Abstract
Background
To analyze the correlation between clinical course and radiographic development on computed tomography (CT) in patients with confirmed coronavirus disease 2019 (COVID-19) and to provide more evidence for treatment.
Methods
This retrospective, observational, cohort study enrolled 49 patients with Reverse transcription-polymerase chain reaction (RT-PCR)-confirmed COVID-19, which included 30 patients admitted to the intensive care unit (ICU) of Wuhan Third Hospital and 19 patients either admitted to or receiving telemedicine consultation from Shanghai General Hospital, Shanghai Xuhui Dahua Hospital, and hospitals in other provinces. CT scans were performed in all enrolled patients and the radiographic features including simple ground-glass opacities (GGOs), GGO with interlobular septal thickening, consolidations with GGO, and consolidations only were monitored by repeating the CT. The progression of these radiographic features was analyzed in combination with their clinical staging and the time interval between onset of symptoms to CT.
Results
Based on illness severity, the 49 patients were classified into four stages: mild (n = 6), moderate (n = 12), severe (n = 16), and critically ill (n = 15). The CT findings were classified into three phases: early (n = 5), progression (n = 39), and recovery (n = 5). Among the 49 patients, 9 had bilateral diffuse GGO or diffuse consolidations (white lungs) and were counted as 18 lesions. Three patients had no abnormal findings on initial CT, but their repeat CT showed new lesions. In all, we identified 892 lesions including simple GGO, GGO with interlobular septal thickening, consolidations with GGO, and consolidations only.
Conclusions
Most patients had pulmonary lesions on the posterior, inferior, and peripheral lung fields on CT. The development of GGO with interlobular septal thickening, GGO with consolidations, and consolidations only happened mainly between day 8 and 14. The emergence of consolidations may suggest the progression to the severe phase of the illness, whereas simple consolidations or “white lung” may suggest a critically ill phase.
Keywords: COVID-19, Lung, Radiology
Introduction
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China. The exact clinical course and development pattern are still poorly understood. Up to 4–11% of COVID-19 patients rapidly progress to acute respiratory distress syndrome (ARDS) and experience acute respiratory failure, eventually dying of multiorgan failure [1], [2]. Early identification and treatment are therefore essential in COVID-19 management. Nucleic acid throat swab tests show false-positive results in identifying COVID-19 [2]. By comparison, computed tomography (CT) is a more repeatable and objective analysis tool. CT abnormalities were identified in 74% of patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [3]. Therefore, radiographic tests can assist in the clinical diagnosis of COVID-19. This retrospective study observed the clinical course of COVID-19 patients along with their radiographic progression on CT. We aim to help in understanding the radiographic development of the new pandemic and to provide more evidence for its treatment by analyzing the correlation between the clinical course of COVID-19 and its radiographic progression on CT.
Methods
Study design and patient enrolment
In this retrospective, observational, cohort study, we enrolled 49 patients with Reverse transcription-polymerase chain reaction (RT-PCR)-confirmed COVID-19. The subjects included 30 patients admitted to the ICU of Wuhan Third Hospital (Wuhan, China) and 19 patients either admitted to or who received telemedicine consultation from Shanghai General Hospital (Shanghai, China), Shanghai Xuhui Dahua Hospital (Shanghai, China), and hospitals in other provinces. The recruitment periods ranged from January 31st to February 10th, 2020. Exposure of patients: COVID-19 patients with complete medical history. Patient follow-up ended on February 16th, 2020. The data collection periods ranged from January 31st to February 16th, 2020. This study was approved by the institutional review boards of Wuhan Third Hospital (reference number: KY2020–007) and Shanghai General Hospital (reference number: 2020[11]). Written informed consent was waived due to the rapid emergence of this infectious disease.
Enrolment criteria
All enrolled patients had clinical symptoms and signs suggestive of COVID-19, and all enrolled patients underwent a CT scan. The RT-PCR results confirmed positive SARS-CoV-2 infection in all patients.
Exclusion criteria
Patients with inadequate clinical data and those without confirmed positive SARS-CoV-2 infection by RT-PCR testing were excluded.
Follow-up
The hospital medical records were used to record the follow-up data; if the patient was discharged, a telephonic follow-up was conducted.
Data collection
We reviewed medical records of the enrolled patients and collected patients' demographic data, clinical information including laboratory results, and administrative information. All patients were tested for SARS-CoV-2 by an upper respiratory throat swab.
