-Z., Mitochondria Liu D-P. endothelial cell function, and vascular diseases. Front. Physiol. 2014;5:175. doi: 10.3389/fphys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang C.-M., Wu Z.-Q., Wang Y., Guo Y.-L., Dai H.-R., Wang X.-H., et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clin. Cancer Res. 2016;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y., Zhang W., Dai H., Wang Y., Shi F., Wang C., et al. An analytical biomarker for treatment of patients with recurrent B-ALL after remission induced by infusion of anti-CD19 chimeric antigen receptor T (CAR-T) cells. Sci. China Life Sci. 2016;59:379–385. doi: 10.1007/s11427-016-5035-4. [DOI] [PubMed] [Google Scholar]
- 154.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 155.Wang Z., Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomarker Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Teachey D.T., Rheingold S.R., Maude S.L., Zugmaier G., Barrett D.M., Seif A.E., et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fitzgerald J.C., Weiss S.L., Maude S.L., Barrett D.M., Lacey S.F., Melenhorst J.J., et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frey N., Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transpl. 2019;25:e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 160.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N., et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thakar M.S., Kearl T.J., Malarkannan S. Controlling cytokine release syndrome to harness the full potential of CAR-based cellular therapy. Front. Oncol. 2020;9 doi: 10.3389/fonc.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chatenoud L., Ferran C., Legendre C., Thouard I., Merite S., Reuter A., et al. In vivo cell activation following OKT3 administration. systemic cytokine release and modulation by corticosteroids. Transplantation. 1990;49:697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 165.Hay K.A., Hanafi L.-A., Li D., Gust J., Liles W.C., Wurfel M.M., et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roberts Z.J., Better M., Bot A., Roberts M.R., Ribas A. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leukemia Lymphoma. 2017;59:1785–1796. doi: 10.1080/10428194.2017.1387905. [DOI] [PubMed] [Google Scholar]
- 167.Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 168.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., et al. Cytokine release syndrome. J. ImmunoTher. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005930. 177ra38-ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 171.Klinger M., Brandl C., Zugmaier G., Hijazi Y., Bargou R.C., Topp M.S., et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell–engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 172.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 174.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 175.Mukohda M., Stump M., Ketsawatsomkron P., Hu C., Quelle F.W., Sigmund C.D. Endothelial PPAR-γ provides vascular protection from IL-1β-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H39–H48. doi: 10.1152/ajpheart.00490.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marin V., Montero-Julian F.A., Grès S., Boulay V., Bongrand P., Farnarier C., et al. The IL-6-soluble IL-6Rα autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J. Immunol. 2001;167:3435–3442. doi: 10.4049/jimmunol.167.6.3435. [DOI] [PubMed] [Google Scholar]
- 177.Choy E., Rose-John S. Interleukin-6 as a multifunctional regulator: inflammation, immune response, and fibrosis. J. Scleroderma Related Disord. 2017;2:S1–S5. [Google Scholar]
- 178.Murakami M., Hirano T. The pathological and physiological roles of IL-6 amplifier activation. Int. J. Biol. Sci. 2012;8:1267–1280. doi: 10.7150/ijbs.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hou T., Tieu B.C., Ray S., Recinos Iii A., Cui R., Tilton R.G., et al. Roles of IL-6-gp130 signaling in vascular inflammation. Curr. Cardiol. Rev. 2008;4:179–192. doi: 10.2174/157340308785160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee J., Nakagiri T., Oto T., Harada M., Morii E., Shintani Y., et al. IL-6 amplifier, NF-κB–triggered positive feedback for IL-6 signaling, in grafts is involved in allogeneic rejection responses. J. Immunol. 2012;189:1928–1936. doi: 10.4049/jimmunol.1103613. [DOI] [PubMed] [Google Scholar]
- 181.Bracaglia C., Prencipe G., De Benedetti F. Macrophage Activation Syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017;15:5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eloseily E.M.A., Minoia F., Crayne C.B., Beukelman T., Ravelli A., Cron R.Q. Ferritin to erythrocyte sedimentation rate ratio: simple measure to identify macrophage activation syndrome in systemic juvenile idiopathic arthritis. ACR Open Rheumatol. 2019;1:345–349. doi: 10.1002/acr2.11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nicholson M.C., Naeije L., Hayden A.R., Mattman A., Dix D., Chen L.Y.C. Etoposide-based treatment of adult HLH is associated with high biochemical response but poor survival outcomes. eJHaem. 2020;1:277–280. doi: 10.1002/jha2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Horne A., von Bahr Greenwood T., Chiang S.C.C., Meeths M., Björklund C., Ekelund M., et al. Efficacy of moderately dosed etoposide in macrophage activation syndrome - hemophagocytic lymphohistiocytosis (MAS-HLH) J. Rheumatol. 2021 doi: 10.3899/jrheum.200941. jrheum.200941. [DOI] [PubMed] [Google Scholar]
- 185.Schulert G.S., Grom A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu. Rev. Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ruscitti P., Cipriani P., Di Benedetto P., Liakouli V., Berardicurti O., Carubbi F., et al. H-ferritin and proinflammatory cytokines are increased in the bone marrow of patients affected by macrophage activation syndrome. Clin. Exp. Immunol. 2018;191:220–228. doi: 10.1111/cei.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bracaglia C., de Graaf K., Pires Marafon D., Guilhot F., Ferlin W., Prencipe G., et al. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann. Rheum. Dis. 2016;76:166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 188.Put K., Avau A., Brisse E., Mitera T., Put S., Proost P., et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology (Oxford) 2015;54:1507–1517. doi: 10.1093/rheumatology/keu524. [DOI] [PubMed] [Google Scholar]
