Abstract
Severe acute pancreatitis (SAP) is a severe acute abdominal disease. Recent evidence shows that intestinal homeostasis is essential for the management of acute pancreatitis. Chitosan oligosaccharides (COS) possess antioxidant activity that are effective in treating various inflammatory diseases. In this study we explored the potential therapeutic effects of COS on SAP and underlying mechanisms. Mice were treated with COS (200 mg·kg−1·d−1, po) for 4 weeks, then SAP was induced in the mice by intraperitoneal injection of caerulein. We found that COS administration significantly alleviated the severity of SAP: the serum amylase and lipase levels as well as pancreatic myeloperoxidase activity were significantly reduced. COS administration suppressed the production of proinflammatory cytokines (TNF-α, IL-1β, CXCL2 and MCP1) in the pancreas and ileums. Moreover, COS administration decreased pancreatic inflammatory infiltration and oxidative stress in SAP mice, accompanied by activated Nrf2/HO-1 and inhibited TLR4/NF-κB and MAPK pathways. We further demonstrated that COS administration restored SAP-associated ileal damage and barrier dysfunction. In addition, gut microbiome analyses revealed that the beneficial effect of COS administration was associated with its ability to improve the pancreatitis-associated gut microbiota dysbiosis; in particular, probiotics Akkermansia were markedly increased, while pathogenic bacteria Escherichia–Shigella and Enterococcus were almost eliminated. The study demonstrates that COS administration remarkably attenuates SAP by reducing oxidative stress and restoring intestinal homeostasis, suggesting that COS might be a promising prebiotic agent for the treatment of SAP.
Keywords: pancreatitis, chitosan oligosaccharides, inflammation, gastrointestinal microbiome, oxidative stress, intestine
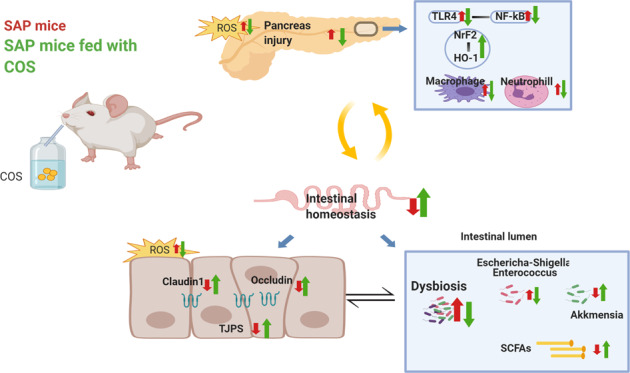
Supplementation of chitosan oligosaccharides(COS) could ameliorate severity of pancreatic injury, prevent intestinal barrier disruption and reduce inflammatory and oxidative injury in severe acute pancreatitis (SAP). The protective mechanism partly attributed to the reshaping of gut microbiota and TLR4 and Nrf2/HO-1 pathway.
Introduction
Severe acute pancreatitis (SAP) is a severe life-threatening disease that is accompanied by a systemic inflammatory response and can damage the function of other organs or systems [1]. Accumulating studies have documented that intestinal homeostasis is essential for the management of acute pancreatitis (AP) [2]. Gut dysbiosis is associated with intestinal barrier dysfunction manifested by increased gut permeability and bacterial translocation, which play a vital role in systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) [3].
Considering the critical role of intestinal barrier dysfunction in the development of pancreatitis, protecting the intestinal barrier has become a key aim in the treatment of SAP [2]. Prebiotics have been demonstrated to have protective effects on the intestinal barrier by reducing intestinal epithelial apoptosis, upregulating tight junction protein expression or promoting probiotic growth [4]. Furthermore, excessive production of inflammatory cytokines and reactive oxygen species (ROS) can cause direct damage to pancreatic cells, and this effect can be reversed by several prebiotics through the regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways [5, 6]. Conclusively, prebiotics show great potential for the treatment of SAP, and it is critical to choose the appropriate prebiotics.
As a degradation product of chitosan, chitosan oligosaccharide (COS) is a β-(1–4)-linked D-glucosamine polymer that originates from the deacetylation of chitin in the exoskeletons of shrimp, crabs and insects [7]. Because of its characteristics, such as water solubility, biocompatibility, intestinal absorption and biological activity, COS has been widely studied as a dietary supplement or health product [8]. To date, COS has been shown to possess a variety of biological activities, including regulation of metabolism, regulation of intestinal flora, and modulation of immunological and antioxidative responses. Evidence has shown that COS can modulate the gut microbiota to protect the host against diabetes, obesity, coronary heart disease (CHD), microbial infection and inflammation [9, 10]. According to these beneficial effects, we hypothesize that COS may have great potential in the treatment of SAP.
Here, we aim to explore whether COS could attenuate SAP and to elucidate its underlying mechanism.
Materials and methods
Experimental design
Male C57BL/6 mice (6–8 weeks, 20–22 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (China) and housed under specific pathogen-free (SPF) conditions with a room temperature of 23 ± 2 °C and a 12-h light/dark cycle. All the animal experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Shanghai Jiao Tong University (SYXK 2013–0050, Shanghai, China).
The SAP model was established according to a previous study [11]. The mice were injected intraperitoneally with caerulein (100 μg/kg) ten times with a 1-h interval between injections. After the final injection of caerulein, LPS (5 mg/kg) was immediately administered by intraperitoneal injection. The mice were randomly divided into four groups (n = 7): the control (CON) group, COS group, SAP group and COS-supplemented SAP (COS/SAP) group. The mice in the CON group and SAP group were orally administered double-distilled water for 4 weeks. Then, the mice in the CON group were intraperitoneally injected with normal saline (NS), and the mice in the SAP group were intraperitoneally injected with caerulein. The mice in the COS and COS/SAP groups were orally supplemented with COS for 4 weeks, and then, the mice in the COS/SAP group were intraperitoneally injected with caerulein. The mice were anesthetized with chloral hydrate and then sacrificed at 12 h after the first injection of caerulein. The distal ileum, pancreas and luminal content of cecum samples were collected.
Dosage information
Chitosan oligosaccharide (<1 kDa, purity of 91.0% as determined by HPLC) was purchased from MedChem Express (Shanghai, China). According to the dosage suggested in previous studies [12, 13], the effective dosage of COS ranges from 10 mg·kg−1·d−1 to 200 mg·kg−1·d−1. Our preliminary experiment showed that 200 mg·kg−1·d−1 has the greatest effect in inhibiting pancreatitis (Fig. S1a, b). Therefore, the mice in the COS and COS/SAP group were orally supplemented with a dosage of 200 mg·kg−1·d−1 (1.0 mg/mL, dissolved in double-distilled water) for 4 weeks. The mice in all the groups were fed standard sterile mouse chow.
Histopathology and immunofluorescence
After the mice were sacrificed, fresh pancreatic, lung, ileum and colon tissues were fixed in 4% paraformaldehyde at 4 °C overnight, embedded in paraffin blocks, cut into 4-μm sections, and stained with hematoxylin and eosin (H&E). Morphological changes were examined by light microscopy (Leica, Germany) at a magnification of ×100 or 200. The histopathological changes to the pancreas were evaluated according to the scoring scale reported by the Schmidt criteria [14], while the pathological damage to the ileum was graded according to Chiu’s standard [15].
For immunofluorescence staining, the antigens in the paraffin-embedded pancreatic sections were recovered with citric acid buffer. The endogenous peroxidase activity was blocked by 3% hydrogen peroxide in methanol for 15 min, and then, the slides were incubated with the primary antibodies at 4 °C overnight. The following antibodies were used for single staining: ZO-1(1;100, ab221547, Abcam, USA), Occludin (1;100, ab216327, Abcam, USA) and Claudin1 (1;100, ab125028, Abcam, USA). After washing, the slides were incubated with fluorescein-labeled secondary antibodies for 30 min and then stained with dihydrochloride (DAPI) for 5 min to visualize the nuclei. A minimum of 5 images/animal were quantified.
