Abstract
There is increasing evidence that coronavirus disease 2019 (COVID-19) is associated with a significant risk of venous thromboembolism. While information are mainly available for deep vein thrombosis of the lower limb and pulmonary embolism, scarce data exist regarding acute splanchnic vein thrombosis (SVT) in this setting. PubMed, EMBASE and Google Scholar English-language articles published up to 30 January 2021 on SVT in COVID-19 were searched. Overall, 21 articles reporting equal number of patients were identified. 15 subjects presented with portal vein thrombosis, 11 with mesenteric vein thrombosis, four with splenic vein thrombosis, and two with Budd-Chiari syndrome. Male sex was prevalent (15 patients), and median age was 43 years (range 26–79 years). Three patients had a history of liver disease, while no subject had known myeloproliferative syndrome. Clinical presentation included mainly gastrointestinal symptoms. Anticoagulation was started in 16 patients. Three patients underwent bowel resection. Ten subjects developed gastric or bowel ischemia, seven of whom underwent bowel resection, and four died after SVT diagnosis.
Although rare, SVT should be seen as a complication of COVID-19. Patients with severe gastrointestinal symptoms should be screened for SVT, as rapid recognition and correct management are essential to improve the outcome of these patients.
Keywords: Anticoagulation, Bowel ischemia, Mesenteric vein thrombosis, Portal vein thrombosis, SARS-CoV-2
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is identified as responsible of coronavirus disease 2019 (COVID-19), a clinical condition ranging from mild symptoms, such as impairment of the smell and taste, to the typical pulmonary manifestations including acute respiratory distress syndrome [1]. The virus may also directly damage the intestinal mucosa [2], as gastrointestinal symptoms have been widely reported [3,4]. Furthermore, increasing evidence showed that COVID-19 might be associated with hemostasis impairment, thus predisposing patients to both venous and arterial thromboembolism [5]. While the association between COVID-19 and deep vein thrombosis of the lower limb and pulmonary embolism has been extensively investigated, less is known about thrombotic events in other districts, such as splanchnic vein thrombosis (SVT). SVT is an uncommon manifestation of venous thromboembolism (VTE) that includes portal vein thrombosis (PVT), mesenteric vein thrombosis (MVT), and thrombosis of the liver veins or vena cava inferior (Budd-Chiari syndrome, BCS). SVT is more often diagnosed in patients with cirrhosis or liver malignancy. Other predisposing factors, which are often observed in patients with non-cirrhotic liver, are intra-abdominal surgery, infections and inflammatory diseases, as well as inherited or acquired thrombophilia [6].
Assuming that the splanchnic venous system could be also affected by the COVID-19-related coagulopathy, we conducted a systematic review of current literature on SVT and COVID-19.
2. Methods
We searched in PubMed, EMBASE and Google Scholar English-language articles published between November 2019 and 30th January 2021 including the following MeSH terms “COVID-19 (and related terms 2019 novel coronavirus, SARS-CoV-2 infection, 2019-nCoV infection) and splanchnic vein thrombosis”, “COVID-19 and portal vein thrombosis”, “COVID-19 and mesenteric vein thrombosis”, “COVID-19 and Budd-Chiari syndrome”, “COVID-19 and bowel ischemia”. Case reports, case series, commentaries, letters to editors, and review articles were considered. We evaluated independently title and abstract for inclusion, double-checking for duplication and extracting the data using the text, tables, and figures of the original published articles. Cross-referencing yielded no additional records and duplicate articles were assessed and subsequently eliminated. All the demographic and clinical characteristics, clinical course and outcomes are reported using median and incidence.
3. Results
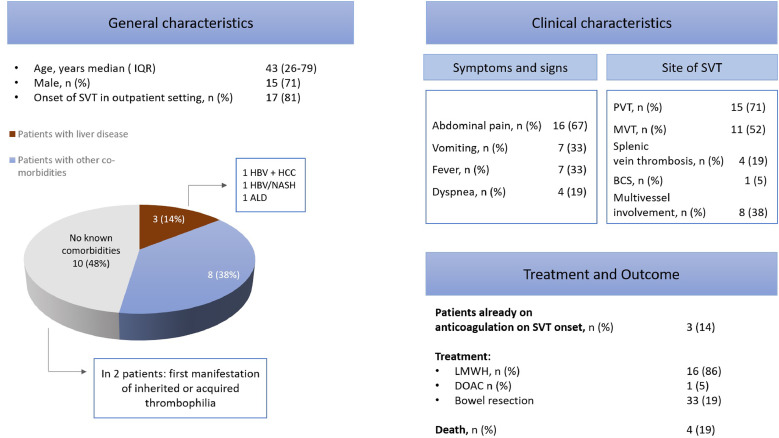
Overall, 641 articles were screened, 21 of which were selected according to the above mentioned criteria [7], [22], [23], [24], [25], [26]–27]. The included articles were all case reports (except for one case series [10]), and contained description of 21 cases. Overall, 15 patients presented with PVT, 11 with MVT, 4 with splenic vein thrombosis, and 2 with BCS. The clinical features, management and outcome are showed in Tables 1 and 2 and summarized in Fig. 2.
Table 1.