- 189.Takada H., Takahata Y., Nomura A., Ohga S., Mizuno Y., Hara T. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin. Exp. Immunol. 2003;133:448–453. doi: 10.1046/j.1365-2249.2003.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Weiss E.S., Girard-Guyonvarc'h C., Holzinger D., de Jesus A.A., Tariq Z., Picarsic J., et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131:1442–1455. doi: 10.1182/blood-2017-12-820852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xu X.-J., Tang Y.-M., Song H., Yang S.-L., Xu W.-Q., Zhao N., et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J. Pediatrics. 2012;160 doi: 10.1016/j.jpeds.2011.11.046. 984-90.e1. [DOI] [PubMed] [Google Scholar]
- 192.McClain K.L., Allen C.E. Fire behind the fury: IL-18 and MAS. Blood. 2018;131:1393–1394. doi: 10.1182/blood-2018-02-828186. [DOI] [PubMed] [Google Scholar]
- 193.Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., De Benedetti F., et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67:3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- 194.Grom A.A., Villanueva J., Lee S., Goldmuntz E.A., Passo M.H., Filipovich A. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediat. 2003;142:292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 195.Villanueva J., Lee S., Giannini E.H., Graham T.B., Passo M.H., Filipovich A., et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res. Therapy. 2005;7:R30–R37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brisse E., Wouters C.H., Matthys P. Hemophagocytic lymphohistiocytosis (HLH): A heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 2015;26:263–280. doi: 10.1016/j.cytogfr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 197.Grom A.A., Mellins E.D. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr. Opin. Rheumatol. 2010;22:561–566. doi: 10.1097/01.bor.0000381996.69261.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sakumura N., Shimizu M., Mizuta M., Inoue N., Nakagishi Y., Yachie A. Soluble CD163, a unique biomarker to evaluate the disease activity, exhibits macrophage activation in systemic juvenile idiopathic arthritis. Cytokine. 2018;110:459–465. doi: 10.1016/j.cyto.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 199.Sumegi J., Barnes M.G., Nestheide S.V., Molleran-Lee S., Villanueva J., Zhang K., et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 2011;117:e151–e160. doi: 10.1182/blood-2010-08-300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The immunology of macrophage activation syndrome. Front. Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McElroy A.K., Shrivastava-Ranjan P., Harmon J.R., Martines R.B., Silva-Flannery L., Flietstra T.D., et al. Macrophage activation marker soluble CD163 associated with fatal and severe ebola virus disease in humans(1) Emerg. Infect. Dis. 2019;25:290–298. doi: 10.3201/eid2502.181326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shimizu M., Yachie A. Compensated inflammation in systemic juvenile idiopathic arthritis: role of alternatively activated macrophages. Cytokine. 2012;60:226–232. doi: 10.1016/j.cyto.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 203.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Minoia F., Davì S., Horne A., Demirkaya E., Bovis F., Li C., et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. (Hoboken, NJ). 2014;66:3160–3169. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 205.Lerkvaleekul B., Vilaiyuk S. Macrophage activation syndrome: early diagnosis is key. Open Access Rheumatol. 2018;10:117–128. doi: 10.2147/OARRR.S151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Grom A.A., Ramanan A.V. Oxford University Press; Oxford Medicine Online: 2016. Macrophage Activation Syndrome. [Google Scholar]
- 207.Hazen S.L., Heinecke J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kaufman K.M., Linghu B., Szustakowski J.D., Husami A., Yang F., Zhang K., et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. (Hoboken, NJ). 2014;66:3486–3495. doi: 10.1002/art.38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vastert S.J., van Wijk R., D’Urbano L.E., de Vooght K.M.K., de Jager W., Ravelli A., et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology. 2009;49:441–449. doi: 10.1093/rheumatology/kep418. [DOI] [PubMed] [Google Scholar]
- 210.Canna S.W., de Jesus A.A., Gouni S., Brooks S.R., Marrero B., Liu Y., et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Romberg N., Al Moussawi K., Nelson-Williams C., Stiegler A.L., Loring E., Choi M., et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 2014;46:1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schulert G.S., Cron R.Q. The genetics of macrophage activation syndrome. Genes Immun. 2020;21:169–181. doi: 10.1038/s41435-020-0098-4. [DOI] [PubMed] [Google Scholar]
- 213.Sheth J., Patel A., Shah R., Bhavsar R., Trivedi S., Sheth F. Rare cause of Hemophagocytic Lymphohistiocytosis due to mutation in PRF1 and SH2D1A genes in two children – a case report with a review. BMC Pediatr. 2019;19:73. doi: 10.1186/s12887-019-1444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.zur Stadt U., Rohr J., Seifert W., Koch F., Grieve S., Pagel J., et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am. J. Hum. Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Henter J.I., Elinder G., Soder O., Hansson M., Andersson B., Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. [PubMed] [Google Scholar]
- 216.Jenkins M.R., Rudd-Schmidt J.A., Lopez J.A., Ramsbottom K.M., Mannering S.I., Andrews D.M., et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J. Exp. Med. 2015;212:307–317. doi: 10.1084/jem.20140964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang M., Bracaglia C., Prencipe G., Bemrich-Stolz C.J., Beukelman T., Dimmitt R.A., et al. A Heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J. Immunol. (Baltimore, Md: 1950) 2016;196:2492–2503. doi: 10.4049/jimmunol.1501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Casciaro R., Cresta F., Favilli F., Naselli A., De Alessandri A., Minicucci L. Macrophage activation syndrome induced by A/H1N1 influenza in cystic fibrosis. Pediatr. Pulmonol. 2014;49:E10–E12. doi: 10.1002/ppul.22778. [DOI] [PubMed] [Google Scholar]
