Abstract
Background:
Every 2 minutes there is a pregnancy related death worldwide, with one third due to severe postpartum hemorrhage (PPH). While international trials demonstrated efficacy of 1,000 mg TXA in treating PPH, to our knowledge there are no dose finding studies of TXA in pregnant women for PPH prevention.
Objective:
To determine the optimal TXA dose needed to prevent PPH.
Methods:
We enrolled 30 pregnant women undergoing scheduled cesarean delivery in an open-label, dose ranging study. Subjects were divided into 3 cohorts receiving 5, 10 or 15 mg/kg (max 1000 mg) of intravenous TXA at umbilical cord clamping. Inclusion criteria were ≥34 week’s gestation and normal renal function. Primary endpoints were pharmacokinetic and pharmacodynamic profiles. TXA plasma concentration greater than 10 μg/mL and maximum lysis less than 17% were defined as therapeutic targets independent to the current study. Rotational thromboelastometry (ROTEM) of tissue plasminogen activator (tPA)-spiked samples was used to evaluate pharmacodynamic profiles at time points up to 24 hours after TXA administration. Safety was assessed by plasma thrombin generation, D-dimer, and TXA concentrations in breast milk.
Results:
There were no serious adverse events including venous thromboembolism. Plasma concentrations of TXA increased in a dose-proportional manner. The lowest dose cohort received an average of 448 ± 87 mg TXA. Plasma TXA exceeded 10 μg/mL and maximum lysis was less than 17% more than 1 hour after administration for all TXA doses tested. Median estimated blood loss for cohorts receiving 5, 10, or 15 mg/kg TXA was 750, 750 and 700 mL, respectively. Plasma thrombin generation did not increase with higher TXA concentrations. D-dimer changes from baseline were not different among the cohorts. Breast milk TXA concentrations were 1% or less than maternal plasma concentrations.
Conclusions:
While large randomized trials are necessary to support clinical efficacy of TXA for prophylaxis, we propose an optimal dose of 600 mg in future TXA efficacy studies to prevent PPH.
Trial Registration:
ClinicalTrials.gov Identifier: NCT03287336; https://clinicaltrials.gov/ct2/show/NCT03287336
Keywords: postpartum hemorrhage, prevention, tranexamic acid, pharmacokinetic, pharmacodynamic
Introduction
Every 2 minutes there is a pregnancy-related death worldwide, with one third attributable to postpartum hemorrhage (PPH).1,2 Uterotonic agents are typically used to prevent PPH, however, about 6.0 per 1000 U.S. delivery hospitalizations require additional medical and surgical interventions, such as blood transfusion, uterine tamponade, uterine artery embolization, or hysterectomy.3,4 The fact that 1.5% patients die from bleeding and worldwide most pregnant women give birth in facilities that do not have ready access to additional interventions necessitates optimizing alternative preventative therapies.
Tranexamic acid (TXA), an anti-fibrinolytic agent routinely used in cardiac, trauma and orthopedic patients to limit hemorrhage and prevent mortality5–8 has shown promise for reducing PPH. National and international guidelines have incorporated use of TXA in PPH management.9,10 The WOMAN Trial showed a 31% reduction in death from bleeding when TXA was given to women for treatment of PPH within 1 to 3 hours after delivery.11 Doses used for the WOMAN Trial were extrapolated from non-pregnant populations.7
Historically, a majority of medications pregnant women receive are used in an “off-label” manner and there are substantial physiologic alternations of pregnancy that may impact drug distribution and clearance.12 The dearth of empiric data in pregnancy has limited the uptake of TXA use in the potentially high impact setting of PPH. Ideally, integrated pharmacokinetic (PK) and pharmacodynamic (PD) data and a pharmacometrics approach should be used to guide drug development and regulatory decisions.13,14 However, few studies have integrated such data and, until now, PK data alone typically have guided dosing recommendations. PD endpoints for fibrinolysis inhibition are not universally agreed upon and the closest in vivo marker is D-dimer, although pregnancy and surgery both cause elevations in normal D-dimer levels. TXA mechanism of action inhibits the degradation (lysis) of fibrin to byproducts such as D-dimer.15–17
Notably, there is wide variation of recommended TXA dosing prophylactically in cardiac18 and non-cardiac19,20 surgery, and no intravenous PK/PD data to guide dosing in peripartum women. PK studies in the cardiac surgery and pediatric literature suggest that when used prophylactically during surgery, low doses of TXA (10 mg/kg) can achieve similar therapeutic effects as higher doses (100 mg/kg).18,21 These data suggest that use of a low dose TXA in the peripartum setting may provide efficacy, while limiting exposures to both mother and fetus/newborn.
Clinical trials using TXA for prevention of PPH have not been large enough to fully address the risk of thrombosis given the baseline rates in the general population.22,23 Since pregnant women have increased thrombotic risk, medications that compromise endogenous fibrinolytic mechanisms, even at standard doses, must be carefully investigated. Moreover, gastrointestinal side effects and seizures have been observed in patients receiving high TXA doses.24 The pressing need for safe utilization of TXA to reduce complications in pregnant women has been identified in comprehensive Cochrane reviews5 as well as by the World Health Organization.25
In this study we evaluated the safety, PK, and PD of TXA administered prophylactically at the time of cesarean delivery. We also have generated new data on the concentration of TXA in breast milk.
Materials and Methods
Study patients
Pregnant women (18 to 50 years of age) were eligible for the study if they were scheduled for cesarean delivery and were ≥ 34+0 weeks gestation. Prior to receiving TXA pregnant women had a documented normal serum creatinine (serum creatinine < 0.9 mg/dL). Women were excluded if they had active thrombotic or thromboembolic disease, a history of arterial or venous thromboembolic events, inherited thrombophilia or preexisting conditions that predisposed them to thromboembolic events (i.e. antiphospholipid antibody syndrome, lupus), a subarachnoid hemorrhage, an acquired defective color vision, history of seizure disorder, known renal dysfunction, multiple gestations, hypersensitivity to TXA or anti-fibrinolytic therapy or a history of liver dysfunction.
