Abstract
Patients with serious COVID infections develop shock frequently. To characterize the hemodynamic profile of this cohort, 156 patients with COVID pneumonia and shock requiring vasopressors had interpretable echocardiography with measurement of ejection fraction (EF) by Simpson's rule and stroke volume (SV) by Doppler. RV systolic pressure (RVSP) was estimated from the tricuspid regurgitation peak velocity. Patients were divided into groups with low or preserved EF (EFL or EFP, cutoff ≤45%), and low or normal cardiac index (CIL or CIN, cutoff ≤2.2 L/min/m2). Mean age was 67 ± 12.0, EF 59.5 ± 12.9, and CI 2.40 ± 0.86. A minority of patients had depressed EF (EFLCIL, n = 15, EFLCIN, n = 8); of those with preserved EF, less than half had low CI (EFPCIL, n = 55, EFPCIN, n = 73). Overall hospital mortality was 73%. Mortality was highest in the EFLCIL group (87%), but the difference between groups was not significant (p = 0.68 by ANOVA). High PEEP correlated with low CI in the EFPCIL group (r = 0.44, p = 0.04). In conclusion, this study reports the prevalence of shock characterized by EF and CI in patients with COVID-19. COVID-induced shock had a cardiogenic profile (EFLCIL) in 9.6% of patients, reflecting the impact of COVID-19 on myocardial function. Low CI despite preservation of EF and the correlation with PEEP suggests underfilling of the LV in this subset; these patients might benefit from additional volume. Hemodynamic assessment of COVID patients with shock with definition of subgroups may allow therapy to be tailored to the underlying causes of the hemodynamic abnormalities.
Patients with COVID-19 and respiratory failure develop shock frequently. Some of that shock is cardiogenic; potential mechanisms of myocardial injury in COVID-19 include direct injury from viral infection, consequences of the immune response to COVID, ischemia or infarction, dysregulation of the renin-angiotensin system, and endothelial dysfunction, which appears to be most prominent in the microcirculation.1, 2, 3 In other patients, shock may be hypovolemic, obstructive, or distributive. The proportion of the various potential etiologies of shock in critically ill patients with COVID-19 is currently unknown. Hemodynamic monitoring in patients with COVID-19 is complicated by logistical and infection control issues. In a European survey use of invasive hemodynamic monitoring was uncommon.4 Echocardiography is noninvasive and can be used for hemodynamic assessment. We report the results of 156 patients with COVID-19 and shock who had interpretable echocardiography with assessment of hemodynamic parameters.
Methods
Hackensack University Medical Center established a comprehensive prospective database of patients admitted with COVID-19 during the first wave of the pandemic from March 2 to May 31,2020, including demographics, clinical features, laboratory values, and clinical outcomes (the RealWorld database). From that database, patients with shock, defined as either MAP <65 mm Hg or need for vasopressors to maintain MAP >65 mm Hg, were identified. Those shock patients who had echocardiograms performed were identified and their echocardiograms were then reviewed by 2 independent readers, who measured ejection fraction (EF), stroke volume (SV), and right ventricular systolic pressure (RVSP). Right ventricular size and function were estimated visually.
This is a retrospective study approved by the Hackensack Meridian Institutional Review Board. All echocardiograms were performed for clinical purposes only and used the hospital's standard protocols. Patient identifiers were removed prior to echocardiographic analysis. The study was approved by the Hackensack Meridian Institutional Review Board.
Ejection fraction (EF) was estimated from apical 4-chamber images and LVEF was calculated using Simpson's rule.5 Right ventricular function was estimated visually and characterized as normal or mildly, moderately, or severely decreased.5
Parasternal long-axis images were used to measure aortic outflow tract diameter (Aodiam) parallel to the valve plane just proximal to aortic valve insertion into the annulus. Doppler echocardiography with the sample volume placed just proximal to the aortic valve was used to measure the velocity-time integral (VTI), and stroke volume (SV) was calculated as VTI x (Aodiam)2 x 0.785.6 Cardiac index (CI) was calculated as SV multiplied by the heart rate on the echo frame from which SV was calculated and divided by body surface area.
