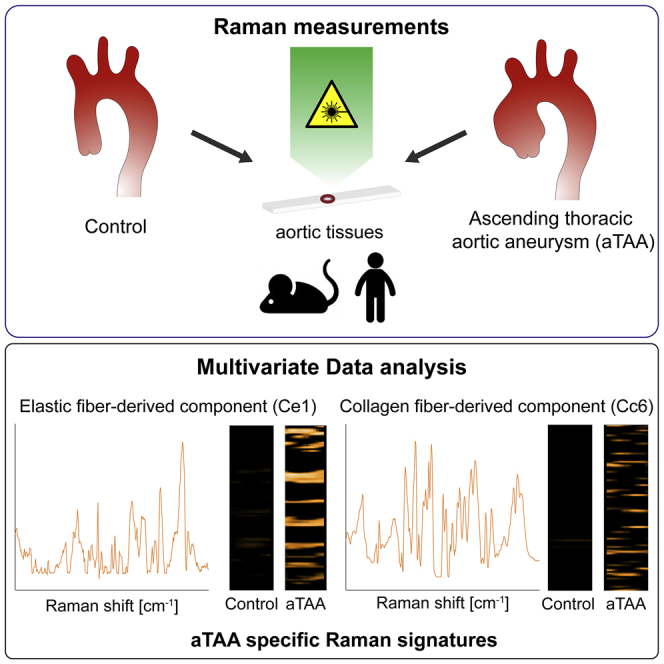
Summary
Aortic rupture and dissection are life-threatening complications of ascending thoracic aortic aneurysms (aTAAs), and risk assessment has been largely based on the monitoring of lumen size enlargement. Temporal changes in the extracellular matrix (ECM), which has a critical impact on aortic remodeling, are not routinely evaluated, and cardiovascular biomarkers do not exist to predict aTAA formation. Here, Raman microspectroscopy and Raman imaging are used to identify spectral biomarkers specific for aTAAs in mice and humans by multivariate data analysis (MVA). Multivariate curve resolution-alternating least-squares (MCR-ALS) combined with Lasso regression reveals elastic fiber-derived (Ce1) and collagen fiber-derived (Cc6) components that are significantly increased in aTAA lesions of murine and human aortic tissues. In particular, Cc6 detects changes in amino acid residues, including phenylalanine, tyrosine, tryptophan, cysteine, aspartate, and glutamate. Ce1 and Cc6 may serve as diagnostic Raman biomarkers that detect alterations of amino acids derived from aneurysm lesions.
Keywords: ascending thoracic aortic aneurysm, Raman imaging, biomarkers, extracellular matrix, multivariate data analysis, collagens, elastic fibers, mouse model
Graphical abstract
Highlights
Label-free Raman imaging of human/murine ascending thoracic aortic aneurysm (aTAA)
Multivariate analysis of Raman spectra allows detection of aTAA molecular features
Identification of spectral biomarkers for aTAA in elastic and collagen fibers
Alterations in amino acid spectra correlate with aTAA formation
Sugiyama et al. performed label-free Raman imaging of human and murine ascending thoracic aortic aneurysms (aTAAs). Using Raman spectra for multivariate data analysis, they identify spectral biomarkers for aTAA in elastic fibers and collagen fibers, Ce1, and Cc6, respectively, that are significantly increased in aTAA lesions.
Introduction
Ascending thoracic aortic aneurysms (aTAAs) are life threatening because of sudden rupture and dissection without any detectable symptoms.1 The aortic diameter in aTAA increases at least 50% compared to the normal thoracic aorta,2 and the maximum aortic diameter is used as a criterion for surgical repair. However, patients with an aortic diameter of <5.5 cm could develop acute dissection,3 indicating an unmet need for diagnostic tools that consider the integrity of the aneurysmal wall.
General risk factors of aortic aneurysms are smoking, hypertension, inflammation, hyperlipidemia, and aging. aTAAs are frequently associated with mutations in components of the extracellular matrix (ECM), including fibrillin-1 in Marfan syndrome,4 type III α1-collagen in Ehlers-Danlos type III,5 lysyl hydroxylase 1 in Ehlers-Danlos type XI,6 and fibulin-4 (FBLN4 encoded by EFEMP2) in cutis laxa syndrome.7,8 Activation of the transforming growth factor β (TGF-β) pathway is observed in Marfan syndrome and Loeys-Dietz syndrome.9 In addition, mutations in the genes involved in the generation of contractile forces in smooth muscle cells (SMCs) have been identified in familial TAAs and dissections.10 Therefore, the causal genes for TAAs encompass the ECM to cellular components along the elastin-contractile units.11
Medial necrosis and disruption of elastic fibers are characteristic pathological features of human aTAA. Massive accumulations of proteoglycans, such as aggrecan and versican,12 and phenotypic switching of SMCs have been observed,13 which can be caused by alterations in ECM components, including FBLN4,14 fibulin-5,15 and collagen VIII.16 These observations suggest that pathological changes involved in aneurysms include cellular and extracellular changes in the vessel wall in addition to increased lumen size.
FBLN4 and fibulin-5 (FBLN5) are major elastic fiber-associated proteins and are essential for elastogenesis in vivo.17 Mutations of FBLN4 and FBLN5 have been identified in autosomal recessive cutis laxa type I patients, and FBLN4 was shown to be associated with aTAA.18 Previously, we generated SMC-specific Fbln4 knockout mice (Fbln4SMKO),14 which develop aortic aneurysms that recapitulate the human condition. We also generated Fbln5 KO mice (Fbln5KO), which develop aortic elongation and tortuosity but do not form aortic aneurysms.19 Lack of Fbln4 resulted in abnormal collagen maturation in addition to abnormal elastic fibers in the Fbln4SMKO aorta,20 which was attributed to decreased lysyl oxidase-mediated cross-linking of collagen fibrils.21,22
Raman microspectroscopy and Raman imaging are marker-independent and non-destructive imaging methods evolving in the biological and biomedical fields.23,24 Raman microspectroscopy can distinguish different components in tissues by detecting molecular vibrations. ECM molecules such as elastin, collagens, or proteoglycans have specific Raman spectra.25,26 In previous studies, Raman microspectroscopy was shown to reveal differences between healthy and cancerous tissues in the skin27,28 and lungs,29 and age-dependent differences in the ECM surrounding pancreatic islets.30 Moreover, Raman microspectroscopy and Raman imaging have the potential to analyze cardiovascular ECM structures on a molecular level.31, 32, 33 At this time, no reports are available regarding the assessment of aortic aneurysm by Raman measurements.
In this study, we used marker-independent Raman microspectroscopy and Raman imaging combined with multivariate data analysis (MVA), including true component analysis (TCA), principal-component analysis (PCA), and multivariate curve resolution (MCR), to examine structural and molecular signatures with a high spatial resolution among Fbln4SMKO, Fbln5KO, and wild-type (WT) aortic tissues. Furthermore, we extended our analyses to human aTAA based on the spectral dataset obtained from murine aneurysmal tissues.
Results
Raman imaging allows marker-independent detection of aortic structural composition
To set the reference spectra of aortic tissues, we performed Raman imaging on cross-sections of murine WT ascending aortas at 1 month of age. We successfully extracted spectra by TCA and five major spectral components were identified, which were assigned to the Raman signatures of elastic fibers, collagen fibers, nuclei, lipids, and residual ECM (Figure S1A, i, ii, iii, vi, and vii).25,34 In addition, reference spectra of lyophilized aggrecan and versican were added as defined components to TCA (Figure S1A, iv and v). In elastic fibers, relevant Raman bands were detected at 528, 957, and 1,108 cm−1 for desmosine and isodesmosine; 904 cm−1 for C-C-N stretch; 1,255 cm−1 for amide III; 1,455 cm−1 for CH3 and CH2 deformation; and 1,666 cm−1 for amide I.25 In collagen fibers, relevant Raman bands were found at 817 cm−1 for C-C stretch35; 855, 878, 921, and 938 cm−1 for proline and hydroxyproline36; and 1,670 cm−1 for amide I.25 In nuclei, nucleotides and phosphate backbone-related Raman bands were detected at 787 cm−1, 1,094 cm−1, and 1,580 cm−1.34 In aggrecan, relevant Raman bands were present at 947 cm−1 for C-C deformation,37 1,066 cm−1 for C-C and C-O stretch,38 1,271 cm−1 for amide III,39 and 1,382 cm−1 for CH2 deformation.37 In versican, 848 cm−1 correlated with glycosaminoglycans,40 918 cm−1 to glycogen,41 and 1,080 cm−1 to SO3− symmetric stretch.37 In lipids, relevant Raman bands were 1,312 cm−1 for CH3CH2 twisting mode,42,43 1,443 cm−1 for CH2 deformation,43 and 1,748 cm−1 for C=O stretch.43 The residual ECM component was dominated by protein Raman bands found at 1,459 and 1,666 cm−1 correlating with CH2 and CH3 deformation and amide I, respectively.36,44 These identified spectra were used as reference spectra for subsequent TCAs (Table S1). Intensity distribution heatmaps for each component were generated for Raman maps of ascending (Figure S1B) and descending (Figure S1C) aortas.
To confirm the sensitivity and specificity of the identified Raman signature for elastic fibers, aortic tissues of postnatal day (P) 1 WT and ElnKO45 pups were analyzed. Previously identified core reference spectra were used to perform TCA on the large area and high-resolution scans on cross-sections of WT and ElnKO aortas (Figures S1D and S1E). Analysis of WT aortas resulted in Raman images that were positive for the applied reference spectra of elastic fibers, whereas signals were undetectable in ElnKO aortas, indicating that the identified spectra were highly specific to elastic fibers.