Pancreas wet weight to dry weight (W/D) ratio
The pancreatic tissue was dried by vacuum and weighed. The tissue was then incubated in an oven at 80 °C for 48 h to obtain a constant weight as the dry weight. The ratio of the wet pancreas weight to the dry pancreas weight (W/D) was calculated to evaluate the degree of tissue edema.
Serum amylase and lipase assays
The serum amylase and lipase activities were determined by enzymatic kinetic chemistry using commercial kits in the Roche/Hitachi modular analysis system according to the manufacturer’s protocol (Roche, Berlin, Germany).
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-1, TNFα, MCP-1, and CXCL2 in the serum, pancreas and ileum were measured by the Luminex Screening Human Magnetic Assay (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturers’ instructions. The oxidative stress-related indexes (malondialdehyde (MDA), superoxide dismutase (SOD) and myeloperoxidase (MPO)) were detected with the appropriate kits (Multisciences (Lianke) Biotech, Co., Ltd., China) according to the manufacturers’ instructions.
Flow cytometry
Flow cytometry was performed based on an established and previously described protocol [16]. Briefly, freshly harvested pancreatic tissues were digested in 0.75 mg/mL collagenase-P solution (Roche Basel, Switzerland) at 37 °C for 15 min; subsequently, the digested pancreatic pieces were dissociated with a gentle MACS Dissociator (Miltenyi Biotechnology, Bergisch Gladbach, NRW, Germany) and immediately filtered through a 75-μm filter screen with phosphate-buffered saline (PBS). Single-cell suspensions were stained with several monoclonal antibodies for 15 min at room temperature. For neutrophil identification, the cells were surface-stained with PE/Cy7 anti-mouse CD45, Alexa Fluor 488 anti-mouse F4/80, Brilliant Violet 421 anti-mouse/human CD11b, and Alexa Fluor 647 anti-mouse Ly6G from Biolegend (CA, USA).
For macrophage identification, the cells were fixed and permeabilized with a Cell Fixation & Permeabilization Kit after being surface-stained with anti-CD45, anti-CD11b, and anti-F4/80 antibodies.
The cells were analyzed on an Attune NxT (Thermo Fisher Scientific, Waltham, MA, USA).
TUNEL staining
Apoptotic cell death in the small intestines was evaluated by TUNEL assay using a kit (Roche,11684817910) according to the manufacturer’s instructions. All the cell counts were performed at ×200 magnification. The apoptotic rate was calculated as the average percentage of positive cells in ten randomly selected areas.
FISH
Bacterial translocation was determined by fluorescence in situ hybridization (FISH) as previously described [17]. Briefly, sections of the distal ileum and pancreas were dewaxed (60 min at 60 °C, 2 × 10 min with 100% xylene, 5 min with 100% ethanol), air dried, and then incubated with specific probes in a humid space at 52 °C for 18 h. We used the following probe: a universal bacterial probe specific for the 16S rRNA gene (EUB338: 5′-Cy3-GCTGCCTCCCGTAGGAGT-3′)16. The sections were washed and counterstained with DAPI. Images were acquired using a confocal microscope (Olympus, Japan).
Intestinal permeability
Intestinal permeability was measured by quantifying the absorption of FITC-dextran (FD4000; Sigma-Aldrich, MO, USA) after orogastric gavage (0.5 g/kg). The mice were sacrificed 4 h after gavage, and the blood levels of FITC-dextran were determined as previously described [18].
Western blot
Pancreatic and ileal tissues were lysed with RIPA buffer supplemented with a complete protease inhibitor cocktail (Yeasen, China). The samples were centrifuged at 4 °C and 10,000 × g for 10 min, and the volumes that contained the same amount of protein (30 μg) were determined by a BCA protein assay kit (Beyotime Biotechnology, China). After heating at 100 °C for 10 min, the protein extracts were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% fat-free milk for 2 h at room temperature, incubated overnight with primary antibodies at 4 °C, and incubated with secondary antibodies conjugated to HRP. The primary antibodies were as follows: Nrf2 (1:1000), HO-1 (1:1000), p-NF-κB (1:500), β-tubulin (1:2000), TLR4 (1:1000), MyD88 (1:500), p-ERK (1:1000), ERK (1:1000), p38 (1:1000), p-p38 (1:1000), p-JNK (1:1000) and JNK (1:1000). The reagents used are listed in the Supplementary Materials and Methods. The protein bands were observed using an enhanced chemiluminescence kit (Pierce, USA). The density of each band was measured by ImageJ software and standardized to the density of the tubulin band.
Fecal microbiota transplantation
Fecal transplantation was performed as previously described [19]. The strategy is shown in Fig. S5a. First, transplant material was collected from donor mice that were fed with or without COS-containing water for 4 weeks. After 4 weeks of feeding, stools from the donor mice were collected and processed. One hundred milligrams of stool was resuspended in 1 mL saline and then centrifuged at 800 × g for 3 min. The supernatant was collected and used as transplant material. The recipient mice were treated for 4 weeks with an antibiotic cocktail [vancomycin (0.5 mg/mL), neomycin (1 mg/mL), ampicillin (1 mg/mL), and metronidazole (1 mg/mL)] in their drinking water. The mice were divided into three groups: the control group, FMT1 group and FMT2 group. The control group received no treatment. The FMT1 group was fed normal mouse stool by oral gavage for 2 weeks, and the FMT2 group was fed fresh transplant material from COS-fed mice (200 μL for each mouse daily) by oral gavage for 2 weeks. Finally, experimental acute pancreatitis was induced in the recipient mice in the FMT1 and FMT2 groups.
Gut microbiota analysis
Genomic DNA was extracted from the microbial community of the luminal contents of the cecum and from the feces samples using the Magnetic Soil And Stool DNA Kit according to the manufacturer’s instructions. Then, high-throughput sequencing was performed by the Major bio cloud. (Shanghai, China). The detailed method is described in the Supplementary Materials and Methods. R language was used to perform alpha diversity, beta diversity, LEfSe, and RDA analyses and plotting.
Short-chain fatty acid (SCFA) analysis
The fecal samples were collected after the induction of AP and then stored at −80 °C until further analysis. The supernatant was harvested from fecal samples that were mixed with 10 μL of internal standards (0.0125 μL/μL 2-ethylbutyric acid, Sigma-Aldrich) and 500 μL of methanol. The concentrations of acetate, propionate and butyrate were detected by a 6890A-5973C GC–MS system (Agilent Technologies, Santa Clara, CA). All the SCFA standards were purchased from Merck (Darmstadt, Germany).
Statistics
All the measured data are expressed as the mean ± standard deviation and were statistically analyzed with GraphPad Prism 7.0 software. A t-test was used for data with a normal distribution and comparisons between two groups. Single-factor analysis of variance (ANOVA) was used for comparison among the three groups, and the Kruskal–Wallis test was used for data without a normal distribution. P < 0.05 indicates that the difference was statistically significant.
Results
COS supplementation reduced the severity of SAP
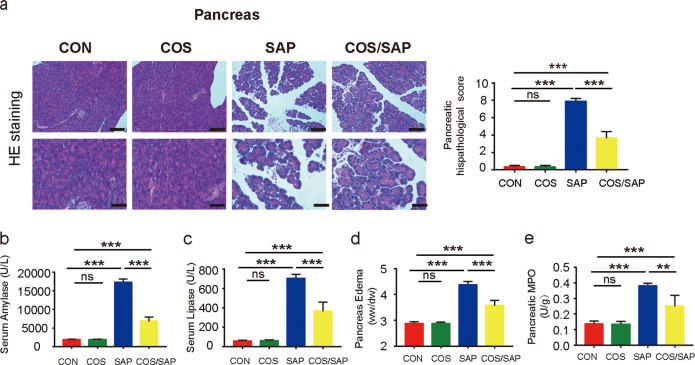
In the COS group (supplementation with COS alone without AP induction), the pancreatic markers were not affected. However, histological injury in the pancreas of the COS/SAP group (4 weeks of COS supplementation) was significantly attenuated compared with that of the SAP group, as evidenced by the reduced levels of inflammatory cell infiltration and acinar necrosis (Fig. 1a). The amylase and lipase levels, pancreatic wet to dry (W/D) weight ratio, MPO level (Fig. 1b–e), and AP-associated lung injury (Fig. S2a, b) were decreased in the COS/SAP group, which further confirmed the protective effects of COS supplementation in SAP. In summary, COS supplementation markedly alleviated the severity of SAP.