Clinical features of the 21 patients included at the time of SVT diagnosis.
| Author | Country | Age, sex | Medical setting on onset | Medical history | Diagnostic test for SARS-CoV-2 infection | Time from COVID-19 to symptoms/signs of SVT | Anticoagulation therapy at the time of SVT diagnosis | Symptoms/signs |
| De Barry et al. [1] | France | 79, F | Outpatient | None | Negative RT-PCR on nasopharynx swab. Suspicion of COVID-19 based on clinical features and pulmonary findings at imaging | Symptoms/signs of SVT at COVID-19 onset | None | Fever, deterioration of general condition, and abdominal pain located in the epigastric area, associated with diarrhea during 8 days |
| Ignat et al. [2] | France | 28, F | Outpatient | None | Not reported | Symptoms/signs of SVT at COVID-19 onset | None | Abdominal pain and vomiting with abdominal guarding at clinical examination |
| Norsa et al. [3] | Italy | 62, M | Outpatient | Obesity, arterial hypertension, T2DM and cirrhosis (NASH + hepatitis B) | Negative RT-PCR on nasopharynx swab. Diagnosis of SARS-CoV-2 infection based on ISH on the resected small bowel (RNAscope technology) | Symptoms/signs of SVT at COVID-19 onset | None | Abdominal pain and bilious vomiting during 3 days, followed by unconsciousness and severe hypotension at admission |
| Dane et al. [4] | US | Not reported | Not reported | No known liver disease or hypercoagulability risk factor (otherwise unknown) | Not reported | Not reported | Not reported | Not reported |
| La Mura et al. [5] | Italy | 72, M | Inpatient (COVID-19 Unit) | Parkinson disease, anxious-depressive syndrome, and mild vascular dementia | Not reported | 6 days | Enoxaparin 4000 UI qd | Fever, jaundice, and obnubilation at admission, followed by mild abdominal pain with bloating and constipation, periumbilical tenderness, and no rebound reaction nor ascites at clinical examination |
| Osofu et al. [6] | US | 55, M | Outpatient | Hyperlipidemia | Not reported | Occasional finding, no symptom/sign of SVT at diagnosis | None | Fever, shortness of breath, and altered mental status during 3 days |
| Franco-Moreno et al. [7] | Spain | 27, M | Outpatient | None | Negative RT-PCR on nasopharynx swab. Diagnosis of SARS-CoV-2 infection based on serological test showing IgG positivity | 21 days | None | Fever and dry cough during 3 days, without nausea, vomiting or diarrhea. Tenderness in the right upper quadrant at clinical examination |
| Del Hoyo et al. [8] | Spain | 61, F | Outpatient | T2DM | Positive RT-PCR on nasopharynx swab and serological test | Symptoms/signs of SVT at COVID-19 onset | None | Severe acute abdominal pain and vomiting |
| Qing Pang et al. [9] | Singapore | 30, M | Outpatient | None | Positive RT-PCR on nasopharynx swab | Symptoms/signs of SVT at COVID-19 onset | None | Colicky abdominal pain and vomiting during 2 days |
| Low et al. [10] | US | 51, M | Not reported | Not reported | Not reported | Not reported | Heparin (not specified) | Large volume of hematemesis following initiation of heparin for a lower extremity deep vein thrombosis |
| Jafari et al. [11] | Iran | 26, M | ICU | Asthma | Positive RT-PCR on nasopharynx swab | 7 days | Not reported | Respiratory distress and fatigue during 7 days, followed by severe abdominal pain located in the right upper quadrant |
| Lari et al. [12] | Kuwait | 38, M | Outpatient | None | Positive RT-PCR on nasopharynx swab | Symptoms/signs of SVT at COVID-19 onset | None | Progressively worsening abdominal pain, nausea, intractable vomiting, and shortness of breath during 2 days. Tachycardia, respiratory distress, and abdominal pain out of proportion to the palpation at clinical examination |
| Filho et al. [13] | Brazil | 33, M | Outpatient | Obesity | Positive RT-PCR on nasopharynx swab | 11 days | None | Dry cough, fever, and fatigue during 11 days, followed by severe low back pain radiating to the hypogastric region |
| Thuluva et al. [14] | Singapore | 29, M | Outpatient | None | Positive RT-PCR on nasopharynx swab | Symptoms/signs of SVT at COVID-19 onset | None | Lefts-side colicky abdominal pain associated with nausea, vomiting, and decreased appetite |
| Abeysekera et al. [15] | UK | 42, M | Outpatient | Chronic hepatitis B (undetectable viral load on Entecavir), and prior trauma-related splenectomy | Negative RT-PCR on nasopharynx swab. Diagnosis of SARS-CoV-2 infection based on serological test | 14 days | None | Fever and cough during 14 days, followed by sudden constant non-radiating right hypochondrial pain |
| Aleman et al. [16] | Ecuador | 44, M | Outpatient | None | Positive RT-PCR on nasopharynx swab | 7 days | None | Severe abdominopelvic pain of progressive and insidious onset, after initial respiratory symptoms |
| Rodriguez-Nakamura et al. [17] | Mexico | 42, F | Outpatient | Extreme obesity, and ventriculoperitoneal shunt due to a partially resected craniopharyngioma | Negative RT-PCR on nasopharynx swab. Suspicion of COVID-19 based on clinical features and pulmonary findings at imaging | Symptoms/signs of SVT at COVID-19 onset | None | Colic abdominal pain associated with a difficulty with passing gases and a weeklong constipation |
| Hambali et al. [18] | Malaysia | 55, M | Outpatient | Active smoking | Positive RT-PCR on nasopharynx swab | Symptoms/signs of SVT at COVID-19 onset | None | Abdominal distension and bilateral leg swelling for 10 days |
| Alharthy et al. [19] | Saudi Arabia | 45, M | Outpatient | None | Positive RT-PCR on nasopharynx swab | Symptoms/signs of SVT at COVID-19 onset | None | Fever, cough, dyspnea, diarrhea, vomiting and abdominal pain |
| Goodfellow et al. [20] | UK | 36, F | Outpatient | Laparoscopic Roux-en-Y Gastric Bypass, asthma and depression | Positive RT-PCR on nasopharynx swab | 5 days | None | Epigastric pain radiating through to the back with nausea |
| Rozenshteyn et al. [21] | US | 50, M | Outpatient | Alcohol-associated cirrhosis | Positive RT-PCR on nasopharynx swab | Not reported | Prophylaxis for deep venous thrombosis (not specified) | Altered mental status, followed by right upper quadrant abdominal pain |
List of abbreviations ICU intensive care unit; ISH immunohistochemistry; NASH non-alcoholic steatohepatitis; RT-PCR real-time reverse transcription polymerase chain reaction; SVT splanchnic vein thrombosis; T2DM type 2 diabetes mellitus.