Transfer of WT spectral components to Fbln5KO and Fbln4SMKO aortic tissues
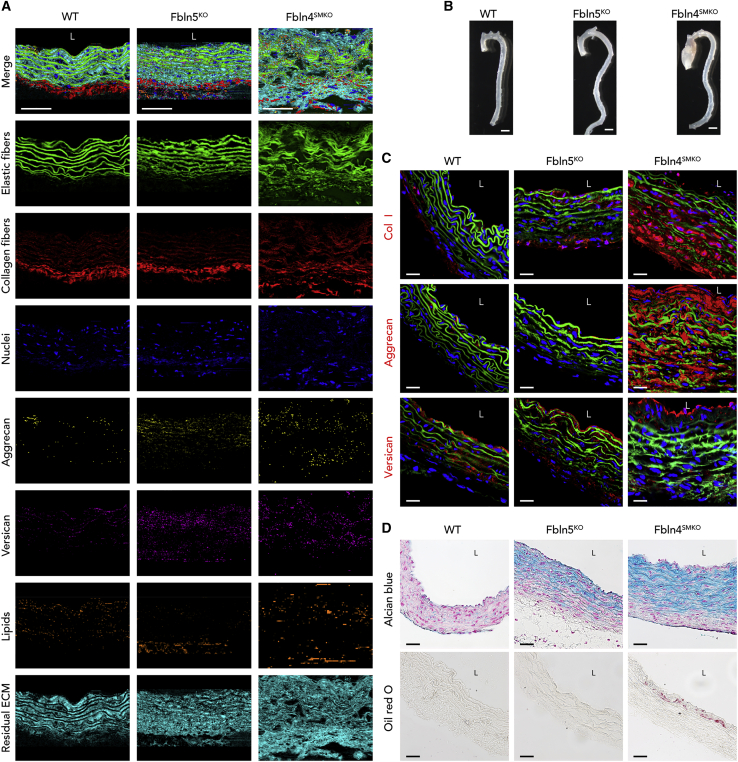
We used two established murine models of aortic disease, Fbln5KO and Fbln4SMKO, to examine whether Raman imaging can detect an alteration of ECM in the diseased aortic wall.14,19 Spectral components identified in the WT aorta were applied as reference spectra to generate Raman images of the mutant aortas and to define the distribution and orientation of each component in ascending (Figure 1A) and descending (Figure S2A) aortas. In Raman imaging, WT aortas contained solid native elastic fibers, whereas Fbln5KO elastic fibers showed disruptions. Fbln4SMKO elastic fibers were disorganized, and elastic layers were markedly increased in the ascending aortas. In WT and Fbln5KO, collagen fibers were predominantly located in the adventitia, whereas collagen fibers were expanded from adventitia to medial layers in Fbln4SMKO aorta. WT, Fbln5KO, and Fbln4SMKO showed a similar distribution of nuclei. Aggrecan, which was previously shown to be increased in TAA patients,12 was markedly accumulated in Fbln4SMKO aortas. In contrast, distribution of versican was similar among genotypes. Lipid signals were marginally increased in the Fbln4SMKO aortas compared with WT. The imaging results obtained from descending aortas were comparable to ascending aortas, except for Fbln4SMKO, which showed a less destructed organization of elastic fibers and a WT-like organization of collagen fibers when compared with ascending aortas (Figure S2A). High-resolution scans were also used to examine the structural details (Figures S2B and S2C) as well as for in-depth spectral analysis by PCA and MCR analysis. Gross observation of Fbln5KO and Fbln4SMKO showed elongation and tortuosity of the aorta in both animals, whereas aneurysm was seen only in the ascending aorta of Fbln4SMKO, as was previously reported (Figure 1B).14,19
Figure 1.
Representative Raman images and immunofluorescence (IF) staining and histochemistry for WT, fibulin-5 knockout (Fbln5KO), and smooth muscle-specific fibulin-4 knockout (Fbln4SMKO) mice
(A) Raman images of large-area scans. Cross-sections of the ascending aorta from WT, Fbln5KO, and Fbln4SMKO. False-color intensity distribution heatmaps for elastic fibers (green), collagen fibers (red), nuclei (blue), aggrecan (yellow), versican (pink), lipids (orange), and residual ECM (cyan). Scale bars, 50 μm.
see also Figure S1.
(B) Gross photos of murine aortas of WT, Fbln5KO, and Fbln4SMKO mice. Fbln5KO exhibits elongation of the ascending aorta and tortuous descending aorta. Fbln4SMKO shows a large aneurysm in the ascending aorta but not in the descending aorta. Scale bars, 1 mm.
(C) IF staining for collagen type I, aggrecan, and versican in red, elastin autofluorescence (green), and nuclei (blue). Scale bars, 20 μm.
(D) Routine histochemical staining for Alcian blue (glycosaminoglycans) and oil red O (lipid) staining.
Scale bars, 20 μm. L, luminal side.
Routine histology complies with Raman imaging results
Immunofluorescence (IF) staining and histological analyses were performed to compare and evaluate the performance of Raman imaging for the identification of tissue structures as well as distribution of matrix components in the aortic tissues. IF identified collagen type I (Col I), aggrecan, versican, elastic fibers (autofluorescence), and nuclei (DAPI) in ascending aortic tissues (Figure 1C). Whereas Col I localized in the adventitia of the WT aorta, its distribution expanded into medial layers of Fbln5KO, and this change was exacerbated in Fbln4SMKO, exhibiting a strong and broad distribution throughout the aortic wall. For aggrecan staining, Fbln4SMKO aorta showed strong signals in the intima as well as in the medial layers, and versican staining was positive in the intima and adventitia of all genotypes.
Next, histochemical staining was performed using Alcian blue and oil red O (ORO), which visualizes glycosaminoglycans and lipids, respectively (Figure 1D). Alcian blue staining exhibited a massive accumulation of proteoglycans in Fbln5KO and Fbln4SMKO, but not in WT. ORO staining showed small lipid deposition in the Fbln4SMKO aortas, but not in other genotypes. Histology and IF staining of the descending aortas were similar to those of the ascending aortas (Figures S2D and S2E). The structures and distribution patterns identified by Raman imaging correlated with routine immunohistochemical staining.
PCA discriminated the molecular composition of the aortic tissues among WT, Fbln5KO, and Fbln4SMKO
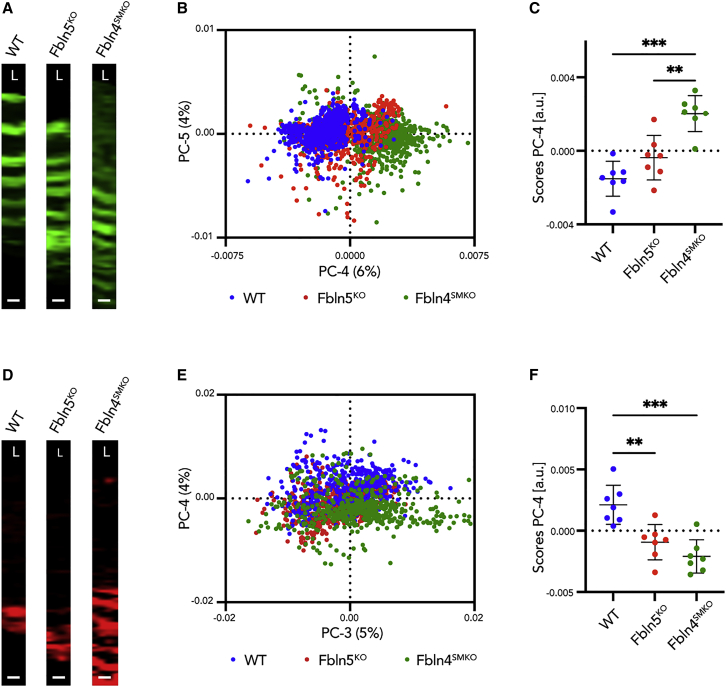
To access spectral patterns in WT, Fbln5KO, and Fbln4SMKO, we focused on elastic fibers. We used the Raman spectra extracted from the high-resolution scanning Raman images of elastic fibers in ascending aortas for PCA to determine the grouping of the datasets (Figure 2A). The scores plot of PC-4 against PC-5 showed a clustering of all genotypes (Figure 2B). Statistical analysis of the PC-4 score indicated a significant difference in WT versus Fbln4SMKO and Fbln5KO versus Fbln4SMKO (Figure 2C; Table S2). The loadings plot, which represents Raman shift values of the selected PC, was further analyzed for the corresponding spectral information that was responsible for the clustering of elastic fibers (Figure S3A) and collagen fibers (Figure S3B). The PC-4 loadings plot represented Raman features in elastic fibers dominating in WT (negative loadings bands) or Fbln4SMKO (positive loadings), respectively (Figure S3A). Those features referred to a decrease in the amide III group (1,240 cm−1) and spectral shifts in the CH2 (1,441–1,473 cm−1) and amide 1 (1,640–1,690 cm−1) bands upon elastic fiber deterioration. PCA on single spectra extracted from the collagen component in ascending aorta Raman images of high-resolution scans showed separation in PC-4 versus PC-3 scores plot (Figures 2D and 2E). PC-4 demonstrated a significant difference between WT and Fbln4SMKO as well as Fbln5KO (Figure 2F). The corresponding molecular differences identified in the PC-4 loadings plot showed collagen-related peaks for proline and hydroxyproline (862 and 944 cm−1) and amide III (1,270 cm−1) and amide III (1,670 cm−1) bands (Figure S3B).
Figure 2.