Outcomes
Radiographic development on CT scan in patients with COVID-19 is the primary outcome. Clinical course in patients with COVID-19 is the secondary outcome.
Diagnostic criteria
Suspected cases should have had at least one of the following etiological or serological evidence [4]: (1) Real-time fluorescent RT-PCR indicates positive for new coronavirus nucleic acids. (2) Viral gene sequence is highly homologous to known new coronaviruses. (3) SARS-CoV-2-specific IgM and IgG are detectable in the patients’ sera; SARS-CoV-2-specific IgG is detectable or reaches a titration of at least a fourfold increase during convalescence compared with the acute phase.
Clinical stage
We used the clinical staging method in Trial Version 7 of the National Health Commission & State Administration of Traditional Chinese Medicine [5]. The following four stages of COVID-19 are described in this study: mild, moderate, severe, and critically ill [5].
-
(1)
Mild type: The clinical symptoms were mild, and no manifestations of pneumonia were observed on imaging.
-
(2)
Moderate type: Patients had a fever, respiratory symptoms, and imaging manifestations of pneumonia.
-
(3)
Severe type: Adult patients with any of the following criteria: (a) Shortness of breath, respiratory rate (RR) ≥30 times/min; (b) Oxygen saturation <93% while inhaling air in the resting state; (c) Arterial partial oxygen pressure (PaO2)/ Fraction of inspiration oxygen (FiO2) ≤300 mmHg; high altitudes (>1000 m) should be corrected for PaO2/FiO2 according to the following formula: PaO2/FiO2 × (760/atmospheric pressure [mmHg]); and (d) Patients with progressive clinical symptoms, pulmonary imaging showed significant progression of >50% within 24–48 h. For children, any of the following criteria were applied: (a) High fever lasting for >3 days; (b) Shortness of breath (<2 months of age, RR ≥60 times/min; 2–12 months of age, RR ≥50 times/min; 1–5 years old, RR ≥40 times/min; >5 years old, RR ≥30 times/min), except during fever and crying; (c) Oxygen saturation <93% while inhaling air in the resting state; (d) Assisted breathing (nasal flapping, three concave signs); (e) Sleepiness and convulsions; and (f) Loss of appetite or feeding difficulties, dehydration symptoms.
-
(4)
Critical type: Any patient who met one of the following conditions: (a) Respiratory failure that requires mechanical ventilation; (b) Shock; and (c) Complicated with other organ failures, when ICU monitoring and treatment become essential.
CT data collection
All patients underwent chest CT from the base of the neck through the upper abdomen with a slice thickness of 0.625–2.000 mm.
The initial CT findings of all patients were collected, including GGO, patchy infiltration, interlobular interstitial fine reticular thickening, interlobular septal thickening, airspace nodules, centrilobular nodules or tree-in-bud sign, thickening of bronchovascular bundles, linear interstitial fibrosis with various thicknesses, scarring lobar atelectasis, interlobar pleural thickening, costal pleural thickening, and pleural effusion. Analysis of location distribution and characteristic distribution was performed for two main CT findings — GGO and patchy consolidation. The association with interlobular septal thickening was also analyzed. If initial CT showed no abnormal findings, the normal findings were reported and a follow-up second CT was performed. CT findings were interpreted and compared longitudinally to monitor disease development.
Characteristics and classification of CT abnormalities:
Class 1. Small lesions: subpleural and with a diameter of ≤15 mm;
Class 2. Coalescing small lesions: subpleural and with a diameter or long diameter ≤25 mm;
Class 3. Subpleural, patchy continuous infiltrate, including circumferential infiltrate of lung peripheries;
Class 4. Continuous infiltrate extending from the peripheral to central lung field, but not more than half of the ipsilateral lung field;
Class 5. Infiltrate involving one entire lobe;
Class 6. Continuous infiltrate extending from the peripheral to central lung field, involving at least half of the ipsilateral lung field (continuous infiltrate involving two-thirds of both lung fields is called “white lung”).
Once the CT results were obtained, we analyzed the correlation between the patient's clinical status and disease severity. In this study, CT findings were classified into four phases: early phase, progression phase, severe phase, and recovery phase based on the Radiologic Diagnosis of Pneumonia Caused by Novel Coronavirus Infection: Chinese Society of Radiology Expert Recommendations (Version 1) [6].