Trial design
In this open-label, dose ranging study women were consecutively assigned to cohort 1, cohort 2 or cohort 3 and received either 5, 10 or 15 mg per kilogram of TXA, respectively, intravenously (IV) at the time of umbilical cord clamping after cesarean delivery (figure 1). A maximum dose of 1000 mg was administered. TXA was administered over 15 minutes using an infusion pump. Timing of blood samples was relative to the completion of the intravenous infusion: within 10 minutes, 30–60 minutes, 1.5–3 hours, 4–6 hours, 7–8 hours and 24 hours after drug administration was completed. A pre-drug sample was also drawn when intravenous access was initially obtained on the day of surgery.
Figure 1. Study profile.
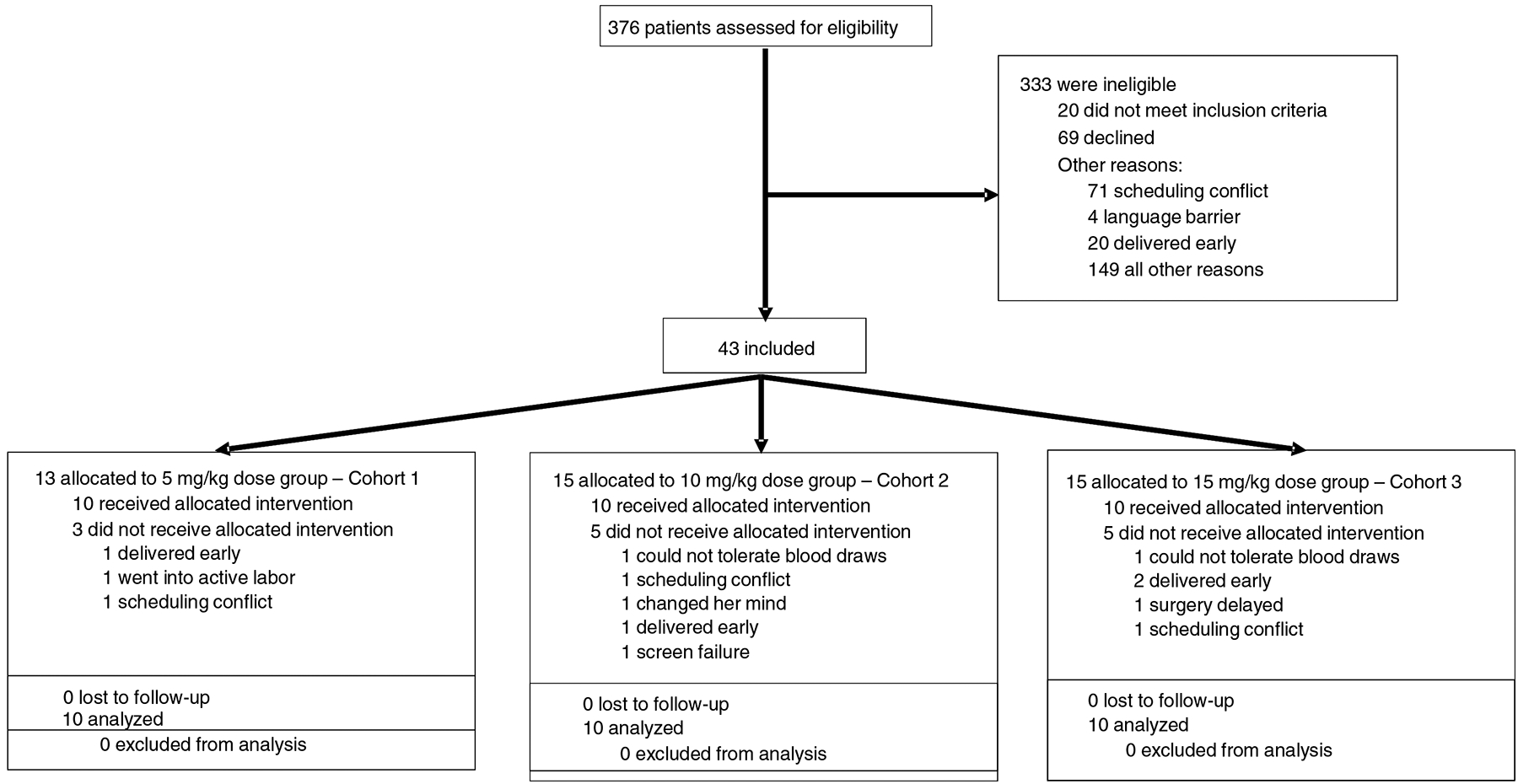
CONSORT diagram of screened, enrolled, and treated tranexamic acid cohort 1, cohort 2 and cohort 3 patients, including reasons for non-enrollment and discontinuation.
Blood samples were drawn into two citrated tubes (Becton Dickinson Vacutainer tubes, Na Citrate 0.109 M, 3.2%); the first one was used for D-dimer measurements and second one for additional coagulation assays. The third tube drawn contained potassium ethylenediaminetetraacetic acid (Becton Dickinson Vacutainer tubes, K2 EDTA 7.2 mg) and was used for TXA measurements. Platelet-poor plasma was obtained by centrifugation (3000xg, 15 minutes) and the plasma was collected, flash frozen on dry ice, and stored at −70°C for further coagulation studies. Breast milk sampling occurred at time points coinciding with when the mother was feeding the newborn and able to provide it.
Bioanalysis
Plasma concentrations of TXA in clinical samples were determined by ultra-high-performance liquid chromatography-tandem mass spectrometry. 4-aminocyclohexanecarboxylic acid was used as the internal standard with some modification compared to the published methods.26,27 Specifically, plasma samples were prepared for analysis using a protein precipitation process with acetonitrile. Sample extracts were analyzed using normal phase chromatography with a Waters BEH HILIC column (2.1*100 mm, 1.7 um, Waters Corp., Milford, MA, USA) followed by detection with a Waters Xevo TQ-XS mass spectrometer (Waters Corp., Milford, MA, USA). The lower limit of quantification for TXA was 0.04 μg/mL. Concentrations over 25 μg/mL were prepared first by dilution with blank plasma before analysis.
