Abstract
The neonatal heart possesses the ability to proliferate and the capacity to regenerate after injury; however, the mechanisms underlying these processes are not fully understood. Melatonin has been shown to protect the heart against myocardial injury through mitigating oxidative stress, reducing apoptosis, inhibiting mitochondrial fission, etc. In this study, we investigated whether melatonin regulated cardiomyocyte proliferation and promoted cardiac repair in mice with myocardial infarction (MI), which was induced by ligation of the left anterior descending coronary artery. We showed that melatonin administration significantly improved the cardiac functions accompanied by markedly enhanced cardiomyocyte proliferation in MI mice. In neonatal mouse cardiomyocytes, treatment with melatonin (1 μM) greatly suppressed miR-143-3p levels. Silencing of miR-143-3p stimulated cardiomyocytes to re-enter the cell cycle. On the contrary, overexpression of miR-143-3p inhibited the mitosis of cardiomyocytes and abrogated cardiomyocyte mitosis induced by exposure to melatonin. Moreover, Yap and Ctnnd1 were identified as the target genes of miR-143-3p. In cardiomyocytes, inhibition of miR-143-3p increased the protein expression of Yap and Ctnnd1. Melatonin treatment also enhanced Yap and Ctnnd1 protein levels. Furthermore, Yap siRNA and Ctnnd1 siRNA attenuated melatonin-induced cell cycle re-entry of cardiomyocytes. We showed that the effect of melatonin on cardiomyocyte proliferation and cardiac regeneration was impeded by the melatonin receptor inhibitor luzindole. Silencing miR-143-3p abrogated the inhibition of luzindole on cardiomyocyte proliferation. In addition, both MT1 and MT2 siRNA could cancel the beneficial effects of melatonin on cardiomyocyte proliferation. Collectively, the results suggest that melatonin induces cardiomyocyte proliferation and heart regeneration after MI by regulating the miR-143-3p/Yap/Ctnnd1 signaling pathway, providing a new therapeutic strategy for cardiac regeneration.
Keywords: melatonin, luzindole, cardiomyocyte proliferation, cardiac repair, miR-143-3p, Yap, Ctnnd1, myocardial infarction
Introduction
Ischemic heart diseases such as myocardial infarction (MI) remain the leading threat to human health worldwide. A loss of cardiomyocytes due to ischemic stimuli and oxidative stress contributes to myocardial remodeling and chronic heart failure. Although the current therapeutic strategies can improve cardiac function after MI, apoptotic or necrotic cardiomyocytes cannot be regenerated. Recently, the neonatal mouse heart was found to possess the ability to proliferate and the potential to regenerate after injury [1, 2]. This provides new hope for identifying effective treatments for cardiovascular disease.
It has been demonstrated that numerous factors participate in the regulation of cardiomyocyte proliferation and heart regeneration. For instance, the neuronal gene transcriptional repressor RE1 silencing transcription factor has been reported to regulate the cardiomyocyte cell cycle by binding and repressing p21 [3]. Gata4 overexpression promotes cardiac regeneration after injury on P7 [4]. The Hippo signaling pathway has also been demonstrated to regulate cardiac regeneration through its involvement in many pathways [5]. Moreover, noncoding RNAs also play a pivotal role in regulating cardiac myocyte proliferation. The long noncoding RNA (lncRNA) CPR acts as a negative regulator of heart regeneration, and cardiac-specific CPR-overexpressing mice exhibit a reduction in cardiac regenerative ability [6]. Silencing of circRNA Nfix in mice increases cardiomyocyte proliferation, promotes angiogenesis, and decreases cardiomyocyte apoptosis after MI [7]. Other important noncoding RNAs that play essential roles in cardiovascular diseases have been found to have the potential to stimulate neonatal cardiomyocyte proliferation [8–10]. For example, injection of miR-19a/19b promotes cardiomyocyte proliferation and induces cardiac regeneration in response to MI [11]. Inhibition of let-7i promotes cardiac myocyte proliferation and improves heart function after injury by targeting CCND2 and E2F2 [12]. Our previous studies found that targeting of Trp53inp1 and Itm2a by miR-296 contributes to cardiomyocyte cell cycle re-entry [13]. Although a variety of factors are involved in the regulation of cardiac repair, few drugs have been found to take part in cardiomyocyte proliferation and cardiac regeneration.
Melatonin is a crucial indoleamine secreted by the pineal gland and is also found in the eye, skin, and heart. It plays an important role in cell apoptosis, proliferation, and differentiation [14, 15]. Melatonin exerts vital protective effects against many diseases, such as cancer [16], glaucoma [17], inflammation [18], autoimmune diseases [19], and Alzheimer’s disease [20]. Moreover, in recent studies, melatonin has emerged as a key regulator of a variety of cardiovascular diseases [21]. Zhang et al. showed that melatonin alleviates diabetic cardiomyopathy-induced cardiac remodeling and dysfunction by increasing autophagy, limiting apoptosis, and modulating mitochondrial integrity and biogenesis [22]. Hung et al. demonstrated that melatonin also protects against IH-induced hypertension by decreasing NADPH oxidase [23]. Furthermore, melatonin induces the secretion of CTRP3 to attenuate obesity-induced cardiomyocyte apoptosis, thus improving cardiac function in mice with heart failure [24]. Moreover, the ability of melatonin to improve myocardial function and cardiomyocyte survival is highly dependent on the AMPK-OPA1 signaling pathways, which are related to mitochondrial fusion/mitophagy [25]. Although many studies have shown that melatonin can improve cardiac function through mitigating oxidative stress, reducing apoptosis, inhibiting mitochondrial fission, and preventing inflammation after myocardial damage [26], it is not clear whether melatonin plays a protective role against myocardial injury by promoting cardiomyocyte proliferation and heart regeneration, and its precise mechanism is still not fully understood.
In this study, we analyzed the effect of melatonin on cardiomyocyte proliferation and heart regeneration. We found that melatonin can induce cardiac regeneration and alleviate MI-induced cardiac injury to preserve cardiac function. Melatonin promotes cardiomyocyte cell cycle re-entry through regulating the miR-143-3p/Ctnnd1/Yap signaling pathway, and the protective effect of melatonin is dependent on the activation of melatonin receptors.
Materials and methods
Cell culture
Cardiomyocytes and cardiac fibroblasts were isolated from neonatal mice using trypsin (Solarbio, Beijing, China), maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 1× penicillin and streptomycin (Life Technologies, Carlsbad, CA, USA), and cultured at 37 °C in a humidified 5% CO2 incubator. Briefly, hearts removed from 1- to 2-day-old neonatal mice were cut into pieces and digested with 0.25% trypsin. The tissues were collected, and the mixture was filtered and centrifuged at 1500 rpm for 5 min. The supernatant was discarded, and the cells were maintained in culture medium for 1.5 h. Then, the cardiac fibroblasts that had adhered to the bottom of the culture flasks were cultured, and the nonadherent cardiomyocytes were transferred to a new flask or plate and cultured under the same atmospheric conditions as mentioned above.