- 219.Brisse E., Wouters C.H., Andrei G., Matthys P. How viruses contribute to the pathogenesis of hemophagocytic lymphohistiocytosis. Front. Immunol. 2017;8:1102. doi: 10.3389/fimmu.2017.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schulert G.S., Zhang M., Fall N., Husami A., Kissell D., Hanosh A., et al. Whole-exome sequencing reveals mutations in genes linked to hemophagocytic lymphohistiocytosis and macrophage activation syndrome in fatal cases of H1N1 influenza. J. Infect. Dis. 2016;213:1180–1188. doi: 10.1093/infdis/jiv550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fall N., Barnes M., Thornton S., Luyrink L., Olson J., Ilowite N.T., et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 222.Pascual V., Allantaz F., Arce E., Punaro M., Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Strippoli R., Caiello I., De Benedetti F. Reaching the threshold: a multilayer pathogenesis of macrophage activation syndrome. J. Rheumatol. 2013;40:761–767. doi: 10.3899/jrheum.121233. [DOI] [PubMed] [Google Scholar]
- 224.Henderson L.A., Cron R.Q. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Paediatr. Drugs. 2020;22:29–44. doi: 10.1007/s40272-019-00367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brandstadter J.D., Yang Y. Natural killer cell responses to viral infection. J. Innate Immun. 2011;3:274–279. doi: 10.1159/000324176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Grom A.A. Natural killer cell dysfunction: A common pathway in systemic-onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis? Arthritis Rheum. 2004;50:689–698. doi: 10.1002/art.20198. [DOI] [PubMed] [Google Scholar]
- 227.Prencipe G., Bracaglia C., Caiello I., Pascarella A., Francalanci P., Pardeo M., et al. The interferon-gamma pathway is selectively up-regulated in the liver of patients with secondary hemophagocytic lymphohistiocytosis. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0226043. e0226043-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Maruyama J., Inokuma S. Cytokine profiles of macrophage activation syndrome associated with rheumatic diseases. J. Rheumatol. 2010;37:967–973. doi: 10.3899/jrheum.090662. [DOI] [PubMed] [Google Scholar]
- 229.Gattorno M., Piccini A., Lasigliè D., Tassi S., Brisca G., Carta S., et al. The pattern of response to anti–interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 230.Sikora K.A., Fall N., Thornton S., Grom A.A. The limited role of interferon-γ in systemic juvenile idiopathic arthritis cannot be explained by cellular hyporesponsiveness. Arthritis Rheum. 2012;64:3799–3808. doi: 10.1002/art.34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ogilvie E.M., Khan A., Hubank M., Kellam P., Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 232.Kip A.E., Wasunna M., Alves F., Schellens J.H.M., Beijnen J.H., Musa A.M., et al. Macrophage activation marker neopterin: a candidate biomarker for treatment response and relapse in visceral leishmaniasis. Front. Cell. Infect. Microbiol. 2018;8 doi: 10.3389/fcimb.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen W., Qiu H., Yang X., Zhang W., Zhang S., Zhang X., et al. The clinical significance of serum neopterin and adenosine deaminase in patients with secondary hemophagocytic lymphohistiocytosis. Zhonghua nei ke za zhi. 2015;54:44–47. [PubMed] [Google Scholar]
- 234.Ibarra M.F., Klein-Gitelman M., Morgan E., Proytcheva M., Sullivan C., Morgan G., et al. Serum neopterin levels as a diagnostic marker of hemophagocytic lymphohistiocytosis syndrome. Clin. Vaccine Immunol. 2011;18:609–614. doi: 10.1128/CVI.00306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shimizu M., Yokoyama T., Yamada K., Kaneda H., Wada H., Wada T., et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology. 2010;49:1645–1653. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 236.Ravelli A., Grom A.A., Behrens E.M., Cron R.Q. Macrophage activation syndrome as part of systemic juvenile idiopathic arthritis: diagnosis, genetics, pathophysiology and treatment. Genes Immun. 2012;13:289–298. doi: 10.1038/gene.2012.3. [DOI] [PubMed] [Google Scholar]
- 237.Hu X., Ivashkiv L.B. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Osugi Y., Hara J., Tagawa S., Takai K., Hosoi G., Matsuda Y., et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89:4100–4103. [PubMed] [Google Scholar]
- 239.F.D. Benedetti, P. Brogan, A. Grom, P. Quartier, R. Schneider, K.D. Graaf, et al., OP0204 Emapalumab, AN INTERFERON GAMMA (IFN-Y)-BLOCKING MONOCLONAL ANTIBODY, IN PATIENTS WITH MACROPHAGE ACTIVATION SYNDROME (MAS) COMPLICATING SYSTEMIC JUVENILE IDIOPATHIC ARTHRITIS (SJIA). Oral Presentations: BMJ Publishing Group Ltd and European League Against Rheumatism (2019)..
- 240.Lounder D.T., Bin Q., de Min C., Jordan M.B. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019;3:47–50. doi: 10.1182/bloodadvances.2018025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Masters S.L., Mielke L.A., Cornish A.L., Sutton C.E., O'Donnell J., Cengia L.H., et al. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 2010;11:640–646. doi: 10.1038/embor.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mayer-Barber K.D., Yan B. Clash of the Cytokine Titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell. Mol. Immunol. 2017;14:22–35. doi: 10.1038/cmi.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schulert G.S., Grom A.A. Macrophage activation syndrome and cytokine-directed therapies. Best Pract Res Clin Rheumatol. 2014;28:277–292. doi: 10.1016/j.berh.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Toplak N., Blazina Š., Avčin T. The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: current status and future perspectives. Drug Des. Deve. Therapy. 2018;12:1633–1643. doi: 10.2147/DDDT.S114532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schulert G.S., Minoia F., Bohnsack J., Cron R.Q., Hashad S., KonÉ-Paut I., et al. Effect of biologic therapy on clinical and laboratory features of macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Arthritis Care Res. 2018;70:409–419. doi: 10.1002/acr.23277. [DOI] [PubMed] [Google Scholar]
- 247.Fitzgerald A.A., LeClercq S.A., Yan A., Homik J.E., Dinarello C.A. Rapid responses to anakinra in patients with refractory adult-onset Still's disease. Arthritis Rheum. 2005;52:1794–1803. doi: 10.1002/art.21061. [DOI] [PubMed] [Google Scholar]