Rotational thromboelastometry
Rotational thromboelastometry (ROTEM) measures viscoelastic changes in blood during clot formation using whole blood. Since women do not typically experience systemic lysis with cesarean delivery, we used a modification of ROTEM28 in which fibrinolysis was stimulated ex vivo using tissue plasminogen activator (tPA). Since plasma tPA concentrations in pregnant women with hyperfibrinolysis are not known, the tPA concentration chosen was based on published values from trauma patients with hyperfibrinolysis.29 Briefly, whole blood was recalcified per manufacture instructions for EXTEM (Instrumentation Laboratory, Bedford, Massachusetts) and tPA (0.3 μg/mL, final concentration, Genentech, San Francisco, California) was added ex vivo prior to starting the ROTEM analysis. A new aliquot of freshly-thawed tPA was used at each time point. The maximum lysis (ML) parameter was the pharmacodynamic target chosen since it reflects the degree of lysis potential.30 We also evaluated the clotting time and maximum clot firmness in reactions performed in the absence of tPA to assess potential hypercoagulable effects of TXA. Software for ROTEM delta machine (version 2.7.1) provided measurements of clotting time, maximum clot firmness and ML.
Calibrated automated thrombography and D-dimer
Thrombin generation in platelet-poor plasma was measured by calibrated automated thrombography, as described.31,32 In this experiment 80 μL of plasma was first mixed with 20 μL solution containing tissue factor and phospholipid vesicles (Synapse Research Institute, Maastricht, the Netherlands). Reaction was initiated by addition of 20 μL solution containing fluorogenic thrombin substrate and CaCl2 (Diagnostica Stago, Parsippany, NJ). Final concentrations were 0.5 pM tissue factor, 4 μM phospholipids, 0.416 mM thrombin substrate and 16.6 mM CaCl2. Thrombin generation results were calculated using Thrombinoscope Analysis software Version 5.0.0.742 (Thrombinoscope BV). D-dimer was measured in the hospital laboratory using an immune-turbidimetric STA Compact by Diagnostica Stago (Parsippany, NJ).
Outcome measures
Primary endpoints were PK and PD profiles. TXA plasma concentrations were used as PK end points and ROTEM ML parameter, D-dimer and thrombin generation were used as PD end points. A concentration of 10 μg/mL was defined as the therapeutic threshold since this is a commonly cited target concentration.15,21,33 Normal reference range for ML (<17%) in the immediate postpartum period was used as the target PD goal range.34 Both PK and PD targets were defined prior to the start of the study. Safety outcomes included adverse events, such as seizures or thromboembolic events, need for blood or platelet transfusion and side effects such as nausea/vomiting. Maternal and neonatal outcomes were assessed upon discharge and at 2 and 6 weeks postpartum. Breast milk TXA concentrations were collected if available. Clinical endpoints, such as estimated blood loss (EBL) and postoperative hemoglobin/hematocrit values, were also obtained. EBL was reported by clinical estimation.
Trial oversight
The institutional review board at George Washington University approved the protocol (IRB# 041737) and prior to study initiation the trial was registered (NCT 03287336). The study was conducted from February 2018 to May 2019. An external data and safety monitoring committee reviewed data acquisition and safety outcomes.
Data analysis
A target of 30 subjects was calculated to be sufficient to allow the precise estimation of the mean of the PK parameters with 20% precision and at least 95% confidence, based on the proposed approach by Wang et al.35,36 For PD ML outcome, assuming a 30% change in ML at the nadir, with a 50% variability or standard deviation in fibrinolysis measurements, we predicted 24 subjects would be required at 80% power and at two-sided significance level of 0.05. Assuming a maximum of 20% dropouts during the study, 30 subjects were enrolled in the study to ensure that data from 24 subjects would be available for the analysis. A population modeling approach carried out by Pumas v0.10.0 (www.pumas.ai)37 was used to characterize the PK and concentration-pharmacologic effect relationship of TXA. The general approach employed for the current analysis was similar to previous reports.38,39 Subsequently, the individual concentration-effect relationships were utilized to derive a dosing regimen to maximize achieving therapeutic targets (Appendix). The probability of patients reaching targets at doses ranging from 300 mg to 800 mg were simulated. Mixed effects model repeated measures analysis was performed using change from baseline for D-dimer concentration and thrombin generation parameters (lag time, time to peak, peak, velocity, endogenous thrombin potential), in order to explore their potential relationship with TXA concentration and time.
Results
Study population
A total of 30 pregnant women, with 10 patients in each cohort, were enrolled prior to cesarean delivery (table 1). The ranges of ages and weights were similar across the cohorts for all patients. One patient in Cohort 1 could not continue with blood draws after the 2nd blood draw following drug administration, and one patient in Cohort 2 missed the 7–8 hour blood draw. All other blood samples were obtained and follow up completed.
Table 1.
Baseline demographic and clinical characteristics included in the primary analysis by study group.
| Total (n=30) | Cohort 1 (n=10) | Cohort 2 (n=10) | Cohort 3 (n=10) | |
|---|---|---|---|---|
| Age (years) | 33 (23–41) | 34 (25–40) | 31 (24–38) | 33 (23–41) |
| Weight (kg) | 86.5 (59.5147.5) | 83.3 (69.4126.6) | 85.4 (59.5125.6) | 93.5 (71.2147.5) |
| Gestational age (weeks) | 39 (34–39) | 39 (34–39) | 39 (37–39) | 39 (37–39) |
| Parity | 1 (0–5) | 2 (1–5) | 1 (0–3) | 2 (0–3) |
| Ethnicity | ||||
| White | 11 (37%) | 4 (40%) | 4 (40%) | 3 (30%) |
| Black | 16 (53%) | 5 (50%) | 5 (50%) | 6 (60%) |
| Hispanic | 1 (3%) | 1 (10%) | 0 | 0 |
| Asian | 1 (3%) | 0 | 0 | 1 (10%) |
| Other | 1 (3%) | 0 | 1 (10%) | 0 |
| Preoperative labs | ||||
| Serum Creatinine (mg/dL) | 0.5 (0.38–0.68) | 0.5 (0.47–0.52) | 0.5 (0.44–0.62) | 0.5 (0.38–0.68) |
| Hematocrit (%) | 33.9 (27.841.9) | 33.1 (30.238.4) | 34.5 (37.340.4) | 36.3 (27.841.9) |
| Platelets (×103) | 210 (93–408) | 226 (93–408) | 212 (174–381) | 251 (167–378) |
| Comorbidity | ||||
| Diabetes | 9 (30%) | 3 (30%) | 2 (20%) | 4 (40%) |
| Hypertension | 5 (17%) | 2 (20%) | 1 (10%) | 2 (20%) |
| Preeclampsia at time of delivery | 4 (13%) | 1 (10%) | 2 (20%) | 1 (10%) |
| Baby aspirin use before delivery | 7 (23%) | 1 (10%) | 3 (30%) | 3 (30%) |
Data were collected at study entry and are shown as median (range), n (%) or n/N (%). SI conversion factor: to convert creatinine to μmol/L multiply by 76.25.
