Abstract
Introduction
Patients with cancer have an increased risk of complications from coronavirus disease 2019 (COVID-19) infection, including death, and thus, they were considered as high-priority subjects for COVID-19 vaccination. We report on the compliance with the COVID-19 vaccine of patients affected by solid tumours.
Materials and methods
Patients with cancer afferent to Medical Oncology 1 Unit of Regina Elena National Cancer Institute in Rome were considered eligible for vaccination if they were receiving systemic immunosuppressive antitumor treatment or received it in the last 6 months or having an uncontrolled advanced disease. The Pfizer BNT162b2 vaccine was proposed to all candidates via phone or during a scheduled visit. The reasons for refusal were collected by administrating a 6-item multiple-choice questionnaire.
Results
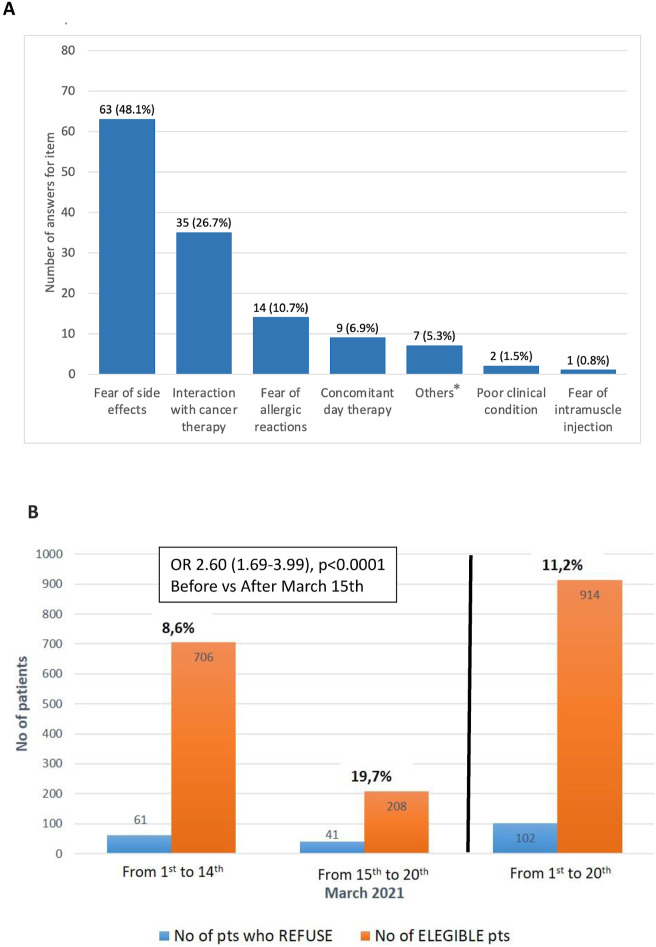
From 1st March to 20th March 2021, of 914 eligible patients, 102 refused vaccination (11.2%, 95% confidence interval [CI] 9.1–13.2). The most frequent (>10%) reasons reported were concerns about vaccine-related adverse events (48.1%), negative interaction with concomitant antitumor therapy (26.7%), and the fear of allergic reaction (10.7%). The refusal rate (RR) after 15th March (date of AstraZeneca-AZD1222 suspension) was more than doubled compared with the RR observed before (19.7% versus 8.6%, odds ratio [OR] 2.60, 95% CI 1.69–3.99; P < 0.0001). ECOG-PS 2 was associated with higher RR compared with ECOG-PS 0-1 (OR 2.94, 95% CI 1.04–8.34; P = 0.04). No statistically significant differences in RR according to other clinical characteristics were found.
Conclusions
Our experience represents the first worldwide report on the adherence of patients with cancer to COVID-19 vaccination and underlines how regulatory decisions and media news spreading could influence the success of the campaign.