- 248.Henter J.-I., Andersson B., Elinder G., Jakobson Å., Lübeck P.-O., Söder O. Elevated circulating levels of interleukin-1 receptor antagonist but not IL-1 agonists in hemophagocytic lymphohistiocytosis. Med. Pediatr. Oncol. 1996;27:21–25. doi: 10.1002/(SICI)1096-911X(199607)27:1<21::AID-MPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 249.Rajasekaran S., Kruse K., Kovey K., Davis A.T., Hassan N.E., Ndika A.N., et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically Ill children*. Pediatric Crit. Care Med. 2014;15:401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 250.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wohlfarth P., Agis H., Gualdoni G.A., Weber J., Staudinger T., Schellongowski P., et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically Ill adult patients with hemophagocytic lymphohistiocytosis. J. Intensive Care Med. 2017;34:723–731. doi: 10.1177/0885066617711386. [DOI] [PubMed] [Google Scholar]
- 252.Ben-Sasson S.Z., Caucheteux S., Crank M., Hu-Li J., Paul W.E. IL-1 acts on T cells to enhance the magnitude of in vivo immune responses. Cytokine. 2011;56:122–125. doi: 10.1016/j.cyto.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ben-Sasson S.Z., Wang K., Cohen J., Paul W.E. IL-1β strikingly enhances antigen-driven CD4 and CD8 T-cell responses. Cold Spring Harb. Symp. Quant. Biol. 2013;78:117–124. doi: 10.1101/sqb.2013.78.021246. [DOI] [PubMed] [Google Scholar]
- 254.Moratal C., Raffort J., Arrighi N., Rekima S., Schaub S., Dechesne C.A., et al. IL-1β- and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci. Rep. 2018;8:17005. doi: 10.1038/s41598-018-35429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Girard C., Rech J., Brown M., Allali D., Roux-Lombard P., Spertini F., et al. Elevated serum levels of free interleukin-18 in adult-onset Still's disease. Rheumatology (Oxford) 2016;55:2237–2247. doi: 10.1093/rheumatology/kew300. [DOI] [PubMed] [Google Scholar]
- 257.Mazodier K. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yasin S., Fall N., Brown R.A., Henderlight M., Canna S.W., Girard-Guyonvarc'h C., et al. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology. 2019;59:361–366. doi: 10.1093/rheumatology/kez282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shimizu M., Nakagishi Y., Inoue N., Mizuta M., Ko G., Saikawa Y., et al. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin. Immunol. (Orlando, Fla). 2015;160:277–281. doi: 10.1016/j.clim.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 260.Novick D., Elbirt D., Dinarello C.A., Rubinstein M., Sthoeger Z.M. Interleukin-18 binding protein in the sera of patients with Wegener’s granulomatosis. J. Clin. Immunol. 2008;29:38–45. doi: 10.1007/s10875-008-9217-0. [DOI] [PubMed] [Google Scholar]
- 261.Novick D., Elbirt D., Miller G., Dinarello C.A., Rubinstein M., Sthoeger Z.M. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J. Autoimmun. 2010;34:121–126. doi: 10.1016/j.jaut.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 262.Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 263.Freeman B.E., Hammarlund E., Raué H.-P., Slifka M.K. Regulation of innate CD8<sup>+</sup> T-cell activation mediated by cytokines. Proc. Natl. Acad. Sci. 2012;109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kim T.-S., Shin E.-C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 2019;51:1–9. doi: 10.1038/s12276-019-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Brzostek J., Gautam N., Zhao X., Chen E.W., Mehta M., Tung D.W.H., et al. T cell receptor and cytokine signal integration in CD8+ T cells is mediated by the protein Themis. Nat. Immunol. 2020;21:186–198. doi: 10.1038/s41590-019-0570-3. [DOI] [PubMed] [Google Scholar]
- 266.Haring J.S., Harty J.T. Interleukin-18-related genes are induced during the contraction phase but do not play major roles in regulating the dynamics or function of the T-cell response to Listeria monocytogenes infection. Infect. Immun. 2009;77:1894–1903. doi: 10.1128/IAI.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Iwai Y., Hemmi H., Mizenina O., Kuroda S., Suda K., Steinman R.M. An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation. PLoS ONE. 2008;3:e2404. doi: 10.1371/journal.pone.0002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Li W., Kashiwamura S., Ueda H., Sekiyama A., Okamura H. Protection of CD8+ T cells from activation-induced cell death by IL-18. J. Leukoc. Biol. 2007;82:142–151. doi: 10.1189/jlb.0706431. [DOI] [PubMed] [Google Scholar]
- 269.Turtle C.J., Swanson H.M., Fujii N., Estey E.H., Riddell S.R. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McCoy S.S., Stannard J., Kahlenberg J.M. Targeting the inflammasome in rheumatic diseases. Transl. Res.: J. Lab. Clin. Med. 2016;167:125–137. doi: 10.1016/j.trsl.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Duncan J.A., Canna S.W. The NLRC4 inflammasome. Immunol. Rev. 2018;281:115–123. doi: 10.1111/imr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cox M.A., Kahan S.M., Zajac A.J. Anti-viral CD8 T cells and the cytokines that they love. Virology. 2013;435:157–169. doi: 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hu Q., Shi H., Zeng T., Liu H., Su Y., Cheng X., et al. Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still's disease. Arthritis Res. Therapy. 2019;21:9. doi: 10.1186/s13075-018-1800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hsieh C.W., Chen Y.M., Lin C.C., Tang K.T., Chen H.H., Hung W.T., et al. Elevated expression of the NLRP3 inflammasome and its correlation with disease activity in adult-onset still disease. J. Rheumatol. 2017;44:1142–1150. doi: 10.3899/jrheum.161354. [DOI] [PubMed] [Google Scholar]
- 275.Chen G., Pedra J.H. The inflammasome in host defense. Sensors (Basel, Switzerland). 2010;10:97–111. doi: 10.3390/s100100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jangra S., Yuen K.-S., Botelho M.G., Jin D.-Y. Epstein-barr virus and innate immunity: friends or foes? Microorganisms. 2019;7:183. doi: 10.3390/microorganisms7060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Khare S., Luc N., Dorfleutner A., Stehlik C. Inflammasomes and their activation. Crit. Rev. Immunol. 2010;30:463–487. doi: 10.1615/critrevimmunol.v30.i5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.de Benedetti F., Massa M., Robbioni P., Ravelli A., Burgio G.R., Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34:1158–1163. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 280.Lasigliè D., Traggiai E., Federici S., Alessio M., Buoncompagni A., Accogli A., et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Billiau A.D., Roskams T., Van Damme-Lombaerts R., Matthys P., Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood. 2005;105:1648–1651. doi: 10.1182/blood-2004-08-2997. [DOI] [PubMed] [Google Scholar]