Pharmacokinetic analysis
The average ± standard deviation (SD) of doses for TXA administered to patients was 447.7 mg (87.1 mg), 831.8 mg (158.8 mg), and 1000 mg (0 mg) for cohorts 1, 2, and 3, respectively. Three patients in Cohort 2 and all patients in Cohort 3 received the maximum of 1000 mg. One patient in Cohort 2 inadvertently received 1022 mg.
The average time to achieve PK threshold was calculated to be 3 minutes (range 1.8–6.6 minutes). Within 5–10 minutes after the infusion was completed, the average ± SD plasma concentration was 19.9 μg/mL (10.2 μg/mL), 30.2 μg/mL (6.13 μg/mL), and 36.9 μg/mL (6.7 μg/mL) for cohorts 1, 2, and 3, respectively. A two-compartment model was used to describe the TXA concentration-time profiles. The PK of TXA was independent of prognostic factors such as body weight, lean body weight, age, serum creatinine and creatinine clearance. The model predictions for each dosing cohort compared with the observations are shown in figure 2a. Based on prior studies of vulnerable populations, we used 10 μg/mL as the target concentration for TXA.21 All patients reached this target concentration for at least one hour after delivery (figure 2b).
Figure 2. Pharmacokinetic data.
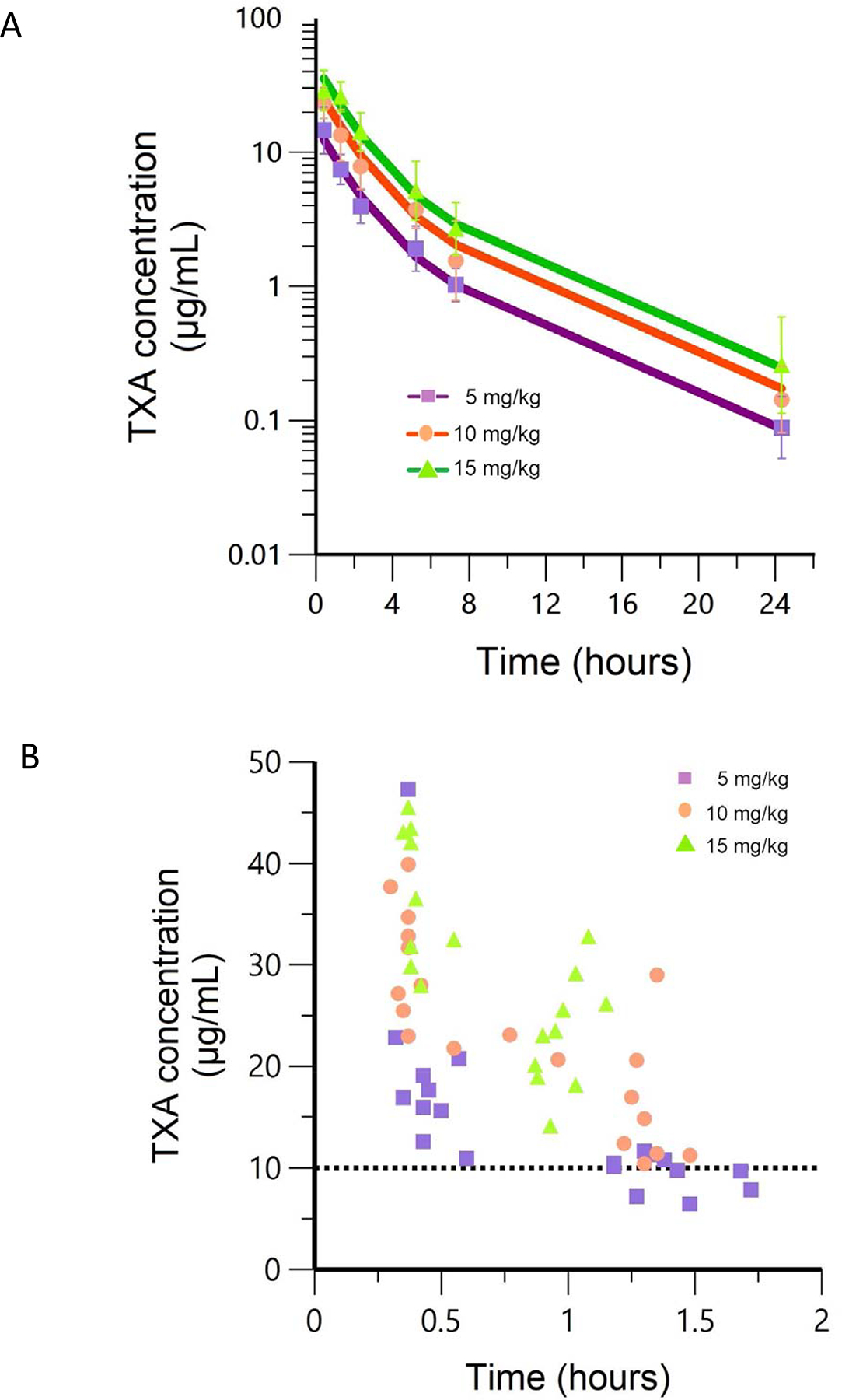
2a. Observed mean (symbols) and population mean (lines) TXA concentration-time profiles after 5 mg/kg, 10 mg/kg and 15 mg/kg IV doses derived from 30 patients. Doses are normalized to a 70 kg patient and shown on log scale. Error bars (1SD) are also shown. 2b. Actual measured TXA concentrations for each dose cohort for up to 2 hours and target pharmacokinetic concentration of 10 μg/mL for reference. All patients maintain over the 10 μg/mL threshold for at least 1 hour after administration.