Principal-component analysis (PCA) on elastic fibers and collagen fibers
(A) Region of interest (ROI) for single spectra extraction defined by elastic fiber TCA component of high-resolution scans in WT, Fbln5 KO, and Fbln4SMKO.
(B) PCA scores plot of PC-4 versus PC-5 shows a clustering between WT (blue), Fbln5KO (red), and Fbln4SMKO (green).
(C) Statistical analysis of PC-4 scores indicated as mean score values ± SD.
(D) ROI for single spectra extraction defined by collagen fiber TCA component of high-resolution scans in WT, Fbln5 KO, and Fbln4SMKO.
(E) PCA scores plot of PC-3 versus PC-4 shows a clustering between WT (blue), Fbln5KO (red), and Fbln4SMKO (green).
(F) Statistical analysis of PC-4 scores indicated as mean score values ± SD.
Scale bars, 5 μm. L, luminal side. N ≥ 6 animals per genotype. Multiple comparison 1-way ANOVA; ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Additional PCAs and statistical analyses were performed to compare elastic fiber and collagen composition between ascending and descending aortas in WT, Fbln5KO, and Fbln4SMKO (Figures S3C and S3D; Table S2). While WT and Fbln5KO showed no differences within the regions, Fbln4SMKO showed significant separation between ascending and descending aorta for both elastic fibers (Figure S3C) and collagen fibers (Figure S3D).
Decomposition of elastic and collagen fibers by MCR
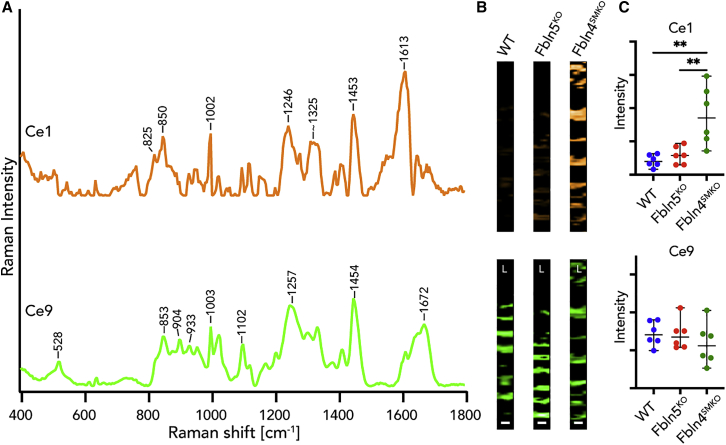
Since PCA clearly differentiated elastic and collagen fibers in WT, Fbln5KO, and Fbln4SMKO, we used MCR to further determine the submolecular structures specific to aneurysm phenotype by Raman microspectroscopy. First, Raman spectra focusing on elastic fibers were decomposed into 16 components (Ce1–Ce16) by MCR and corresponding Raman images were generated (Figure S3E). Statistical analysis of the relative intensity of MCR Raman images revealed that Ce1 was significantly different in Fbln4SMKO compared with WT and Fbln5KO (Figure 3; Table S2). As Figure 3 and Table S2 show, Ce1 and Ce9 contained Raman bands arising from proteins, such as phenylalanine (1,002 cm−1), amide III (1,246–1,257 cm−1), amide I (1,662–1,672 cm−1), and CH2/CH3 deformation (1,450–1,454 cm−1).34 Ce1 is assigned to a non-elastin-related substructure of the elastic fiber because of a lack of desmosine and isodesmosine bands. Ce9 demonstrated a similar Raman spectrum to the native elastic fiber component identified by TCA (Figure S1A) and protein-related bands (Table S1).25,34
Figure 3.
Multivariate curve resolution (MCR) decomposed spectra and images of elastic fibers in WT, Fbln5KO, and Fbln4SMKO
(A) Most relevant MCR spectra in Fbln4SMKO compared with WT and Fbln5KO. Ce1 is specifically found in Fbln4SMKO (orange) and Ce9 represents the standard elastic fiber signature (green).
(B) MCR images of Ce1 and Ce9 components.
(C) Statistical analysis of relative intensities of MCR images in Ce1 and Ce9.
Scale bars, 5 μm. L, luminal side. N = 6 per genotype, data points are represented as mean intensity values ± SD, statistical analysis by 1-way ANOVA; ∗∗p < 0.01.
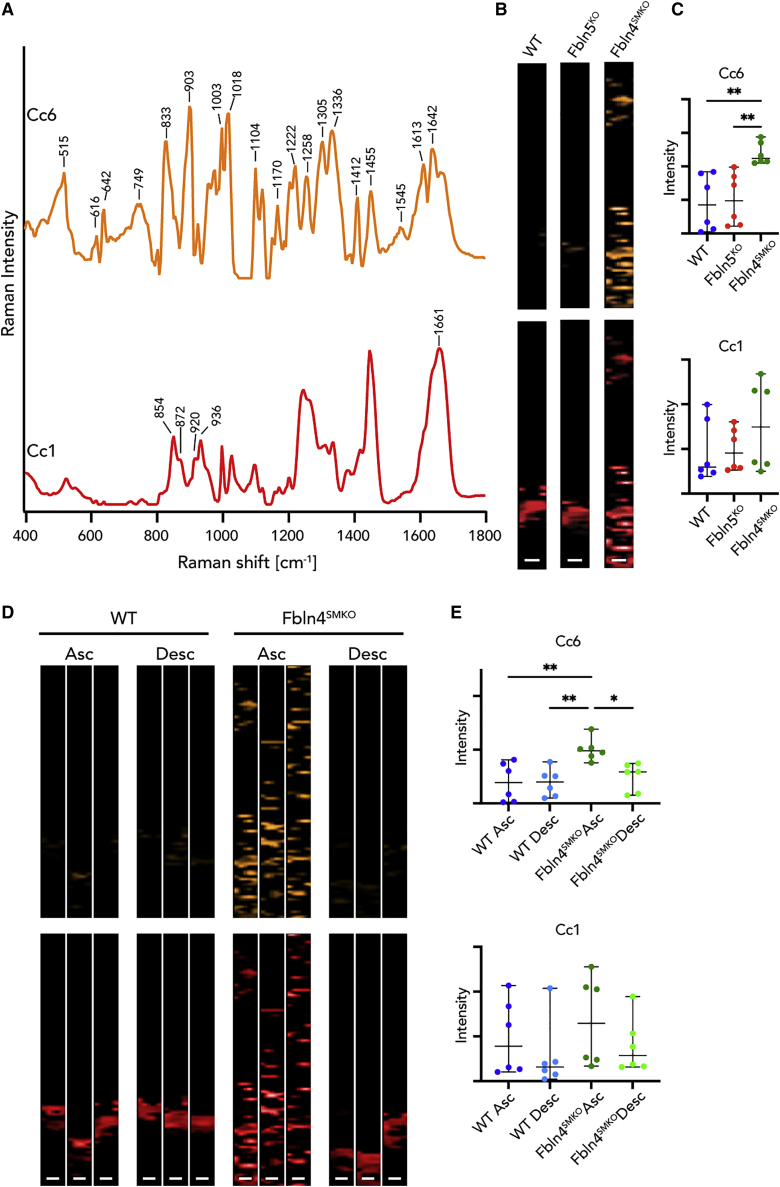
We then analyzed collagen fibers for differences in molecular signatures among genotypes. MCR extracted TCA-derived collagen fibers to 12 components (Cc1–Cc12) in ascending aortas of WT, Fbln5KO, and Fbln4SMKO (Figure S3F). Cc1 was comparable to collagen fibers indicated by proline and hydroxyproline bands (Figure 4A; Table S2).25,36 Statistical analysis of the intensity of each MCR image showed significant differences in Cc6 (Table S2). Cc6 was only detectable in Fbln4SMKO, indicating the abnormal molecular modification specific to Fbln4SMKO (Figures 4B and 4C). This suggests that the different structure of collagen fibers is related to aortic aneurysm phenotype.
Figure 4.
MCR decomposed spectra and images of collagen fibers in ascending (Asc) and descending (Desc) aortas of WT, Fbln5KO, and Fbln4SMKO
(A) Most relevant MCR spectra in Fbln4SMKO compared with WT and Fbln5KO. Cc6 was identified as a specific Raman spectrum in Fbln4SMKO (orange), and Cc1 represents the native signature of collagen fibers (red).
(B) MCR images of Cc6 and Cc1 components.
(C) Statistical analysis of relative intensities of MCR images in Cc6 and Cc1.
(D) MCR images of Cc6 and Cc1 in WT and Fbln4SMK in ascending and descending aortas. Three representative images per genotype.
(E) Statistical analysis of relative intensities of MCR images in Cc6 and Cc1.
Scale bars, 5 μm. L, luminal side. N = 6 animals per genotype, data points represent mean intensity values of 3 images ± SD, 1-way ANOVA (Cc6) or Kruskal-Wallis test (Cc1) were performed; ∗p < 0.05, ∗∗p < 0.01.