Table 2.
Management and outcomes of the 21 patients included.
| Author | Imaging test for SVT diagnosis | Sites of SVT | Other sites | Other findings at imaging | Diagnostic workup for inherited or acquired thrombophilia | Therapy | Outcome |
| De Barry et al. [1] | CT scan | Right portal vein thrombosis originating from the distal part of the upper mesenteric vein extended to the spleno-mesenteric trunk | Proximal thrombosis of the upper mesenteric and jejunal arteries | Features of bowel ischemia of the cecum and small intestine, small amount of liquid in the peritoneal cavity | Not reported | Bowel resection, thrombolysis and thrombectomy of the upper mesenteric artery | Death |
| Ignat et al. [2] | CT scan | Superior mesenteric vein and portal vein thrombosis | None | Signs of segmental portal hypertension with gastric varices and portal cavernoma | The diagnosis of essential thrombocythemia was established | Anticoagulation (not specified) | Clinical worsening due to segmental small bowel ischemia necessitating resection. Patient discharged thereafter |
| Norsa et al. [3] | CT scan | Superior mesenteric vein thrombosis | Inferior vena cava thrombosis | High suspicion of small bowel ischemia | Not reported | Bowel resection | Death |
| Dane et al. [4] | DUS | Main portal vein thrombosis extending to the right and left portal veins | Not reported | Not reported | Not reported | Not reported | Not reported |
| La Mura et al. [5] | CT scan | Total occlusion of the left portal venous system and the secondary branches of the right portal vein | None | Large area of transient hepatic attenuation differences in the liver segments supplied by thrombosed branches | Protein C, Antithrombin, Factors II and VII were normal. Otherwise, the authors report that inherited and acquired thrombophilia was excluded with no further specification | Enoxaparin 100 UI/kg bid | Not reported |
| Osofu et al. [6] | CT scan | Thrombosis of the main right anterior and posterior divisions of the right portal vein | None | Wedge-shaped peripheral defect suggestive of ischemia | Antithrombin, Lupus anticoagulant, Proteins C and S were normal | Apixaban 5 mg bid | Discharge |
| Franco-Moreno et al. [7] | CT scan | Thrombosis of the right branch of the portal vein | None | None | JAK2, Factor V Leiden, and prothrombin G20210A mutations, antiphospholipid antibodies, Proteins C and S, Antithrombin and Factor VIII levels, flow cytometric testing for paroxysmal nocturnal hemoglobinuria were negative | Enoxaparin 100 UI/kg bid, followed by acenocoumarin | Discharge |
| Del Hoyo et al. [8] | CT scan | Right hepatic vein thrombosis and complete thrombosis of the spleno-portal axis | None | Ileo-jejunal and right colon wall edema as signs of tissue hypoperfusion changes | Lupus anticoagulant antibodies were detectable at low titer, whereas V617F JAK2, Factor V Leiden, prothrombin G20210A mutations, anticardiolipin IgG and antibeta2-glycoprotein antibodies were negative | Enoxaparin 100 UI/kg bid | Rectal bleeding and death |
| Qing Pang et al. [9] | CT scan | Superior mesenteric vein thrombosis | None | Diffuse mural thickening and fat stranding of multiple jejunal loops | Lupus anticoagulant was positive | Enoxaparin 100 UI/kg bid | Clinical worsening due to tight stenosis of mid jejunum caused by congenital adhesion band necessitating excision and bowel resection. Patient discharged thereafter to a community isolation facility |
| Low et al. [10] | CT scan | Non-occlusive thrombus in the right and left portal veins | Lower extremity deep vein thrombosis | Gastric pneumatosis, portal venous gas | Not reported | Nasogastric decompression and intravenous heparin | Unknown. According to the authors, the patient had resolution of the intramural gastric and portal venous gas, with no residual portal vein thrombosis at imaging one week later |
| Jafari et al. [11] | CT scan | Portal vein thrombosis | None | Intraperitoneal fluid | Not reported | Continuous intravenous heparin infusion (1000 UI/h) | Discharged |
| Lari et al. [12] | CT scan | Extensive thrombosis of the portal, splenic, superior and inferior mesenteric veins | Pulmonary embolism | High suspicion of ischemia of the mid-portion of the small bowel | According to the authors, the patient was tested for coagulopathies by serological testing, which were negative with low/clinically insignificant titers (with no further specification) | Heparin therapy (not specified), bowel resection, ECMO | Still in ICU at the time of manuscript submission |
| Filho et al. [13] | CT scan | Inferior mesenteric vein thrombosis | None | Infiltration of the adjacent adipose planes | Not reported | Enoxaparin (therapeutic dose), warfarin after 5 days | Discharged |
| Thuluva et al. [14] | CT scan | Superior mesenteric vein thrombosis | None | Diffuse small bowel wall thickening involving the jejunal loops, with adjacent mesenteric fat stranding secondary to mesenteric venous congestion with no bowel wall ischemia, minor ascites | Not reported | Low molecular weight heparin 100 UI/kg bid | Unknown. According to the authors, the patient showed an improvement of abdominal pain, and resumed a normal diet by day 6 of hospitalization |