Drug treatment
Cardiomyocytes were exposed to different concentrations of melatonin (Sigma-Aldrich, St Louis, MO, USA) for 24 h. Cardiomyocytes in the melatonin plus luzindole group were pretreated with luzindole (Sigma-Aldrich, St Louis, MO, USA) for 2 h before being exposed to melatonin and cocultured with melatonin for an additional 24 h. Cardiomyocytes in the other groups were treated with solvent.
Transfection
Chemically synthesized miR-143-3p mimic, miR-143-3p inhibitor, Yap siRNA, MT1 siRNA, and MT2 siRNA, and a scrambled control were purchased from GenePharma Co., Ltd. (Shanghai, China). Their sequences are listed in Table 1. These RNAs and siRNAs were transiently transfected into cardiomyocytes using Lipofectamine 3000 or RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The scrambled control was transfected into the cardiomyocytes in the other groups.
Table 1.
Sequences for RNAs and siRNAs.
| sense (5′-3′) | antisense (5′-3′) | |
|---|---|---|
| Yap siRNA | GAUGAAUUCUGCCUCAGGATT | UCCUGAGGCAGAAUUCAUCTT |
| MT1 siRNA | CAAAUGAAGAAGCAGAUAATT | UUAUCUGCUUCUUCAUUUGTT |
| MT2 siRNA | GAGAACAGCUCAAUCCCUATT | UAGGGAUUGAGCUGUUCUCTT |
| miR-143-3p mimic | UGAGAUGAAGCACUGUAGCUC | GAGCUACAGUGCUUCAUCUCA |
| miR-143-3p inhibitor | GAGCUACAGUGCUUCAUCUCA |
Immunofluorescence
Cardiomyocytes were fixed with 4% paraformaldehyde (P1110; Solarbio, Beijing, China) for 15 min at room temperature. After being washed with PBS, the cells were permeabilized with 0.4% Triton X-100 (T8200; Solarbio, Beijing, China) in PBS for 60 min, blocked with goat serum (AR0009; Boster, Wuhan, China) for 30 min, and incubated with primary antibodies against phospho-Histone H3 (pH3; #06-570; Millipore, Billerica, MA, USA), Aurora B (ab2254; Abcam, Cambridge, UK), α-actinin (ab9465, Abcam, Cambridge, UK) at 4 °C overnight followed by Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibody for 1 h. The cells were counterstained with DAPI (C0065; Solarbio, Beijing, China) to label the nuclei. The pH3- and Aurora B-positive cardiomyocytes were then evaluated with a confocal laser scanning microscope (FV10i; Olympus, Tokyo, Japan).
EdU incorporation assay
After treatment with appropriate reagents and incubation with EdU for 12 h, cardiomyocytes were fixed and labeled with EdU using an In Cell-Light EdU Apollo 567 In Vitro Kit (RiboBio, Guangzhou, China) according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cardiomyocytes using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from the total RNA according to the manufacturer’s protocol with random primers or miRNA-specific primers. Real-time PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems) and carried out on an ABI Prism 7500 system. The threshold cycle (Ct) value for each gene was normalized to the Ct value of U6. Relative mRNA expression was calculated using the ΔΔCt method.
Western blotting
Protein samples (70–80 μg) were loaded onto SDS-PAGE gels for protein electrophoresis and then transferred onto nitrocellulose membranes by an electroblotting apparatus. Antibodies against Ctnnd1 (HPA015955; Sigma, St Louis, MO, USA), Yap (13584-1-AP; Proteintech, Chicago, IL, USA), and GAPDH (AC033; ABclonal, Wuhan, China) were used as primary antibodies. Mouse or rabbit IgG antibodies coupled to horseradish peroxidase were used as secondary antibodies. Western blot quantification was performed with an Odyssey system (LI-COR Biosciences, Lincoln, NE, USA).
Myocardial infarction
Adult C57BL/6 mice (6–8 weeks old) were purchased from the Experimental Animal Center of the Affiliated Second Hospital of Harbin Medical University (Harbin, China). Food and water were freely available throughout the experiments. Our experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of Harbin Medical University. The procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
The mice were administered melatonin (10 mg· kg−1· d−1) and luzindole (1 mg ·kg−1· d−1) or solvent by intraperitoneal injection for 2 days before MI and for 14 days after myocardial infarction (MI). An MI model was established by ligation of the left anterior descending coronary artery. Briefly, adult mice underwent tracheal intubation and were anesthetized with 3% isoflurane for induction and 2% isoflurane for maintenance of anesthesia. Then, the left anterior descending coronary artery was ligated with a 7-0 Prolene suture. After completion of the surgery, the chest was closed. The mice were then warmed for several minutes until recovery. Hearts were collected 14 days after the operation for immunofluorescence.
Echocardiography
Cardiac function was evaluated in conscious mice by two-dimensional M-mode echocardiography at week 4 after MI. Echocardiography was performed using a Vevo 1100 VisualSonics device (VisualSonics, Toronto, ON, Canada). Fractional shortening (FS) and ejection fraction (EF) were measured from M-mode images taken from the parasternal short-axis view. Echocardiography data were analyzed by investigators blinded to treatment and genotype. The average of at least three measurements was used for each single data point.
Immunohistochemistry
After harvest, the hearts were snap-frozen in liquid nitrogen with optimal cutting temperature (OCT) compound and cut into 6-μm sections. Immunohistochemical and immunofluorescence analyses were performed as previously described [27].
Wheat germ agglutinin
Hearts were harvested and snap-frozen in liquid nitrogen with OCT compound and sliced into 6-μm sections. Then, the sections were fixed in acetone, washed with PBS and incubated with wheat germ agglutinin (WGA) conjugated to Alexa 488 (#W11261; Thermo Fisher, Carlsbad, CA, USA). Cell size was measured using ImageJ software. The observer was blinded to group identity.