CT reading
Every chest CT in this study was reviewed and read by two radiologists with >10 years of experience. Any disagreements were resolved by discussion with a third radiologist who had >35 years of experience. The radiologists who read the CT images were blinded to the patient's clinical information, but the radiographic staging of CT was based on the combination of CT findings, rapidity of disease progression, and clinical staging. Patients’ clinical staging was determined by two experienced internists based on individual patient's clinical conditions.
Efforts to address potential sources of bias
The inclusion and exclusion criteria were strictly followed, and it was a multicenter research.
Study size
We did not calculate the sample size, because this was an observational descriptive study, and the number of COVID-19 bases increased rapidly.
Statistical methods
Descriptive analysis was performed by indicating the mean and standard deviation for quantitative variables and the percentage for qualitative variables. The analyses were carried out using Excel software. There was no follow-up loss and no missing data.
Results
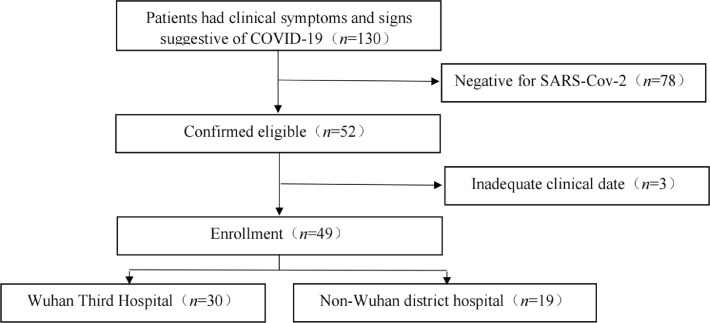
There were 130 potentially eligible patients for this study, and we examined them for eligibility; 78 patients were negative for SARS-CoV-2, and 3 patients had inadequate clinical data. The study enrolled 49 patients [23 males (46.9%) and 26 females (53.1%), mean age: 55 (24–84) years] with RT-PCR-confirmed SARS-CoV-2. All the patients completed follow-up [Fig. 1]. Characteristics of study participants: The symptom onset in patients was as follows: fever (n = 48), cough (n = 49), fatigue (n = 20), myalgia (n = 10), rhinorrhea (n = 45), sore throat (n = 39), and diarrhea (n = 2).
Fig. 1.
Research flowchart. COVID-19: Coronavirus disease 2019.
The interval days between symptom onset to first CT study were as follows: 0–3 days in 9 patients, 4–7 days in 14 patients, 8–14 days in 21 patients, and 15–20 days in 5 patients. Two patients had an initial negative CT: one patient had negative CT on day 0 of symptoms, but a repeat CT on day 4 showed bilateral lower lobe peripheral GGO; the other patient had negative CT on day 4 of symptoms, but a repeat CT on day 8 showed bilateral lower lobe large consolidations with worsening of symptoms. Further, 14 patients had at least one follow-up repeat CT. Among the 49 cases, 9 patients experienced clinical deterioration and 9 patients died. The average follow-up time was 7 days, and no participant had missing data for any variable of interest.
Table 1 shows the location and characteristics of CT abnormalities. Twenty-two patients had simple GGO; of these, bilateral and inferior lung involvement was observed in 19 (86.4%) and 19 (86.4%) patients, respectively. All 22 patients had posterior and peripheral lung involvement. Thirty patients had GGO with interlobular septal thickening, among which 26 (86.7%) had bilateral lung involvement, 28 (93.3%) had posterior lung involvement, 23 (76.7%) had inferior lung involvement, and 29 (96.7%) had peripheral lung involvement. Thirty patients had GGO with consolidation, among which 28 (93.3%) had bilateral lung involvement, 28 (93.3%) had posterior lung involvement, 21 (70.0%) had inferior lung involvement, and 29 (96.7%) had peripheral lung involvement. Twenty-four patients had simple lung consolidation, among which 22 (91.6%) had bilateral lung involvement, 23 (95.8%) had posterior lung involvement, and 17 (70.8%) had inferior lung involvement; all 24 had peripheral lung involvement.
Table 1.
Location of CT abnormalities.
| Characteristic | Unilateral/bilateral | Anterior/posterior | Superior/middle/inferior | Peripheral/central |
|---|---|---|---|---|
| Simple GGO | 3/19 | 3/22 | 2/15/19 | 22/4 |
| GGO with interlobular septal thickening | 4/26 | 4/28 | 3/19/23 | 29/12 |
| GGO with consolidation | 2/28 | 5/28 | 3/19/21 | 29/15 |
| Simple consolidation | 2/22 | 2/23 | 4/13/17 | 24/16 |
Data are expressed as numbers.