Keywords: Cancer patients, COVID-19, Vaccine, BNT162b2, Refusal, SARS-CoV-2, Public health
1. Introduction
Patients with cancer have an increased risk of complications from coronavirus disease 2019 (COVID-19) infection, including a mortality rate of 30% if hospitalised [1]. In Italy, the Ministry of Health and FOCE confederation of oncologists/cardiologists/haematologists work together to offer COVID-19 vaccine with high priority to frail patients affected by these diseases. The troubled authorisation iter of COVID-19 vaccines, such as occurred for AstraZeneca AZD1222, which was even suspended temporarily during 15th March to 19th March 2021 [2], and the great attention reserved by media to vaccine safety information, may influence adherence to vaccination. We report on the compliance of patients affected by cancer with COVID-19 vaccine based on a large population from a single institutional experience.
2. Materials and Methods
Accordingly to the government's plan, patients affected by solid tumour and afferent to Medical Oncology 1 Unit of Regina Elena National Cancer Institute in Rome were considered eligible for vaccination if they were receiving systemic immunosuppressive/myelosuppressive antitumor treatment or received it in the last 6 months or having an uncontrolled advanced disease. Pfizer BNT162b2 vaccine with a fixed schedule was proposed to all candidates by phone contact or during a scheduled visit. After receiving adequate information from the physicians on the benefits/risks ratio, patients who refused vaccination answer anonymously a 6-item multiple-choice questionnaire (Supplementary material) to collect the specific reasons for withdrawal. The survey was conducted according to the rules of the local ethics committee.
3. Results
From 1 st March to 20th March 2021, we proposed Pfizer BNT162b2 to 914 patients with cancer. Females were 61%, and the median age was 62 (range 21–97) years. Breast cancer (31.2%), lung cancer (19.7%), and melanoma (14.7%) were the most frequent (>10%) tumour subtypes. Most patients were on active treatment or received it within the last 6 months (96%), and 4% were under surveillance with progressive disease. Of 914 eligible patients, 102 refused vaccination (11.2%, 95% confidence interval [CI] 9.1–13.2). The most frequent (>10%) reasons reported were concerns about vaccine-related adverse events, negative interaction with concomitant antitumor therapy, and the fear of allergic reaction, recorded in 48.1%, 26.7%, and 10.7% of answers, respectively (Fig. 1 A). The refusal rate (RR) after 15th March (date of AstraZeneca AZD1222 suspension) was more than doubled compared with the RR observed before (19.7% versus 8.6%, OR 2.60, 95% CI 1.69–3.99; P < 0.0001; Fig. 1B). ECOG-PS 2 was associated with higher RR compared with ECOG-PS 0-1(OR 2.94, 95% CI 1.04–8.34; P = 0.04). No statistically significant differences in RR according to other clinical characteristics were found (Table 1 ).
Fig. 1.
(A) Reasons reported by patients with cancer who refused COVID-19 vaccination in the questionnaire ∗.Others are self-reported reasons by patients (data not shown). (B) Refusal rate of COVID-19 vaccination in patients with cancer observed between 1st March and 14th March, 15th March and 20th March, and 1st March and 20th March. COVID-19, coronavirus disease 2019. No, number; Pts, Patients
Table 1.
Refusal rate of COVID-19 vaccine according to clinical characteristics.