Statistical analysis
The data are expressed as the mean ± SEM. Data distribution was evaluated by the Kolmogorov–Smirnov test. An F-test was used to evaluate the homogeneity of variance. Normally distributed data with only one variable were analyzed by parametric analysis: an unpaired (two-tailed) Student’s t test (with Welch correction when variance was unequal) was used for two groups, and one-way analysis of variance (ANOVA) with post hoc Newman–Keuls test was used for more than two groups. Nonnormally distributed data with only one variable were analyzed by nonparametric analysis: the Mann–Whitney U test (two-tailed) was used for two groups, and one-way analysis of variance (ANOVA) with a Kruskal–Wallis test was used for more than two groups. The data were analyzed using GraphPad Prism software (version 5.0, GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
Melatonin promotes cell cycle activity in neonatal cardiomyocytes
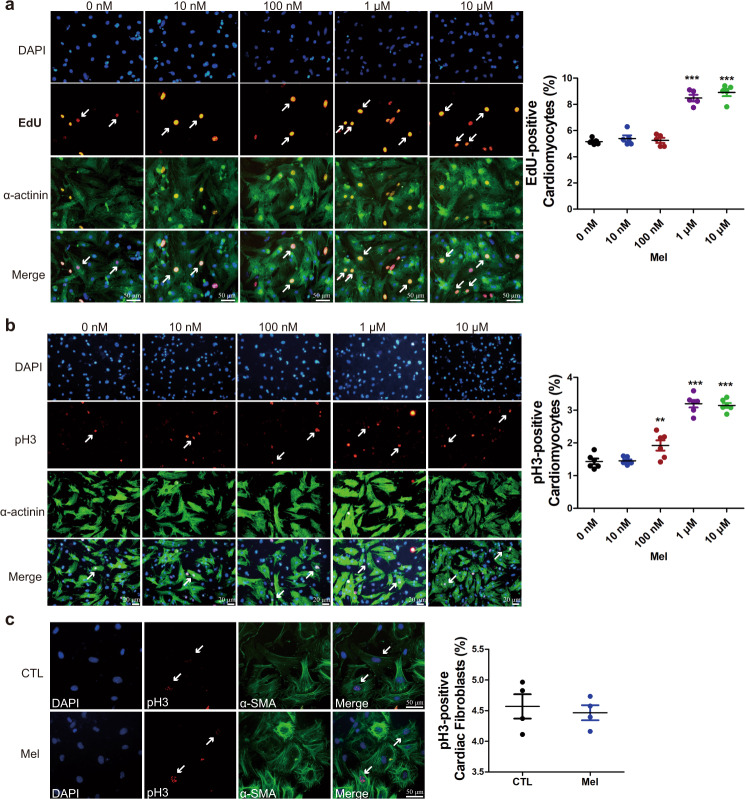
To explore whether melatonin affects cardiomyocyte proliferation, we treated neonatal cardiomyocytes with different concentrations of melatonin. Cardiomyocyte proliferative activity was measured by using EdU as an indicator of DNA synthesis and pH3 as an indicator of mitosis. The results showed that compared to control treatment, 1 and 10 μM melatonin increased the number of EdU-positive cardiomyocytes (Fig. 1a). The number of pH3-positive cardiomyocytes was also increased after treatment with 100 nM, 1 μM, and 10 μM melatonin compared with control treatment (Fig. 1b). Since the effect of 10 μM melatonin in promoting cardiomyocyte proliferation was not different from that of 1 μM melatonin, we used 1 μM melatonin in subsequent experiments. These findings indicate that melatonin is capable of promoting the proliferation of cardiomyocytes. Furthermore, we also detected the proliferative effect of melatonin on cardiac fibroblasts by pH3 staining. Interestingly, melatonin did not change the percentage of pH3-positive cardiac fibroblasts (Fig. 1c). These data suggest that melatonin promotes cardiomyocyte proliferation but has no effect on cardiac fibroblast duplication.
Fig. 1. Melatonin promotes cell cycle activity in neonatal cardiomyocytes.
a EdU staining was used to detect the proliferative effect of different concentrations of melatonin on cardiomyocytes. n = 5. b Mitosis was visualized with an antibody specific for phospho-histone H3 (pH3). n = 6. c The influence of melatonin on the proliferation of cultured cardiac fibroblasts, as determined by pH3 staining. n = 4. The data represent the mean ± SEM. The data were analyzed by one-way ANOVA followed by Dunnett’s post hoc test. **P < 0.01, ***P < 0.001 versus 0 nM.
Administration of melatonin enhances cardiac regenerative capacity in adult mice after MI
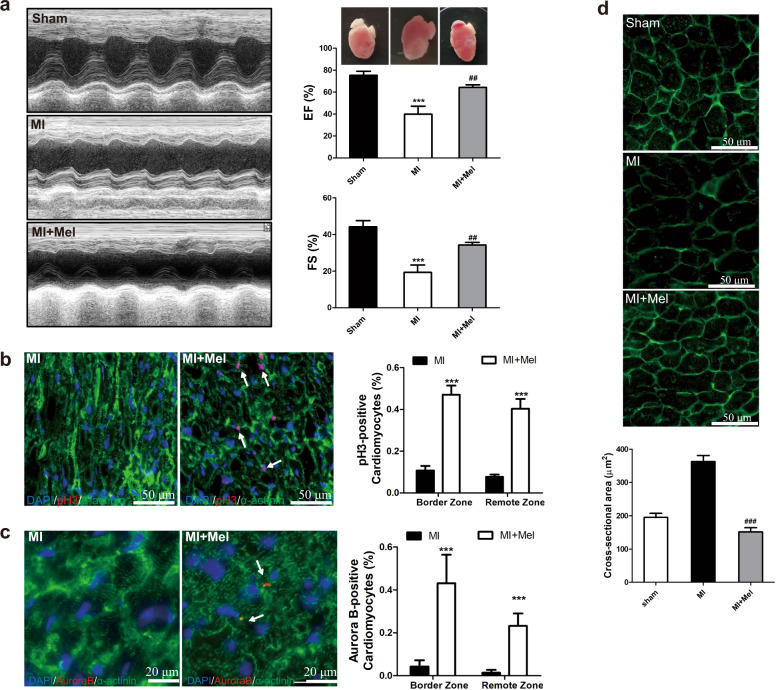
It has been reported that melatonin protects the heart against myocardial injury by regulating apoptosis, autophagy, and inflammation [26]. The in vitro results of this study showed that melatonin induced cardiomyocyte cell cycle re-entry. Thus, we further investigated whether the protective effect of melatonin on MI mice is due to its proproliferative function. To answer this question, we administered melatonin to mice by intraperitoneal injection. The echocardiography results showed that compared to control treatment, melatonin administration elevated the ejection fraction (EF%) and fractional shortening (FS%) of mice with MI (Fig. 2a), which is in good agreement with recent reports [28, 29]. Then, we performed immunohistochemical staining to detect the expression level of proliferation-related genes. As indicated by increases in the number of pH3- and Aurora B-positive cardiomyocytes, pretreatment with melatonin improved cardiac myocyte mitosis and cytokinesis activity (Fig. 2b, c). WGA staining was used to observe the cross-sectional area of cardiomyocytes as an indicator of the number of cardiomyocytes in the heart. As shown in Fig. 2d, melatonin treatment attenuated the increase in cardiomyocyte cross-sectional area induced by MI. These data indicate that melatonin improves cardiac function, at least in part by enhancing heart regeneration capacity.
Fig. 2. Administration of melatonin enhances cardiac regenerative capacity in adult mice after MI.
a Cardiac function was measured by echocardiography (n = 9, 5, 6). The data were analyzed by one-way ANOVA followed by the Newman–Keuls multiple comparison test. b Representative heart sections stained with pH3 for evaluating mitosis. The Mann–Whitney two-tailed U test was used for nonparametric analysis. c Representative heart sections stained with Aurora B for evaluating cytokinesis. The Mann–Whitney two-tailed U test was used for nonparametric analysis. d WGA staining for determining the cross-sectional area of cells. The Mann–Whitney two-tailed U test was used for nonparametric analysis. The data represent the mean ± SEM, ***P < 0.001 versus the sham group, ##P < 0.05, ###P < 0.001 versus the MI group.