CT: Computed tomography; GGO: Ground-glass opacity.
Table 2 shows the characteristic distribution of CT abnormalities. In patients with simple GGO, 50.0% were classified as Class 1. Patients with GGO with interlobular septal thickening were mainly Class 3 (27.6%) and Class 6 (27.6%). Patients with GGO with consolidation were mainly Class 3 (37.9%) and Class 6 (34.5%). Patients with simple consolidation were mainly Class 6 (41.7%) .
Table 2.
Characteristic distribution of CT abnormalities.
| Characteristic | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | Class 6 |
|---|---|---|---|---|---|---|
| Simple GGO | 11 | 6 | 5 | 0 | 0 | 0 |
| GGO with interlobular septal thickening | 6 | 3 | 8 | 2 | 2 | 8 |
| GGO with consolidation | 3 | 1 | 11 | 2 | 2 | 10 |
| Simple consolidation | 1 | 0 | 6 | 5 | 2 | 10 |
Data are expressed as numbers.
Class 1. Small lesions: subpleural and with a diameter of ≤15 mm; Class 2. Coalescing small lesions: subpleural and with a diameter or long diameter ≤25 mm; Class 3. Subpleural, patchy continuous infiltrate, including circumferential infiltrate of lung peripheries; Class 4. Continuous infiltrate extending from the peripheral to central lung field, but not more than half of the ipsilateral lung field; Class 5. Infiltrate involving one entire lobe; Class 6. Continuous infiltrate extending from the peripheral to central lung field, involving at least half of the ipsilateral lung field (continuous infiltrate involving two-thirds of both lung fields is called “white lung”).
CT: Computed tomography; GGO: Ground-glass opacity.
Table 3 shows the CT features along with the clinical course. Simple GGO had a similar occurrence rate in 0–3 days, 4–7 days, and 8–14 days groups (31.8%, 27.2%, and 31.8%, respectively). GGO with interlobular septal thickening (43.3%) and GGO with consolidation (41.4%) developed mainly in the 8–14 days group. Simple consolidation also developed mainly in the 8–14 days group (50.0%) . Owing to the small sample size, there was no significant difference after statistical analysis.There was no observed airspace nodules, centrilobular nodules, or tree-in-bud sign, or cavity formation. There were no observed large pleural effusions or mediastinal lymph node enlargement.
Table 3.
CT features along the clinical course.
| Characteristics | 0–3 days | 4–7 days | 8–14 days | >14 days | P-value |
|---|---|---|---|---|---|
| Simple GGO | 7 | 6 | 7 | 2 | 0.741 |
| GGO with interlobular septal thickening | 3 | 8 | 13 | 5 | 0.172 |
| GGO with consolidation | 3 | 8 | 12 | 6 | 0.269 |
| Simple consolidation | 1 | 5 | 12 | 6 | 0.072 |
| Interlobular septal thickening | 2 | 3 | 4 | 4 | NA |
| Thickening of bronchovascular bundles | 3 | 3 | 7 | 1 | NA |
| Linear interstitial fibrosis with various thicknesses | 1 | 3 | 5 | 2 | NA |
| Interlobar pleural thickening | 1 | 3 | 0 | 0 | NA |
| Costal pleural thickening with small pleural effusion | 0 | 3 | 1 | 1 | NA |
| Scarring lobar atelectasis | 0 | 1 | 1 | 1 | NA |
| Interstitial emphysema with pneumomediastinum | 0 | 0 | 1 | 0 | NA |
Data are expressed as numbers.
CT: Computed tomography; GGO: Ground-glass opacity; NA: not available.
Table 4 shows the CT features and clinical stages of all patients. Simple GGO was mostly found in mild stage patients (54.5%), and simple GGO and critically ill stage were negatively correlated (P = 0.031, Pearson's correlation = −0.969). GGO with interlobular septal thickening was mostly found in severe stage patients (41.4%). GGO with consolidation was mostly found in severe stage patients (48.3%) . Simple consolidation was mostly found in critically ill stage patients (45.8%), and simple consolidation and critically ill stage were positively correlated (P = 0.034, Pearson's correlation = −0.966).