| Clinical charactheristics | Pts who refuse vaccine, % (no/total no) | Pts who accept vaccine, %(no/total no) | Odds ratio (95% CI) |
|---|---|---|---|
| AGE (≥65 years versus < 65 years) | 0.83 (0.55–1.26); P = 0.39 | ||
| ≥65 years | 10.2 (43/422) | 89.9 (379/422) | |
| <65 years | 12 (59/492) | 88 (433/492) | |
| Sex (male versus female) | 0.71 (0.46–1.15); P = 0.13 | ||
| Male | 9.2 (33/359) | 90.8 (326/359) | |
| Female | 12.4(69/555) | 87.6 (486/555) | |
| ECOG-PS (2 versus 0-1) | 2.94 (1.04–8.34); P = 0.04 | ||
| 2 | 26.3 (5/19) | 73.7 (14/19) | |
| 0-1 | 10.8 (97/895) | 89.2 (798/895) | |
| CV comorbiditiesa (yes versus no) | 0.80 (0.52–1.22); P = 0.30 | ||
| Yes | 9.9 (38/384) | 90.1 (346/384) | |
| No | 12.1 (64/530) | 87.9 (466/530) | |
| Chronic steroid useb (yes versus no) | 1.61 (0.94–2.77); P = 0.08 | ||
| Yes | 15.8 (19/120) | 84.2 (101/120) | |
| No | 10.5 (83/794) | 89.5 (711/794) | |
| Tumor subtypes | |||
| Breast cancer versus others | 1.17 (0.76–1.81); P = 0.47 | ||
| Breast cancer | 12.3 (35/285) | 87.7 (250/285) | |
| Others | 10.7 (67/629) | 89.3 (562/629) | |
| Lung cancer versus others | 0.80 (0.46–1.38); P = 0.42 | ||
| Lung cancer | 9.4 (17/180) | 90.6 (163/180) | |
| Others | 11.6 (85/734) | 88.4 (649/734) | |
| Melanoma versus others | 0.99 (0.56–1.78); P = 0.98 | ||
| Melanoma | 11.1 (15/135) | 88.9 (120/135) | |
| Others | 11.2 (87/779) | 88.8 (692/779) | |
| Anticancer treatment | |||
| Chemotherapyd versus others | 1.20 (0.79–1.83); P = 0.39 | ||
| Chemotherapy | 12.3 (41/332) | 87.7 (291/332) | |
| Others | 10.5 (61/582) | 89.5 (521/582) | |
| Immunotherapyd versus others | 0.73 (0.40–1.31); P = 0.29 | ||
| Immunotherapy | 8.8 (14/160) | 91.2 (146/160) | |
| Others | 11.7 (88/754) | 88.3 (666/754) | |
| Target therapy versus others | 0.82 (0.51–1.33); P = 0.43 | ||
| Target therapy | 9.8 (24/245) | 90.2 (221/245) | |
| Others | 11.7 (78/669) | 88.3 (591/812) | |
| Date (after versus before 15th March) | 2.60 (1.69–3.99); P < 0.0001 | ||
| After 15th March | 19.7 (41/208) | 80.3 (167/208) | |
| Before 15th March | 8.6 (61/706) | 91.4 (645/706) | |
| Previous COVID-19c (yes versus no) | 0.56 (0.07–4.34); P = 0.58 | ||
| Yes | 6.7 (1/15) | 93.3 (14/15) | |
| No | 11.2 (101/899) | 88.8 (798/899) | |
| Setting (f-up versus active treatment) | 1.06(0.37–3.08); P = 0.91 | ||
| Surveillance | 11.8(4/34) | 88.2 (30/34) | |
| Active treatmente | 11.1 (98/880) | 88.9 (782/880) | |
CI, confidence interval; COVID-19, coronavirus disease 2019; CV, cardiovascular; F-up, follow-up; No, number; Pts, patients.
Cardiovascular comorbidities include heart disease, chronic obstructive pulmonary disease (asthma), diabetes, and chronic kidney disease.
Chronic steroid use was defined as started at least 30 days before vaccine administration.
Previous COVID-19 was defined as laboratory confirmed infection occurred before the vaccine administration.
Alone or in combination with other treatment.
Active treatment include pts under ongoing therapy or who have received treatment within the last 6 months.
4. Discussion
To our knowledge, our experience represents the first worldwide report on the withdrawal rate of COVID-19 vaccination in patients with cancer.
A cross-sectional survey conducted on French patients with cancer before the starting of the vaccination campaign reported the unwillingness to get vaccinated in 16.6% of subjects [3]. The rate observed was higher than that reported in our study, which was otherwise based on effective withdrawals. Furthermore, in the French study, more than half of the patients were under surveillance or receiving hormone therapy in contrast with our study population mostly including patients on active treatment.