Based on the differential MCR analysis, we further searched for aneurysm-specific and region-specific molecular signatures focusing on collagen fibers in WT and Fbln4SMKO (Figure 4D). Statistical analysis (Table S2) revealed significant differences between ascending and descending aortas of WT and Fbln4SMKO in Cc6, indicating that these bands detected the aneurysm-specific changes in Fbln4SMKO aortas (Figures 4D and 4E). In addition, Cc6 was only detected in the ascending aortas of Fbln4SMKO, and the overall spectral pattern of Cc6 includes protein-indicating bands such as CH2/CH3 deformation and amide I/III (Table S1). The Cc6 spectrum contains amino acid residues, including phenylalanine at 616, 1,003, 1,222, and 1,613 cm−1; tyrosine at 642, 833, 1,170, and 1,222 cm−1; tryptophan at 749, 1,018, 1,336, and 1,455 cm−1; cysteine at 515 cm−1; and aspartic and glutamic acid at 1,412 cm−1 (tentative assignments).34,46 Interestingly, Cc6 showed relatively intense peaks of tryptophan, suggesting dysregulated tryptophan metabolisms or a substitution of tryptophan in Col I, which has been observed in abdominal aortic aneurysm (AAA).47,48
Identification of disease-related MCR Raman signature in human aTAA
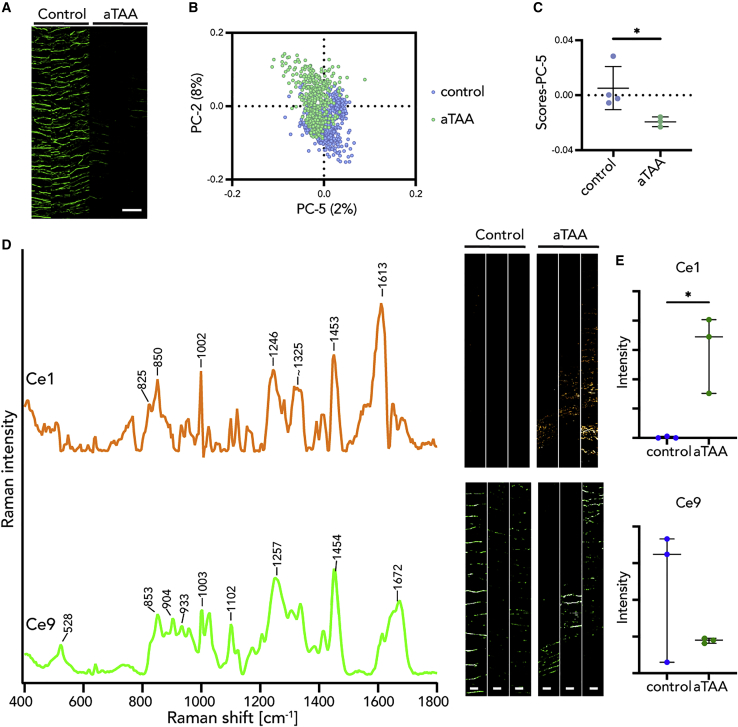
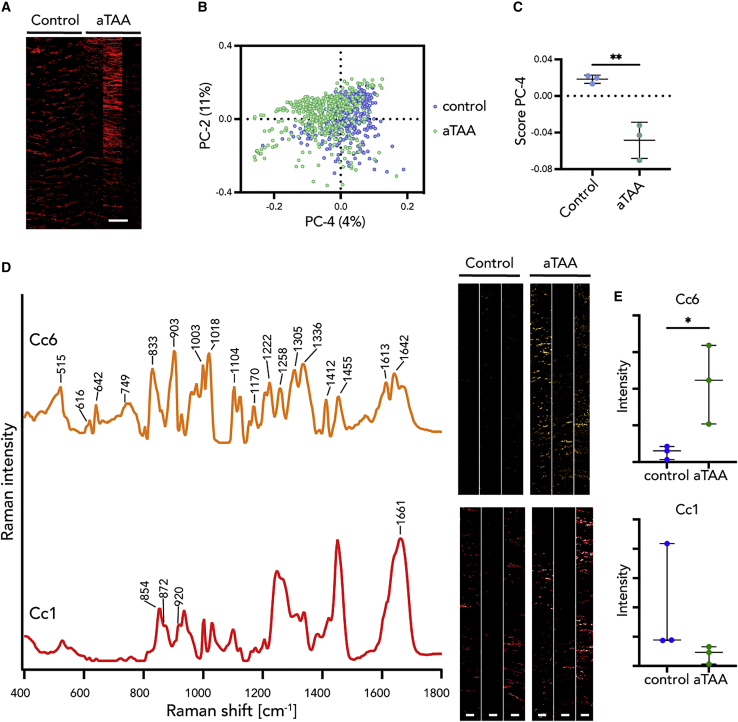
To further examine whether the aTAA-relevant Raman signature in murine aneurysms is applicable to human aTAAs, we analyzed tissues from aTAA patients. TCA reference components defined in the murine WT tissues described in Figure S1A were applied for TCA and subsequent Raman image generation of human control and aTAA tissues (Figure S4A). Spectra of elastic fibers and collagen fibers were then extracted based on the TCA intensity distribution heatmaps. The PCA results showed a separation between control and aTAA in both elastic fibers (Figures 5A– 5C) and collagen fibers (Figures 6A–6C, S4B, and S4C).
Figure 5.
Multivariate analysis (MVA) of elastic fibers revealed similar structural patterns in murine and human aneurysm tissues
(A) Intensity distribution heatmaps of elastic fibers. Scale bar, 50 μm.
(B and C) PC-5 score values showed a separation between aTAA and human control.
(D and E) By MCR, the murine aneurysm-specific spectral marker Ce1 (orange) was confirmed in human TAA tissue and could not be found in human control tissues, whereas the native elastic fiber signature Ce9 (green) was localized in both groups.
Scale bars, 20 μm. N = 3, data points represent mean score (C) or intensity (E) values ± SD, non-paired t test (C and Ce9) or non-parametric Mann-Whitney test (Ce1) were performed; ∗p < 0.05.
Figure 6.
MVA on collagen fibers revealed similar structural patterns in murine and human aneurysm tissues
(A) Intensity distribution heatmaps of collagen fibers in human control and ascending thoracic aortic aneurysm (aTAA). Scale bar equals 50 μm.
(B and C) PCA showing a clustering between control and aneurysm in PC-4.
(D and E) MCR decomposed Raman spectra and imaging of murine Cc6 (aneurysm feature, orange) and Cc1 (native collagen feature, red) components applied to human control and TAA tissues indicate a significant difference in the aneurysm-specific signature (Cc6) but not in the native collagen signature (Cc1).
Scale bars equal 5 μm. N = 3, data points are represented as mean score (C) or intensity (E) values ± SD, non-paired t tests were performed; ∗p < 0.05, ∗∗p < 0.01.
For MCR analysis, the murine MCR components from elastic fibers (Ce1–Ce16) were used as reference spectra and additional spectral components, which were only detected in human tissues (Ce17–Ce20; Figure S4D), were extracted. As Table S2 shows, Ce1 in aTAA samples is significantly different from control samples. In particular, Ce1 was increased in both murine and human aneurysms (Figures 5D and 5E).
Similarly, we examined aneurysm lesion-specific spectra in collagen fibers (Figure 6). The murine collagen MCR components (Cc1–12) were used as reference spectra for human analysis. Similar to the elastic fiber analysis, additional components were extracted only from the human data (Cc13–15; Figure S4D). Significant differences were revealed in Cc6 between control and aTAA (Table S2). Cc6 was increased in human aneurysms similar to murine aneurysms (Figure 6E).
In addition, we used PCA to compare changes in elastic fibers and collagen fibers in murine aneurysm and human aTAA. Although significant species differences were detected (Figure S4C), aneurysm tissues from mice and humans were separated from healthy human controls (Figure S4E). A significant separation of control human aortas and aTAA, as well as Fbln4SMKO, was shown in PC-5 for elastic fibers and in PC-4 for collagen fibers. Moreover, the corresponding loadings recapitulated the aneurysm-specific peaks identified by MCR. Elastin loadings demonstrated peaks at ~822 and 1,613 cm−1 similar to Ce1 (Figure 5D). Collagen loadings demonstrated peaks at 642, 831, 1,334 and 1,613 cm−1 characteristic for bands identified in MCR component Cc6 (Figure 6D). MCR further decomposed elastic fiber and collagen fiber components and identified aneurysm-specific molecular signatures in human aTAA, which have the potential for use as diagnostic markers.
Discussion
In this study, we used Raman microspectroscopy and Raman imaging to perform marker-independent analyses of ascending aortic aneurysms in a murine model and human aTAA. Raman imaging allowed the visualization of cellular and extracellular components with a high spatial resolution. Structural features of aortic tissues in their native conditions were identified without the need for using specific labeling. The obtained data correlated to the distributional patterns demonstrated by IF staining and histochemistry, and sample preparation was simple and less time-consuming compared with conventional staining procedures. MVA further allowed for submolecular discrimination between healthy aortas and aneurysmal lesions, and successfully identified human aneurysm-specific marker signatures in elastic fibers (Ce1) and collagen fibers (Cc6) that can be used as biomarkers for aTAA diagnosis.
Raman microspectroscopy has the ability to evaluate tissues in situ in their native conditions.49 For ECM molecules, which are usually incorporated in a complex tissue surrounded by assembled and complex fibrous structures, Raman microspectroscopy may facilitate examination in a more native-like state. Marker-independent biomedical methods, such as echocardiography and X-ray microtomography, are a gold standard in clinical settings, but are limited to image-based analysis. Raman measurements are advantageous among optical methods because they provide hyperspectral images with additional information on molecular structures. By combining massive spectral datasets with deep learning techniques, the accuracy of disease-specific signatures and the definition of tissue borders is increasing.