Table 4.
CT features and clinical stages.
| Characteristics | Mild stage | Moderate stage | Severe stage | Critically ill stage | P-value | Pearson correlation |
|---|---|---|---|---|---|---|
| Simple GGO | 12 | 6 | 3 | 1 | 0.031 | −0.969 |
| GGO with interlobular septal thickening | 7 | 6 | 12 | 4 | 0.886 | −0.114 |
| GGO with consolidation | 4 | 4 | 14 | 7 | 0.480 | 0.520 |
| Simple consolidation | 0 | 6 | 7 | 11 | 0.034 | 0.966 |
Data are expressesd as numbers.
If the patient's initial CT was negative, subsequent follow-up CT abnormalities were recorded. P-value associated with critically ill stage, Pearson correlation analysis.
CT: Computed tomography; GGO: Ground-glass opacity.
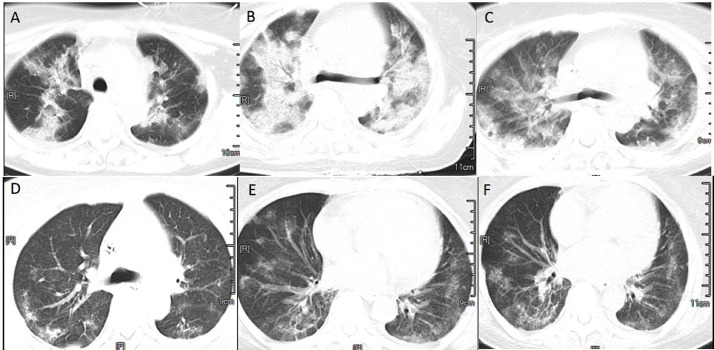
Fig. 2 shows the CT images of a 66-year-old woman who had COVID-19 onset on January 7th and was admitted on January 14th. Her clinical classification was a severe infection, and the image classification showed progressive stage and bilateral lobe lesions, which were more in the middle and less in the posterior lobe, GGO, interlobular interstitial thickening, interlobular septal thickening, more lung consolidation with a bronchogenic phase, and blurred and minor thickening of bronchovascular bundle. Reexamination showed changes in fibrous exudation and gradual absorption, and improved fibrosis.This case contained most of the imaging findings of COVID-19 with a typical complete course of disease.
Fig. 2.
CT images of a typical complete course of COVID-19 .(A)Imaging findings of COVID-19 on day 7 of onset,CT conducted on January 14th, (B) On day 8 of onset,the image classification showed progressive stage and bilateral lobe lesions, CT conducted on January 15th, (C)On day 10 of onset,fibrous exudation and gradual absorption,CT conducted on January 17th, (D)On day 13 of onset, improved fibrosis.CT conducted on January 20th, (E) On day 24 of onset, improved fibrosis.CT conducted on January 31st, (F) On day 26 of onset, before discharge.CT conducted on February 2nd. COVID-19: Coronavirus disease 2019; CT: Computed tomography.
Discussion
Our study showed that GGO is the main radiographic feature of COVID-19 on CT. Most patients had pulmonary lesions on the posterior, inferior, and peripheral lung fields on CT. The development of GGO with interlobular septal thickening, GGO with consolidations, and consolidations only occurred mainly between day 8 and 14. The subsequent strong immune response causes quick development of consolidations, which suggests entering the progression phase. Pleural effusions were rarely seen. Lymph node enlargement or cavitary lesions were not observed.
For elderly patients or patients with baseline comorbidities such as diabetes or immunocompromised status, there was an increased risk of multiple infections. In these patients, the CT findings are relatively more complex and may show a combination of multiple features. Severe pneumonia develops rapidly and may progress into diffuse bilateral consolidations (e.g., “white lungs”). Small pleural effusions are also seen in these patients. The diseased lungs are unable to perform gas exchange, thereby causing ARDS and suffocation.