Fig. 1. COS supplementation alleviated SAP.
a Histopathological changes of pancreatic samples observed by HE staining. Original magnification, 100× (the upper figures) or 200× (the lower figures). Pancreatic histopathological scores were evaluated by Schmidt criteria. b, c Serum levels of amylase and lipase. d Pancreas edema wet-dry (W/D) ratio. e Pancreatic level of MPO. The data are provided as the mean ± standard error of the mean (SEM) (n = 6 per group). Symbol (*) means P < 0.05, **means P < 0.01, ***means P < 0.001, ns means P > 0.05.
COS supplementation ameliorated inflammatory infiltration and oxidative stress in SAP
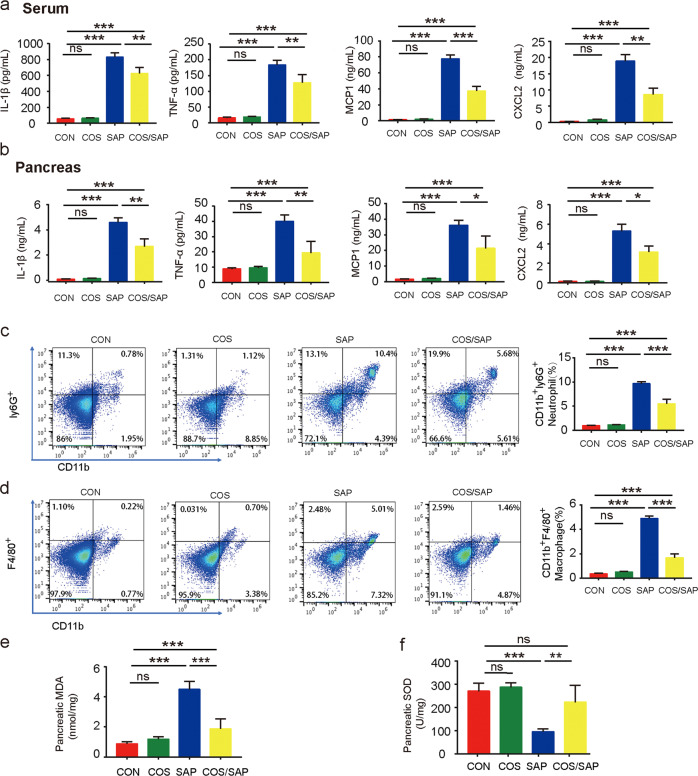
To assess the degree of inflammation in SAP, proinflammatory cytokines (IL-1β, TNF-α, CXCL2 and MCP-1) in the serum and pancreatic tissues were detected by ELISA. MCP1 has been proven to recruit macrophages under inflammatory conditions. CXCL2, which is secreted by macrophages, is a chemo attractant for polymorphonuclear leukocytes and hematopoietic stem cells. These two biomarkers were used to represent the level of macrophage infiltration at the molecular level. We found robust increases in inflammatory cytokines (IL-1β, TNF-α, CXCL2 and MCP-1) in the SAP group. However, the systemic inflammation was attenuated in the COS/SAP group, as evidenced by the decline in serum inflammatory cytokines (Fig. 2a). Consistent with these findings, the inflammatory cytokines in the pancreatic tissues of the COS/SAP group were also decreased (Fig. 2b).
Fig. 2. COS supplementation ameliorated inflammatory cell infiltration and oxidative stress in the pancreas.
a Serum levels of IL-1β, TNF-α, MCP-1 and CXCL2. b Pancreatic levels of IL-1β, TNF-α, MCP-1 and CXCL2. c, d The frequencies of neutrophils (CD45+ CD11b+ Ly6G+) and total macrophages (CD45+ CD11b+ F4/80+) in the pancreas were detected by flow cytometry. e Pancreatic level of MDA. f Pancreatic level of SOD. The results are represented as the mean ± SEM, n = 6 per group, *means P < 0.05 **means P < 0.01 ***means P < 0.001 ns means P > 0.05.
Our study further examined the protective effects of COS in reducing inflammatory cell infiltration levels in the pancreas. We subsequently examined neutrophil infiltration and macrophage polarization by immunostaining. COS supplementation significantly reduced the infiltration of neutrophils (Ly6G) in the pancreas of the SAP group (Fig. 2c). In addition, COS supplementation in SAP mice significantly reduced the number of macrophages in the pancreas (Fig. 2d). These phenomena suggested that COS exerted immunomodulatory and anti-inflammatory effects in the pancreas.
Intriguingly, we also found that COS supplementation remarkably reduced the elevated levels of oxidative stress in the SAP group, as evidenced by the reduction in the MDA levels and the increase in the SOD levels (Fig. 2d, e).
COS supplementation attenuated ileal injury in SAP
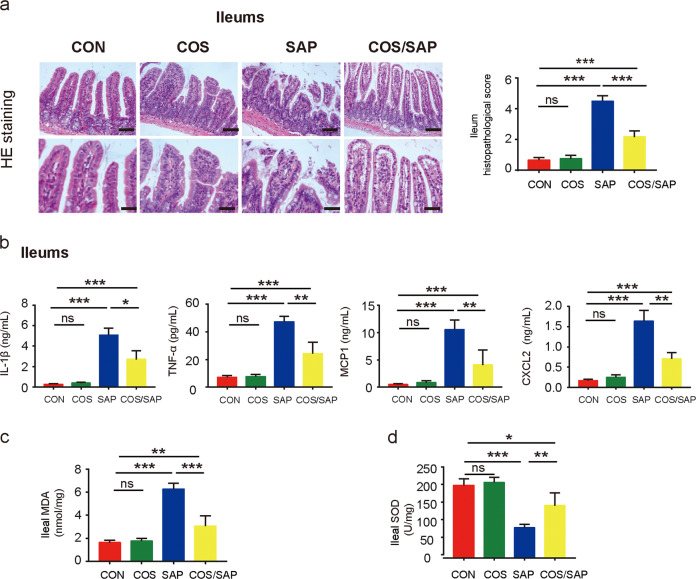
Considering the critical role of intestinal barrier dysfunction in the development of pancreatitis [2], we next investigated intestinal damage in SAP. Histopathological examination clearly showed ileal and colon damage in the SAP group (Figs. 3a and S3a). The COS/SAP group exhibited less intestinal damage and a lower histopathological score than the SAP group (Figs. 3a and S3a). However, bacterial translocation from the gut lumen has been suggested to be the main source of the bacteria that contribute to pancreatic necrosis [20]. Although the bacteria in the colon are the most abundant, recent studies have found that the small intestine, not the colon, is the main source of pancreatic pathogens in AP [21, 22]. Therefore, we chose to further examine ileal dysfunction rather than colonic dysfunction in SAP. We observed a lower level of inflammation in the ileal tissues in the COS/SAP group compared with that in the SAP group, which was reflected by the reduction in inflammatory factors such as IL-1β, TNF-α, CXCL2 and MCP-1 (Fig. 3b). We observed a tremendous decrease in the levels of oxidative stress in the ileal tissues after COS supplementation, as evidenced by the reduction in the MDA levels and the increase in the SOD levels, which was consistent with the reduced level of oxidative stress in the pancreas (Fig. 3c, d). The results demonstrated that COS exerted a protective effect against inflammation and oxidants not only in the pancreas but also in SAP-related intestinal injury.