Structural differences of aortic tissues revealed by TCA and PCA
Elastic fibers in Fbln5KO and Fbln4SMKO appeared similarly disrupted at a light microscopic level; however, Raman imaging and PCA separated the genotypes at PC-4 (Figure 2C). Likewise, PCA separated ascending aortas from descending aortas in Fbln4SMKO (Figures S3C and S3D). The molecular analysis of elastic fibers by PCA identified differences in Raman bands of amide I and amide III between Fbln5KO and Fbln4SMKO. Shifts in amide I and III have been previously reported in relation to an increase in α-helical content in elastic fibers due to structural deterioration.50 Since electron microscopic observation failed to distinguish the morphological differences in elastic fibers between ascending and descending aortas in Fbln4SMKO (data not shown), this suggests that Raman microspectroscopy can be a powerful tool to study the alteration of elastic fiber components from the view of secondary structural alteration.51
For collagen fibers, differences in distributional patterns identified by IF staining in WT, Fbln5KO, and Fbln4SMKO were also identified by marker-independent spectral scans. Raman imaging combined with PCA not only provides semiquantitative information on vascular collagen fibers but also allows the simultaneous assessment of qualitative molecular-level differences. PCA revealed a separation of WT, Fbln5KO, and Fbln4SMKO, and PC loadings showed different structures of proteins in Fbln5KO and Fbln4SMKO, which are possibly due to differences in proline and hydroxyproline content in collagen fibers.25,36 As for elastic fibers, alterations in the features of collagen fibers were apparent between aneurysmal ascending aortas and non-aneurysmal descending aortas in Fbln4SMKO. In addition to providing information regarding molecule localization and quantity, which can also be accessed by routine staining, Raman measurements allow for the identification of minor alterations in ECM structure, indicating the potential as an early diagnostic tool to identify pre-aneurysmal lesions in the aortic wall.
In normal aortas, proteoglycans such as versican are distributed transmurally and contribute to the regulation of residual stress.52 Alcian blue staining demonstrated the increased deposition of glycosaminoglycans (GAGs) in Fbln5KO and Fbln4SMKO, suggesting that disrupted elastic fibers may facilitate the deposition of GAGs or that accumulation of GAG affects the structural integrity of elastic fibers. Aggrecan accumulation was massive in Fbln4SMKO compared with Fbln5KO and WT. Aggrecan is specifically expressed in vascular SMCs and is involved in vascular remodeling.53 Since the deposition of GAGs causes the distortion of mechanical stress and can create a spot for aortic dissection,54 early detection of GAG accumulation and pathogenic proteoglycan within the aortic wall may be a potential diagnostic tool for diseased aorta at risk of dissection.
Identification of aneurysm-specific spectra by MCR
MCR effectively decomposed TCA elastic fibers and collagen fibers and identified aneurysm-specific Raman signals. In elastic fibers, Ce1 appears to be derived from abnormal proteins associated with aneurysmal lesions, which is detectable only in aTAA and Fbln4SMKO. Although the comparison of Raman spectra and accurate assignment to authentic proteins are needed, the 1,613 cm−1 band in Ce1 could be a characteristic marker for aneurysm, since the band does not overlap with amide I or phenylalanine.
Although elastic fibers have been suggested as a major site of pathological alteration in Fbln4SMKO aortas,14 MCR identified the region-specific features of aTAA for murine and human aortas not only in elastic structures but also in collagen fibers. Previously, we reported that different types of collagens showed distinctive peaks related to CH2 deformation at 1,453 and 1,456 cm−1.30 Intriguingly, Cc6, a protein-like component, was detected only in murine and human aTAA regions (Figures 4 and 6). The spectrum of Cc6 features high intensities of several amino acid residues, indicating compositional changes of collagen molecules. Amino acid profiles are shown to be associated with aortic aneurysm and dissection in human patients.48 MCR has successfully captured distinct marker spectra related to aTAA, enabling the specification of structural and compositional changes in elastin and collagen molecules.
Raman measurement as a diagnostic tool for aTAA
In the present study, PCA revealed distinct Raman signatures between ascending and descending aortas in Fbln4SMKO based on the molecular structure of ECM components, highlighting the sensitivity of Raman microspectroscopy for the detection of lesion-specific biochemical differences that are not accessible by other routine techniques. In addition, two-step decompositions in TCA and MCR were efficient ways to analyze the details and proper features of spectral components. Although there were differences in species and tissue conditioning such as paraffin embedding and cryosection, TCA was robust enough to localize ECM structures with a single set of reference components. PCA and MCR enabled the extraction and discrimination of structural alterations on a submolecular level beyond species differences.
Raman microspectroscopy previously detected changes in the Raman signatures of fibrillin-1 microfibrils and elastic fiber networks in the skin of healthy mice and a murine model of Marfan syndrome.28 In the present study, we demonstrated that the two identified signatures in elastic and collagen fibers have high potential as diagnostic biomarkers for aortic aneurysms. By increasing the components of Raman spectra and improving the resolution, Raman imaging can quickly evaluate vessel wall integrity and biochemical components of the diseased vessel wall. Future research may explore other TAA models that develop in adulthood, including fibrillin-1 mutant mice (Fbn1mgR/mgR) and apolipoprotein E-deficient mice.55,56
Recently, several applications for marker-independent Raman imaging have been reported; however, the imaging itself does not provide sufficient information to identify Raman marker spectra associated with cardiovascular diseases.32,57,58 The Fbln4SMKO model recapitulates ascending aneurysms of cutis laxa type I, which is a rare disease. However, the spectral data obtained from Fbln4SMKO aortas remarkably depicted ECM changes in human aTAA, indicating the versatile application of in vivo experimental data to human conditions.
For clinical implementation, Raman spectroscopy probe techniques have already been reported in determining structural features in cancer, skin, and oral tissues.59, 60, 61 Raman measurements are now used for rapid intraoperative diagnosis by stimulated Raman histology for brain tumors.62, 63, 64 Combined with automated spectral decomposition methods, Raman techniques have the potential to extract target signals from big datasets without the need for extensive training of the end user. Fiberoptic confocal Raman probes have already been applied in preclinical setups. Wang et al.65 used Raman endoscopy to measure epithelial tissues. Recently, fiberoptic Raman endoscopy has been used in patients with recurrent nasopharyngeal carcinoma to follow up on tumor progression.66 The field of endoscopy is rapidly evolving, as the first robotic magnetic flexible endoscope systems are now available.67 Therefore, fiberoptic Raman endoscopy for the aorta has the potential as a research tool as well as in a clinical setting. Furthermore, a combination of Raman measurements and artificial intelligence to identify molecular tissue patterns specific to (pre-)aneurysm will be a robust tool for modeling and monitoring the risk for aneurysm rupture/dissection.
Limitations of study
We are aware that the number of human aTAA patient samples is relatively small and needs to be increased to translate the established method into clinical applications. At this stage, human samples served as a proof-of-principle approach to evaluate the relevance of the findings in the aneurysm mouse model. In addition, the character of the study is mainly readout oriented. We used this highly molecular-sensitive technique but focused on identifying an aneurysm-specific signature as a spectral marker rather than a complex biomolecular characterization of aneurysm tissue. Structural differences were mainly linked to Col I and elastic fibers; however, the availability of data on spectral references of elastic fiber substructures (e.g., tropoelastin, fibrillin, fibulins) remains restricted and needs to be further elaborated for mechanistic investigations on aneurysm formation.
STAR★Methods
Key resources table
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-mouse collagen type I polyclonal antibody | Millipore | Cat#: AB765P, RRID:AB_92259 |
| Rabbit anti-mouse versican (GAG beta domain) polyclonal antibody | Millipore | Cat#: AB1033, RRID: AB_90462 |
| Anti-aggrecan polyclonal antibody | Millipore | Cat#: AB1031, RRID:AB_90460 |
| Alexa Fluor 546, anti-mouse | Thermo Fisher Scientific | Cat# A-11003, RRID:AB_2534071) |
| Alexa Fluor 546, anti-rabbit | Thermo Fisher Scientific | Cat# A-11035, RRID:AB_2534093 |
| VECTASHIELD Mounting Medium antibody | Vector Laboratories | Cat#: H-1000, RRID:AB_2336789 |
| Biological samples | ||
| Human aorta block | New York University Langone Medical Center | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Aggrecan from bovine articular cartilage | Millipore Sigma | Cat#: A1960 |
| Mouse VCAN / versican protein (Recombinat His, N-Terminal) (aa3058-3299) | LifeSpan BioSciences Inc. | Cat#: LS-G14317-10 |
| Oil Red O | FUJIFILM Wako Pure Chemical Corporation | Cat#: 1320-06-5 |
| Alcian Blue Solution | FUJIFILM Wako Pure Chemical Corporation | Cat#: 75881-23-1 |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664 |
| Mouse: 129S6/SvEVTac | Taconic Biosciences | 129SVE |
| Mouse: Fbln4SMKO | Huang et al., 201014 | N/A |
| Mouse: Fbln5KO | Yanagisawa et al., 200219 | N/A |
| Mouse: ElnKO | Li et al., 199845 | N/A |
| Software and algorithms | ||
| Igor Pro | Wave Metrics |
https://www.wavemetrics.com/products/igorpro/igorpro.htm RRID:SCR_000325 |
| Python | Python Software | https://www.python.org/, RRID:SCR_008394 |
| MCR-ALS | Ando and Hamaguchi, 201468 | https://github.com/mshrAndo/PyMCR |
| Project FIVE 5.0 software | WiTec GmbH | https://www.witec.de/jp/products/accessories/software-witec-suite/ |
| GraphPad Prism 8 | GraphPad Software | RRID:SCR_002798 https://www.graphpad.com/ |
| Unscrambler X | CAMO Software | http://www.stjapan.co.jp/products/1658 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Hiromi Yanagisawa (hkyanagisawa@tara.tsukuba.ac.jp).
Materials availability
This study did not generate unique reagents. Genetic mouse models will be available upon request after completion of a Materials Transfer Agreement.