Interestingly, most of our patients only developed very thin subpleural GGO around day 3 of the illness. Some patients even had completely normal initial CT. Our initial thought was that GGO develops rapidly after SARS-CoV-2 infection, and the thin GGO without septal thickening represents new progressive lesions. However, our findings showed that GGO did not emerge until several days after onset of symptoms followed by requirement of hospitalization and progression into severe stage disease. Despite a positive SARS-CoV-2 swab test, many patients only had mild-to-moderate symptoms. We believe that a small group of patients had a self-limiting course of upper respiratory infection, whereas most patients developed lung involvement with a small amount of them progressing into a severe or critical ill stage. Therefore, we believe it is critical to repeat the chest CT on day 5–7 of symptoms to monitor lung involvement. Some patients showed rapid progression from peripheral GGO to diffuse central GGO within 24–48 h. One patient in this study progressed rapidly since day 3 of symptom onset and developed both GGO and infiltrates involving the bilateral central and peripheral lung fields. The diseased lungs demonstrated butterfly-shaped distribution. On day 14 of the illness, linear interstitial fibrosis was developed on top of GGO, consolidations, and air bronchograms.
A previous study suggested that the lung injury caused by COVID-19 is mainly from alveolar injury and proliferation of type II epithelial cells [7]. One study suggests that in COVID-19, there are no significant alveolar wall or interlobular septal changes, rather desquamating inflammation and bronchitis. Furthermore, there is pulmonary hyaline membrane formation, a large number of alveolar infiltrates of inflammatory cells, and patchy hemorrhagic focal necrosis, and the alveolar infiltrate tends to organize in patients with prolonged clinical course [8]. Another study had similar findings of diffuse alveolar damage with cellular fibromyxoid exudates, desquamation of pneumocytes and hyaline membrane formation, and interstitial mononuclear inflammatory infiltrates [7]. Histological findings of previous studies, to some extent, explained why interlobular septal thickening was not observed in initial CTs but only on follow-up CTs together with reticular appearance (cobblestone sign).
In this study, we found that bilateral lung involvement is common in COVID-19. Simple GGO was seen in 44.8% of patients, among which 86.4% had bilateral lung involvement and 86.4% had inferior lung involvement. All simple GGO patients had posterior and peripheral lung involvement. GGO with interlobular septal thickening was seen in 61.2% of patients, most had posterior lung involvement or peripheral lung involvement. GGO with consolidation was seen in 61.2% of patients, among which most had bilateral lung involvement, posterior lung involvement, or peripheral lung involvement. Simple lung consolidation was seen in 48.9% of patients, among which most had bilateral lung involvement or posterior lung involvement, and all had peripheral lung involvement. In sum, posterior, inferior, and peripheral lung fields were those mainly involved in SARS-CoV-2 infection. This is consistent with results from previous studies [9], [10], [11], [12], [13]. This study enrolled patients from multiple centers, and hence, the results are likely more convincing than those from single-center analyses.
In terms of the correlation between CT findings and clinical course, we found that simple GGO had a similar occurrence rate in 0–3 days (31.8%), 4–7 days (27.2%), and 8–14 days (31.8%). GGO with interlobular septal thickening presented mainly between day 8 and 14 (50.0%). Due to the small sample size, there was no significant statistical difference after statistical analysis. In a study by Pan et al. [14], CT lung abnormality due to COVID-19 was found most prominent around day 10 after symptom onset. Studies with larger samples are needed for further investigation.
COVID-19 has various presentations and progresses rapidly. Chest CT is essential for timely diagnosis, severity evaluation, and guiding further follow-up [15]. Whether CT findings can help clinical staging still needs further discussion. In this study, we found that GGO was most common in patients with mild disease (54.5%), whereas GGO with interlobular thickening and GGO with consolidation were often seen in severe disease (41.4% and 48.3%, respectively). Simple consolidation was mainly seen in critically ill patients. Therefore, clinicians should be warned by the appearance of simple consolidation on CT. The laboratory data and patient's signs and symptoms should be used in combination with CT in guiding clinical management.
To conclude, in our COVID-19 patient sample, GGO is the earliest finding on CT. As the disease progresses, thickening of the interalveolar septum and alveolar sac septum develops, and fine reticular opacities start to show up on the CT. Then, consolidation develops followed by fibrosis or absorption. Fibrosis on CT presents as highly dense patchy shadows, especially when mixed with linear or reticular opacities with various thicknesses. The more severe cases would develop scarring atelectasis and reduction of the local lung volume. On CT, we also observed a pattern of progression of opacities. Initially, COVID-19 presents as scattered small GGOs of lung periphery (involving the costal subpleural areas as well as interlobar, mediastinal, and peridiaphragmatic subpleural areas). The small GGOs start fusing together and form a continuous circumferential infiltrate of lung peripheries. Then, the infiltrates progress to the central lung field and spread to the entire lung. Another observed feature is that posterior and inferior lung fields usually develop infiltrates first and also worsen first. It is only at the “white lung” phase that infiltrates begin to appear in the anterior/apical upper lobe or basal lower lobe. Pleural effusion is uncommon and only small pleural effusion was observed in the late stage of the disease. Interlobular septal wall thickening was rarely seen. This is consistent with the description in the first pathological report of COVID-19 wherein no interlobular septal thickness was observed [7].