Fig. 3. COS supplementation attenuated SAP-associated ileal injury.
a Histopathological changes of ileal samples observed by HE staining. Original magnification, 100× (the upper figures) or 200× (the lower figures). COS supplementation reduced (b) the ileal levels of IL-1β, TNF-α, MCP-1 and CXCL2 and changed the ileal levels of (c) MDA and (d) SOD. The data are provided as the mean ± SEM (n = 6 per group). *means P < 0.05 **means P < 0.01 ***means P < 0.001 ns means P > 0.05.
COS protected the integrity of the intestinal barrier and reduced bacterial translocation in SAP
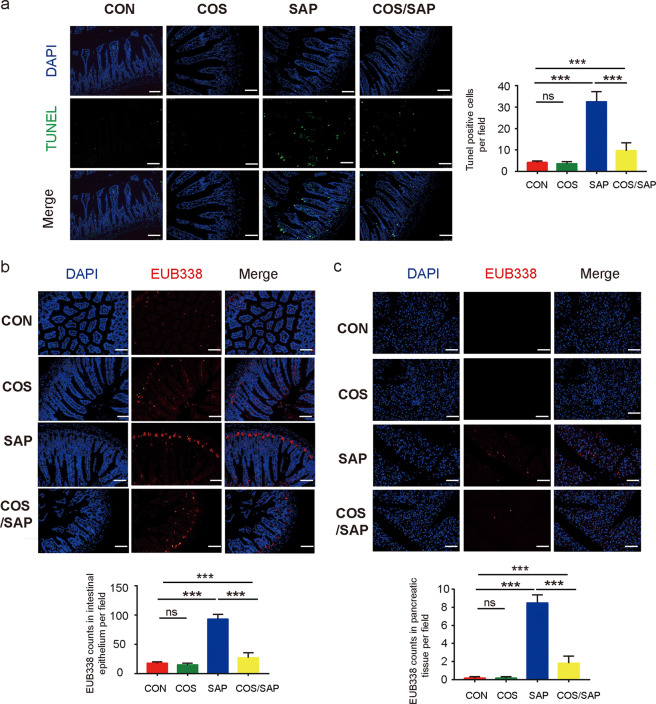
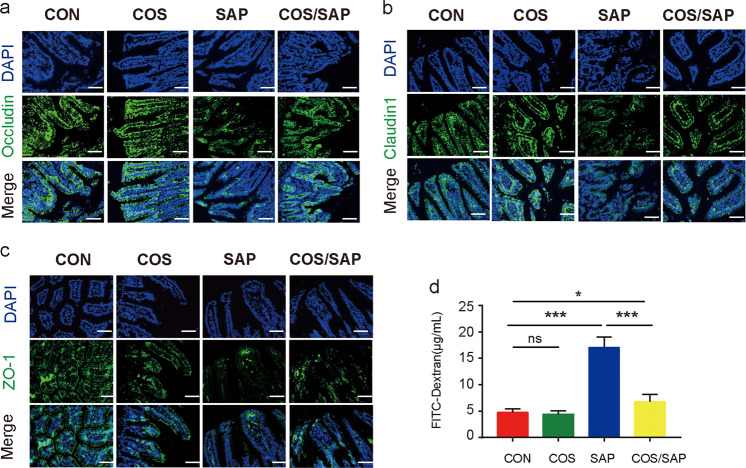
We next investigated intestinal barrier function in SAP. TUNEL staining showed that induction of SAP caused severe apoptosis of the intestinal epithelium, which was significantly reduced by supplementation with COS (Fig. 4a). Bacterial translocation in the intestinal epithelium and pancreas was detected by FISH assay using the EUB338 probe. We found that the SAP group exhibited higher levels of bacterial translocation in the ileum and pancreas than the CON group, while in the COS/SAP group, the level of bacterial translocation was decreased (Fig. 4b); these results suggest that COS unequivocally inhibits bacterial translocation from the ileum to the pancreas. Moreover, the tight junctions of IECs were assessed by immunofluorescence. Although the expression level of ZO-1 did not change significantly, the levels of Occludin and Claudin1 in the COS/SAP group were increased compared with those in the SAP group (Fig. 5a–c). The intestinal permeability was detected by FITC. The intestinal permeability was also increased in the SAP group, and this effect could be reversed by COS supplementation (Fig. 5d). To summarize, our results suggested that COS could protect the intestinal barrier and prevent the translocation of gut bacteria during SAP.
Fig. 4. COS reduced intestinal apoptosis and decreased bacterial translocation in SAP.
a Apoptosis in the small intestines was assayed by TUNEL. The number of TUNEL-positive cells (green) per field of view was quantified. (100× magnification). (b, c) Positive hybridizing signal of total bacteria detected by the EUB338 probe. The EUB338 counts in the intestinal epithelium (100× magnification) or pancreas (100× magnification) per field were quantified. *means P < 0.05 **means P < 0.01 ***means P < 0.001 ns means P > 0.05.
Fig. 5. COS protected the integrity of the intestinal barrier and reduced intestinal permeability in SAP.
Photomicrographs of (a) Occludin, (b) Claudin1 and (c) ZO-1 immunofluorescence in the ileum (200× magnification). d The level of FITC-dextran was detected. The data are provided as the mean ± SEM (n = 6 per group). *means P < 0.05, **means P < 0.01, ***means P < 0.001 ns means P > 0.05.
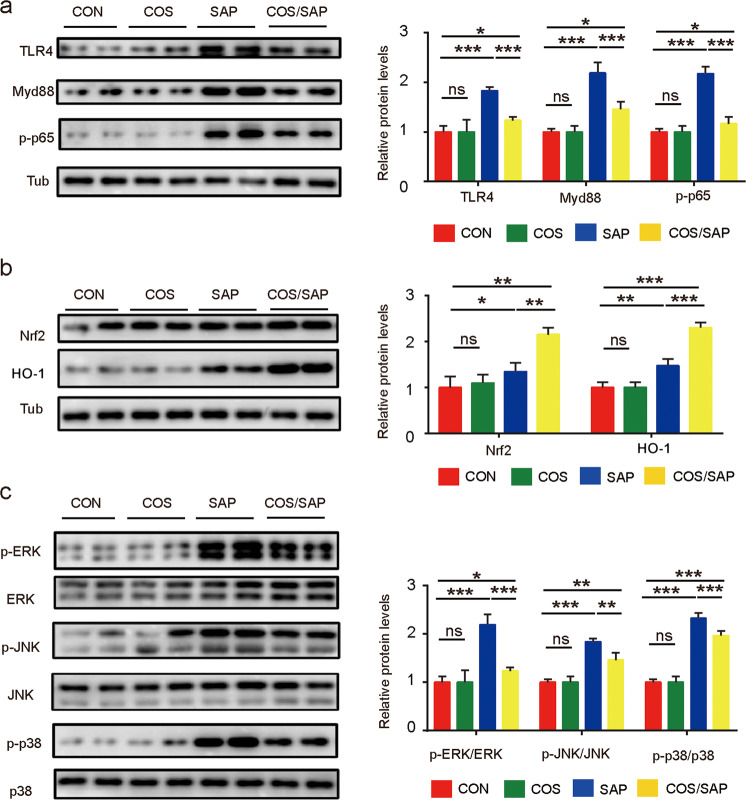
COS supplementation downregulated proteins in the TLR4/MyD88/NF-κb and MAPK pathways and upregulated proteins Nrf-2/HO-1 signaling in the pancreas
TLR4 is able to recognize LPS and has been shown to contribute to the inflammatory response. The immunoblotting results showed that the induction of SAP induced the upregulation of TLR4 expression in the pancreas. COS supplementation mitigated the upregulation of TLR4/MyD88/NF-κB in the mice with SAP (Fig. 6a), indicating that COS reduced the production of inflammatory factors by downregulating the expression of the molecules in this signaling pathway. The Nrf2/HO-1 pathway is closely associated with oxidative stress, and the upregulation of Nrf2 leads to higher levels of antioxidants. We further examined the expression levels of proteins in this pathway, and the levels were slightly increased in the SAP group. Interestingly, COS supplementation significantly increased the expression of Nrf2/HO-1 (Fig. 6b), consistent with the increased antioxidative capacity. Moreover, recent studies indicated that COS activates Nrf2 by modulating the MAPK signaling pathway [23, 24]. We further examined this pathway in the pancreas. COS supplementation significantly suppressed the phosphorylation of p38 MAPK, JNK and ERK during SAP, indicating that COS may upregulate proteins in the Nrf2/HO-1 pathway by blocking the MAPK pathway (Fig. 6c). Our results suggested that COS attenuated the inflammatory response and reduced oxidative stress levels partly by regulating the TLR4/MyD88/NF-κB, MAPK and Nrf2/HO-1 pathways.