| Abeysekera et al. [15] | Suspected at DUS, confirmed at CT scan | Portal vein and proximal superior mesenteric vein thrombosis | None | Expansion and surrounding inflammatory stranding | According to the authors, the patient was tested for thrombophilia, which excluded inherited and acquired conditions like antiphospholipid syndrome, myeloproliferative disorders and paroxysmal nocturnal hematuria. | Apixaban 5 mg bid | Unknown. According to the authors, an imaging 6 weeks later showed an established portal vein thrombosis with collateralization extending into the upper abdomen, the patient being asymptomatic |
| Aleman et al. [16] | DUS and CT scan | Superior mesenteric, splenic, and portal vein thrombosis | None | Small bowel loop dilatation and mesenteric fat edema | Not reported | Enoxaparin, followed by warfarin | Discharge |
| Rodriguez-Nakamura et al. [17] | CT scan | Portal vein and mesenteric veins thrombosis | None | Ileum, wall edema and perfusion alterations due to stress, absence of a defined transition zone, peritoneal fat stripes, and abdominopelvic collection in the mesentery | Not reported | Bowel resection | Death |
| Hambali et al. [18] | CT scan | Portal vein thrombosis | None | Multifocal liver lesions | Not reported | No anticoagulation therapy reported | Discharge |
| Alharthy et al. [19] | CT scan | Portal vein thrombosis | Pulmonary embolism | Thickened bowel wall | According to the authors, the patient was tested negative for lupus anticoagulant, antiphospholipid antibodies, anti-neutrophil cytoplasmic antibodies and thrombophilia screening (i.e. levels of proteins C and S, homocysteine, factor V Leiden) | Bowel resection, followed by continuous renal replacement therapy, full anticoagulation therapy (not specified) | Discharge |
| Goodfellow et al. [20] | CT scan | Superior mesenteric vein thrombosis | None | Diffuse infiltration of the mesentery suggestive of mesenteric edema and wall thickening in the small bowel | According to the authors, the patient was tested negative for JAK-2, Calreticulin, and MPL, lupus, anti-phospholipid syndrome, and paroxysmal nocturnal hemoglobinuria | Continuous intravenous heparin infusion, followed by dalteparin | Discharge |
| Rozenshteyn et al. [21] | Suspected at DUS, confirmed at CT scan | Extensive veno-occlusive disease involving the inferior vena cava and hepatic veins, consistent with Budd-Chiari syndrome | None | None | Not reported | Variceal band ligation prior to initiation of anticoagulation therapy (not specified) | Not reported |
List of abbreviations CT computed tomography; DUS Doppler ultrasound; ECMO extracorporeal membrane oxygenation; SVT splanchnic vein thrombosis
Fig. 2.
General and clinical features, management and outcomes in the SVT patients presented in this systematic review. List of abbreviations BCS Budd-Chiari syndrome; LMWH low molecular weight heparin; MVT mesenteric vein thrombosis; PVT portal vein thrombosis; SVT splanchnic vein thrombosis; HCC hepatocellular carcinoma, HBV hepatits B virus; ALD alcohol liver disease
3.1. Comorbidities
Male sex was prevalent (15 patients), and median age was 43 years (range 26–79 years).
Comorbidities included cardiovascular risk factors like obesity (3 patients), type 2 diabetes (2 patients), arterial hypertension and hyperlipidemia (one patient each), as well as asthma (2 patients), Parkinson disease and vascular dementia (one patient each). Three patients had a history of known liver disease (non-alcoholic steatohepatitis (NASH) plus hepatitis B virus (HBV) cirrhosis, chronic hepatitis B, and alcohol-associated cirrhosis). Except for one patient with operated craniopharyngioma, none of the patients had a history of neoplasia or myeloproliferative neoplasms (MPN). In one case, imaging tests performed at admission documented multifocal liver lesions consistent with hepatocellular carcinoma in the setting of newly discovered chronic hepatitis B, whereas no neoplastic thrombus was reported in the patient with known cirrhosis on NASH and HBV on the investigations performed. For ten patients, no comorbidities were reported.
3.2. Presentation
Symptoms and/or signs of SVT were present at COVID-19 onset in ten cases, whereas in seven patients SVT represented a belated manifestation (range 5–21 days). For one subject, SVT was diagnosed as occasional finding with imaging performed in the setting of COVID-19.
Clinical presentation included mainly gastrointestinal symptoms, such as abdominal pain (16 patients), and vomiting (7 patients). Jaundice and diarrhea were reported in one case each. Fever was present for 7 patients, while dyspnea, cough and altered mental status were reported in 4 cases each. One patient had a large volume hematemesis following initiation of heparin for a lower extremity deep vein thrombosis during COVID-19.