- 282.Musso T., Espinoza-Delgado I., Pulkki K., Gusella G.L., Longo D.L., Varesio L. Transforming growth factor beta downregulates interleukin-1 (IL-1)- induced IL-6 production by human monocytes. Blood. 1990;76:2466–2469. [PubMed] [Google Scholar]
- 283.Netea M.G., Kullberg B.J., Verschueren I., Meer J.W.M.Vd. Interleukin-18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1β. Eur. J. Immunol. 2000;30:3057–3060. doi: 10.1002/1521-4141(200010)30:10<3057::AID-IMMU3057>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 284.Sterba Y., Ilowite N. Biologics in pediatric rheumatology: quo vadis? Curr. Rheumatol. Rep. 2016;18 doi: 10.1007/s11926-016-0593-9. [DOI] [PubMed] [Google Scholar]
- 285.Grom A.A., Horne A., De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016;12:259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Correll C.K., Binstadt B.A. Advances in the pathogenesis and treatment of systemic juvenile idiopathic arthritis. Pediatr. Res. 2014;75:176–183. doi: 10.1038/pr.2013.187. [DOI] [PubMed] [Google Scholar]
- 287.Makay B., Yılmaz Ş., Türkyılmaz Z., Ünal N., Ören H., Ünsal E. Etanercept for therapy-resistant macrophage activation syndrome. Pediatr. Blood Cancer. 2007;50:419–421. doi: 10.1002/pbc.21019. [DOI] [PubMed] [Google Scholar]
- 288.Buonuomo P.S., Campana A., Insalaco A., Bracaglia C., Pardeo M., Cortis E. Necrotizing fasciitis in a pediatric patient treated with etanercept and cyclosporine for macrophage activation syndrome. Rheumatol. Int. 2011;33:1097–1098. doi: 10.1007/s00296-011-2319-7. [DOI] [PubMed] [Google Scholar]
- 289.Jordan M.B., Hildeman D., Kappler J., Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 290.Sterba G., Sterba Y., Isea L., Torres M., Iglesias-Gamarra A. Macrophage activation syndrome in nine patients: three adults and six children. JCR J. Clin. Rheumatol. 2006;12:S8. [Google Scholar]
- 291.Stern A., Riley R., Buckley L. Worsening of macrophage activation syndrome in a patient with adult onset still’s disease after initiation of etanercept therapy. JCR J. Clin. Rheumatol. 2001;7:252–256. doi: 10.1097/00124743-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 292.Agbanoma G., Li C., Ennis D., Palfreeman A.C., Williams L.M., Brennan F.M. Production of TNF-α in macrophages activated by T cells, compared with lipopolysaccharide, uses distinct IL-10–dependent regulatory mechanism. J. Immunol. 2012;188:1307–1317. doi: 10.4049/jimmunol.1100625. [DOI] [PubMed] [Google Scholar]
- 293.Brach M.A., Henschler R., Mertelsmann R.H., Herrmann F. Regulation of M-CSF expression by M-CSF: role of protein kinase C and transcription factor NF kappa B. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 1991;59:284–288. doi: 10.1159/000163664. [DOI] [PubMed] [Google Scholar]
- 294.Kogan M., Haine V., Ke Y., Wigdahl B., Fischer-Smith T., Rappaport J. Macrophage colony stimulating factor regulation by nuclear factor kappa B: a relevant pathway in human immunodeficiency virus type 1 infected macrophages. DNA Cell Biol. 2012;31:280–289. doi: 10.1089/dna.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wolf Y., Shemer A., Polonsky M., Gross M., Mildner A., Yona S., et al. Autonomous TNF is critical for in vivo monocyte survival in steady state and inflammation. J. Exp. Med. 2017;214:905–917. doi: 10.1084/jem.20160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ding J., Song D., Ye X., Liu S.F. A pivotal role of endothelial-specific NF-κB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J. Immunol. 2009;183:4031–4038. doi: 10.4049/jimmunol.0900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liao J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013;123:540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mussbacher M., Salzmann M., Brostjan C., Hoesel B., Schoergenhofer C., Datler H., et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sehnert B., Burkhardt H., Wessels J.T., Schröder A., May M.J., Vestweber D., et al. NF-κB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-κB in immune-mediated diseases. Proc. Natl. Acad. Sci. 2013;110:16556–16561. doi: 10.1073/pnas.1218219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.van der Poll T., van de Veerdonk F.L., Scicluna B.P., Netea M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 301.Grinberg-Bleyer Y., Caron R., Seeley J.J., De Silva N.S., Schindler C.W., Hayden M.S., et al. The alternative NF-κB pathway in regulatory T cell homeostasis and suppressive function. J. Immunol. (Baltimore, Md: 1950) 2018;200:2362–2371. doi: 10.4049/jimmunol.1800042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Krishna S., Xie D., Gorentla B., Shin J., Gao J., Zhong X.-P. Chronic activation of the kinase IKKβ impairs T cell function and survival. J. Immunol. 2012;189:1209–1219. doi: 10.4049/jimmunol.1102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Oh H., Ghosh S. NF-κB: roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev. 2013;252:41–51. doi: 10.1111/imr.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) – Mol. Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 305.Yarosz E.L., Chang C.-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 2018;18:e14. doi: 10.4110/in.2018.18.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Johnson B.L., 3rd, Goetzman H.S., Prakash P.S., Caldwell C.C. Mechanisms underlying mouse TNF-alpha stimulated neutrophil derived microparticle generation. Biochem. Biophys. Res. Commun. 2013;437:591–596. doi: 10.1016/j.bbrc.2013.06.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kim C.H., Lee K.-H., Lee C.-T., Kim Y.W., Han S.K., Shim Y.-S., et al. Aggregation of β2 integrins activates human neutrophils through the IκB/NF-κB pathway. J. Leukoc. Biol. 2004;75:286–292. doi: 10.1189/jlb.0103038. [DOI] [PubMed] [Google Scholar]
- 308.Lapponi M.J., Carestia A., Landoni V.I., Rivadeneyra L., Etulain J., Negrotto S., et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J. Pharmacol. Exp. Therap. 2013;345:430–437. doi: 10.1124/jpet.112.202879. [DOI] [PubMed] [Google Scholar]