The gradient between the right atrium and the pulmonary artery was measured where possible by using the peak velocity of tricuspid valve regurgitation (VTR), calculating the gradient by the modified Bernoulli equation as (VTR)2 x 4.6 This was attempted in 82/156 patients and was interpretable in 76. Pulmonary artery systolic pressure was estimated by adding 10 mm Hg to the TR gradient; this method was used because IVC diameter and compressibility does not provide a reliable estimate of right atrial pressure in intubated patients, and positive end-expiratory pressure (PEEP) contributes to right atrial pressure to some extent.7 , 8
Patients were characterized by EF and cardiac index and divided into groups with low or preserved EF using a cutoff of ≤45% and low or normal CI using a cutoff of ≤2.2 L/min/m2 for low CI. Statistical comparisons were made using ANOVA with post-hoc Tukey testing for pair-wise comparisons. Correlations were evaluated using Pearson correlation coefficients.
Results
Of 1,275 patients hospitalized at Hackensack Meridian University Hospital with COVID pneumonia between March 2 and May 31, 2020, 215 met shock criteria of whom 160 had echocardiography to assess ventricular function and stroke volume. Four patients were excluded due to inadequate images. Mean age was 67 ± 12.0, mean EF 59.5 ± 12.9, and mean CI 2.40 ± 0.86. Five patients had obstructive shock, defined by normal LVEF, low CI, and RV dysfunction; 4 of them had massive pulmonary embolism and one had a hemodynamically significant pneumothorax; they were excluded from further analysis since their shock related to right ventricular rather than left ventricular failure. No patients had significant obstructive valvular disease or left ventricular outflow tract obstruction. The overall hospital mortality of the remaining 151 patients that constitute the study group was 73%.
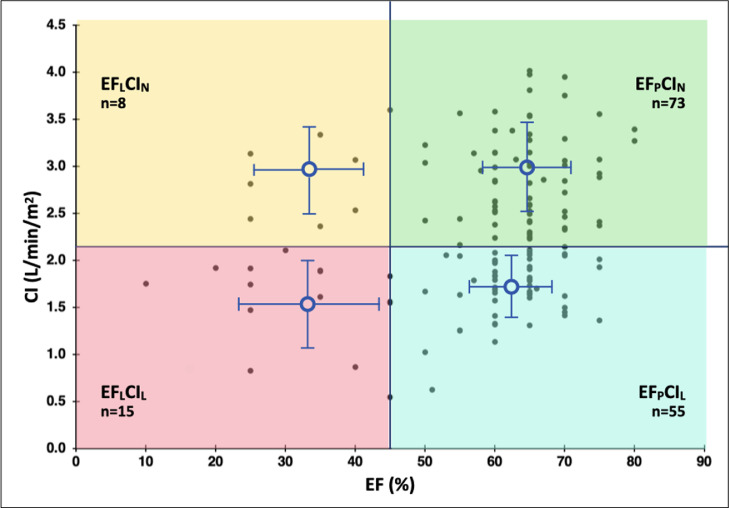
The patients were divided into 4 subgroups defined by EF and CI: 15 had low EF and low CI (EFLCIL), 8 had low EF and normal CI (EFLCIN), 55 had preserved EF and low CI (EFPCIL), and 73 had preserved EF and normal CI (EFPCIN). A scatterplot of EF and CI is shown in Figure 1 . The characteristics of the patients broken down by group is shown in Table 1 . Heart failure, CAD, and diabetes were more common in patients the low ejection fraction groups, EFLCIL and EFLCIN. At the time of the echocardiogram, 47% of patients were on vasopressor support, using norepinephrine 96% of the time; 2% were on inotropes. Patients in the 2 low CI groups had lower CI than those in the 2 normal CI groups as expected (1.69 ± 0.17 vs 3.05 ± 0.29, L/min/m2 p < 0.001), and also had lower stroke volume index (24.9 ± 3.2 vs 34.8 ± 3.6 ml/m2, p < 0.001). Serum lactate levels were higher in the groups with low CI than normal CI (Table 1, p < 0.01).
Figure 1.