Pharmacodynamic analysis by ROTEM
In samples collected prior to TXA infusion, tPA-spiked ROTEM showed 98–100% ML. Immediately post-infusion, the ML approached zero, showing reversal of the ex vivo tPA by in vivo TXA34 (figure 3a). There was partial inhibition of fibrinolysis even up to 6 hours after TXA administration. There was a more sustained impact on fibrinolysis inhibition with Cohort 2 and 3 but even in Cohort 1 doses the target PD ML of less than 17% was reached for at least one hour after delivery (figure 3b). Notably, the median clotting time 58.0 sec (IQR 12.0 sec) and median maximum clot firmness 71.0 mm (IQR 7.0 mm) on ROTEM without tPA did not change with increasing concentrations of measured TXA (data not shown).
Figure 3. Pharmacodynamic data.
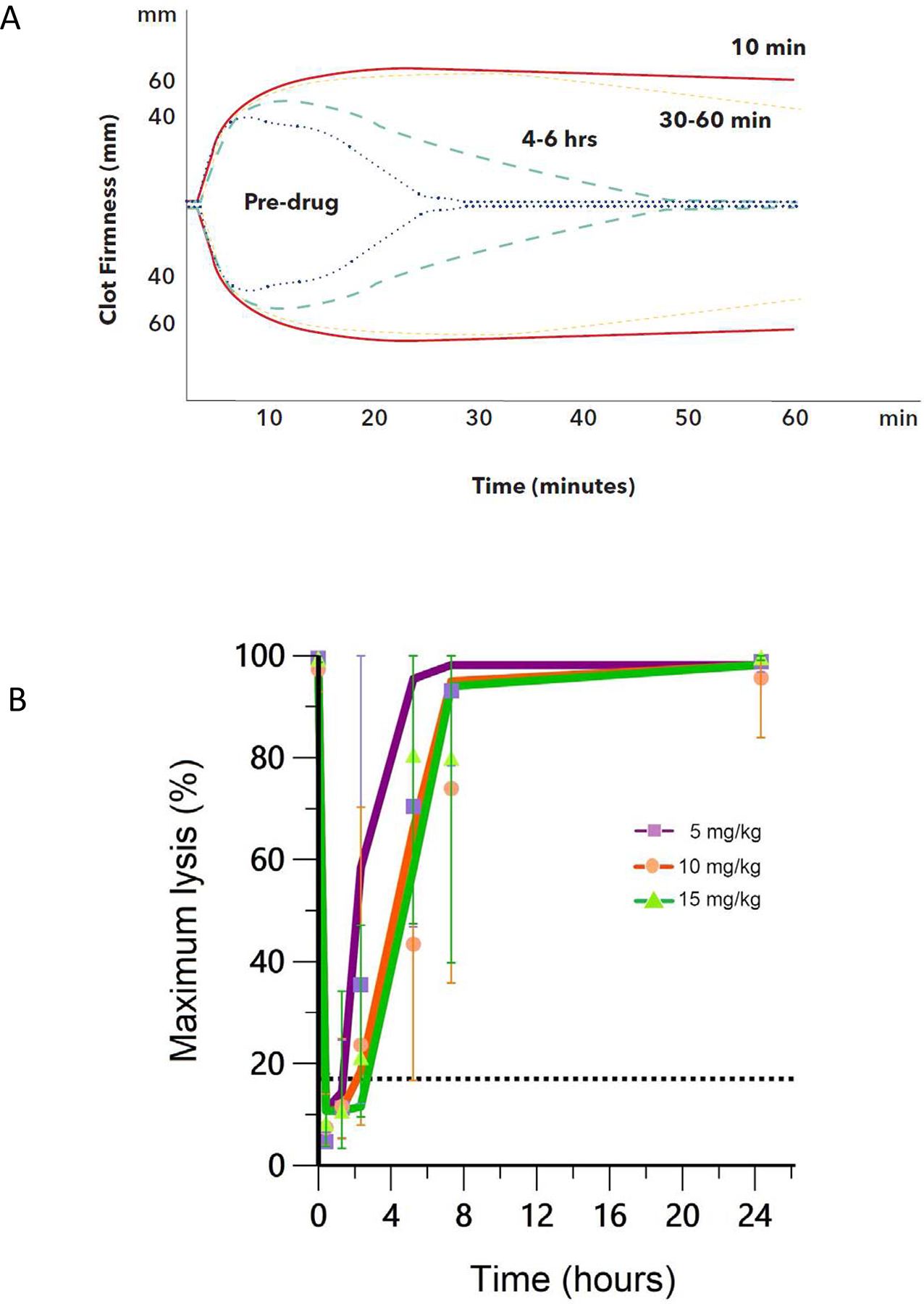
3a. Example of ROTEM profiles observed for one patient in 5 mg/kg dose group at different time points in study (pre-TXA and post-TXA within 10 minutes, 30–60 minutes and 4–6 hours). Maximum lysis is 100% prior to drug administration then approaches 0% immediately after infusion. Even at 4–6 hours post administration there are some partial inhibitory effects of TXA seen on modified tPA ROTEM assay. 3b. Summary of maximum lysis data where observed mean (symbols) and population mean (lines) of maximum lysis-time profiles after 5 mg/kg, 10 mg/kg and 15 mg/kg IV doses derived from 30 patients. Maximum lysis at 17% is shown as the reference line. Error bars (1SD) are also shown.
Simulations to derive optimal dosing
The simulations suggested that 77% of patients would maintain 10 μg/mL or higher for 30 minutes at a TXA dose of 300 mg (Table 3). A dose of 600 mg achieved this target in 97% of the patients for 60 minutes. Further, the ML is maintained below 17% for 30 minutes in 87% of the patients at the 600 mg dose.
Table 3.