- 309.Mohammed B.M., Fisher B.J., Kraskauskas D., Farkas D., Brophy D.F., Fowler A.A., 3rd, et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5:3131–3151. doi: 10.3390/nu5083131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Targeted Therapy. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O’Neil A., et al. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2021;264:118617. doi: 10.1016/j.lfs.2020.118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. (London, England: 1979) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 313.Liang J., Nagahashi M., Kim E.Y., Harikumar K.B., Yamada A., Huang W.-C., et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 2013;23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Theiss A.L. Sphingosine-1-phosphate. JAKSTAT. 2013;2 doi: 10.4161/jkst.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Li S., Zhou Y., Zheng X., Wu X., Liang Y., Wang S., et al. Sphk1 promotes breast epithelial cell proliferation via NF-κB-p65-mediated cyclin D1 expression. Oncotarget. 2016;7:80579–80585. doi: 10.18632/oncotarget.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Nguyen A.V., Wu Y.-Y., Lin E.Y. STAT3 and sphingosine-1-phosphate in inflammation-associated colorectal cancer. World J. Gastroenterol. 2014;20:10279–10287. doi: 10.3748/wjg.v20.i30.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Pyne N.J., McNaughton M., Boomkamp S., MacRitchie N., Evangelisti C., Martelli A.M., et al. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul. 2016;60:151–159. doi: 10.1016/j.jbior.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 318.Song L., Xiong H., Li J., Liao W., Wang L., Wu J., et al. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2011;17:1839–1849. doi: 10.1158/1078-0432.CCR-10-0720. [DOI] [PubMed] [Google Scholar]
- 319.Murakami M., Hirano T. A Four-step model for the IL-6 amplifier, a regulator of chronic inflammations in tissue-specific MHC class II-associated autoimmune diseases. Front. Immunol. 2011;2 doi: 10.3389/fimmu.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Singh M., Prasad N., Rai M.K., Behera M.R., Agarwal V., Misra D.P. Role of IL-6 and IL-17 mediated inflammation amplifier loop in chronic antibody-mediated rejection (CABMR) Transplantation. 2020;104:S54. [Google Scholar]
- 321.Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) pathway. Sci. Signaling. 2010;3 doi: 10.1126/scisignal.3105cm1. cm1-cm. [DOI] [PubMed] [Google Scholar]
- 323.Verstrepen L., Bekaert T., Chau T.L., Tavernier J., Chariot A., Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell. Mol. Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zhuang Z., Ju H.Q., Aguilar M., Gocho T., Li H., Iida T., et al. IL1 Receptor Antagonist Inhibits Pancreatic Cancer Growth by Abrogating NF-κB Activation. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2016;22:1432–1444. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Buckley L.F., Wohlford G.F., Ting C., Alahmed A., Van Tassell B.W., Abbate A., et al. Role for anti-cytokine therapies in severe coronavirus disease 2019. Crit. Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Nomura A., Gupta V.K., Dauer P., Sharma N.S., Dudeja V., Merchant N., et al. NFκB-mediated invasiveness in CD133(+) pancreatic TICs is regulated by autocrine and paracrine activation of IL1 signaling. Mol. Cancer Res. 2018;16:162–172. doi: 10.1158/1541-7786.MCR-17-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Dinarello C., Novick D., Kim S., Kaplanski G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Rada B., Park J.J., Sil P., Geiszt M., Leto T.L. NLRP3 inflammasome activation and interleukin-1β release in macrophages require calcium but are independent of calcium-activated NADPH oxidases. Inflammation Res.: Off. J. Eur. Histamine Res. Soc. [et al]. 2014;63:821–830. doi: 10.1007/s00011-014-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cahill C.M., Rogers J.T. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mohr T., Haudek-Prinz V., Slany A., Grillari J., Micksche M., Gerner C. Proteome profiling in IL-1β and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0179065. e0179065-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Puhlmann M., Weinreich D.M., Farma J.M., Carroll N.M., Turner E.M., Alexander H.R. Interleukin-1β induced vascular permeability is dependent on induction of endothelial Tissue Factor (TF) activity. J Transl Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ridker P.M., MacFadyen J.G., Thuren T., Libby P., Group obotCT Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2019;41:2153–2163. doi: 10.1093/eurheartj/ehz542. [DOI] [PubMed] [Google Scholar]
- 333.Otsuka R., Seino K-i. Macrophage activation syndrome and COVID-19. Inflamm Regen. 2020;40:19. doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ikonomidis I., Lekakis J.P., Nikolaou M., Paraskevaidis I., Andreadou I., Kaplanoglou T., et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 335.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Su H., Lei C.-T., Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hu B., Elinav E., Huber S., Strowig T., Hao L., Hafemann A., et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl. Acad. Sci. U S A. 2013;110:9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Doeleman M.J.H., van Maarseveen E.M., Swart J.F. Immunogenicity of biologic agents in juvenile idiopathic arthritis: a systematic review and meta-analysis. Rheumatology. 2019;58:1839–1849. doi: 10.1093/rheumatology/kez030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Si S., Teachey D.T. Spotlight on tocilizumab in the treatment of CAR-T-cell-induced cytokine release syndrome: clinical evidence to date. Ther. Clin. Risk Manag. 2020;16:705–714. doi: 10.2147/TCRM.S223468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N. Engl. J. Med. 2020;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Horby P.W., Pessoa-Amorim G., Peto L., Brightling C.E., Sarkar R., Thomas K., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021 2021.02.11.21249258. [Google Scholar]