SARS-CoV-2 is transmitted through the respiratory tract and 82% of the droplets range from 0.74 μm to 2.12 μm in size [16]. In most cases, the virus hits the subpleural areas of the peripheral lung first where gas exchange takes place. Very rarely do central lung lesions develop first without subpleural area involvement. Hence, we infer that viral particles first arrive at the lung periphery where there are rich alveoli, and damage the alveolar wall; this presents on CT as interstitial (interalveolar septum and septum of alveolar sac) inflammatory infiltrates and edema. At this time, lung parenchyma can also be damaged, presenting as alveoli filled with half air and half infiltrates. The severe disease would affect gas exchange and causes significant hypoxia. We found that there is a correlation between the clinical course and radiographic development on CT in COVID-19. The disease severity (clinical stage) is associated with the rapidity of disease progression and is thereby associated with the progression of CT findings that range from mild to severe illness.
Limitations
This study was a retrospective, observational study. Some potentially useful clinical data such as blood gas results were not collected. However, we endeavored to collect other data such as white blood cell and lymphocyte counts. Moreover, because of the study period, we were unable to analyze the long-term prognosis of the enrolled patients; therefore, we analyzed the short-term prognosis. We also enrolled only 49 patients; thus, further studies with a larger sample size are needed to validate our findings. Although this was a multicenter study, we thought the CT characteristics that cannot totally represent COVID-19.
Conclusions
Posterior, inferior, and peripheral lung involvements were the key CT findings in COVID-19 patients in our study. GGO with interlobular septal thickening, GGO with consolidations, and simple consolidation were mainly seen between days 8 and 14 from symptom onset. Lung consolidation may represent disease progression to a severe stage. Simple consolidation or “white lung” may suggest a critically ill stage. CT characteristics of COVID-19 are varied and change quickly. Chest CT is essential in early diagnosis, severity evaluation, and guiding further follow-up patients with COVID-19.
Ethical statement
The authors are accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review boards of Wuhan Third Hospital (reference number: KY2020–007) and Shanghai General Hospital (reference number: 2020[11]). Written informed consent was waived given the rapid emergence of this infectious disease.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Dr. Li Yi for his help with the English translation of this article. We also thank the hospital staff for their efforts in recruiting patients.
Managing Editor: Jingling Bao
Contributor Information
Jun Liu, Email: liujun-sgh@sjtu.edu.cn.
Zhiyan He, Email: zhiyan85@hotmail.com.
Ruilan Wang, Email: wangyusun@hotmail.com.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO (2020) Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- 5.National Health Commission of the People's Republic of China and Traditional Chinese Medicine of the People's Republic of China (2020) Guidelines for the Diagnosis and Treatment of Coronavirus Disease 2019 (Trial Version 7). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 6.Radiology of the Chinese Medical Association Radiological diagnosis of COVID-19: expert recommendation from the Chinese Society of Radiology (First edition) Chin J Radiol. 2020;54(4):279–285. doi: 10.3760/cma.j.cn112149-20200205-00094. [DOI] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y.Q., Bian X.W. [Analysis of coronavirus disease-19 (COVID-19) based on SARS autopsy] Zhonghua Bing Li Xue Za Zhi. 2020;49(4):291–293. doi: 10.3760/cma.j.cn112151-20200211-00114. [DOI] [PubMed] [Google Scholar]
- 9.Lei J., Li J., Li X., Qi X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295(1):18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295(1):16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang Y., Zhang H., Xu Y., Xie J., Pang P., Ji W. CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):208–209. doi: 10.1148/radiol.2020200280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H., Han X., Zheng C. Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology. 2020;295(1):20. doi: 10.1148/radiol.2020200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T., et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S., Lee G.W., Chen C.M., Wu C.C., Yu K.P. The size and concentration of droplets generated by coughing in human subjects. J Aerosol Med. 2007;20(4):484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]