Melatonin reactivates cardiomyocyte proliferation by decreasing miR-143-3p expression
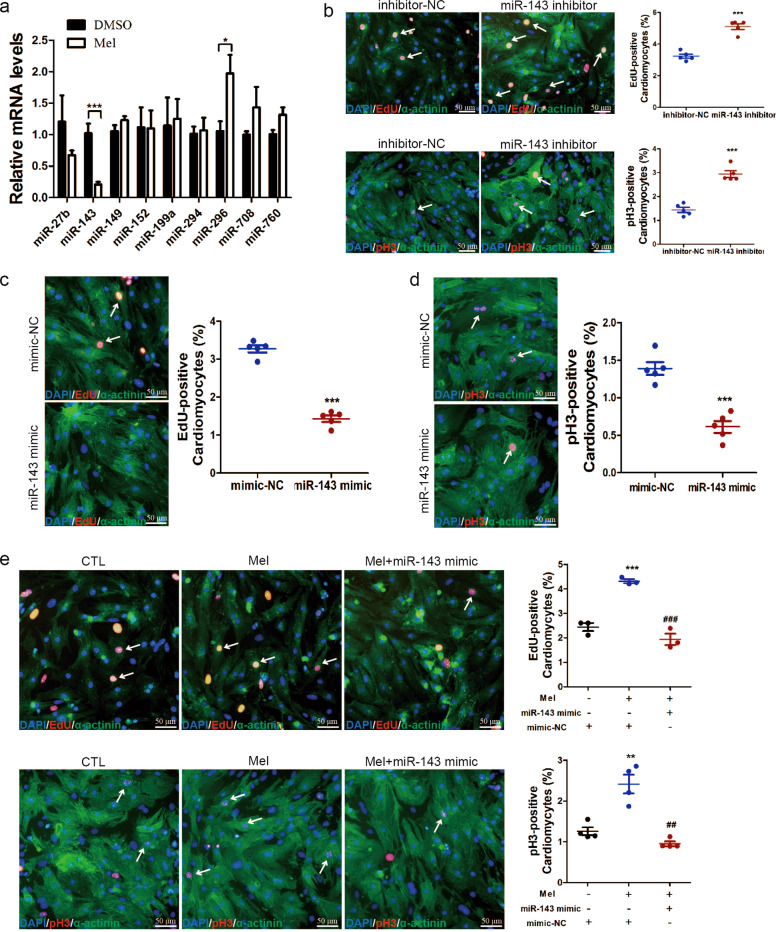
To illustrate the mechanism underlying the ability of melatonin to regulate cardiac regeneration, we measured the expression level of some miRNAs that play important roles in cardiac regeneration and cardiac vascular diseases [8, 30]. Among these miRNAs, we found that miR-143-3p was significantly decreased (Fig. 3a). These results indicate that miR-143-3p may be involved in the process of melatonin-induced cardiomyocyte proliferation. First, we observed the effect of miR-143-3p inhibition on the proliferative ability of ventricular myocytes. The results revealed that the silencing of miR-143-3p increased the numbers of EdU- and pH3-positive cardiomyocytes (Fig. 3b). In contrast, overexpression of miR-143-3p blocked cardiomyocyte entry into the cell cycle (Fig. 3c, d). Then, to study the role of miR-143-3p in melatonin-treated ventricular myocytes, cardiomyocytes were exposed to both melatonin and miR-143-3p, and their proliferation capacity was detected by EdU and pH3 staining. The data showed that overexpression of miR-143-3p attenuated the enhancement of cardiomyocyte proliferation ability induced by melatonin (Fig. 3e). This indicates that melatonin promotes cardiac myocyte proliferation by regulating miR-143-3p.
Fig. 3. Melatonin reactivates cardiomyocyte proliferation by decreasing miR-143-3p expression.
a MiRNA expression levels were analyzed by qRT-PCR after treatment with melatonin. b The influence of miR-143-3p inhibitor on the proliferation of cultured cardiomyocytes, as determined by EdU and pH3 staining. n = 5. c, d Cardiomyocytes transfected with miR-143-3p mimic were fixed 48 h posttransfection and immunostained for EdU to evaluate DNA synthesis and for pH3 to evaluate mitosis. n = 5. e EdU (n = 3) and pH3 (n = 4) staining were performed to examine the effect of cotreatment with miR-143-3p mimic and melatonin on cardiomyocyte proliferation. The data represent the mean ± SEM. The data were analyzed by two-tailed Student’s t test with or without Welch’s correction. *P < 0.05, ***P < 0.001 versus the control group, ##P < 0.01, ###P < 0.001 versus the mel group.
Ctnnd1 and Yap as targets of miR-143-3p regulate cardiomyocyte proliferation induced by melatonin
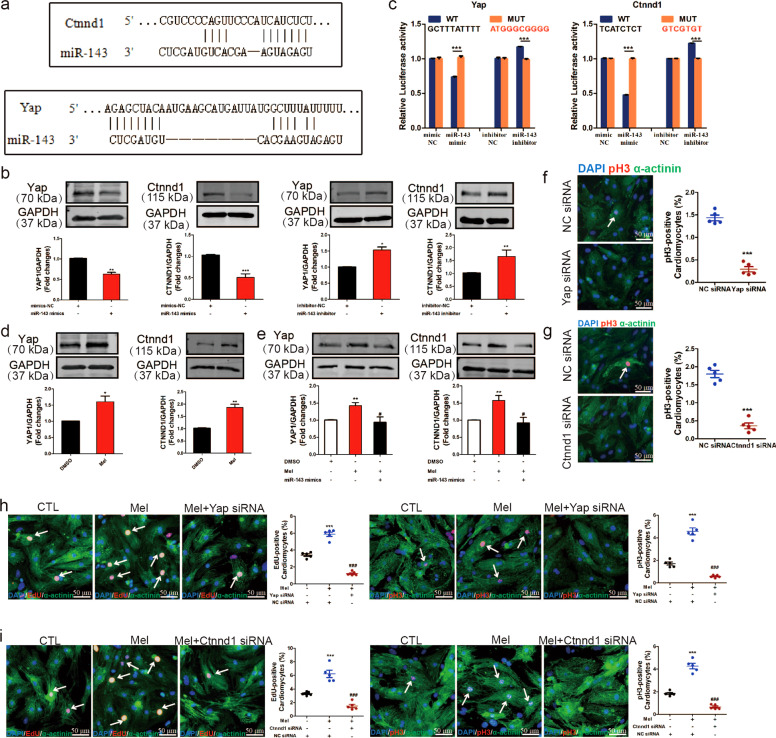
To further investigate how miR-143-3p affects the proproliferative effects of melatonin on ventricular myocytes, the direct target genes of miR-143-3p were explored. We found that Ctnnd1 and Yap have a binding site for miR-143-3p (Fig. 4a). To identify whether these two genes could act as targets of miR-143-3p, we observed changes in Ctnnd1 and Yap at the protein level after overexpression or silencing of miR-143-3p by Western blotting. The results showed that forced expression of miR-143-3p inhibited the protein expression of Ctnnd1 and Yap (Fig. 4b). Ctnnd1 and Yap expression were increased by inhibition of miR-143-3p (Fig. 4b). Furthermore, a luciferase assay also confirmed that miR-143-3p could bind Ctnnd1 and Yap (Fig. 4c). This indicates that Ctnnd1 and Yap are indeed the direct target genes of miR-143-3p. Interestingly, as shown in Fig. 4d, melatonin administration also elevated Ctnnd1 and Yap protein levels. To further determine whether the increases in Ctnnd1 and Yap protein levels induced by melatonin involved miR-143-3p, we expressed miR-143-3p in the presence of melatonin. The Western blot results showed that miR-143-3p abolished the positive effect of melatonin on Ctnnd1 and Yap expression (Fig. 4e). Taken together, these data suggest that Ctnnd1 and Yap are targets of miR-143-3p and that melatonin regulates Ctnnd1 and Yap expression by targeting miR-143-3p.