Fig. 6. COS supplementation downregulated proteins in the TLR4/MyD88/NF-κB and MAPK pathways and upregulated proteins in the Nrf2/Ho-1 pathway in the pancreas.
The protein levels of (a) TLR4, MyD88, p-NF-κB, (b) Nrf2, HO-1 and β-tubulin, (c) p-ERK, ERK, p-JNK, JNK, p-p38, and p38 in the pancreas were analyzed by Western blotting (WB). The relative protein levels were quantified. The data are provided as the mean ± SEM (n = 6 per group). *means P < 0.05 **means P < 0.01 ***means P < 0.001 ns means P > 0.05.
Moreover, COS supplementation reduced the upregulation of proteins in the TLR4/MyD88/NF-κB pathway, but not TLR2, in the ileums and colons during SAP (Fig. S4a, b), indicating that COS attenuated the inflammatory response in ileums and colons during SAP partly by regulating the TLR4/NF-κB pathway.
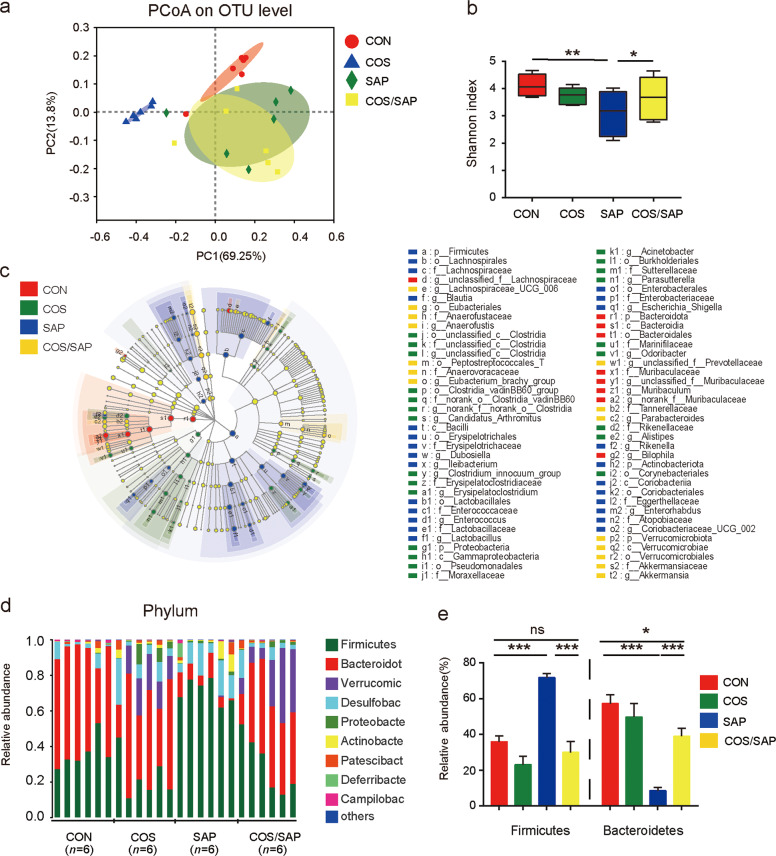
COS supplementation modulated the gut microbiota composition in SAP
Gut dysbiosis is closely associated with the progression of SAP [2]. To investigate the changes in the intestinal microbial in SAP, we performed 16S RNA sequencing of mouse fecal DNA. Principal component analysis (PCoA) revealed that the gut microbiota composition of the COS-treated group was clearly different from that of the SAP group (Fig. 7a). The macrobiotic diversity in the SAP-treated mice exhibited a profound decrease compared with that in the CON mice, which was demonstrated by the lower rarefaction data (shown by Shannon index) (Fig. 7b). However, the decreased diversity in mice with SAP was partly rescued by supplementation with COS. We further investigated the alterations in the composition of the gut microbiota at different taxonomic levels. LEfSe analysis was applied to identify the bacteria that were altered. The results showed that the composition of the gut microbiota was greatly altered from the phylum level to the genus level in the SAP and COS/SAP groups (Fig. 7c). Bacteroidetes and Firmicutes were found to be two dominant bacteria at the phylum level (Fig. 7d). After the induction of SAP, the abundance of Firmicutes was markedly increased, while the abundance of Bacteroidetes was decreased (Fig. 7e), leading to an increase in the Firmicutes/Bacteroidetes ratio that could be reversed by COS supplementation.
Fig. 7. COS supplementation modulated the gut microbiota composition in SAP.
a Principle coordination analysis (PCoA) based on OTU abundance. b α-diversity analysis between four groups using the Shannon index. c The cladograms generated by LEfSe show the differences in the taxa between the four groups (from the phylum level to the genus level). d The taxonomic composition distribution among the three groups of feces at the phylum level. e The relative abundances of Firmicutes and Bacteroidetes are shown (phylum level). The data are provided as the mean ± SEM (n = 6 per group). *P < 0.05 **P < 0.01 ***P < 0.001 ns means P > 0.05.
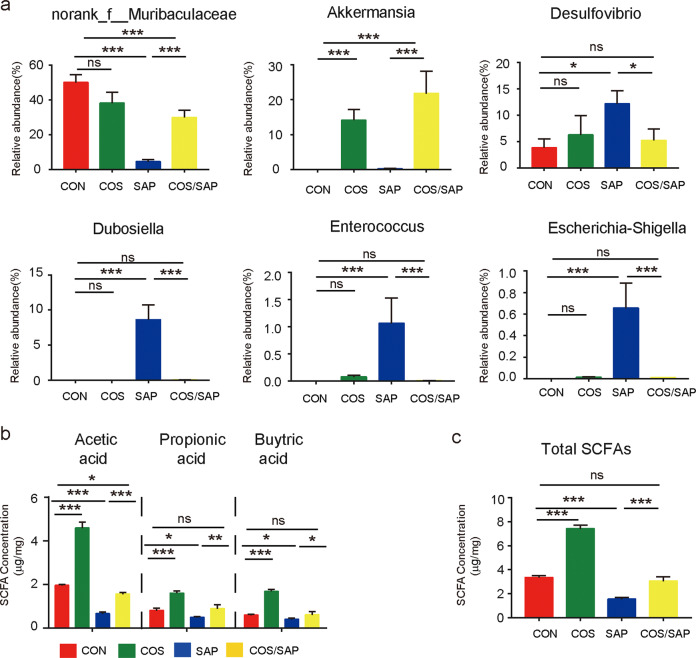
At the genus level, the results demonstrated that some probiotics, such as norank_f_Muribaculaceae and Akkermansia were increased in the mice supplemented with COS (Fig. 8a). Moreover, Desulfovibrio and Dubosiella, known proinflammatory bacteria, were increased in the SAP group (Fig. 8a), and this increase was significantly reversed by COS supplementation. In addition, Escherichia–Shigella and Enterococcus, which are closely correlated with SAP progression [3], were also increased in the SAP group. COS supplementation significantly reduced the abundance of these genera to levels similar to those in the CON group (Fig. 8a).