Data and code availability
The data of this study are available without restriction. MCR bioinformatics code can be found online (https://github.com/mshrAndo/PyMCR).
Experimental model and subject details
Mice and tissue sections
Fbln5KO, Fbln4SMKO, and Eln null (ElnKO) mice were previously described and maintained on a C57/Bl6;129SvEv background.14,19,45 Animals at 1 to 3 months of age were used as adult mice for Fbln5KO, Fbln4SMKO, and wild-type (WT). For ElnKO and WT control, postnatal day (P) 1 neonates were used. Six to seven mice per genotype of adult mice for Fbln5KO, Fbln4SMKO, WT and thee mice per genotype of P1 mice for ElnKO and WT were used in all experiments and aortic tissues were harvested, embedded in Tissue Tek ® O.C.T. medium (Tissue Tek, Sakura), and snap-frozen in liquid nitrogen or embedded in paraffin. Mice were housed in the specific pathogen-free condition under a 12 h/12 h light/dark cycle. All animal protocols were approved by the Institutional Animal Experiment Committee of the University of Tsukuba.
Human tissue sections
Human ascending aorta aTAA samples were collected from open surgical repairs. Control aortic tissues were obtained from multi-organ donors confirmed as brain-dead. aTAA and control aortic tissues were analyzed for three different patients and donors. All studies were approved by New York University Langone Medical Center Institutional Review Board (IRB). Tissues were formalin-fixed and paraffin embedded prior to sectioning and Raman microscopy analysis.
Method details
Immunofluorescence staining
Ten-micrometer cross sections of the murine aorta prepared in Tissue Tek® O.C.T frozen blocks were fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature (RT). Sections were incubated with blocking buffer containing 5% normal goat serum in PBS and permeabilized with 0.1% Triton-X for 30 min at RT. Primary antibodies used were: anti-mouse collagen type I antibody (1:100; AB765P, Merck Millipore), rabbit anti-mouse versican (GAG beta domain) polyclonal antibody (1:200; AB1033, Chemicon international), rabbit anti-aggrecan polyclonal antibody (1:200; AB1031, Chemicon international). All antibodies were diluted in 5% BSA with 0.1% Triton-X in PBS and incubated overnight at 4°C. Secondary antibodies (Alexa 546 mouse or rabbit) were incubated for 1 hr at RT. Vectashield with DAPI (Vector Laboratories) was used to mount the slides and samples were analyzed with a laser scanning microscope LSM 710 (Zeiss GmbH). At least three mice per genotype were used for analysis.
Histochemistry
Paraffin-embedded sections were stained with Alcian blue for glycosaminoglycans following the standard protocol.20 Cross sections of aortas from Tissue Tek® O.C.T frozen block were fixed with 4% PFA for 10 min at RT and stained with Oil Red O (ORO) (Wako, Japan) to visualize lipids.69 Three mice per genotype were used for analysis.
Raman measurements
Raman measurements were performed with a confocal Raman microscope (WiTec alpha 300 R, WiTec GmbH, Ulm, Germany) equipped with a green laser (532 nm) as described previously.70 Cross sections of aortic tissues were rinsed with PBS and a 63x Apochromat water dipping objective (N.A. 1.0; Carl Zeiss GmbH) was utilized for data acquisition. Large area scans were performed on areas of 100 × 200 μm with 2 × 2 μm pixel resolution and acquisition time of 0.25 s per spectrum. High-resolution scans were performed on areas of 10 × 150 μm with 1 × 1 μm pixel resolution and 0.5 s acquisition time per spectrum. Laser power was set to 50 mW. Reference spectra were obtained from lyophilized aggrecan from bovine articular cartilage (A1960, Sigma-Aldrich/Merck, Darmstadt Germany) and recombinant lyophilized murine versican (LG-G14317, LifeSpan BioSciences Inc., Seattle, USA). Single spectra were acquired with a laser power of 50 mW and an acquisition time of 5 s. For the murine tissues, samples were prepared with as frozen sections, washed, and kept under PBS during the measurements. Seven mice per genotype were used and at least three areas were examined per tissue sample. For the human tissues, samples were deparaffinized, rehydrated, and kept under PBS during the entire measurement. High-resolution scans of an area of 50 × 500 μm were acquired at a pixel resolution of 1 × 1 μm, an integration time of 0.5 s, and a laser power of 50 mW. Three control and three aTAA samples were obtained, and at least three areas per section were analyzed.
Raman imaging analysis
Spectral data were pre-processed by cosmic ray removal and background subtraction. The spectral maps were analyzed by True Component Analysis (TCA), a tool from Project FIVE 5.0 software (WiTec GmbH) that identified different spectral information within the dataset, as previously described.71 Briefly, TCA is a non-negative matrix factorization-based algorithm that defines similar spectra as the same component and allows for the generation of color-coded intensity distribution heatmaps of the identified structures. TCA components identified in the murine WT aortas were applied as reference signatures for the TCA analysis of the KO animals as well as the human tissues.
Principal-component analysis (PCA)
Raman spectral datasets were extracted for elastic and collagen fiber components identified by TCA. The data were normalized and the Raman shift range was cropped to the fingerprint region between 400 and 1800 cm-1. The Unscrambler X10.5 (CAMO Software, AS, Oslo, Norway) was applied to perform principal component analysis (PCA) using NIPALS algorithm. PCA is commonly implemented in chemometrics and allows for the identification and interpretation of spectral differences within a Raman dataset.28,33 Briefly, in PCA, spectral information is described by vectors, so-called principal components (PCs). PCA results are visualized by a scores plot, where one PC is plotted against another, with every spectrum represented as an individual dot. The corresponding PC loadings plot displays peaks that have a great impact on the scores values and allows for the interpretation of molecular differences among the groups.28,43 The average of the score values in each animal was calculated and used for statistical analysis.
Multivariate curve resolution (MCR)
Multivariate curve resolution by alternating least-squares (MCR-ALS) was effectively used to extract chemically interpretable spectra.68 In MCR-ALS, an original data matrix (A) can be decomposed into two matrices—W, spectral components, and H, their intensity contribution profiles—as given below.
Here E is the error matrix. In the present study, the elastic fiber and collagen fiber datasets were preselected by TCA and whole area intensity normalized elastic fiber/collagen fiber datasets were used as A. In the optimization calculation, non-negativity constraints (Wij ≥ 0 and Hij ≥ 0) are introduced in order to conduct a physically adaptable decomposition; Raman scattering intensities and molecular concentrations are always non-negative. Furthermore, in the present study, l1-norm regularization (Lasso regression) was applied to enable the extraction of specific spectral components, via sparser matrix factorization, that contribute to the difference among samples, which were mice aortas in WT, Fbln5KO, and Fbln4SMKO, and human aortas in control and aTAA patients.72 Under these constraints, using singular value decomposition (SVD)-based initialization (applied to fingerprint region between 400 to 1800 cm-1), MCR-ALS optimization was performed by minimizing . The hyperparameter λ for Lasso regression was determined as 0.002 by cross validation. For murine analysis, SVD initialization was utilized to identify MCR components, and for human analysis, the results of murine MCR analysis were utilized as initial spectra. All optimization calculations were performed using an in-house program written in Python code.68 The average intensity of hi vector for each mapping image was calculated and used for the statistical analysis.
Statistical analysis
Data were represented as mean ± SD. Statistical analysis was performed using Prism 9 software (GraphPad, La Jolla, CA, USA). Shapiro-Wilk tests were conducted to examine whether the data followed normal distribution. If the data followed normal distribution, statistical significance was determined by unpaired or paired t test for two-group comparisons and one-way/two-way analysis of variance (ANOVA) for comparison among three or more groups followed by Bonferroni’s correction for multiple comparison tests. If the normality assumption was violated, nonparametric tests (Mann-Whitney or Kruskal-Wallis) were conducted. Data were considered statistically significant for p values of 0.05 or less.
Acknowledgments
This work was supported in part by MEXT KAKENHI (grant nos. JP 17H04289 and JP 20H03762) and SENSHIN Medical Research Foundation, Japan, to H.Y., as well as the Deutsche Forschungsgemeinschaft (INST 2388/64-1 and Germany’s Excellence Strategy, EXC 2180-390900677), the Ministry of Science, Research, and the Arts of Baden-Württemberg (33-729.55-3/214 and SI-BW 01222-91, to K.S.-L.), and the State Ministry of Baden-Württemberg for Economic Affairs, Labour and Housing Construction. M.A. was supported by JST, PRESTO (grant no. JPMJPR1671), Japan.
Author contributions
The experiments were designed by K.S., K.S.-L., and H.Y. The Raman experiments were measured by K.S., J.A. (murine samples), and J.M. (human samples). The data analysis was executed by K.S., J.M., and M.A. TCA was analyzed by K.S. and J.M. PCA was mainly analyzed by J.M. MCR was mainly analyzed by K.S. and M.A. The murine samples were prepared by K.S., Y.Y., and H.Y. The human samples were provided by B.R. K.S. and J.M. wrote the manuscript, which was revised by E.M.B., M.A., K.S.-L., and H.Y. All of the authors commented on the paper.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. It takes all kinds of minds to uncover new scientific discoveries.
Published: April 28, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100261.
Contributor Information
Katja Schenke-Layland, Email: katja.schenke-layland@uni-tuebingen.de.
Hiromi Yanagisawa, Email: hkyanagisawa@tara.tsukuba.ac.jp.