Fig. 4. Ctnnd1 and Yap, as targets of miR-143-3p, regulate cardiomyocyte proliferation induced by melatonin.
a The predicted binding site of miR-143-3p for Yap or Ctnnd1. b Yap and Ctnnd1 levels in cardiomyocytes with miR-143-3p upregulation or downregulation were determined by Western blotting. c The luciferase assay was used to analyze the binding between miR-143-3p and Yap1 or Ctnnd1. d Yap and Ctnnd1 levels after melatonin treatment. e Western blotting was used to evaluate the expression of Yap and Ctnnd1. f, g Cardiomyocyte proliferative ability was evaluated by pH3 staining. n = 5. h, i Yap and Ctnnd1 siRNAs abrogated the influence of melatonin on the proliferation of neonatal cardiomyocytes, as determined by EdU and pH3 staining. n = 5. The data represent the mean ± SEM. The data were analyzed by two-tailed Student’s t test with or without Welch’s correction, and the Mann–Whitney U test was used for nonnormally distributed data from two groups. One-way ANOVA with post hoc Newman–Keuls test was used to analyze data from more than two groups. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, #P < 0.05, ###P < 0.001 versus the mel group.
We subsequently clarified whether Ctnnd1 and Yap mediate the regulatory role of melatonin in cardiac regeneration. First, we observed the effect of Ctnnd1 and Yap silencing on the proliferation of cardiomyocytes. As indicated in Fig. 4f, g, Ctnnd1 and Yap siRNA inhibited cardiomyocyte proliferation. Then, we coadministered melatonin and Ctnnd1 siRNA or Yap siRNA to ventricular myocytes and measured cardiomyocyte DNA synthesis and mitosis. The results showed that Ctnnd1 siRNA and Yap siRNA blocked the proproliferative effects of melatonin, as indicated by reductions in the numbers of EdU- and pH3-positive cells (Fig. 4h, i). These data indicate that melatonin promotes cardiomyocyte proliferation and cardiac regeneration by regulating miR-143-3p/Ctnnd1/Yap signaling.
Melatonin regulation of cardiomyocyte proliferation and cardiac regeneration is dependent on melatonin receptor activation
Increasing evidence has suggested that the beneficial effect of melatonin is largely dependent on the activation of the melatonin receptor [28]. Thus, we extensively investigated whether the favorable effects of melatonin on cardiomyocyte proliferation are dependent on the melatonin receptor both in vitro and in vivo. For the in vitro study, we pretreated cardiomyocytes with the melatonin receptor antagonist luzindole, and the results showed that the proproliferative effect of melatonin was impeded by luzindole (Fig. 5a). We also confirmed the function of luzindole in vivo (Fig. 5b–d). The data showed that melatonin administration failed to improve cardiac function upon exposure to luzindole (Fig. 5b). Furthermore, luzindole abrogated melatonin-induced cardiac regeneration, as evidenced by a decrease in the number of pH3-positive cells and an increase in cell size (Fig. 5c, d). This suggests that the administration of melatonin can alleviate myocardial damage and that melatonin-induced cardiac regeneration and cardiac repair are dependent on melatonin receptor activation.
Fig. 5. Luzindole blocks the effect of melatonin on cardiomyocyte proliferation and cardiac regeneration.
a EdU and pH3 staining for cardiomyocyte proliferation in the presence of melatonin and luzindole. n = 5. One-way ANOVA with the Newman–Keuls multiple comparison test was used for analysis. ***P < 0.001 versus the control group, ###P < 0.001 versus the mel group. b Representative echocardiography and serial echocardiographic measurements of ejection fraction (EF) and fraction shortening (FS) (n = 6, 6, 4, 4). Mann–Whitney two-tailed U test was used for nonparametric analysis. **P < 0.01 versus the MI+mel group. c Representative heart sections stained with pH3 for evaluating mitosis. The Mann–Whitney two-tailed U test was used for nonparametric analysis. ***P < 0.001 versus the MI group, ###P < 0.001 versus the MI+mel group. d Representative heart sections stained with WGA for evaluating the cross-sectional area of the cells. Two-tailed Student’s t test with Welch’s correction. The data are shown as the mean ± SEM, ***P < 0.001 versus the MI group, ##P < 0.01, ###P < 0.001 versus the MI+mel group.
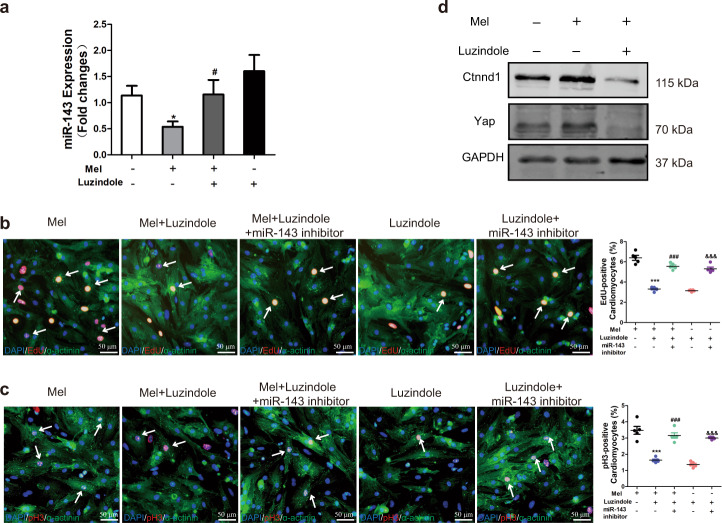
Silencing of miR-143-3p reverses the inability of cardiomyocytes to re-enter the cell cycle induced by luzindole
We have demonstrated that melatonin stimulates ventricular myocyte cell cycle re-entry by regulating miR-143-3p expression. Moreover, the melatonin receptor antagonist luzindole abolished the beneficial effect of melatonin on cardiomyocyte proliferation and cardiac repair after MI. Hence, we proposed that luzindole exerts its unfavorable effect on cardiomyocytes by regulating miR-143-3p. To confirm this hypothesis, we first verified the changes in miR-143-3p in ventricular myocytes after treatment with luzindole. We isolated neonatal cardiomyocytes and exposed them to luzindole and melatonin, and the expression level of miR-143-3p was measured by RT-PCR. The results showed that melatonin decreased the miR-143-3p expression level. However, the inhibitory effect was reversed by luzindole (Fig. 6a). Then, we explored whether inhibition of miR-143-3p eliminates the inhibitory effect of luzindole on cardiomyocyte proliferation. As indicated in Fig. 6b, c, luzindole abolished the favorable effect of melatonin in cardiomyocytes, whereas silencing of miR-143-3p reversed the inhibitory effect of luzindole on cardiomyocyte proliferation. Additionally, Yap and Ctnnd1 expression in cardiomyocytes cotreated with melatonin and luzindole were analyzed using Western blotting (Fig. 6d). The results showed that the melatonin-induced increases in Yap and Ctnnd1 expression levels were abrogated by luzindole (Fig. 6d). These data show that inactivation of the melatonin receptor blocks the proproliferative effect of melatonin by regulating Yap and Ctnnd1 via miR-143-3p.