3.3. Diagnosis of SVT and of COVID-19
SVT was diagnosed in the outpatient setting in 17 cases. Almost all the diagnosis of SVT required a computed tomography (CT) scan to be confirmed, except in one case (abdominal Doppler ultrasound, DUS). Eight patients displayed multivessel involvement, including upper mesenteric artery for one patient. At the time of SVT diagnosis, features of gastric or bowel ischemia were reported in ten cases.
Diagnostic tests for COVID-19 included real-time reverse transcription polymerase chain reaction (RT-PCR) on nasopharynx swab for 16 patients, confirming COVID-19 in eleven cases, and serological test in 3 cases, all of which were positive. For one subject, SARS-CoV-2 infection was confirmed by immunohistochemistry on a specimen of the resected small bowel, while for two patients the disease was suspected based on clinical features and pulmonary findings at imaging, despite a negative RT-PCR on nasopharynx swab. For 5 patients, the diagnostic test used was not reported by the authors.
Diagnostic workup for inherited or acquired thrombophilia was reported in 11 cases, two of which had positive lupus anticoagulant (LAC) (one at low titer), and one an established diagnosis of essential thrombocythemia (ET). Further tests performed were reported as negative by the authors.
3.4. Treatment and outcomes
Before SVT was diagnosed, three patients were taking anticoagulation therapy (one at therapeutic dose). After the diagnosis, an anticoagulation treatment was started in 16 cases, including unfractionated heparin (UFH) or low molecular weight heparin (LMWH) (11 patients), and Apixaban (2 patients). In three case, the anticoagulant treatment introduced was not specified. Seven patients with radiological features of intestinal ischemia underwent bowel resection, one of whom had also thrombolysis and thrombectomy in the context of upper mesenteric artery thrombosis.
Outcome was available only for 14 patients, four of whom died. These were 2 males and 2 females, aged 79, 62, 61, and 42, respectively. One subject had no known comorbidities, another one had only diabetes reported, the third one had diabetes, obesity, arterial hypertension and cirrhosis, while the last one had extreme obesity and a ventriculoperitoneal shunt due to a partially resected craniopharyngioma. All these patients had multivessel involvement, including upper mesenteric artery, as well as bowel ischemia complicating SVT.
Two subjects had clinical worsening following anticoagulant therapy (one due to subsequent bowel ischemia, and the other one due to tight stenosis of mid-jejunum caused by congenital adhesion band). Both underwent bowel resection and were discharged thereafter. In one case, a 6-week follow-up imaging showed an established PVT with collateralization extending into the upper abdomen. At last, two patients had gastrointestinal bleeding after anticoagulant treatment introduction.
4. Discussion
Venous thromboembolism is a common complication of inpatients with COVID-19, with a prevalence as high as 21% in a recent meta-analysis of over 8 thousand patients [28]. The mechanisms underpinning the strong relationship between SARS-CoV-2 infection and venous and arterial thromboembolism are not clear yet, and likely include endothelial dysfunction, excessive inflammatory response, and hemodynamic components (stasis) [29], [30], [31], [32]–33].
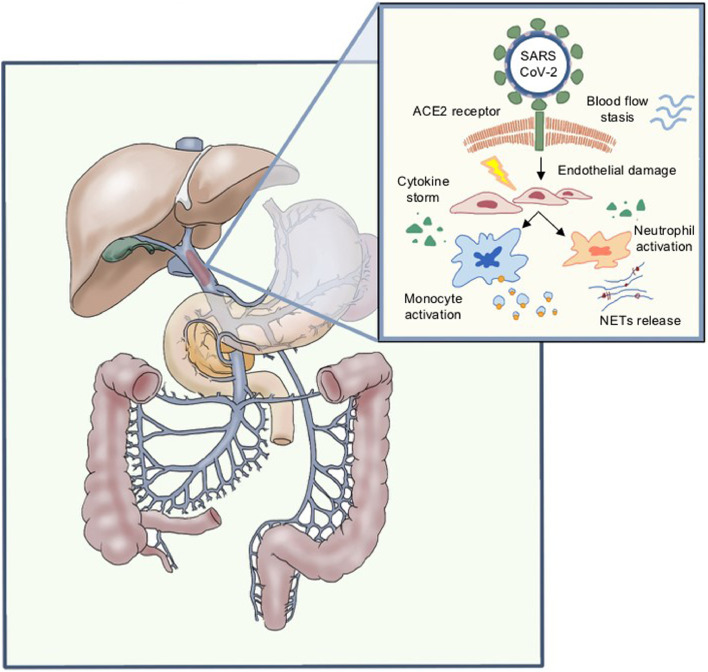
There is no specific data regarding SVT, but it can be hypothesized that the above-mentioned mechanisms in patients with predisposing conditions such as chronic liver disease or MPD can lead to a preferential splanchnic location of thrombosis (Fig. 1 ). Interestingly, however, in a meta-analysis by Diaz et al. on histopathological reports from deceased COVID-19 patients undergoing autopsy or liver biopsy, almost 30% of cases presented hepatic vascular thrombosis in spite of a low prevalence of known chronic liver disease [34].
Fig. 1.
Hypothesis of pathological mechanisms of SARS-CoV-2 infection and splanchnic vein thrombosis. List of abbreviations ACE2 Angiotensin-converting enzyme II; NETs neutrophil extracellular traps; SARS-CoV-2 Severe acute respiratory syndrome coronavirus 2
In the here reviewed cases, male gender was prevalent with a median age of 43 years. Current literature shows that the mean age of patients developing venous thromboembolic events other than SVT during COVID-19 ranges between 60 and 70 years [35,36]. Concerning SVT in other clinical scenarios, the mean age of patients is estimated between 50 and 60 years in case of PVT [37], and between 70 and 79 years for MVT [38]. Therefore, the data reported herein seem to suggest an uncommonly young age in patients developing SVT and concomitant COVID-19.