Scatterplot of ejection fraction and cardiac index. Groups were characterized by low (EFL) or preserved EF (EFP) using a cutoff of ≤45% for low EF and low (CIL) or normal (CIN) using a cutoff of ≤2.2 L/min/m2 for low CI. Mean and standard deviation is shown for each group. Abbreviations: CI, cardiac index; EF, ejection fraction.
Table 1.
Patient characteristics
| Variable | Low ejection fraction low cardiac index (n = 15) | Low ejection fraction high cardiac index (n = 8) | High ejection fraction low cardiac index (n = 55) | High ejection fraction high cardiac index (n = 73) | P value |
|---|---|---|---|---|---|
| Age (years) | 72.0 (12.1) | 71.1 (7.8) | 66.9 (11.8) | 65.3 (13.4) | 0.203 |
| Male | 11 (73%) | 6 (75%) | 30 (55%) | 37 (51%) | 0.276 |
| Body Mass index (kg/m2) | 31.6 (8.7) | 27.4 (5.5) | 31.8 (6.5) | 30.7 (7.1) | 0.376 |
| History of Heart Failure | 4 (27%) | 1 (13%) | 1 (2%) | 5 (7%) | 0.011 |
| History of Hypertension | 12 (80%) | 6 (75%) | 35 (64%) | 49 (67%) | 0.647 |
| Left Ventricular Hypertrophy | 5 (33%) | 3 (38%) | 17 (31%) | 28 (38%) | 0.848 |
| History of Coronary Artery Disease | 7 (47%) | 4 (50%) | 12 (22%) | 14 (19%) | 0.043 |
| Prior PCI | 4 (27%) | 2 (25%) | 4 (7%) | 7 (10%) | 0.104 |
| Prior CABG | 4 (27%) | 1 (13%) | 3 (5%) | 3 (4%) | 0.018 |
| History of CKD | 3 (20%) | 2 (25%) | 2 (4%) | 7 (10%) | 0.090 |
| Serum Creatinine (mg/dL) | 2.87 (2.48) | 3.05 (3.72) | 2.71 (8.17) | 1.70 (2.04) | 0.672 |
| History of Chronic Lung Disease | 3 (20%) | 1 (13%) | 6 (11%) | 8 (11%) | 0.788 |
| Current Smoker | 1 (7%) | 1 (13%) | 3 (5%) | 3 (4%) | 0.779 |
| History of Diabetes Mellitus | 10 (67%) | 5 (63%) | 19 (35%) | 25 (34%) | 0.049 |
| Sequential Organ Failure Assessment (SOFA) Score | 8.4 (2.30) | 7.9 (3.2) | 7.5 (2.3) | 7.4 (2.0) | 0.453 |
| Mechanical Ventilation | 14 (93%) | 3 (38%) | 46 (84%) | 46 (63%) | 0.002 |
| Right Ventricular Systolic Pressure (mmHg) | 35 (17) | 27 (0) | 37 (14) | 38 (15) | 0.428 |
| Serum Lactate (mmol/L) | 1.86 (0.72) | 3.92 (5.11) | 1.72 (0.91) | 1.73 (0.79) | 0.004 |
| Vasopressor Support | 10 (67%) | 3 (38%) | 32 (49%) | 32 (45%) | 0.199 |
| Inotropic Support | 2 (13%) | 0 | 0 | 1 (2%) | 0.081 |
Patients were characterized by EF and cardiac index and divided into groups with low preserved EF using a cutoff of ≤45% and low or normal CI using a cutoff of ≤2.2 L/min/m2 for low CI. Values are mean (standard deviation) or n (%)
Abbreviations: CABG = coronary artery bypass grafting; CKD = chronic kidney disease; PCI = percutaneous coronary intervention.