Fig. 6. Silencing of miR-143-3p inhibits the loss of cardiomyocyte cell cycle re-entry ability induced by luzindole.
a miR-143-3p expression was detected by qRT-PCR. Two-tailed Student’s t test was used for analysis. *P < 0.05 versus the control group, #P < 0.05 versus the mel group. b EdU staining for evaluating DNA synthesis. c pH3 staining for evaluating mitosis. The data are shown as the mean ± SEM. n = 5. One-way ANOVA with the Newman–Keuls multiple comparison test was used for analysis. ***P < 0.001 versus the mel group, ###P < 0.001 versus mel+luzindole group, &&&P < 0.001 versus the luzindole group. d Western blotting for evaluating the expression of Yap and Ctnnd1.
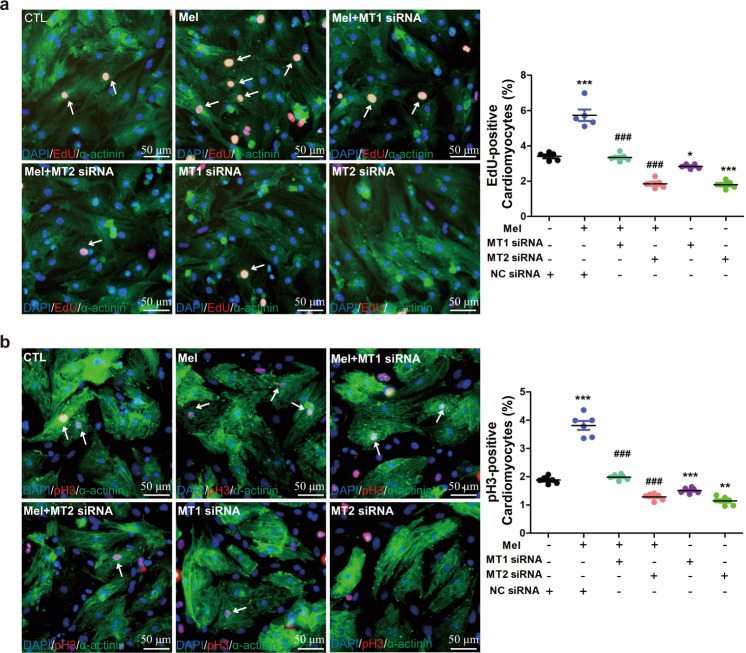
Melatonin receptor 1 and 2 are involved in melatonin-induced cardiac repair
Evidence has shown that melatonin receptors, including the melatonin receptor 1 (MT1, melatonin receptor 1a or MTNR1A) and melatonin receptor 2 (MT2, melatonin receptor 1b or MTNR1B) subtypes in mammals and different receptor subtypes may have different functions [26, 28]. A recent report proved that MT2 is involved in the beneficial effects of melatonin against myocardial ischemia/reperfusion damage [28]. In our study, we found that melatonin promoted cardiac regeneration by activating the melatonin receptor. Which receptor subtype is responsible for regulating cardiomyocyte proliferation? To answer this question, we produced MT1 siRNA and MT2 siRNA and exposed cardiomyocytes to melatonin receptor siRNA in the presence of melatonin. The results showed that both MT1 and MT2 siRNA decreased the numbers of EdU- and pH3-positive cardiomyocytes (Fig. 7). This indicates that both MT1 and MT2 siRNA can abrogate the increase in cardiomyocyte proliferation ability induced by melatonin.
Fig. 7. Melatonin receptor 2 plays an important role in melatonin-induced cardiac regeneration.
a, b EdU (n = 5) and pH3 (n = 6) staining for evaluating cardiomyocyte proliferation. The data are shown as the mean ± SEM. One-way ANOVA with the Newman–Keuls multiple comparison test was used for analysis. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group, ###P < 0.001 versus the mel group.
Discussion
It is well known that myocardial infarction is the leading cause of death worldwide, and the induction of cardiac regeneration has recently emerged as a new method for promoting cardiac function [1, 2]. Many factors have been found to exert regulatory functions on cardiac regeneration [5–7, 9]. While melatonin has been demonstrated to protect against cardiac diseases by regulating oxidation and cardiomyocyte apoptosis, there are no data regarding the influence of melatonin on cardiomyocyte proliferation and heart regeneration [23–25]. In the current study, we proved that melatonin can enhance cardiomyocyte proliferation ability in vitro. The protective effects of melatonin on the infarcted heart also arise from its ability to activate cardiomyocytes into the cell cycle. To our knowledge, this is the first study to analyze the function of melatonin in cardiac regeneration. Furthermore, we provide evidence that the proproliferative and proregenerative effects of melatonin are associated with the miR-143-3p/Yap/Ctnnd1 signaling pathway. In addition, the beneficial effect of melatonin is dependent on the activation of melatonin receptors, mainly melatonin receptor 2.
Melatonin, as a vital agent, is involved in many cardiovascular diseases [21]. Melatonin can alleviate diabetic cardiomyopathy [22], protect against hypertension [23], improve cardiac function in heart failure [24], prevent myocardial injury [26], and attenuate myocardial ischemia-reperfusion-induced damage [25]. Mechanistically, in previous studies, melatonin was shown to exert its protective effect against cardiac injury mainly by inhibiting cell apoptosis, promoting autophagy, attenuating mitochondrial fission, preventing inflammation, and regulating oxidative stress [22, 24, 26]. In our study, we observed that cardiomyocyte proliferative ability was enhanced after treatment with melatonin in vitro and in vivo. (Figs. 1 and 2). Moreover, we also found that melatonin was cardioprotective in myocardial infarction mice, which is consistent with the findings of other studies, as indicated by improved cardiac contractile function (Fig. 2). These data prompted us to propose a new mechanism by which melatonin protects heart function: melatonin plays a protective role against myocardial injury by promoting cardiomyocyte proliferation and cardiac regeneration.
A series of factors have been found to have the ability to regulate cardiomyocyte proliferation and heart regeneration [3–7, 9, 10, 13]. MiRNAs play a major role in cardiac diseases. Researchers have already observed that some miRNAs can enhance cardiomyocyte cell cycle activity, thus improving heart function after cardiac injury [11, 12]. MiR-143-3p is a cardiovascular disease-related miRNA that has been found to be markedly increased in the myocardium after MI in both humans and mice [30, 31]. Inhibition of miR-143-3p attenuates MI-induced cell apoptosis and necrosis by targeting PKCe, thus protecting cardiomyocytes against mitochondrial damage [30]. In this study, we found for the first time that melatonin treatment decreased miR-143-3p expression levels and that overexpression of miR-143-3p attenuated the enhancement of cardiomyocyte proliferation ability induced by melatonin (Fig. 3). In our study, we not only demonstrated that miR-143-3p has the potential to regulate cardiac regeneration but also identified Yap and Ctnnd1 as targets of miR-143-3p (Fig. 4a–c). Moreover, we found that melatonin affected the expression levels of Yap and Ctnnd1 by modulating miR-143-3p (Fig. 4d, e) and that melatonin-induced cardiomyocyte proliferation was blocked by Yap siRNA and Ctnnd1 siRNA (Fig. 4h, i).