Supplemental information
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
References
- 1.Isselbacher E.M. Trends in thoracic aortic aneurysms and dissection: out of the shadows and into the light. Circulation. 2014;130:2267–2268. doi: 10.1161/CIRCULATIONAHA.114.013603. [DOI] [PubMed] [Google Scholar]
- 2.Norman P.E., Powell J.T. Site specificity of aneurysmal disease. Circulation. 2010;121:560–568. doi: 10.1161/CIRCULATIONAHA.109.880724. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi S., Jonker F.H., Hutchison S., Isselbacher E.M., Pape L.A., Patel H.J., Froehlich J.B., Muhs B.E., Rampoldi V., Grassi V. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J. Thorac. Cardiovasc. Surg. 2011;142:e101–e107. doi: 10.1016/j.jtcvs.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Dietz H.C., Pyeritz R.E. Mutations in the human gene for fibrillin-1 (FBN1) in the Marfan syndrome and related disorders. Hum. Mol. Genet. 1995;4:1799–1809. doi: 10.1093/hmg/4.suppl_1.1799. [DOI] [PubMed] [Google Scholar]
- 5.Pope F.M., Martin G.R., Lichtenstein J.R., Penttinen R., Gerson B., Rowe D.W., McKusick V.A. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc. Natl. Acad. Sci. USA. 1975;72:1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeowell H.N., Walker L.C., Farmer B., Heikkinen J., Myllyla R. Mutational analysis of the lysyl hydroxylase 1 gene (PLOD) in six unrelated patients with Ehlers-Danlos syndrome type VI: prenatal exclusion of this disorder in one family. Hum. Mutat. 2000;16:90. doi: 10.1002/1098-1004(200007)16:1<90::AID-HUMU19>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Dasouki M., Markova D., Garola R., Sasaki T., Charbonneau N.L., Sakai L.Y., Chu M.L. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am. J. Med. Genet. A. 2007;143A:2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- 8.Renard M., Holm T., Veith R., Callewaert B.L., Adès L.C., Baspinar O., Pickart A., Dasouki M., Hoyer J., Rauch A. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isselbacher E.M., Lino Cardenas C.L., Lindsay M.E. Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation. 2016;133:2516–2528. doi: 10.1161/CIRCULATIONAHA.116.009762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milewicz D.M., Trybus K.M., Guo D.C., Sweeney H.L., Regalado E., Kamm K., Stull J.T. Altered Smooth Muscle Cell Force Generation as a Driver of Thoracic Aortic Aneurysms and Dissections. Arterioscler. Thromb. Vasc. Biol. 2017;37:26–34. doi: 10.1161/ATVBAHA.116.303229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi A., Milewicz D.M. Structure of the Elastin-Contractile Units in the Thoracic Aorta and How Genes That Cause Thoracic Aortic Aneurysms and Dissections Disrupt This Structure. Can. J. Cardiol. 2016;32:26–34. doi: 10.1016/j.cjca.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cikach F.S., Koch C.D., Mead T.J., Galatioto J., Willard B.B., Emerton K.B., Eagleton M.J., Blackstone E.H., Ramirez F., Roselli E.E., Apte S.S. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018;3:97167. doi: 10.1172/jci.insight.97167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petsophonsakul P., Furmanik M., Forsythe R., Dweck M., Schurink G.W., Natour E., Reutelingsperger C., Jacobs M., Mees B., Schurgers L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019;39:1351–1368. doi: 10.1161/ATVBAHA.119.312787. [DOI] [PubMed] [Google Scholar]
- 14.Huang J., Davis E.C., Chapman S.L., Budatha M., Marmorstein L.Y., Word R.A., Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer J.A., Hacker S.L., Davis E.C., Mecham R.P., Knutsen R.H., Li D.Y., Gerard R.D., Richardson J.A., Olson E.N., Yanagisawa H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. USA. 2005;102:2946–2951. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adiguzel E., Hou G., Sabatini P.J., Bendeck M.P. Type VIII collagen signals via β1 integrin and RhoA to regulate MMP-2 expression and smooth muscle cell migration. Matrix Biol. 2013;32:332–341. doi: 10.1016/j.matbio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Papke C.L., Yanagisawa H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 2014;37:142–149. doi: 10.1016/j.matbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iascone M., Sana M.E., Pezzoli L., Bianchi P., Marchetti D., Fasolini G., Sadou Y., Locatelli A., Fabiani F., Mangili G., Ferrazzi P. Extensive arterial tortuosity and severe aortic dilation in a newborn with an EFEMP2 mutation. Circulation. 2012;126:2764–2768. doi: 10.1161/CIRCULATIONAHA.112.119883. [DOI] [PubMed] [Google Scholar]
- 19.Yanagisawa H., Davis E.C., Starcher B.C., Ouchi T., Yanagisawa M., Richardson J.A., Olson E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 20.Papke C.L., Tsunezumi J., Ringuette L.J., Nagaoka H., Terajima M., Yamashiro Y., Urquhart G., Yamauchi M., Davis E.C., Yanagisawa H. Loss of fibulin-4 disrupts collagen synthesis and maturation: implications for pathology resulting from EFEMP2 mutations. Hum. Mol. Genet. 2015;24:5867–5879. doi: 10.1093/hmg/ddv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horiguchi M., Inoue T., Ohbayashi T., Hirai M., Noda K., Marmorstein L.Y., Yabe D., Takagi K., Akama T.O., Kita T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc. Natl. Acad. Sci. USA. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhury N., Bouchot O., Rouleau L., Tremblay D., Cartier R., Butany J., Mongrain R., Leask R.L. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc. Pathol. 2009;18:83–91. doi: 10.1016/j.carpath.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Camp C.H., Jr., Lee Y.J., Heddleston J.M., Hartshorn C.M., Hight Walker A.R., Rich J.N., Lathia J.D., Cicerone M.T. High-Speed Coherent Raman Fingerprint Imaging of Biological Tissues. Nat. Photonics. 2014;8:627–634. doi: 10.1038/nphoton.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith R., Wright K.L., Ashton L. Raman spectroscopy: an evolving technique for live cell studies. Analyst (Lond.) 2016;141:3590–3600. doi: 10.1039/c6an00152a. [DOI] [PubMed] [Google Scholar]
- 25.Frushour B.G., Koenig J.L. Raman scattering of collagen, gelatin, and elastin. Biopolymers. 1975;14:379–391. doi: 10.1002/bip.1975.360140211. [DOI] [PubMed] [Google Scholar]
- 26.Pudlas M., Koch S., Bolwien C., Thude S., Jenne N., Hirth T., Walles H., Schenke-Layland K. Raman spectroscopy: a noninvasive analysis tool for the discrimination of human skin cells. Tissue Eng. Part C Methods. 2011;17:1027–1040. doi: 10.1089/ten.tec.2011.0082. [DOI] [PubMed] [Google Scholar]
- 27.Qian X., Peng X.H., Ansari D.O., Yin-Goen Q., Chen G.Z., Shin D.M., Yang L., Young A.N., Wang M.D., Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 28.Brauchle E., Bauer H., Fernes P., Zuk A., Schenke-Layland K., Sengle G. Raman microspectroscopy as a diagnostic tool for the non-invasive analysis of fibrillin-1 deficiency in the skin and in the in vitro skin models. Acta Biomater. 2017;52:41–48. doi: 10.1016/j.actbio.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z., McWilliams A., Lui H., McLean D.I., Lam S., Zeng H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer. 2003;107:1047–1052. doi: 10.1002/ijc.11500. [DOI] [PubMed] [Google Scholar]
- 30.Spiers R.M., Marzi J., Brauchle E.M., Cross S.E., Vaughan R.H., Bateman P.A., Hughes S.J., Schenke-Layland K., Johnson P.R.V. Donor age significantly influences the Raman spectroscopic biomolecular fingerprint of human pancreatic extracellular matrix proteins following collagenase-based digestion. Acta Biomater. 2019;99:269–283. doi: 10.1016/j.actbio.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Chaichi A., Prasad A., Gartia M.R. Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs. Biosensors (Basel) 2018;8:E107. doi: 10.3390/bios8040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You A.Y.F., Bergholt M.S., St-Pierre J.P., Kit-Anan W., Pence I.J., Chester A.H., Yacoub M.H., Bertazzo S., Stevens M.M. Raman spectroscopy imaging reveals interplay between atherosclerosis and medial calcification in the human aorta. Sci. Adv. 2017;3:e1701156. doi: 10.1126/sciadv.1701156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzi J., Brauchle E.M., Schenke-Layland K., Rolle M.W. Non-invasive functional molecular phenotyping of human smooth muscle cells utilized in cardiovascular tissue engineering. Acta Biomater. 2019;89:193–205. doi: 10.1016/j.actbio.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Movasaghi Z., Rehman S., Rehman I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007;42:493–541. [Google Scholar]
- 35.Frank C.J., McCreery R.L., Redd D.C.B. Raman spectroscopy of normal and diseased human breast tissues. Anal. Chem. 1995;67:777–783. doi: 10.1021/ac00101a001. [DOI] [PubMed] [Google Scholar]
- 36.Cheng W.T., Liu M.T., Liu H.N., Lin S.Y. Micro-Raman spectroscopy used to identify and grade human skin pilomatrixoma. Microsc. Res. Tech. 2005;68:75–79. doi: 10.1002/jemt.20229. [DOI] [PubMed] [Google Scholar]
- 37.Ellis R., Green E., Winlove C.P. Structural analysis of glycosaminoglycans and proteoglycans by means of Raman microspectrometry. Connect. Tissue Res. 2009;50:29–36. doi: 10.1080/03008200802398422. [DOI] [PubMed] [Google Scholar]