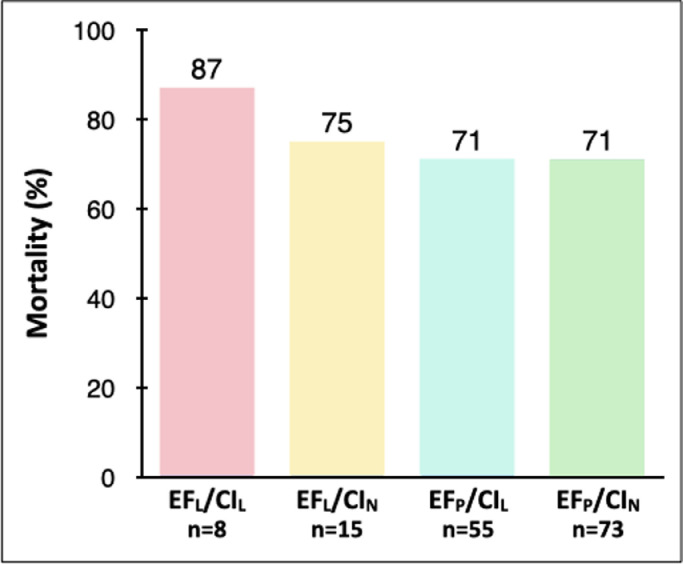
Mortality in the EFLCIL group was numerically higher (87%), than in groups EFLCIN (75%), EFPCIL (71%) and EFPCIN (71%), but these differences did not reach statistical significance (p = 0.68 by ANOVA) (Figure 2 ). Sequential Organ Failure Assessment (SOFA) scores did not differ among the groups (Table 1).
Figure 2.
Mortality in subgroups classified by ejection fraction (low, ≤45%) and cardiac index (low, ≤2.2 L/min/m2). No statistically significant differences were present. Abbreviations: CI, cardiac index; EF, ejection fraction; EFLCIL, low EF and low CI; EFLCIN, low EF and normal CI; EFPCIL, preserved EF and low CI; EFPCIN, preserved EF and normal CI.
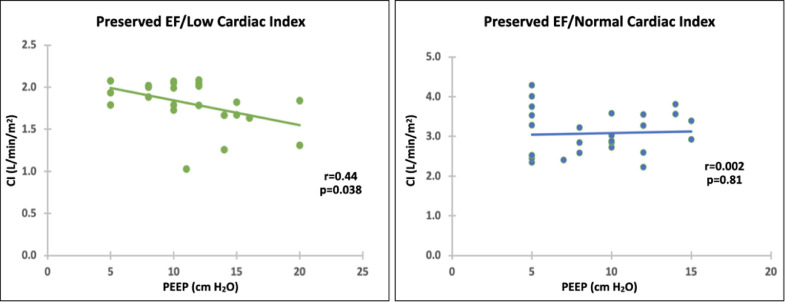
RV systolic pressure (RVSP) was measurable in 76 patients, 56 of whom (74%) were on mechanical ventilation, all with PEEP. Mean RVSP did not differ among groups (Table 1, p = 0.57 by ANOVA). PEEP levels also did not differ (Table 1, p = 0.83 by ANOVA). Patient numbers were too small in the low EF groups to permit analysis, but in patients with preserved EF, those on mechanical ventilation with PEEP had lower CI (2.39 ± 0.84 L/min/m2) than those not on mechanical ventilation (2.78 ± 0.87 L/min/m2, p = 0.02). In patients with preserved EF on PEEP, High PEEP correlated with low CI in patients in the EFPCIL group (n = 23, r = 0.44, p = 0.038) but not in the EFPCIN group (n = 27, r = 0.04, p = 0.81). See Figure 3 .
Figure 3.
Cardiac index as a function of PEEP in patients with preserved EF A. Low CI B. Normal CI. Abbreviations: CI, cardiac index; EF, ejection fraction; PEEP, positive end-expiratory pressure.