Yap acts as a major transcriptional regulator in the Hippo signaling pathway, activating the transcription of genes involved in cell proliferation and anti-apoptotic genes. The Hippo signaling pathway was found to participate in cardiac development in recent studies [5]. In addition, melatonin can alleviate idiopathic pulmonary fibrosis by targeting Yap/Hippo signaling [32]. Furthermore, Mst1, another important factor of the Hippo signaling pathway, is regulated by melatonin. Zhang and colleagues demonstrated that melatonin attenuates the phosphorylation of Mst1 to promote autophagy, inhibit apoptosis, and thus improve cardiac function in diabetic cardiomyopathy [22]. In this study, we found that melatonin regulated Yap and thus affected heart regeneration. The above studies indicate that melatonin has a vital regulatory effect on the Hippo pathway and thus plays a role in various diseases.
Ctnnd1, which is upregulated in many cancer tissues, binds to E-cadherin through its juxtamembrane domain and activates Wnt/β-catenin signaling to promote cancer cell proliferation, migration and invasion [33–36]. Wnt/β-catenin signaling plays an important role in heart development. Studies have demonstrated that the absence of β-catenin in the heart leads to ventricular hypoplasia and fetal death [37]. Researchers have also proven that activation of Wnt/β-catenin signaling is essential for promoting mature cardiomyocyte proliferation [38]. Furthermore, the conditional knockout of Salvador (Salv), a component of the Hippo signaling pathway, can increase the expression of Yap in the nucleus and enhance cardiomyocyte cell cycle re-entry. Investigators also found that Salv CKO upregulates the level of Wnt target genes and promotes β-catenin nuclear localization. Thus, the interaction between Yap and β-catenin in the nucleus plays a vital role in promoting cardiac cell proliferation [39]. However, there are currently no studies on the direct interaction between Ctnnd1 and Yap. In this study, both Yap and Ctnnd1 were identified as direct targets of miR-143-3p, and Ctnnd1 may exert its role in promoting cardiac regeneration by activating the Wnt/β-catenin signaling pathway, thereby promoting the formation of the β-catenin and Yap complex. Further studies are needed to confirm the relationship between Ctnnd1 and Yap.
Previous studies have suggested that the melatonin level is decreased after MI [40]. Ours and other studies have found that melatonin administration can alleviate the reduction in heart function following MI. Furthermore, it has been demonstrated that the mRNA expression of MT1/2 is increased after MI, which may be a response to MI [41]. This indicates that melatonin receptors might be involved in the cardioprotective actions of melatonin. Han demonstrated that MT2 contributes to the beneficial effects of melatonin against myocardial ischemia/reperfusion damage [28]. In our study, we found that the function of melatonin in cardiac regeneration was blocked by luzindole, which is a nonspecific melatonin membrane receptor inhibitor. Our results also illustrated that melatonin-mediated cardiomyocyte proliferation was dependent on the activation of both MT1 and MT2. These results lay the foundation for the clinical application of melatonin for the treatment of cardiovascular diseases.
In conclusion, this study provides a new mechanism by which melatonin protects the heart against myocardial injury by regulating cardiomyocyte proliferation and cardiac regeneration. The proproliferative ability of melatonin was mediated via Yap and Ctnnd1, which were regulated by miR-143-3p (Fig. 8). Furthermore, the beneficial effect of melatonin was dependent on the activation of melatonin receptors, especially melatonin receptor 2. Given the favorable effect of melatonin on cardiac regeneration, melatonin has the potential to protect the cardiovascular system.
Fig. 8. Underlying mechanisms of melatonin promoting cardiomyocyte proliferation and heart repair.
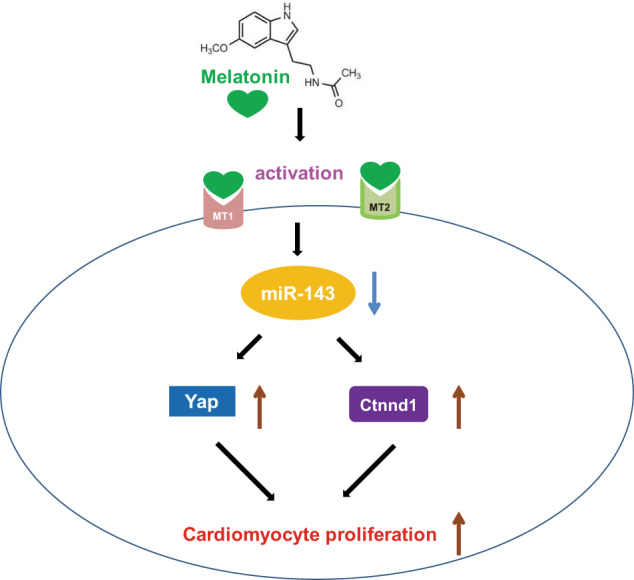
Schematic representation showing that melatonin regulates cardiomyocyte proliferation by upregulating Yap and Ctnnd1 through inhibition of miR-143-3p via activation of the melatonin receptor.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81872857, 81573434, 81170096, 81773733], the China Postdoctoral Science Foundation [2018M641867], and the Heilongjiang Postdoctoral Fund [LBH-Z18126].