Fig. 8. COS treatment affected the abundance of intestinal bacterial flora and SCFAs.
a The relative abundances of norank_f_Muribaculaceae, Akkermansia, Desulfovibrio, Dubosiella, Enterococcus and Escherichia–Shigellaare shown (genus level). b, c The fecal levels of SCFAs (acetic acid, propionic acid, and butyric acid and their sum) in the four groups of mice were measured by GC-MS, and the data are provided as the mean ± SEM (n = 6 per group). *means P < 0.05 **means P < 0.01 ***means P < 0.001 ns means P > 0.05.
As microbial metabolites, we also detected the concentrations of SCFAs in the different groups (Fig. 8b, c). COS supplementation significantly elevated the levels of SCFAs, including propionic acid, acetic acid, butyric acid and their sum, in the mice with or without SAP. Collectively, our results indicated that COS supplementation could regulate the composition of the gut microbiota and enhance the production of SCFAs to protect the gut barrier.
To further confirm the importance of the gut microbiota in the therapeutic effects of COS in SAP, we transplanted the fecal microbiota of COS-fed mice to mice depleted of gut bacteria (Fig. S5a) and then induced SAP. Our results showed that pancreatic injury (assessed by histopathology and serum amylase) was markedly decreased in mice receiving feces from the COS-fed mice compared with those receiving feces from the saline-treated mice (Fig. S5b, c).
RDA, also known as redundancy analysis, is a PCA constrained by environmental factors. Samples and environmental factors can be reflected on the same two-dimensional sequence diagram, from which the relationship between sample distribution and environmental factors can be intuitively seen. RDA of the microbiota at the genus level showed that the gut microbiota in the COS/SAP group were positively related to Nrf2 expression and total SCFA concentration, while those in the SAP group were closely related to TLR4 expression (Fig. 9), indicating that COS mediates signaling pathways partly by reshaping the gut microbiota.
Fig. 9. Redundancy analysis (RDA) of the microbial community structure based on 16S rRNA gene amplicon sequencing data.
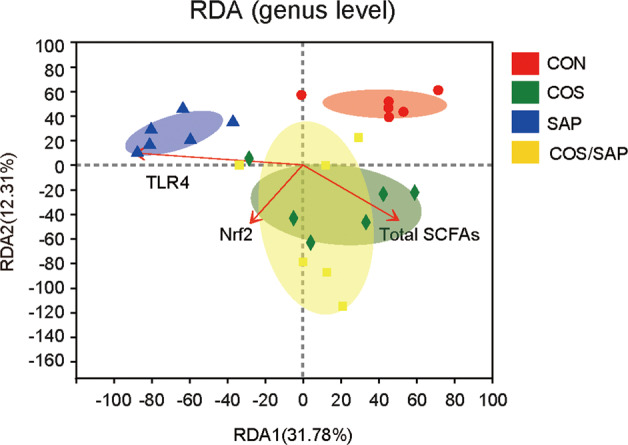
The red arrows represent quantitative environmental factors (pancreatic expression of TLR4, Nrf2 and total SCFAs), and the length of the arrows represents the degree of influence of the environmental factors on the species data. The angle between the arrows of the environmental factors represents positive and negative correlations (acute angle: positive correlation; Obtuse angle: negative correlation; Right angle: no correlation).
Discussion
In this study, we determined the protective effects of chitosan oligosaccharides (COS) against caerulein-induced SAP in mice from different aspects. We demonstrated that COS supplementation not only modulated the TLR4/NF-κB and Nrf2 signaling pathways in the pancreas but also protected the integrity of the intestinal epithelial barrier by restoring intestinal permeability, reshaping the intestinal flora and enhancing SCFA production. Moreover, intestinal injury during SAP was ameliorated by reduced bacterial translocation, thus attenuating pancreatitis. Therefore, the antioxidant capacity of COS and its ability to maintain intestinal homeostasis contribute to the alleviation of acute pancreatitis.
It has been widely verified that gut microbiota dysbiosis is critical for the development of SAP, for the stimulation of innate and adaptive immunity and for contributing to intestinal barrier dysfunction [3]. Because of their abilities to preserve intestinal barrier integrity and maintain gut microbiota homeostasis, prebiotics have been considered a prospective treatment for SAP [25]. For instance, a plethora of evidence has shown that early enteral nutrition with probiotic supplementation conclusively prevents gut dysbiosis, reduces infection rates and improves clinical outcomes [26]. As a prebiotic with full solubility, COS has been associated with various health-promoting effects, including regulating glucose and lipid metabolism, promoting calcium absorption and maintaining gut barrier integrity [27, 28]. Moreover, COS has the ability to reshape the gut microbiota and reduce intestinal damage in mice, thus improving diabetes status [12]. To date, whether COS could be a potential candidate for the treatment of SAP has not been determined. Therefore, we conducted a study to investigate the direct mechanism of COS in pancreatitis, and this was the first study of the use of COS in pancreatitis.
During SAP, the disrupted pancreatic acinar cells and activated inflammatory cells that infiltrate into the pancreas release high amounts of oxidants, such as ROS, and decrease the release of antioxidants, leading to exacerbated damage due to the original injury [29, 30]. The substantial antioxidative capacity of COS has been demonstrated in various disease models [31]. Our results also verified that COS inhibited oxidative stress in the mice with SAP, as shown by the reduced MDA levels and increased SOD levels. Previous studies demonstrated that ROS-related injury in pancreatitis could be prevented by activating the Nrf2/HO-1 signaling pathway [29, 32]. Increasing evidence indicates that the induction of Nrf2/HO-1 is closely related to many kinase signaling pathways, such as the p38 MAPK pathway [33]. Inhibiting the MAPK signaling pathway could reverse the upregulation of Nrf2/HO-1 [34]. Consistent with these studies, our results first showed that COS treatment could increase Nrf2/HO-1 partly through the MAPK pathway in a pancreatitis model.
Previous studies have shown that the intestine is the organ that is most easily affected during pancreatitis [35]. The damaged intestinal barrier provides favorable conditions for bacterial translocation, which exacerbates the original injury to the pancreas [36]. Many studies have shown that protecting the intestinal barrier is closely related to the prognosis of acute pancreatitis [37]. In the current study, the decreased number of necrotic IECs and the enhanced expression of intestinal tight junction proteins (Occludin and Claudin1) in the COS-supplemented groups suggested the beneficial effects of COS on the integrity of the physical intestinal barrier, thus attenuating intestinal permeability and preventing bacterial translocation.
The translocation of bacteria and endotoxins due to SAP-associated intestinal barrier injury is key to causing secondary damage and further leading to SIRS [22]. Consistent with previous studies [38], the TLR4/NF-κB pathway, which leads to a proinflammatory cascade in immune cells after recognizing lipopolysaccharide (LPS) from translocated bacteria, was downregulated in both the intestine and pancreas in the COS/SAP group. A similar decline in the inflammatory levels was shown by the decreased inflammatory cytokine production in the COS supplementation group, indicating that COS alleviates the LPS-induced inflammatory response partly by suppressing the TLR4/NF-κB signaling pathway.
Consistent with previous studies, we demonstrated that COS also had the ability to increase the production of SCFAs [12]. SCFAs, mainly including acetate, propionate and butyrate, are important fermentation products of the gut microbiota and serve as effectors of biological activities, such as increasing energy expenditure and metabolic parameters and regulating immune responses [39, 40]. Not restricted to the intestine, the beneficial effects of SCFAs, such as acetate and propionate, extend to peripheral regions of the body by entering into circulation. SCFAs have been shown to reduce inflammation of the pancreas [41]. In this study, the increased amount of SCFAs in the COS group suggested that the protective effect of COS was attributed to the production of short-chain fatty acids.
Moreover, redundancy analysis showed that the gut microbiota in the COS/SAP group are positively related to total SCFA concentration and Nrf2 expression, while those in the SAP group are closely related to TLR4 expression, suggesting that COS regulates TLR4 and Nrf2 partly through reshaping the gut microbiota.