The overall RR of 11.2% observed in our study could be considered relevant if compared with that reported by the press for Italian health operators (ranges from 1% to 3.5%), even if this estimate seems to be in contrast with the peak of 11–15% reported by some regional authorities, such as in Lombardy and Puglia [4].
Interestingly, the RR had more than doubled after the ban of AZD1222-vaccine, demonstrating how decisions by the regulatory agencies and the news spreading by media could influence the willingness of the patient to get vaccinated. This increase of RR appears much higher than that (of 5 percentage points) reported by a pool conducted on a random sample from Italian people in the same period [5]. It is conceivable that patients with cancer could be more negatively influenced by information about vaccine safety because concerns related to their frailty were more elevated. In fact, the RR was also significantly higher in patients with poorer clinical conditions (ECOG-PS 2), although they represent a small subset of the study population.
Our survey study is affected by some limitations, such as the potential risk of underestimation due to untraceable patients, whose number was however limited, and the single institutional design. However, the large sample size permits a reliable estimate of the adherence of patients although external validation could be warranted for confirmation.
Given the vulnerability of patients with cancer for severe complications and mortality related to COVID-19, the dramatic drop of adherence to vaccination observed would translate into an unacceptably high cost in otherwise preventable deaths. Health care authorities have been called to action to overcome hesitancy on vaccination in the oncological and general population by promoting adequate education and psychological interventions [6]. To pursue a successful vaccination campaign, with the appropriate consideration for frail patients, we also suggest more clarity in the decisions by the regulatory agencies and more caution in the news spreading of potential safety alarm, whose release too often occurred before a valid scientific revision. Drug safety and information transparency should be guaranteed to avoid the diffusion of threatening uncertainty in public opinion.
Funding
No funding to declare.
Conflict of interest statement
V.D.N. received speaker’s fee by AstraZeneca, MSD, BMS, Istituto Gentili, Boehringer Ingelheim. V.D.N. received grant consultancies by AstraZeneca, MSD, BMS, Boehringer Ingelheim and travel fee from MSD and Boehringer Ingelheim. V.D.N. received institutional research grants from Roche. F.C. was a member of advisory board of GSK, Roche, AstraZeneca, and Eli-Lilly. F.C. received speaker’s fee by GSK, Roche, AstraZeneca, Eli-Lilly, Novartis, Amgen, Pfizer, MSD, BMS, Astellas, and Eli-Lilly. All remaining authors have declared no conflicts of interest.
Acknowledgements
The authors thank medical oncologists, residents, nurses, hospital pharmacists, and data managers (all affiliated with Medical Oncology of Regina Elena National Cancer Institute, Rome, Italy) for their commitment to the COVID-19 vaccination campaign for patients with cancer. The authors thank the patients afferent to our Unit and their families.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.05.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agenzia Italiana del Farmaco. COVID-19 vaccines communications. https://www.aifa.gov.it/en/web/guest/-/covid-19-vaccine-astrazeneca-benefits-still-outweigh-the-risks-despite-possible-link-to-rare-blood-clots-with-low-blood-platelets. Accessed April 15th, 2021.
- 3.Barrière J., Gal J., Hoch B., et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32 doi: 10.1016/j.annonc.2021.01.066. 773-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Open Online. Quanti sono i medici e infermieri No Vax negli ospedali? 35 mila non risultano ancora vaccinati: cosa non torna nelle stime basse dei sindacati https://www.open.online/2021/03/27/covid-19-medici-infermieri-no-vax-cosa-non-torna-stime-sindacati/. Accessed April 15th, 2021.
- 5.Corriere della Sera. Vaccini, il sondaggio di Pagnoncelli: italiani ora più cauti, ma il 52% è pronto a farlo subito https://www.corriere.it/politica/21_marzo_20/vaccini-italiani-ora-piu-cauti-ma-52percento-pronto-farlo-subito-73dbdf34-88e5-11eb-9214-48facb37773c.s.html. Accessed April 15th, 2021.
- 6.Corti C., Crimini E., Tarantino P., Pravettoni G., Eggermont A.M.M., Delaloge S., et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Canc. 2021 May;148:316–327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.