- 343.Rubin E.J., Longo D.L., Baden L.R. Interleukin-6 receptor inhibition in covid-19 — cooling the inflammatory soup. N. Engl. J. Med. 2021 doi: 10.1056/NEJMe2103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lan S.-H., Lai C.-C., Huang H.-T., Chang S.-P., Lu L.-C., Hsueh P.-R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Shimizu M., Nakagishi Y., Kasai K., Yamasaki Y., Miyoshi M., Takei S., et al. Tocilizumab masks the clinical symptoms of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome: the diagnostic significance of interleukin-18 and interleukin-6. Cytokine. 2012;58:287–294. doi: 10.1016/j.cyto.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 346.Masiá M., Fernández-González M., Padilla S., Ortega P., García J.A., Agulló V., et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: a prospective cohort study. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Böttcher J.P., Schanz O., Garbers C., Zaremba A., Hegenbarth S., Kurts C., et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. 2014;8:1318–1327. doi: 10.1016/j.celrep.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 348.Dienz O., Rincon M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. (Orlando, Fla). 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kang S., Tanaka T., Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 2014;27:21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- 350.Malemud C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Therap. Adv. Musculoskeletal Dis. 2018;10:117–127. doi: 10.1177/1759720X18776224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tanaka Y. The JAK inhibitors: do they bring a paradigm shift for the management of rheumatic diseases? Rheumatology. 2019;58:i1–i3. doi: 10.1093/rheumatology/key280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kotyla P.J. Are Janus kinase inhibitors superior over classic biologic agents in RA patients? Biomed Res. Int. 2018;2018:7492904. doi: 10.1155/2018/7492904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cantini F., Niccoli L., Nannini C., Matarrese D., Natale M.E.D., Lotti P., et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford) 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Titanji B.K., Farley M.M., Mehta A., Connor-Schuler R., Moanna A., Cribbs S.K., et al. Use of baricitinib in patients with moderate and severe COVID-19. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Valenzuela-Almada M.O., Putman M.S., Duarte-García A. The protective effect of rheumatic disease agents in COVID-19. Best Pract. Res. Clin. Rheumatol. 2021;101659 doi: 10.1016/j.berh.2021.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Han M.K., Ignacio R.A.B., Bernus A.L., Milger-Kneidinger K., Nyaku A.N., Parker B., et al. Abstract PO-002: RUXCOVID: A phase 3, randomized, placebo-controlled study evaluating the efficacy and safety of ruxolitinib in patients with COVID-19–associated cytokine storm. Clin. Cancer Res. 2020;26 PO-002-PO- [Google Scholar]
- 359.Migita K., Izumi Y., Jiuchi Y., Kozuru H., Kawahara C., Izumi M., et al. Effects of Janus kinase inhibitor tofacitinib on circulating serum amyloid A and interleukin-6 during treatment for rheumatoid arthritis. Clin. Exp. Immunol. 2014;175:208–214. doi: 10.1111/cei.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.O'Shea J.J., Kontzias A., Yamaoka K., Tanaka Y., Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013;72(Suppl 2) doi: 10.1136/annrheumdis-2012-202576. ii111-ii5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Okano T., Inui K., Tada M., Sugioka Y., Mamoto K., Wakitani S., et al. Levels of interleukin-1 beta can predict response to tocilizumab therapy in rheumatoid arthritis: the PETITE (predictors of effectiveness of tocilizumab therapy) study. Rheumatol. Int. 2016;36:349–357. doi: 10.1007/s00296-015-3379-x. [DOI] [PubMed] [Google Scholar]
- 362.G.E. Fragoulis, I.B. McInnes, S. Siebert, JAK-inhibitors, New players in the field of immune-mediated diseases, beyond rheumatoid arthritis, Rheumatology. 58 (2019) i43–i54.. [DOI] [PMC free article] [PubMed]
- 363.McInnes I.B., Byers N.L., Higgs R.E., Lee J., Macias W.L., Na S., et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res. Therapy. 2019;21:183. doi: 10.1186/s13075-019-1964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Fleming S.B. Viral Inhibition of the IFN-induced JAK/STAT signalling pathway: development of live attenuated vaccines by mutation of viral-encoded IFN-antagonists. Vaccines (Basel). 2016;4:23. doi: 10.3390/vaccines4030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Raftery N., Stevenson N.J. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell. Mol. Life Sci.: CMLS. 2017;74:2525–2535. doi: 10.1007/s00018-017-2520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kuchipudi S.V. The complex role of STAT3 in viral infections. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/272359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Chang Z., Wang Y., Zhou X., Long J.E. STAT3 roles in viral infection: antiviral or proviral? Future Virol. 2018;13:557–574. doi: 10.2217/fvl-2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Mehta P., Ciurtin C., Scully M., Levi M., Chambers R.C. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur. Respir. J. 2020;56:2001919. doi: 10.1183/13993003.01919-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Gram H. The long and winding road in pharmaceutical development of canakinumab from rare genetic autoinflammatory syndromes to myocardial infarction and cancer. Pharmacol. Res. 2020;154 doi: 10.1016/j.phrs.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 370.Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Shah S.R., Abbasi Z., Fatima M., Ochani R.K., Shahnawaz W., Asim Khan M., et al. Canakinumab and cardiovascular outcomes: results of the CANTOS trial. J. Community Hosp Intern. Med. Perspect. 2018;8:21–22. doi: 10.1080/20009666.2018.1428023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Hoy S.M. Canakinumab: a review of its use in the management of systemic juvenile idiopathic arthritis. BioDrugs. 2015;29:133–142. doi: 10.1007/s40259-015-0123-8. [DOI] [PubMed] [Google Scholar]