Disruption of the gut microbiota has been associated with the pathogenesis of both intestinal and extraintestinal disorders, such as colitis and cardiovascular disease [42, 43]. Consistent with previous reports that a loss of microbiome diversity is frequently observed in patients with SAP, our current study showed that the abundance of the intestinal flora and the composition of the gut microbiota were significantly affected by SAP [3]. For example, at the phylum level, Firmicutes sharply increased while Bacteroidetes significantly decreased in the mice with SAP. Notably, treatment of mice with COS exerted a profound protective effect on the gut microbiota, preserving the abundance of the microbiota and SCFA-producing bacteria in the mice with SAP. Moreover, our data indicated that the induction of SAP caused a marked decrease in norank_f_Muribaculaceae and Akkermansia, which are bacteria thought to generate SCFAs. These results suggested that the impairment of SCFA-producing bacteria might constitute a primary mechanism of SAP and thus a potential therapeutic target. In support of this notion, our results showed that at the phylum level, COS supplementation significantly increased the abundance of Bacteroidetes, bacteria that have been suggested to maintain gut homeostasis and are positively correlated with the production of propionate [44]. An elevated Firmicutes/Bacteroidetes ratio has been observed in some inflammatory conditions, such as diabetes, obesity and high-fat diet treatment [12, 45, 46]. A similar trend was observed in our mice with SAP, and this trend could be markedly reversed by COS pretreatment, indicating the anti-inflammatory capacity of COS. Furthermore, at the genus level, our results showed that COS supplementation markedly increased norank_f_Muribaculaceae and Akkermansia and caused a dramatic decrease in Desulfovibrio, Dubosiella, Enterococcus and Escherichia–Shigella, indicating that COS creates an intestinal microbiological environment that is conducive to the growth of probiotics and that inhibits the growth of pathogenic bacteria.
As a recognized probiotic, Akkermansia was proven to degrade mucin and release SCFAs, and the consumption of intestinal mucus stimulates goblet cells to secrete more mucin to strengthen the physical gut barrier, leading to a reduced level of endotoxemia [47]. Moreover, by interacting with intestinal epithelial cells, Akkermansia shows great potential in improving insulin resistance and alleviating obesity [48]. We hypothesized that supplementation with COS maintained intestinal barrier integrity and reduced bacterial translocation mainly by increasing the abundance of Akkermansia. Norank_f_Muribaculaceae also serves as a potential probiotic, has been proven to be correlated with propionate concentration, and promotes the fermentation of dietary fiber in the intestine [49]. COS enhanced the anti-inflammatory capacity of the intestinal microbiota by reducing proinflammatory bacteria, such as Desulfovibrio and Dubosiella.
The increase in the abundance of several kinds of pathogens, including Escherichia–Shigella and Enterococcus, was closely related to the severity of AP [3]. Escherichia–Shigella are gram-negative bacteria and can produce endotoxins, such as LPS, which leads to a severe inflammatory response by binding to receptors such as TLR4 on intestinal epithelial cells [17]. As a dominant intestinal microbial species in SAP, Enterococcus could serve as a predictor of the gut microbiota abundance in SAP [50]. As bacteria known to be involved in SAP, both Enterococcus and Escherichia–Shigella are closely related to intestinal oxidative stress. Research has shown that the abundance of Escherichia–Shigella is positively correlated with the production of ROS, and more Escherichia–Shigella are observed under high oxidation levels [51, 52]. Enterococcus was proven to play a dominant role in generating extracellular superoxide and intracellular ROS because of the incomplete respiratory chain, which contributes to critical damage to cellular DNA [53, 54]. According to these studies, we speculated that the level of oxidative stress in SAP was partly caused by the abnormally increased abundance of Enterococcus and Escherichia–Shigella, and COS administration protected the intestinal barrier from oxidative stress by decreasing the abundance of these genera.
Conclusion
The present study found that supplementation with COS ameliorated the severity of pancreatic injury, prevented intestinal barrier disruption and reduced inflammatory and oxidative injury in SAP. The protective mechanism was partly attributed to the reshaping of the gut microbiota and the regulation of the TLR4 and Nrf2/HO-1 pathways. Recently, the protective effects of COS have attracted public interest, and our study provided experimental evidence for further clinical strategies.
Supplementary information
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81970555), the Clinical Cultivation Research Project of Shanghai Shenkang Hospital Development Center (SHDC12017X09), the Foundation of Shanghai Jiaotong University School of Medicine for the Research-oriented Doctors (No. 20181813).
Author contributions
QXM and JHH designed the research plan, QXM and ZHH performed the research and generated the draft of the manuscript, CLH and JJF assisted in the animal experiments and YYL interpreted the results. XPW and YZ edited and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Qi-xiang Mei, Jun-hui Hu, Ze-hua Huang
Contributor Information
Xing-peng Wang, Email: richardwangxp@163.com.
Yue Zeng, Email: zengyue1592@yahoo.com.
Supplementary information
The online version of this article (10.1038/s41401-020-00581-5) contains supplementary material, which is available to authorized users.
References
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–52. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Cen ME, Wang F, Su Y, Zhang WJ, Sun B, Wang G. Gastrointestinal microecology: a crucial and potential target in acute pancreatitis. Apoptosis. 2018;23:377–87. doi: 10.1007/s10495-018-1464-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y, et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 2019;54:347–58. doi: 10.1007/s00535-018-1529-0. [DOI] [PubMed] [Google Scholar]
- 4.Kanwal S, Joseph TP, Owusu L, Xiaomeng R, Meiqi L, Yi X. A polysaccharide isolated from dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients. 2018;10:1003. doi: 10.3390/nu10081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasari LP, Khurana A, Anchi P, Aslam Saifi M, Annaldas S, Godugu C. Visnagin attenuates acute pancreatitis via Nrf2/NFkappaB pathway and abrogates associated multiple organ dysfunction. Biomed Pharmacother. 2019;112:108629. doi: 10.1016/j.biopha.2019.108629. [DOI] [PubMed] [Google Scholar]
- 6.Ma R, Yuan F, Wang S, Liu Y, Fan T, Wang F. Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-kappaB signal pathways in mice. Biomed Pharmacother. 2018;105:599–605. doi: 10.1016/j.biopha.2018.05.080. [DOI] [PubMed] [Google Scholar]
- 7.Yuan X, Zheng J, Jiao S, Cheng G, Feng C, Du Y, et al. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr Polym. 2019;220:60–70. doi: 10.1016/j.carbpol.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 8.Naveed M, Phil L, Sohail M, Hasnat M, Baig M, Ihsan AU, et al. Chitosan oligosaccharide (COS): an overview. Int J Biol Macromol. 2019;129:827–43. doi: 10.1016/j.ijbiomac.2019.01.192. [DOI] [PubMed] [Google Scholar]
- 9.Jiang T, Xing X, Zhang L, Liu Z, Zhao J, Liu X. Chitosan oligosaccharides show protective effects in coronary heart disease by improving antioxidant capacity via the increase in intestinal probiotics. Oxid Med Cell Longev. 2019;2019:7658052. doi: 10.1155/2019/7658052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng QY, Wang H, Cui ZB, Yu WG, Lu XZ. Chitosan oligosaccharides attenuate amyloid formation of hIAPP and protect pancreatic beta-cells from cytotoxicity. Molecules. 2020;25:1314. doi: 10.3390/molecules25061314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, Li B, Ye X, Mulatibieke T, Wu J, Dai J, et al. Dopamine D2 receptor signalling controls inflammation in acute pancreatitis via a PP2A-dependent Akt/NF-kappaB signalling pathway. Br J Pharmacol. 2017;174:4751–70. doi: 10.1111/bph.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Yuan X, Cheng G, Jiao S, Feng C, Zhao X, et al. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr Polym. 2018;190:77–86. doi: 10.1016/j.carbpol.2018.02.058. [DOI] [PubMed] [Google Scholar]