Clinical presentation of SVT was often unspecific, especially in a context of multiorgan involvement and failure, making its prompt identification challenging. Typical presentation for patients without COVID-19 includes abdominal pain, reported in almost half of cases, gastrointestinal bleeding and ascites [39]. Abdominal pain was also the main reported symptom in the reported series, followed by vomiting and fever.
As for the known risk factors for SVT, only two patients had known liver cirrhosis. In one case, imaging tests performed at admission documented multifocal liver lesions consistent with hepatocellular carcinoma in the setting of newly discovered chronic hepatitis B. Advanced liver disease is itself a risk factor for SVT. Defining a clear etiological role of SARS-CoV-2 different in this population from SVT in non-cirrhotic patients remains unclear. However, it is likely that SARS-CoV-2 represents a further trigger in this context as well. Indeed, a worsening effect of COVID-19 on the prognosis of patients with cirrhosis is well established and progression of PVT in patients despite prophylactic therapy with LMWH has been reported [40].
Interestingly, in one case COVID-19 diagnosis was possible with RNAs in situ hybridization technique applied to the resected small bowel, suggesting that local inflammation due to SARS-CoV-2 infection might be a major trigger in the development of SVT for these patients.
The diagnostic workup for inherited or acquired thrombophilia led in one case to a new diagnosis of ET, while positive LACs were found in two patients. In non-cirrhotic SVT, the mean prevalence of JAK2 V617F mutation ranges between 41.1 and 27.7%, while the mean prevalence of MPN is estimated at 40.9% and 31.5% for BCS and PVT, respectively. Additionally, for almost 20% of the subjects with SVT, JAK2 V617F screening identified MPN in patients otherwise with no typical feature of MPN [41].
With regard to LAC positivity, recent observations have suggested that single LAC positivity is a common finding during the acute phase of SARS-CoV-2 infection, without a clear causal relationship with thrombotic events. Conversely, other high-risk thrombophilia conditions, such as triple antiphospholipid antibodies positivity or high anticardiolipin/antibeta2-glycoprotein I antibodies, have rarely been described in this setting [42]. Importantly, since these findings have often been transient, unconfirmed at later measurements and not consistently associated with thrombotic events, they may not be an adequate screening tool for acquired thrombophilia in the acute phase of the disease [43].
In the here reviewed cases, SVT was assessed using DUS imaging only for one patient, whereas in the rest of the cases CT scans ruled out the diagnosis. One patient, a young woman who was later on diagnosed of ET, showed signs of cavernous transformation of the portal vein and large porto-systemic collaterals, suggesting the presence of an acute on chronic thrombosis. DUS is a validated technique in detecting SVT with an accuracy up to 90% for diagnosing PVT, cavernous transformation of the PV and BCS [44], although the sensitivity of DUS is lower for mesenteric and splenic veins [45]. CT scan or similar cross-sectional imaging should be always considered as diagnostic tool, which may also be used to investigate the presence of signs of bowel ischemia [46] and assess the extent of involvement of all the vessels of the PV system, since involvement of more than one vessel indicates a worse prognosis [47,48]. In 8 of the SVT here reviewed cases, thrombosis involved more than one vessel.
Of note, three patients were on anticoagulation at SVT diagnosis, of whom one at therapeutic dose for concomitant deep venous thrombosis (unclear if in the outpatient or inpatient setting). One patient was receiving LMWHs at standard prophylactic dose in a COVID-19 Unit, and another one in the outpatient setting. In the only reported case of SVT occurring during ICU stay, the presence of pharmacological prophylaxis of thrombosis was not reported. Previous research suggested that the incidence of thrombotic complications in ICU patients with COVID-19 infections is remarkably high despite anticoagulant therapy at standard prophylactic dose [49]. Notwithstanding this, recent evidence on patients admitted to the ICU with COVID-19 showed that intermediate-dose prophylactic anticoagulation did not result in a significant difference in the primary outcome of a composite of adjudicated venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days [50]. This might also hold true as for SVT. Ongoing trials should clarify the role of different prophylaxis strategies in the outpatient setting, including LMWHs at standard prophylactic dose, DOACs at both low and high intensity, aspirin, and sulodexide [51].
The goal of treatment of acute SVT is to achieve the patency of the vein, thus preventing bowel infarction, liver injury, and late complications of portal hypertension. The timing for starting an anticoagulant therapy is crucial in order to avoid potentially life-threatening gastrointestinal bleeding. All but five patients included in the present systematic review received anticoagulant therapy. However, four patients who underwent urgent intestinal resection have died, thus supporting the importance of immediate surgical evaluation in subjects with severe abdominal clinical presentation (e.g. bowel infarction at imaging, peritonitis, septic shock) even before considering the anticoagulant treatment. Preventive evaluation of signs of portal hypertension including gastroesophageal varices should be evaluated in a case-by-case basis in this context [46].