Discussion
This study is the first to report the prevalence of different types of shock in patients with COVID-19. The incidence of classic cardiogenic shock, with low EF and low CI, was 10%, similar to that with septic cardiomyopathy in other settings,9 and in keeping with the known propensity for COVID to affect the heart.2 Potential mechanisms by which COVID-19 may impact myocardial function include myocarditis,10 effects of inflammatory cytokines, MI, and microcirculatory dysfunction with the potential for ischemia.1 , 3 Several patients had pulmonary emboli and pericardial tamponade, which have also been reported as complications of COVID.2 , 11
The most striking finding of the study is the prevalence of patients with preserved ejection fraction and low cardiac index in COVID, which represented 43% (55/128) of the patients with normal EF in our sample. In classic distributive shock, ejection fraction and cardiac index are preserved, and hypotension is usually felt to result from vasodilation.12, 13, 14 Patients with normal ejection fraction and low stroke volume have lower end-diastolic volumes, and so their left ventricles are underfilled. Several factors operative in critically ill intubated patients with COVID pneumonia could contribute to the underfilling of the LV. These include ventilatory strategies, fluid management aiming for “dry” Adult Respiratory Distress Syndrome, and effects of PEEP. Our study showed that CI was lower in patients on mechanical ventilation and PEEP than those not on mechanical ventilation, and higher PEEP levels correlated with lower CI in patients with COVID and normal LV systolic function. This suggests that PEEP and mechanical ventilation contribute to LV underfilling, possibly by decreasing venous return.15 , 16
The mortality of patients with “classic” cardiogenic shock (EFLCIL) was quite high, which is not surprising given the development of cardiac insufficiency superimposed on respiratory failure. The study contained relatively few patients with classic septic cardiomyopathy9 (EFLCIN) but mortality was not significantly increased in these patients compared to those with preserved EF and normal CI, consistent with prior reports.17, 18, 19 What was most striking was that mortality did not differ in patients with preserved EF whether their cardiac output was low or normal. Fluid resuscitation might increase stroke volume and cardiac output in these patients, but those patients with evidence of clinical hypoperfusion are likely to benefit most; in other patients, the course of the pulmonary disease may be the major contributor to mortality.
The study has a number of limitations, including some resulting from its retrospective design, and several small subgroups. Echocardiography was performed based on clinical indications, and so this population may not be entirely representative of all patients with COVID pneumonia. Only the clinical data collected in the RealWorld database are available for analysis, and some of those data may not be fully synchronous with the timing of the echocardiograms. Some of the patients may have had more than one echocardiogram; we limited analysis to the first study when the patient was in shock. None of the patients in this report had cardiac catheterization for acute coronary syndromes with ECG changes, although that has been reported in COVID as well.11 Although ejection fraction has its limitations as a measure of left ventricular performance,20 we chose a threshold generally considered to reflect clinically significant ventricular dysfunction,9 particularly in the setting of vasoactive support.
This study reports ejection fractions and cardiac outputs obtained by echocardiography in a large cohort of patients with COVID-19 pneumonia and shock. Some of the patients had decreased EF, most of whom had low cardiac output. Out of the majority of patients with preserved EF, a substantial proportion had a lower cardiac output than expected, which is contrary to the classic distributive shock pattern in which patients present with normal or high CI. This could not be attributed to right heart failure, since patients with RV dysfunction were excluded from the analysis. Decreased stroke volume and cardiac index with preserved ejection fraction indicates that these patients had underfilled left ventricles. Most of the patients were mechanically ventilated, and the correlation of PEEP with cardiac index in only this group suggests that positive pressure ventilation might be contributing by decreasing venous return.
Our data suggest potential benefits of careful measurement of hemodynamics using echocardiography in patients with COVID pneumonia and shock. Identification of patients with low EF might select those who could conceivably benefit from inotropic support. Whether patients with underfilled ventricles and preserved EF but low CI might benefit from fluid administration will require further study, as fluids might increase cardiac output but also cause increased lung edema and thus potentially worsen oxygenation. Serial assessment with evaluation of stroke volume responses to fluid might be advisable in this setting. Hemodynamic assessment of COVID patients with shock, with definition of its subgroups, may help tailor therapy to the underlying causes of the hemodynamic abnormalities.