- 13.Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther. 2017;170:80–97. doi: 10.1016/j.pharmthera.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Shiratori K, Sawada T, Kobayashi M, Hayashi N, Saotome H, et al. Recombinant human interleukin-11 decreases severity of acute necrotizing pancreatitis in mice. Pancreas. 2000;21:134–40. doi: 10.1097/00006676-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Chiu C-J, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states: I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–83. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Fang X, Wang F, Li H, Niu W, Liang W, et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol. 2019;176:4446–61. doi: 10.1111/bph.14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J, Lou L, Fan J, Huang C, Mei Q, Wu J, et al. Commensal escherichia coli aggravates acute necrotizing pancreatitis through targeting of intestinal epithelial cells. Appl Environ Microbiol. 2019;85:e00059–19. doi: 10.1128/AEM.00059-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji J, Ge X, Chen Y, Zhu B, Wu Q, Zhang J, et al. Daphnetin ameliorates experimental colitis by modulating microbiota composition and T/T17 balance. FASEB J. 2019;33:9308–22. doi: 10.1096/fj.201802659RR. [DOI] [PubMed] [Google Scholar]
- 20.Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepato-Biliary-Pancreat Surg. 2003;10:415–8. doi: 10.1007/s00534-002-0727-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Huang L, Luo M, Xia X. Bacterial translocation in acute pancreatitis. Crit Rev Microbiol. 2019;45:539–47. doi: 10.1080/1040841X.2019.1621795. [DOI] [PubMed] [Google Scholar]
- 22.Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200:111–7. doi: 10.1016/j.amjsurg.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Hyung JH, Ahn CB, Il Kim B, Kim K, Je JY. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur J Pharmacol. 2016;793:43–8. doi: 10.1016/j.ejphar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Ahn CB, Je JY, Kim YS, Park SJ, Kim BI. Induction of Nrf2-mediated phase II detoxifying/antioxidant enzymes in vitro by chitosan-caffeic acid against hydrogen peroxide-induced hepatotoxicity through JNK/ERK pathway. Mol Cell Biochem. 2017;424:79–86. doi: 10.1007/s11010-016-2845-4. [DOI] [PubMed] [Google Scholar]
- 25.Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, et al. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr Polym. 2020;235:115957. doi: 10.1016/j.carbpol.2020.115957. [DOI] [PubMed] [Google Scholar]
- 26.Tian X, Pi YP, Liu XL, Chen H, Chen WQ. Supplemented use of pre-, pro-, and synbiotics in severe acute pancreatitis: an updated systematic review and meta-analysis of 13 randomized controlled trials. Front Pharmacol. 2018;9:690. doi: 10.3389/fphar.2018.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y, Zheng J, Yuan X, Jiao S, Feng C, Du Y, et al. Chitosan oligosaccharides improve glucolipid metabolism disorder in liver by suppression of obesity-related inflammation and restoration of peroxisome proliferator-activated receptor gamma (PPARγ) Mar Drugs. 2018;16:455. doi: 10.3390/md16110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung WK, Moon SH, Kim SK. Effect of chitooligosaccharides on calcium bioavailability and bone strength in ovariectomized rats. Life Sci. 2006;78:970–6. doi: 10.1016/j.lfs.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Zhu Q, Zhang M, Yin T, Xu R, Xiao W, et al. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2018;2018:7161592. doi: 10.1155/2018/7161592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D, et al. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018;293:8032–47. doi: 10.1074/jbc.RA118.003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu HT, Li WM, Xu G, Li XY, Bai XF, Wei P, et al. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol Res. 2009;59:167–75. doi: 10.1016/j.phrs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Pan Y, Gao L, Zhang J, Xie X, Tong Z, et al. Naringenin protects against acute pancreatitis in two experimental models in mice by NLRP3 and Nrf2/HO-1 pathways. Mediators Inflamm. 2018;2018:3232491. doi: 10.1155/2018/3232491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang HJ, Kim YM, Tsoyi K, Park EJ, Lee YS, Kim HJ, et al. Ethyl pyruvate induces heme oxygenase-1 through p38 mitogen-activated protein kinase activation by depletion of glutathione in RAW 264.7 cells and improves survival in septic animals. Antioxid Redox Signal. 2012;17:878–89. doi: 10.1089/ars.2011.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Ka SO, Lee Y, Park BH, Bae EJ. Butein induction of HO-1 by p38 MAPK/Nrf2 pathway in adipocytes attenuates high-fat diet induced adipose hypertrophy in mice. Eur J Pharmacol. 2017;799:201–10. doi: 10.1016/j.ejphar.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Cui QR, Ling YH, Wen SH, Liu KX, Xiang YK, Yang WJ, et al. Gut barrier dysfunction induced by aggressive fluid resuscitation in severe acute pancreatitis is alleviated by necroptosis inhibition in rats. Shock. 2019;52:e107–e16. doi: 10.1097/SHK.0000000000001304. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Li Q, Ren J. Microbiota-immune interaction in the pathogenesis of gut-derived infection. Front Immunol. 2019;10:1873. doi: 10.3389/fimmu.2019.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101:1644–56. doi: 10.1002/bjs.9665. [DOI] [PubMed] [Google Scholar]
- 38.Shi L, Fang B, Yong Y, Li X, Gong D, Li J, et al. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway. Carbohydr Polym. 2019;219:269–79. doi: 10.1016/j.carbpol.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 39.Mandaliya DK, Seshadri S. Short chain fatty acids, pancreatic dysfunction and type 2 diabetes. Pancreatology. 2019;19:280–4. doi: 10.1016/j.pan.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7:2360. doi: 10.1038/s41598-017-02546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanika G, Khan S, Jena G. Sodium butyrate ameliorates l-arginine-induced pancreatitis and associated fibrosis in wistar rat: role of inflammation and nitrosative stress. J Biochem Mol Toxicol. 2015;29:349–59. doi: 10.1002/jbt.21698. [DOI] [PubMed] [Google Scholar]
- 42.Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018;214:153–7. doi: 10.1016/j.lfs.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 43.Ke J, Li Y, Han C, He R, Lin R, Qian W, et al. Fucose ameliorate intestinal inflammation through modulating the crosstalk between bile acids and gut microbiota in a chronic colitis murine model. Inflamm Bowel Dis. 2020;26:863–73. doi: 10.1093/ibd/izaa007. [DOI] [PubMed] [Google Scholar]
- 44.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terzo S, Mule F, Caldara GF, Baldassano S, Puleio R, Vitale M, et al. Pistachio consumption alleviates inflammation and improves gut microbiota composition in mice fed a high-fat diet. Int J Mol Sci. 2020;21:365. doi: 10.3390/ijms21010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17:120. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Wang N, Tan H-Y, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, Immune Resp Gut Syst. 2020;11:219. [DOI] [PMC free article] [PubMed]
- 49.Smith BJ, Miller RA, Ericsson AC, Harrison DC, Strong R, Schmidt TM. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19:130. doi: 10.1186/s12866-019-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S, Xiong Y, Xu J, Liang X, Fu Y, Liu D, et al. Identification of dysfunctional gut microbiota through rectal swab in patients with different severity of acute pancreatitis. Dig Dis Sci. 2020;65:3223–37. doi: 10.1007/s10620-020-06061-4. [DOI] [PubMed] [Google Scholar]
- 51.Peralta DR, Adler C, Corbalan NS, Paz Garcia EC, Pomares MF, Vincent PA. Enterobactin as part of the oxidative stress response repertoire. PLoS One. 2016;11:e0157799. doi: 10.1371/journal.pone.0157799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge Y, Lin S, Li B, Yang Y, Tang X, Shi Y, et al. Oxidized pork induces oxidative stress and inflammation by altering gut microbiota in mice. Mol Nutr Food Res. 2020;64:e1901012. doi: 10.1002/mnfr.201901012. [DOI] [PubMed] [Google Scholar]
- 53.Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–36. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- 54.Strickertsson JAB, Rasmussen LJ, Friis-Hansen L. Enterococcus faecalis infection and reactive oxygen species down-regulates the miR-17-92 cluster in gastric adenocarcinoma cell culture. Genes. 2014;5:726–38. doi: 10.3390/genes5030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.