Evidence on which anticoagulant therapy should be used in patients with SVT is limited and choice is based mostly on clinical experience. UFH, LMWH and vitamin K antagonists are commonly used. The use of direct oral anticoagulants (DOACs) still remains off-label in most countries for SVT [46]. Potential malabsorption in case of intestinal ischemia should be always considered as a potential risk of lack of efficacy for oral therapy. Current recommendations for in-hospital patients with COVID-19 requiring anticoagulation suggest LMWH as first-line treatment [52], emphasizing its higher stability compared with UFH, particularly during the cytokine storm phase, and its reduced risk of interaction with antiviral drugs compared with DOACs. Indeed, antiviral therapy for COVID-19 has been reported to dramatically increase DOACs plasma levels [53]. Accordingly, the use of LMWH may constitute the most effective and safe strategy also for patients with SVT during COVID-19. Future research is needed to clarify these aspects.
Limitations of the present systematic review include the small number of cases reported, and reporting bias (likely reporting of the most severe cases). The conclusions of the present study rely on the quality and accuracy of the reports included in the analysis.
5. Conclusions
SVT has been reported in 21 COVID-19 cases so far, and as such it can be considered as an uncommon manifestation of SARS-CoV-2 infection. However, SVT is often fatal, thereby requiring prompt recognition and treatment. Young patients and subjects without known comorbidities may be at risk of developing this complication. A high level of warning should be raised in presence of SVT-compatible symptoms in the setting of COVID-19.
Particular attention should be given to screening of inherited or acquired thrombophilia, bearing in mind the correct timing for testing. DUS and cross-sectional imaging remains essential for diagnosis and mapping of thrombosis extent in the portal venous system. Careful monitoring of potential signs of bowel ischemia should be performed, in order to provide appropriate treatment at early stages.
Future perspectives might embrace long-term follow-up of patients with SVT and COVID-19, aiming to evaluate its natural history, including development of late portal hypertension, specific histological alterations of the liver parenchyma, and vessels recanalization rates. In this sense, an international registry could be an extremely useful tool to document and group such sporadic cases, and follow-up the clinical evolution of these patients over time. Identifying the pathophysiological mechanisms underlying the relationship between SARS-CoV-2 infection and SVT may also be of great interest.
Declaration of Competing Interest
Giacomo Buso, Chiara Becchetti, Annalisa Berzigotti have no conflict of interest to declare.
Funding
Chiara Becchetti received financial support from the Stiftung für Leberkrankheiten.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in china summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Xiao F, Tang M, Zheng X, et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158 doi: 10.1053/j.gastro.2020.02.055. 1831–1833 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, et al. Extra pulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 4.Gulen M, Satar S. Uncommon presentation of COVID-19 gastrointestinal bleeding. Clin Res Hepatol Gastroenterol. 2020;44:e72–e76. doi: 10.1016/j.clinre.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease implications for prevention, antithrombotic therapy, and follow-Up JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the study of the liver. Electronic address. EASL clinical practice guidelines vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 7.de Barry O, Mekki A, Diffre C, et al. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep. 2020;15:1054–1057. doi: 10.1016/j.radcr.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignat M, Philouze G, Aussenac-Belle L, et al. Small bowel ischemia and SARS-CoV-2 infection an underdiagnosed distinct clinical entity. Surgery. 2020;168:14–16. doi: 10.1016/j.surg.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norsa L, Valle C, Morotti D, et al. Intestinal ischemia in the COVID-19 era. Dig Liver Dis. 2020;52:1090–1091. doi: 10.1016/j.dld.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dane B, Smereka P, Wain R, et al. Hypercoagulability in patients with coronavirus disease (COVID-19) identification of arterial and venous thromboembolism in the abdomen, pelvis, and lower extremities. AJR Am J Roentgenol. 2021;216:104–105. doi: 10.2214/AJR.20.23617. [DOI] [PubMed] [Google Scholar]
- 11.La Mura V, Artoni A, Martinelli I, et al. Acute portal vein thrombosis in SARS-CoV-2 infection a case report. Am J Gastroenterol. 2020;115:1140–1142. doi: 10.14309/ajg.0000000000000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ofosu A, Ramai D, Novikov A, et al. Portal vein thrombosis in a patient with COVID-19. Am J Gastroenterol. 2020;115:1545–1546. doi: 10.14309/ajg.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco-Moreno A, Piniella-Ruiz E, Montoya-Adarraga J, et al. Portal vein thrombosis in a patient with COVID-19. Thromb Res. 2020;194:150–152. doi: 10.1016/j.thromres.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Hoyo J, Lopez-Munoz P, Fernandez-de la Varga M, et al. Hepatobiliary and pancreatic a fatal case of extensive splanchnic vein thrombosis in a patient with Covid-19. J Gastroenterol Hepatol. 2020;35:1853. doi: 10.1111/jgh.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang JHQ, Tang JH, Eugene-Fan B, et al. A peculiar case of small bowel stricture in a coronavirus disease 2019 patient with congenital adhesion band and superior mesenteric vein thrombosis. Ann Vasc Surg. 2021;70:286–289. doi: 10.1016/j.avsg.2020.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low SW, Swanson KL, McCain JD, et al. Gastric ischemia and portal vein thrombosis in a COVID-19-infected patient. Endoscopy. 2020;52:E465–E466. doi: 10.1055/a-1230-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafari SH, Naseri R, Khalili N, et al. Portal vein thrombosis associated with COVID-19 points to consider. BJR Case Rep. 2020;6 doi: 10.1259/bjrcr.20200089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lari E, Lari A, AlQinai S, et al. Severe ischemic complications in Covid-19-a case series. Int J Surg Case Rep. 2020;75:131–135. doi: 10.1016/j.ijscr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmo Filho A, Cunha BDS. Inferior mesenteric vein thrombosis and COVID-19. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0412-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thuluva SK, Zhu H, Tan MML, et al. A 29-year-old male construction worker from India who presented with left- sided abdominal pain due to isolated superior mesenteric vein thrombosis associated with SARS-CoV-2 infection. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.926785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abeysekera KW, Karteszi H, Clark A, et al. Spontaneous portomesenteric thrombosis in a non-cirrhotic patient with SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleman W, Cevallos LC. Subacute mesenteric venous thrombosis secondary to COVID-19 A late thrombotic complication in a nonsevere patient. Radiol Case Rep. 2021;16:899–902. doi: 10.1016/j.radcr.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Nakamura RM, Gonzalez-Calatayud M, Martinez Martinez AR. Acute mesenteric thrombosis in two patients with COVID-19. Two cases report and literature review. Int J Surg Case Rep. 2020;76:409–414. doi: 10.1016/j.ijscr.2020.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hambali NL, Mohd Noh M, Paramasivam S, et al. A Non-severe coronavirus disease 2019 patient with persistently high interleukin-6 level. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.584552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alharthy A, Balhamar A, Faqihi F, et al. Rare case of COVID-19 presenting as acute abdomen and sepsis. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodfellow M, Courtney M, Upadhyay Y, et al. Mesenteric venous thrombosis due to coronavirus in a post Roux-en-Y gastric bypass patient a case report. Obes Surg. 2021;31:2308–2310. doi: 10.1007/s11695-020-05214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozenshteyn F, Shah M, Adeyemo O, et al. Budd-chiari syndrome secondary to SARS-COV-2 infection. Am J Gastroenterol. 2020;115:1292. SUPPL. [Google Scholar]
- 28.Malas MB, Naazie IN, Elsayed N, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasecka A, Borovac JA, Guerreiro RA, et al. Thrombotic complications in patients with COVID-19 pathophysiological mechanisms. Diagnosis Treat Cardiovasc Drugs Ther. 2021;35:215–229. doi: 10.1007/s10557-020-07084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9:1652. doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS) an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allegra A, Innao V, Allegra AG, et al. Coagulopathy and thromboembolic events in patients with SARS-CoV-2 infection pathogenesis and management strategies. Ann Hematol. 2020;99:1953–1965. doi: 10.1007/s00277-020-04182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz LA, Idalsoaga F, Cannistra M, et al. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19 a systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693–7706. doi: 10.3748/wjg.v26.i48.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients a French multicenter cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ageno W, Dentali F, Pomero F, et al. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari Syndrome. Thromb Haemost. 2017;117:794–800. doi: 10.1160/TH16-10-0781. [DOI] [PubMed] [Google Scholar]
- 38.Acosta S, Alhadad A, Svensson P, et al. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245–1251. doi: 10.1002/bjs.6319. [DOI] [PubMed] [Google Scholar]
- 39.Ageno W, Riva N, Schulman S, et al. Long-term clinical outcomes of splanchnic vein thrombosis results of an international registry. JAMA Intern Med. 2015;175:1474–1480. doi: 10.1001/jamainternmed.2015.3184. [DOI] [PubMed] [Google Scholar]
- 40.Iavarone M, D'Ambrosio R, Soria A, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis a meta-analysis. Blood. 2012;120:4921–4928. doi: 10.1182/blood-2011-09-376517. [DOI] [PubMed] [Google Scholar]
- 42.Devreese KMJ, Linskens EA, Benoit D, et al. Antiphospholipid antibodies in patients with COVID-19 A relevant observation? J Thromb Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatto M, Perricone C, Tonello M, et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection findings from a multicenter study on 122 cases. Clin Exp Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 44.Berzigotti A, Piscaglia F, Education E, et al. Ultrasound in portal hypertension–part 2–and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012;33:8–32. doi: 10.1055/s-0031-1299145. quiz 30–1. [DOI] [PubMed] [Google Scholar]
- 45.Bradbury MS, Kavanagh PV, Chen MY, et al. Noninvasive assessment of portomesenteric venous thrombosis current concepts and imaging strategies. J Comput Assist Tomogr. 2002;26:392–404. doi: 10.1097/00004728-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology. 2019;156 doi: 10.1053/j.gastro.2019.01.265. 1582–1599 e1. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues SG, Maurer MH, Baumgartner I, et al. Imaging and minimally invasive endovascular therapy in the management of portal vein thrombosis. Abdom Radiol. 2018;43:1931–1946. doi: 10.1007/s00261-017-1335-9. [DOI] [PubMed] [Google Scholar]
- 48.Berzigotti A, Garcia-Criado A, Darnell A, et al. Imaging in clinical decision-making for portal vein thrombosis. Nat Rev Gastroenterol Hepatol. 2014;11:308–316. doi: 10.1038/nrgastro.2013.258. [DOI] [PubMed] [Google Scholar]
- 49.Jenner WJ, Kanji R, Mirsadraee S, et al. Thrombotic complications in 2928 patients with COVID-19 treated in intensive care a systematic review. J Thromb Thromb. 2021;51:595–607. doi: 10.1007/s11239-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Investigators I, Sadeghipour P, Talasaz AH, et al. Effect of intermediate-dose vs. standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19 JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerotziafas GT, Catalano M, Colgan MP, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19 position paper from VAS-European Independent Foundation in angiology/vascular medicine. Thromb Haemost. 2020;120:1597–1628. doi: 10.1055/s-0040-1715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels' striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents The Cremona experience. J Thromb Haemost. 2020;18:1320–1323. doi: 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]