Author contributions
BZC and WYM designed the study and drafted the article. RJS performed the experiments and analyzed the data. BBX, YX, XXW, HYS, SNL, SZL, MXY, FY, RG, ZBH, YY, DYY, and DB performed the experiments. NW and ZWP revised the paper. All authors read and approved the final paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Wen-ya Ma, Rui-jie Song, Bin-bin Xu
References
- 1.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 2.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D, Wang Y, Lu P, Wang P, Yuan X, Yan J, et al. REST regulates the cell cycle for cardiac development and regeneration. Nat Commun. 2017;8:1979. doi: 10.1038/s41467-017-02210-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Malek Mohammadi M, Kattih B, Grund A, Froese N, Korf-Klingebiel M, Gigina A, et al. The transcription factor GATA4 promotes myocardial regeneration in neonatal mice. EMBO Mol Med. 2017;9:265–79. doi: 10.15252/emmm.201606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mia MM, Singh MK. The Hippo signaling pathway in cardiac development and diseases. Front Cell Dev Biol. 2019;7:211. doi: 10.3389/fcell.2019.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponnusamy M, Liu F, Zhang YH, Li RB, Zhai M, Liu F, et al. Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation. 2019;139:2668–84. doi: 10.1161/CIRCULATIONAHA.118.035832. [DOI] [PubMed] [Google Scholar]
- 7.Huang SL, Li XZ, Zheng H, Si XY, Li B, Wei GQ, et al. Loss of super-enhancer-regulated circrna nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation. 2019;139:2857–76. doi: 10.1161/CIRCULATIONAHA.118.038361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colpaert RMW, Calore M. Micrornas in cardiac diseases. Cells. 2019;8:737. doi: 10.3390/cells8070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrini C, Cubero RJ, Dirkx E, Braga L, Ali H, Prosdocimo G, et al. Common regulatory pathways mediate activity of micrornas inducing cardiomyocyte proliferation. Cell Rep. 2019;27:2759–71. doi: 10.1016/j.celrep.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. Functional screening identifies mirnas inducing cardiac regeneration. Nature. 2012;492:376–81. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 11.Gao F, Kataoka M, Liu N, Liang T, Huang ZP, Gu F, et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat Commun. 2019;10:1802. doi: 10.1038/s41467-019-09530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu YL, Jin GQ, Li B, Chen YM, Zhong LT, Chen GJ, et al. Suppression of miRNA let-7i-5p promotes cardiomyocyte proliferation and repairs heart function post injury by targetting CCND2 and E2F2. Clin Sci (Lond) 2019;133:425–41. doi: 10.1042/CS20181002. [DOI] [PubMed] [Google Scholar]
- 13.Cai BZ, Ma WY, Ding FZ, Zhang L, Huang Q, Wang XX, et al. The long noncoding RNA CAREL controls cardiac regeneration. J Am Coll Cardiol. 2018;72:534–50. doi: 10.1016/j.jacc.2018.04.085. [DOI] [PubMed] [Google Scholar]
- 14.Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iggena D, Winter Y, Steiner B. Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J Pineal Res. 2017;62:e12397. 10.1111/jpi.12397. [DOI] [PubMed]
- 16.Capote-Moreno A, Ramos E, Egea J, Lopez-Munoz F, Gil-Martin E, Romero A. Potential of melatonin as adjuvant therapy of oral cancer in the era of epigenomics. Cancers (Basel) 2019;11:1712. doi: 10.3390/cancers11111712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkozi HA, Navarro G, Franco R, Pintor J. Melatonin and the control of intraocular pressure. Prog Retin Eye Res. 2020;75:100798. doi: 10.1016/j.preteyeres.2019.100798. [DOI] [PubMed] [Google Scholar]
- 18.Ma N, Zhang J, Reiter RJ, Ma X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalK: A THERAPeutic target to reduce intestinal inflammation. Med Res Rev. 2020;40:606–32. doi: 10.1002/med.21628. [DOI] [PubMed] [Google Scholar]
- 19.Zhao CN, Wang P, Mao YM, Dan YL, Wu Q, Li XM, et al. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019;48:1–10. doi: 10.1016/j.cytogfr.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Dunnett S, Ho YS, Chang RC. The role of sleep deprivation and circadian rhythm disruption as risk factors of Alzheimer’s disease. Front Neuroendocrinol. 2019;54:100764. doi: 10.1016/j.yfrne.2019.100764. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Gusdon AM, Qu S. Effects of melatonin on cardiovascular diseases: progress in the past year. Curr Opin Lipido. 2016;27:408–13. doi: 10.1097/MOL.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang MM, Lin J, Wang SJ, Cheng Z, Hu JQ, Wang TT, et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res. 2017;63:e12418. [DOI] [PubMed]
- 23.Hung MW, Kravtsov GM, Lau CF, Poon AM, Tipoe GL, Fung ML. Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J Pineal Res. 2013;55:247–56. doi: 10.1111/jpi.12067. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Li LN, Guo S, Zhao XY, Liu YZ, Liang C, et al. Melatonin improves cardiac function in a mouse model of heart failure with preserved ejection fraction. Redox Biol. 2018;18:211–21. doi: 10.1016/j.redox.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang Y, Xu JN, Tian F, Hu SY, Chen YD, et al. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res. 2019;66:e12542. doi: 10.1111/jpi.12542. [DOI] [PubMed] [Google Scholar]
- 26.Singhanat K, Apaijai N, Chattipakorn SC, Chattipakorn N. Roles of melatonin and its receptors in cardiac ischemia-reperfusion injury. Cell Mol Life Sci. 2018;75:4125–49. doi: 10.1007/s00018-018-2905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai BZ, Ma WY, Wang XX, Sukhareva N, Hua BJ, Zhang L, et al. Targeting LncDACH1 promotes cardiac repair and regeneration after myocardium infarction. Cell Death Differ. 2020;27:2158–75. doi: 10.1038/s41418-020-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han D, Wang YJ, Chen JW, Zhang JB, Yu P, Zhang R, et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J Pineal Res. 2019;67:e12571. doi: 10.1111/jpi.12571. [DOI] [PubMed] [Google Scholar]
- 29.Pei HF, Du J, Song XF, He L, Zhang YF, Li XC, et al. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic Biol Med. 2016;97:408–17. doi: 10.1016/j.freeradbiomed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Hong H, Tao T, Chen S, Liang CQ, Qiu Y, Zhou YH, et al. MicroRNA-143 promotes cardiac ischemia-mediated mitochondrial impairment by the inhibition of protein kinase cepsilon. Basic Res Cardiol. 2017;112:60. doi: 10.1007/s00395-017-0649-7. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Li J, Xue K, Zhang J, Wang C, Zhang QQ, et al. MicroRNA-143-3p promotes human cardiac fibrosis via targeting sprouty3 after myocardial infarction. J Mol Cell Cardiol. 2019;129:281–92. doi: 10.1016/j.yjmcc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhao XG, Sun J, Su W, Shan HT, Zhang BW, Wang YN, et al. Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. Int J Mol Sci. 2018;19:1118. doi: 10.3390/ijms19041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao XH, Zhang YL, Zhang ZY, Guo SS, Chen XB, Guo YZ. MicroRNA-96-5p represses breast cancer proliferation and invasion through Wnt/beta-catenin signaling via targeting CTNND1. Sci Rep. 2020;10:44. doi: 10.1038/s41598-019-56571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Q. Delta-catenin dysregulation in cancer: interactions with E-cadherin and beyond. J Pathol. 2010;222:119–23. doi: 10.1002/path.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang B, Tang F, Wang ZR, Qi GY, Liang XS, Li B, et al. Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/beta-catenin signaling. J Exp Clin Cancer Res. 2016;35:82. doi: 10.1186/s13046-016-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YX, Liu CZ, Luo M, Zhang ZY, Gong JN, Li JJ, et al. Chemotherapy-induced miRNA-29c/catenin-delta signaling suppresses metastasis in gastric cancer. Cancer Res. 2015;75:1332–44. doi: 10.1158/0008-5472.CAN-14-0787. [DOI] [PubMed] [Google Scholar]
- 37.Ye B, Hou N, Xiao L, Xu YF, Boyer J, Xu HD, et al. APC controls asymmetric Wnt/beta-catenin signaling and cardiomyocyte proliferation gradient in the heart. J Mol Cell Cardiol. 2015;89(Pt B):287–96. doi: 10.1016/j.yjmcc.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Y, Ho BX, Pang JKS, Pek NMQ, Hor JH, Ng SY, et al. Wnt/beta-catenin-mediated signaling re-activates proliferation of matured cardiomyocytes. Stem Cell Res Ther. 2018;9:338. doi: 10.1186/s13287-018-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misaka T, Yoshihisa A, Yokokawa T, Sato T, Oikawa M, Kobayashi A, et al. Plasma levels of melatonin in dilated cardiomyopathy. J Pineal Res. 2019;66:e12564. doi: 10.1111/jpi.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallinen P, Manttari S, Leskinen H, Ilves M, Vakkuri O, Ruskoaho H, et al. The effect of myocardial infarction on the synthesis, concentration and receptor expression of endogenous melatonin. J Pineal Res. 2007;42:254–60. doi: 10.1111/j.1600-079X.2006.00413.x. [DOI] [PubMed] [Google Scholar]