- 373.Kedor C., Listing J., Zernicke J., Weiß A., Behrens F., Blank N., et al. Canakinumab for Treatment of adult-onset still’s disease to achieve reduction of arthritic manifestation (CONSIDER): phase II, randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial. Ann. Rheum. Dis. 2020;79:1090–1097. doi: 10.1136/annrheumdis-2020-217155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ucciferri C., Auricchio A., Di Nicola M., Potere N., Abbate A., Cipollone F., et al. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020;2 doi: 10.1016/S2665-9913(20)30167-3. e457-ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sheng C.C., Sahoo D., Dugar S., Prada R.A., Wang T.K.M., Abou Hassan O.K., et al. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: The three C study) Clin. Cardiol. 2020;43:1055–1063. doi: 10.1002/clc.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Chioato A., Noseda E., Felix S.D., Stevens M., Del Giudice G., Fitoussi S., et al. Influenza and meningococcal vaccinations are effective in healthy subjects treated with the interleukin-1 beta-blocking antibody canakinumab: results of an open-label, parallel group, randomized, single-center study. Clin. Vaccine Immunol. 2010;17:1952–1957. doi: 10.1128/CVI.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ozen S., Ben-Cherit E., Foeldvari I., Amarilyo G., Ozdogan H., Vanderschueren S., et al. Long-term efficacy and safety of canakinumab in patients with colchicine-resistant familial Mediterranean fever: results from the randomised phase III CLUSTER trial. Ann. Rheum. Dis. 2020;79:1362–1369. doi: 10.1136/annrheumdis-2020-217419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ridker P.M., MacFadyen J.G., Thuren T., Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2020;41:2153–2163. doi: 10.1093/eurheartj/ehz542. [DOI] [PubMed] [Google Scholar]
- 379.Wong C.C., Baum J., Silvestro A., Beste M.T., Bharani-Dharan B., Xu S., et al. Inhibition of IL1β by canakinumab may be effective against diverse molecular subtypes of lung cancer: an exploratory analysis of the CANTOS trial. Cancer Res. 2020;80:5597–5605. doi: 10.1158/0008-5472.CAN-19-3176. [DOI] [PubMed] [Google Scholar]
- 380.Harnack U., Johnen H., Pecher G. IL-1 receptor antagonist anakinra enhances tumour growth inhibition in mice receiving peptide vaccination and β-(1–3), (1–6)-D-glucan. Anticancer Res. 2010;30:3959–3965. [PubMed] [Google Scholar]
- 381.Giacomelli R., Sota J., Ruscitti P., Campochiaro C., Colafrancesco S., Dagna L., et al. The treatment of adult-onset Still's disease with anakinra, a recombinant human IL-1 receptor antagonist: a systematic review of literature. Clin. Exp. Rheumatol. 2020 doi: 10.55563/clinexprheumatol/fsq5vq. [DOI] [PubMed] [Google Scholar]
- 382.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Waugh J., Perry C.M. Anakinra. BioDrugs. 2005;19:189–202. doi: 10.2165/00063030-200519030-00005. [DOI] [PubMed] [Google Scholar]
- 384.Hedrich C.M., Bruck N., Fiebig B., Gahr M. Anakinra: a safe and effective first-line treatment in systemic onset juvenile idiopathic arthritis (SoJIA) Rheumatol. Int. 2012;32:3525–3530. doi: 10.1007/s00296-011-2249-4. [DOI] [PubMed] [Google Scholar]
- 385.Mertens M., Singh J.A. Anakinra for rheumatoid arthritis: a systematic review. J. Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 386.Ramírez J., Cañete J.D. Anakinra for the treatment of rheumatoid arthritis: a safety evaluation. Expert Opin. Drug Saf. 2018;17:727–732. doi: 10.1080/14740338.2018.1486819. [DOI] [PubMed] [Google Scholar]
- 387.Mehta P., Cron R.Q., Hartwell J., Manson J.J., Tattersall R.S. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2:e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Wampler Muskardin T.L. Intravenous anakinra for macrophage activation syndrome may hold lessons for treatment of cytokine storm in the setting of coronavirus disease 2019. ACR Open Rheumatol. 2020;2:283–285. doi: 10.1002/acr2.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020;79:1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 390.Balkhair A., Al-Zakwani I., Al Busaidi M., Al-Khirbash A., Al Mubaihsi S., BaTaher H., et al. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int. J. Infect. Dis. 2021;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Kooistra E.J., Waalders N.J.B., Grondman I., Janssen N.A.F., de Nooijer A.H., Netea M.G., et al. Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit. Care. 2020;24:688. doi: 10.1186/s13054-020-03364-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Gonçalves N.P., Vieira P., Saraiva M.J. Interleukin-1 signaling pathway as a therapeutic target in transthyretin amyloidosis. Amyloid. 2014;21:175–184. doi: 10.3109/13506129.2014.927759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Coates B.M., Staricha K.L., Ravindran N., Koch C.M., Cheng Y., Davis J.M., et al. Inhibition of the NOD-like receptor protein 3 inflammasome is protective in juvenile influenza a virus infection. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Lindblad A., Persson K., Demirel I. IL-1RA is part of the inflammasome-regulated immune response in bladder epithelial cells and influences colonization of uropathogenic E. coli. Cytokine. 2019;123:154772. doi: 10.1016/j.cyto.2019.154772. [DOI] [PubMed] [Google Scholar]
- 395.Petrasek J., Bala S., Csak T., Lippai D., Kodys K., Menashy V., et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Niu X., He D., Deng S., Li W., Xi Y., Xie C., et al. Regulatory immune responses induced by IL-1 receptor antagonist in rheumatoid arthritis. Mol. Immunol. 2011;49:290–296. doi: 10.1016/j.molimm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 397.Cavalli G., Dinarello C.A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 2018;9:1157. doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Vallejo S., Palacios E., Romacho T., Villalobos L., Peiró C., Sánchez-Ferrer C.F. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2014;13:158. doi: 10.1186/s12933-014-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 400.Loo J., Spittle D.A., Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-216243. thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 401.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020 2020.06.22.20137273. [Google Scholar]
- 402.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Al-Samkari H., Gupta S., Leaf R.K., Wang W., Rosovsky R.P., Brenner S.K., et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically Ill patients with COVID-19. Ann. Intern. Med. 2021 doi: 10.7326/M20-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Lemos A.C.B., do Espírito Santo D.A., Salvetti M.C., Gilio R.N., Agra L.B., Pazin-Filho A., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb. Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Investigators I. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]