Simulation modeling data showing percent of patients reaching PK (10 μg/mL) and PD targets (17% maximum lysis) 30 and 60 minutes after infusion start.
| Dose (mg) | PK after 30 minutes | PD after 30 minutes | PK after 60 minutes | PD after 60 minutes |
|---|---|---|---|---|
| 300 | 77% | 70% | 13% | 23% |
| 350 | 93% | 73% | 30% | 50% |
| 400 | 100% | 87% | 43% | 60% |
| 450 | 100% | 83% | 63% | 67% |
| 500 | 100% | 90% | 83% | 77% |
| 550 | 100% | 90% | 93% | 83% |
| 600 | 100% | 90% | 97% | 87% |
| 650 | 100% | 90% | 100% | 87% |
| 700 | 100% | 90% | 100% | 90% |
| 750 | 100% | 93% | 100% | 90% |
| 800 | 100% | 100% | 100% | 90% |
Efficacy outcomes
Median EBL in Cohort 1, 2 and 3 respectively (with ranges) was 750 mL (600–2000 mL), 750 mL (518–1000 mL) and 700 mL (400–800 mL). Median difference in hematocrit between preoperative state and postoperative day 1 value was not different between the three cohorts (2.8, 2.6 and 3.9%, respectively). The median EBL and hematocrit were unchanged with increasing doses of the drug. One patient received a blood transfusion, though this was in the setting of known placenta accreta. One patient received methergine, other than the standard oxytocin uterotonic at delivery. Of note, our study was not powered or designed to look into clinical efficacy or safety.
Safety outcomes
Intravenous administration of TXA after cord clamping had an acceptable safety profile at all doses studied. A total of 5 adverse events were observed in 30 patients (table 2). Two neonates had adverse events reported several weeks after delivery; these events were judged to be unrelated to drug. No serious maternal or neonatal serious adverse events were observed. There was no significant change in thrombin generation parameters (lag time, time to peak, peak, velocity, endogenous thrombin potential) or D-dimer with increasing concentrations of plasma TXA (p>0.4). D-dimer data showed a peak approximately at 1–2 hours and no difference in the three cohorts looking at the change from baseline (Figure 4). Breast milk TXA concentrations were available for 7 out of 30 patients and ranged from 0.06 to 0.47 μg/mL, with three patients having concentrations that were below limits of detection. Concentrations did not exceed 1% of the maximum maternal concentration measured.
Table 2.
Summary of adverse events and nausea/vomiting side effects with tranexamic acid administration.
| Cohort 1 (n=10) | Cohort 2 (n=10) | Cohort 3 (n=10) | |
|---|---|---|---|
| Any event | 9 | 8 | 6 |
| Nausea/vomiting | 8 | 6 | 4 |
| Diarrhea | 0 | 0 | 0 |
| Seizure | 0 | 0 | 0 |
| Allergic reaction | 0 | 0 | 0 |
| Maternal thrombosis | 0 | 0 | 0 |
| Facial rash | 1 | 0 | 0 |
| Itching | 0 | 1 | 0 |
| Bradycardia | 0 | 1 | 0 |
| Blood in neonate’s stool | 0 | 0 | 1 |
| Neonatal thrombosisa | 0 | 0 | 1 |
This event was judged by the investigator to be unrelated to tranexamic acid administrated.
Figure 4. Measured D-dimer change from baseline.
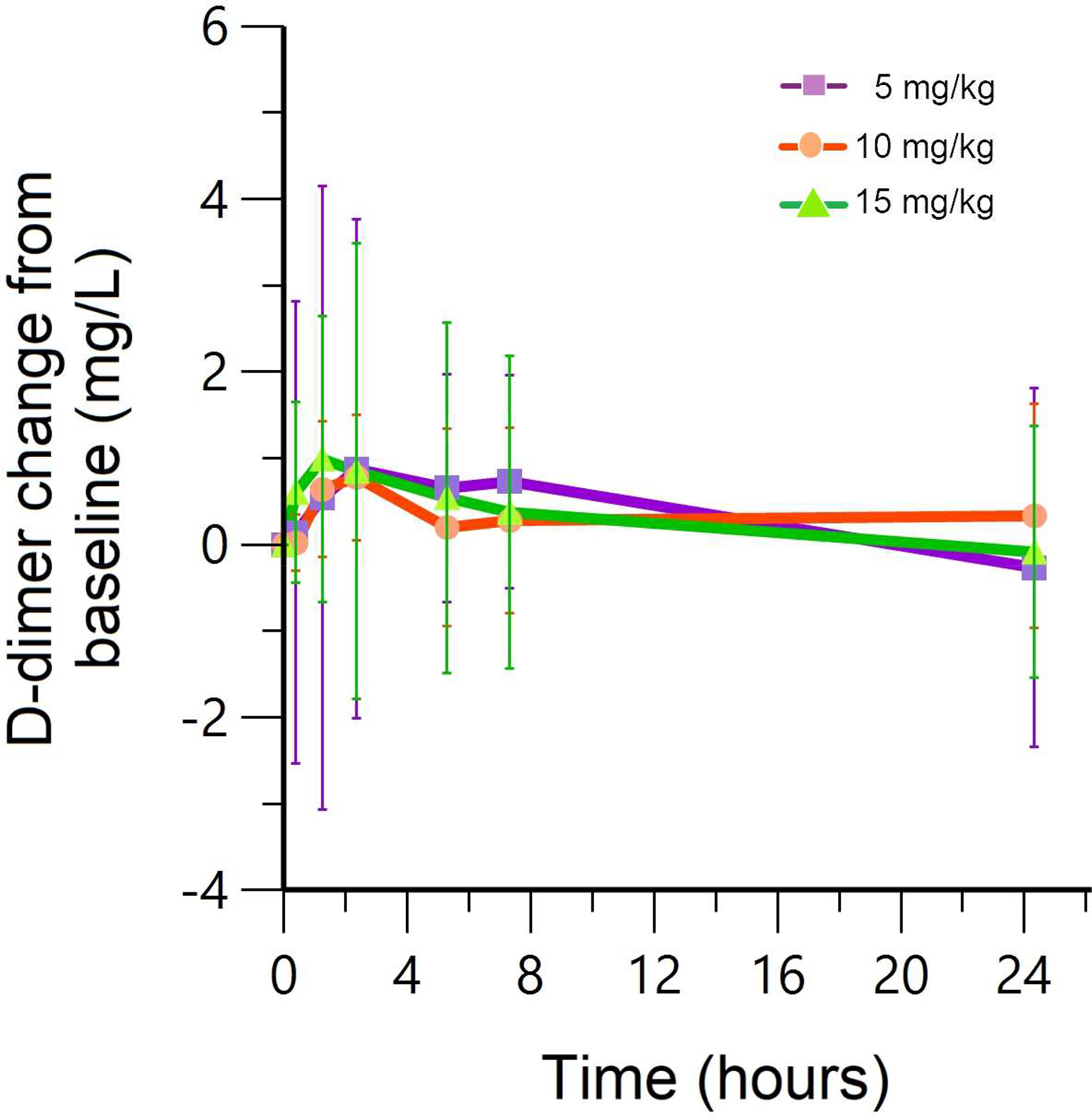
Observed mean (symbols) and population mean (lines) D-dimer concentration-time profiles after 5 mg/kg, 10 mg/kg and 15 mg/kg IV doses derived from 30 patients. Error bars (1SD) are also shown. There is no significant difference in D-dimer change from baseline with increasing TXA concentrations.