- 38.Notingher I., Green C., Dyer C., Perkins E., Hopkins N., Lindsay C., Hench L.L. Discrimination between ricin and sulphur mustard toxicity in vitro using Raman spectroscopy. J. R. Soc. Interface. 2004;1:79–90. doi: 10.1098/rsif.2004.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigurdsson S., Philipsen P.A., Hansen L.K., Larsen J., Gniadecka M., Wulf H.C. Detection of skin cancer by classification of Raman spectra. IEEE Trans. Biomed. Eng. 2004;51:1784–1793. doi: 10.1109/TBME.2004.831538. [DOI] [PubMed] [Google Scholar]
- 40.Shetty G., Kendall C., Shepherd N., Stone N., Barr H. Raman spectroscopy: elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer. 2006;94:1460–1464. doi: 10.1038/sj.bjc.6603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sajan D., Binoy J., Pradeep B., Venkata Krishna K., Kartha V.B., Hubert Joe I., Jayakumar V.S. NIR-FT Raman and infrared spectra and ab initio computations of glycinium oxalate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004;60:173–180. doi: 10.1016/s1386-1425(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 42.Stone N., Kendall C., Shepherd N., Crow P., Barr H. Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J. Raman Spectrosc. 2002;33:564–573. [Google Scholar]
- 43.Stone N., Kendall C., Smith J., Crow P., Barr H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004;126:141–157. doi: 10.1039/b304992b. discussion 169–183. [DOI] [PubMed] [Google Scholar]
- 44.Hanlon E.B., Manoharan R., Koo T.W., Shafer K.E., Motz J.T., Fitzmaurice M., Kramer J.R., Itzkan I., Dasari R.R., Feld M.S. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000;45:R1–R59. doi: 10.1088/0031-9155/45/2/201. [DOI] [PubMed] [Google Scholar]
- 45.Li D.Y., Brooke B., Davis E.C., Mecham R.P., Sorensen L.K., Boak B.B., Eichwald E., Keating M.T. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 46.Tuma R. Raman spectroscopy of proteins: from peptides to large assemblies. J. Raman Spectrosc. 2005;36:307–319. [Google Scholar]
- 47.Black K.M., Masuzawa A., Hagberg R.C., Khabbaz K.R., Trovato M.E., Rettagliati V.M., Bhasin M.K., Dillon S.T., Libermann T.A., Toumpoulis I.K. Preliminary biomarkers for identification of human ascending thoracic aortic aneurysm. J. Am. Heart Assoc. 2013;2:e000138. doi: 10.1161/JAHA.113.000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q., Ding Y., Song P., Zhu H., Okon I., Ding Y.N., Chen H.Z., Liu D.P., Zou M.H. Tryptophan-Derived 3-Hydroxyanthranilic Acid Contributes to Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice In Vivo. Circulation. 2017;136:2271–2283. doi: 10.1161/CIRCULATIONAHA.117.030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergholt M.S., St-Pierre J.P., Offeddu G.S., Parmar P.A., Albro M.B., Puetzer J.L., Oyen M.L., Stevens M.M. Raman Spectroscopy Reveals New Insights into the Zonal Organization of Native and Tissue-Engineered Articular Cartilage. ACS Cent. Sci. 2016;2:885–895. doi: 10.1021/acscentsci.6b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mithieux S.M., Rasko J.E., Weiss A.S. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials. 2004;25:4921–4927. doi: 10.1016/j.biomaterials.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 51.Debelle L., Alix A.J., Wei S.M., Jacob M.P., Huvenne J.P., Berjot M., Legrand P. The secondary structure and architecture of human elastin. Eur. J. Biochem. 1998;258:533–539. doi: 10.1046/j.1432-1327.1998.2580533.x. [DOI] [PubMed] [Google Scholar]
- 52.Azeloglu E.U., Albro M.B., Thimmappa V.A., Ateshian G.A., Costa K.D. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1197–H1205. doi: 10.1152/ajpheart.01027.2007. [DOI] [PubMed] [Google Scholar]
- 53.Suna G., Wojakowski W., Lynch M., Barallobre-Barreiro J., Yin X., Mayr U., Baig F., Lu R., Fava M., Hayward R. Extracellular Matrix Proteomics Reveals Interplay of Aggrecan and Aggrecanases in Vascular Remodeling of Stented Coronary Arteries. Circulation. 2018;137:166–183. doi: 10.1161/CIRCULATIONAHA.116.023381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roccabianca S., Bellini C., Humphrey J.D. Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J. R. Soc. Interface. 2014;11:20140397. doi: 10.1098/rsif.2014.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daugherty A., Cassis L.A. Mouse models of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 56.Bellini C., Bersi M.R., Caulk A.W., Ferruzzi J., Milewicz D.M., Ramirez F., Rifkin D.B., Tellides G., Yanagisawa H., Humphrey J.D. Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. J. R. Soc. Interface. 2017;14:20161036. doi: 10.1098/rsif.2016.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei L., Chen Z., Shi L., Long R., Anzalone A.V., Zhang L., Hu F., Yuste R., Cornish V.W., Min W. Super-multiplex vibrational imaging. Nature. 2017;544:465–470. doi: 10.1038/nature22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogt N. Super-resolution Raman imaging. Nat. Methods. 2019;16:1202. doi: 10.1038/s41592-019-0677-3. [DOI] [PubMed] [Google Scholar]
- 59.Stevens O., Iping Petterson I.E., Day J.C.C., Stone N. Developing fibre optic Raman probes for applications in clinical spectroscopy. Chem. Soc. Rev. 2016;45:1919–1934. doi: 10.1039/c5cs00850f. [DOI] [PubMed] [Google Scholar]
- 60.Jermyn M., Mok K., Mercier J., Desroches J., Pichette J., Saint-Arnaud K., Bernstein L., Guiot M.C., Petrecca K., Leblond F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015;7:274ra19. doi: 10.1126/scitranslmed.aaa2384. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho L.F.C.S., Nogueira M.S., Bhattacharjee T., Neto L.P.M., Daun L., Mendes T.O., Rajasekaran R., Chagas M., Martin A.A., Soares L.E.S. In vivo Raman spectroscopic characteristics of different sites of the oral mucosa in healthy volunteers. Clin. Oral Investig. 2019;23:3021–3031. doi: 10.1007/s00784-018-2714-5. [DOI] [PubMed] [Google Scholar]
- 62.Orringer D.A., Pandian B., Niknafs Y.S., Hollon T.C., Boyle J., Lewis S., Garrard M., Hervey-Jumper S.L., Garton H.J.L., Maher C.O. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 2017;1:0027. doi: 10.1038/s41551-016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollon T.C., Lewis S., Pandian B., Niknafs Y.S., Garrard M.R., Garton H., Maher C.O., McFadden K., Snuderl M., Lieberman A.P. Rapid Intraoperative Diagnosis of Pediatric Brain Tumors Using Stimulated Raman Histology. Cancer Res. 2018;78:278–289. doi: 10.1158/0008-5472.CAN-17-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Livermore L.J., Isabelle M., Bell I.M., Scott C., Walsby-Tickle J., Gannon J., Plaha P., Vallance C., Ansorge O. Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neurooncol. Adv. 2019;1:vdz008. doi: 10.1093/noajnl/vdz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J., Bergholt M.S., Zheng W., Huang Z. Development of a beveled fiber-optic confocal Raman probe for enhancing in vivo epithelial tissue Raman measurements at endoscopy. Opt. Lett. 2013;38:2321–2323. doi: 10.1364/OL.38.002321. [DOI] [PubMed] [Google Scholar]
- 66.Shu C., Zheng W., Lin K., Lim C., Huang Z. Label-Free Follow-Up Surveying of Post-Treatment Efficacy and Recurrence in Nasopharyngeal Carcinoma Patients with Fiberoptic Raman Endoscopy. Anal. Chem. 2021;93:2053–2061. doi: 10.1021/acs.analchem.0c03778. [DOI] [PubMed] [Google Scholar]
- 67.Martin J.W., Scaglioni B., Norton J.C., Subramanian V., Arezzo A., Obstein K.L., Valdastri P. Enabling the future of colonoscopy with intelligent and autonomous magnetic manipulation. Nat. Mach. Intell. 2020;2:595–606. doi: 10.1038/s42256-020-00231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ando M., Hamaguchi H.O. Molecular component distribution imaging of living cells by multivariate curve resolution analysis of space-resolved Raman spectra. J. Biomed. Opt. 2014;19:0110161. doi: 10.1117/1.JBO.19.1.011016. [DOI] [PubMed] [Google Scholar]
- 69.Arai S., Shelton J.M., Chen M., Bradley M.N., Castrillo A., Bookout A.L., Mak P.A., Edwards P.A., Mangelsdorf D.J., Tontonoz P., Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Marzi J., Biermann A.C., Brauchle E.M., Brockbank K.G.M., Stock U.A., Schenke-Layland K. Marker-Independent In Situ Quantitative Assessment of Residual Cryoprotectants in Cardiac Tissues. Anal. Chem. 2019;91:2266–2272. doi: 10.1021/acs.analchem.8b04861. [DOI] [PubMed] [Google Scholar]
- 71.Zbinden A., Marzi J., Schlünder K., Probst C., Urbanczyk M., Black S., Brauchle E.M., Layland S.L., Kraushaar U., Duffy G. Non-invasive marker-independent high content analysis of a microphysiological human pancreas-on-a-chip model. Matrix Biol. 2020;85-86:205–220. doi: 10.1016/j.matbio.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Hoyer P.O. Non-negative matrix factorization with sparseness constraints. J. Mach. Learn. Res. 2004;5:1457–1469. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Data Availability Statement
The data of this study are available without restriction. MCR bioinformatics code can be found online (https://github.com/mshrAndo/PyMCR).