Credit Author Statement
Steven Hollenberg: Conceptualization, Methodology, Formal Analysis, Writing- Original Draft, Lucy Safi: Methodology, Formal Analysis, Joseph E Parrillo: Conceptualization, Methodology, Writing – Review and Editing, Supervision, Matthew Fata: Validation, Data Curation, Brent Klinkhammer: Formal Analysis, Data Curation, Noha Gayed: Data Curation, Taya Glotzer: Writing – Review and Editing, Ronaldo Go: Investigation, Data Curation, Elli Gourna-Paleoudis: Data Curation, David Landers Conceptualization, Methodology, Writing – Review and Editing, Sameer Jamal: Writing – Review and Editing, Neel Shah: Data Curation, Roshan Shah Data Curation, Jana Tancredi: Data Curation, Zoltan Turi: Conceptualization, Methodology, Writing – Review and Editing.
Footnotes
Conflicts of Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Hemodynamics in 151 COVID patients with shock characterized by echo and divided into subsets by EF and CI.
References
- 1.Jasiński T, Stefaniak J. COVID-19 and haemodynamic failure: a point of view on mechanisms and treatment. Anaesthesiol Intensive Ther. 2020;52:409–417. doi: 10.5114/ait.2020.101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, Weber C, Bochaton-Piallat ML, Back M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michard F, Malbrain ML, Martin GS, Fumeaux T, Lobo S, Gonzalez F, Pinho-Oliveira V, Constantin JM. Haemodynamic monitoring and management in COVID-19 intensive care patients: an International survey. Anaesth Crit Care Pain Med. 2020;39:563–569. doi: 10.1016/j.accpm.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiograph. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 6.Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, Troughton RW. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:40–56. doi: 10.1016/j.echo.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Pinsky M, Vincent JL, De Smet JM. Estimating left ventricular filling pressure during positive end-expiratory pressure in humans. Am Rev Respir Dis. 1991;143:25–31. doi: 10.1164/ajrccm/143.1.25. [DOI] [PubMed] [Google Scholar]
- 8.Jue J, Chung W, Schiller NB. Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation? J Am Soc Echocardiogr. 1992;5:613–619. doi: 10.1016/s0894-7317(14)80327-1. [DOI] [PubMed] [Google Scholar]
- 9.Hollenberg SM, Singer M. Septic Cardiomyopathy. Nature Rev Cardiol. 2021;18:424–434. doi: 10.1038/s41569-020-00492-2. [DOI] [PubMed] [Google Scholar]
- 10.Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT, Chahal CAA. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. 2020;226:29–44. doi: 10.1016/j.ahj.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollenberg SM. Vasopressor support in septic shock. Chest. 2007;132:1678–1687. doi: 10.1378/chest.07-0291. [DOI] [PubMed] [Google Scholar]
- 13.Beale RJ, Hollenberg SM, Vincent JL, Parrillo JE. Vasopressor and inotropic support in septic shock: an evidence-based review. Crit Care Med. 2004;32:S455–S465. doi: 10.1097/01.ccm.0000142909.86238.b1. [DOI] [PubMed] [Google Scholar]
- 14.Hollenberg SM, Parrillo JE. In: Current Therapy in Critical Care Medicine. Parrillo JE, editor. Mosby; St Louis: 1997. Septic shock; pp. 89–95. [Google Scholar]
- 15.Kyhl K, Ahtarovski KA, Iversen K, Thomsen C, Vejlstrup N, Engstrom T, Madsen PL. The decrease of cardiac chamber volumes and output during positive-pressure ventilation. Am J Physiol Heart Circ Physiol. 2013;305:H1004–H1009. doi: 10.1152/ajpheart.00309.2013. [DOI] [PubMed] [Google Scholar]
- 16.Berger D, Moller PW, Weber A, Bloch A, Bloechlinger S, Haenggi M, Sondergaard S, Jakob SM, Magder S, Takala J. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol. 2016;311:H794–H806. doi: 10.1152/ajpheart.00931.2015. [DOI] [PubMed] [Google Scholar]
- 17.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 18.Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care. 2013;17:R96. doi: 10.1186/cc12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sevilla Berrios RA, O'Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29:495–499. doi: 10.1016/j.jcrc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Liu PP, Al-Khalaf M, Blet A. Time to reframe ejection fraction in light of new pathophysiological insights into heart failure. J Am Coll Cardiol. 2020;76:1995–1998. doi: 10.1016/j.jacc.2020.09.012. [DOI] [PubMed] [Google Scholar]