Comment
Principal Findings
In this dose ranging study, prophylactic use of low dose (5 mg/kg) intravenous TXA at the time of delivery sustained effective PK (10 μg/mL) and PD (<17% ML) targets for at least one hour after administration. Mean dose for the 5 mg/kg group was 448 mg, with a range of doses used for Cohort 1 of 347 mg to 632 mg. Our modeling data shows that while 97% of subjects treated with 600 mg reached the PK target for one hour after infusion start, 26 of 30 subjects (87%) of the patients reach the PD target. Since recent data suggest lower concentrations of plasma TXA (5–10 μg/mL) may be effective for inhibition of fibrinolysis,15 the estimates provided for effective PK and PD targets are likely conservative. In addition, there was partial inhibition of fibrinolysis (figure 3a) even up to 4–6 hours after delivery. Furthermore, PK endpoints may be more reliable given they are validated over many studies compared to limited PD data.18,21 Practically, TXA in generic form comes in vials containing 100 mg/mL; 5–10 mL vials are most readily available worldwide. Taking a holistic perspective of the safety, experience and relative reliance of PK and PD targets, we propose 600 mg of TXA be used for PPH prophylaxis in future efficacy studies.
Results
Based on dosing from the largest clinical study to date, current practice for TXA used prophylactically at delivery due to a high risk situation is 1000 mg administered IV at the time of cord clamp.11 A majority of PPH cases are diagnosed within 1 hour after delivery, and blood loss can accumulate rapidly given the average 500 mL of blood flow per minute to the uterus at full term gestation. Calling for treatment medications during an unexpected hemorrhage adds at least several minutes and may compound a coagulopathy in severe PPH. Thus, low dose prophylactic TXA, particularly for high risk groups at delivery seems especially justified, since benefits outweigh known risks.
Clinical Implications
The TRAAP efficacy study used 1000 mg of TXA for PPH prevention at delivery which did not increase risk of thrombosis or other serious adverse events.23 However, their sample size could not definitely prove safety. Our study adds reassurance from a mechanistic perspective by using safety parameters such as thrombin generation, clot formation on ROTEM and change in D-dimer levels. The peak D-dimer seen approximately 1–2 hours after infusion is consistent with existing literature and reflects the physiologic response to blood loss with cesarean delivery.40 In addition, our study showed extremely low concentrations of TXA in breast milk. These data are consistent with the findings from the only paper published to date on this topic,41 in which clinical outcomes of 21 neonates with TXA exposure through breast milk compared to 42 controls did not show any adverse long term outcomes.
Our study is similar to the TRACES protocol42 in terms of the TXA dose ranges used (500 mg and 1000 mg IV), but their study is focusing on treatment of PPH, defined as > 800 mL blood loss at cesarean delivery. Recently, oral TXA given 1 hour after delivery was shown to reach a PK target of 5 μg/mL 0.87 hour after administration.43 Thus, unless given prior to delivery, oral TXA is unlikely to effectively prevent PPH. A large ongoing efficacy study worldwide will assess if prophylactic TXA, given as 1000 mg IV at delivery will reduce major morbidity in women with severe anemia.44
Research Implications
Unfortunately, many low or middle income countries do not have ready access to intravenous materials or trained providers, so intramuscular (IM) administration is a practical alternative route. The plasma TXA concentration appears almost immediately after an IM TXA and reaches therapeutic target of 10 μg/mL less than 15 minutes after administration.45 Future studies are needed in pregnant women to determine bioavailability of IM dosing to confirm these predictions.
Strengths and Limitations
A major strength in our study design was a novel application for the modified ROTEM assay with ex vivo tPA to monitor PD response. Dirkman et al. used a similar approach to characterize hemostatic agents that may benefit a pediatric patient during surgery with factor XI deficiency.28 This modified ROTEM approach may be developed into a real-time diagnostic test to determine ‘therapeutic’ levels for antifibrinolytic therapy, where plasma TXA concentrations are more difficult to obtain.
Notably, our patient population focused on two important vulnerable groups (pregnant women and neonates) whom are often neglected in drug studies.46 Further clinical studies are also needed in the areas of alternative routes (IM, rectal, oral, or inhaled), timing of administration (at delivery vs. prior to delivery) and specific at risk populations such as pregnant women with renal impairment.43,45
Our study has limitations. We did not have a cohort that received placebo, and it is likely that surgery and undergoing childbirth both affect PD outcomes. One patient withdrew after the first three blood draws due to inability to tolerate repeated blood draws and one patient missed the 7–8 hour blood draw; both patients were included in analysis as planned. Our cohort of pregnant women on average had a higher weight than most parts of the world so we normalized our data for an average 70 kg pregnant patient. This study was not primarily designed to study efficacy given the small sample size and known inaccuracies with EBL. Lastly, the PD assay’s requirement for freshly thawed tPA (due to loss of activity at room temperatures with time) potentially limits its use in a clinical setting.
Conclusion
In conclusion, we propose an optimal dose of 600 mg in future TXA efficacy studies to prevent PPH. Large randomized trials are still needed to support clinical efficacy of TXA for prophylaxis.
Supplementary Material
Condensation.
A dose of 600 mg tranexamic acid given intravenously at cesarean delivery achieved pharmacokinetic and pharmacodynamics targets approximately 30–60 minutes after delivery.
AJOG at a Glance:
-
Why was the study conducted?
Although tranexamic acid has been proven to effectively treat postpartum hemorrhage, there is no study that has defined the optimal dose of tranexamic acid to prevent postpartum hemorrhage at delivery.
-
What are the key findings?
In this dose ranging finding study in 30 pregnant women using tranexamic acid at cesarean delivery, a dose of 600 mg given intravenously at the time of cord clamp achieved pharmacokinetic and pharmacodynamics targets approximately 30–60 minutes after delivery.
-
What does this study add to what is already known?
Future clinical efficacy studies using tranexamic acid to prevent postpartum hemorrhage should use a dose of 600 mg. This study provides a framework for defining optimal doses of tranexamic acid other than intravenous (i.e. intramuscular, rectal, oral).
Acknowledgements
Alexandra North and Ebonie Carter helped with study recruitment, Elyes Dahmanes helped with sample size calculations, Jamil Kazma helped with editing of the final manuscript, Nancy Gaba and Charles Macri supported the departmental research infrastructure at George Washington University where the study was conducted. AN, EC, JK, NG, CM during study conduct were employed by George Washington University Medical Faculty Associates and AN, EC, JK received funding support from HKA NIH awards. ED is employed by University of Maryland School of Pharmacy and no financial support was provided for services.
Funding:
The project was funded by National Institutes of Health (K23HL141640 and KL2TR001877/UL1TR001876 to HKA; R61HL141791 to ASW; T32HD087969 to JVDA). Of note, this publication was supported by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Appendix
Pharmacokinetics model
A population approach was adopted to characterize the tranexamic acid plasma concentrations, using non-linear mixed effect modeling (Phoenix 8.0; Certara, Mountain View, CA, USA). 1-compartment and 2-compartment models with first-order estimation were fitted to the data. Clearance (CL) and volume of distribution (V) were used to characterize 1- compartment model and additional parameters, including intercompartmental clearance and peripheral volume of distribution (Vt), were added in the 2-compartment model. Exponential models were used to describe the interpatient variability in the PK parameters. Additive, proportional and combined (additive and proportional) residual error models were tested to describe the residual unexplained variability on TXA concentrations. The effect of physiological and clinically relevant covariates, including body weight, age, and renal function (serum creatinine and serum creatinine) were considered to explain the variability in PK parameters.
Pharmacodynamics model
Exploratory analysis was conducted for ROTEM ML data, D-dimer data and thrombin generation data. After that, ROTEM ML data were modeled to perform the pharmacodynamics modeling. The model was parameterized with baseline lysis (E0), the maximal fractional inhibition (Imax), and the concentration of TXA causing 50% of maximal fractional inhibition (IC50). The interindividual variability in the parameters were assumed to be log normally distributed. The residual unexplained variability was described using an additive model.
Simulation to derive optimal dosing
Simulations to explore the various doses were performed using the population in the original data based on the empirical Bayes estimated parameters. The therapeutic thresholds for TXA concentration, 10 μg/mL, and ML threshold, 17%, were used to project the optimal dose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The ROTEM device was borrowed from the manufacturer. No input on the study protocol or interpretation of the findings was sought from the instrumentation company. Adam Miszta is employed by Synapse Research Institute, a member of the STAGO Diagnostic group that produces calibrated automated thrombography for thrombin generation measurements in plasma. Otherwise, the authors report no conflict of interest.
Meeting or Conference:
An oral presentation on part of these research findings was presented at the Society of Maternal-Fetal Medicine (SMFM) conference in February 2020 and additional findings were presented as an oral presentation at the virtual conference for International Society of Thrombosis and Hemostasis (ISTH), July 2020.
References
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Glob Heal. 2014;2(6):323–333. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 2.Weeks A Secondary prevention: a new era for postpartum haemorrhage? BJOG An Int J Obstet Gynaecol. 2016;123(1):128–128. doi: 10.1111/1471-0528.13606 [DOI] [PubMed] [Google Scholar]
- 3.James AH, McLintock C, Lockhart E. Postpartum hemorrhage: When uterotonics and sutures fail. Am J Hematol. 2012;87(SUPPL. 1):16–22. doi: 10.1002/ajh.23156 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Data on selected pregnancy complications in the United States. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications-data.htm. Published 2019.
- 5.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane database Syst Rev. 2011;(3):CD001886. doi: 10.1002/14651858.CD001886.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ker K, Prieto-Merino D, Roberts I, Ker MK. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br Surg. 2013;100(10):1271–1279. doi: 10.1002/bjs.9193 [DOI] [PubMed] [Google Scholar]
- 7.Olldashi F, Kerçi M, Zhurda T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 8.Bidolegui F, Arce G, Lugones A, Pereira S, Vindver G. Tranexamic Acid Reduces Blood Loss and Transfusion in Patients Undergoing Total Knee Arthroplasty without Tourniquet: A Prospective Randomized Controlled Trial. Open Orthop J. 2014;8(July 2012):250–254. doi: 10.2174/1874325001408010250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130(4):e168–e186. doi: 10.1097/AOG.0000000000002351 [DOI] [PubMed] [Google Scholar]
- 10.Dept. of Reproductive Health and Research W. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage.; 2012. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548502/en/. [PubMed]
- 11.Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105–2116. doi: 10.1016/S0140-6736(17)30638-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaffidi J, Mol BW, Keelan JA. The pregnant women as a drug orphan: a global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG An Int J Obstet Gynaecol. 2017;124(1):132–140. doi: 10.1111/1471-0528.14151 [DOI] [PubMed] [Google Scholar]
- 13.Powell JR, Gobburu JVS. Pharmacometrics at FDA: Evolution and impact on decisions. Clin Pharmacol Ther. 2007;82(1):97–102. doi: 10.1038/sj.clpt.6100234 [DOI] [PubMed] [Google Scholar]
- 14.Bhattaram VA, Booth BP, Ramchandani RP, et al. Impact of pharmacometrics on drug approval and labeling decisions: A survey of 42 new drug applications. AAPS J. 2005;7(3):503–512. doi: 10.1208/aapsj070351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picetti R, Shakur-Still H, Medcalf RL, Standing JF, Roberts I. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis. 2019;30(1):1–10. doi: 10.1097/MBC.0000000000000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: A reference table for clinicians. Obstet Gynecol. 2009;114(6):1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8 [DOI] [PubMed] [Google Scholar]
- 17.Hedengran KK, Andersen MR, Stender S, Szecsi PB. Large D-Dimer Fluctuation in Normal Pregnancy: A Longitudinal Cohort Study of 4,117 Samples from 714 Healthy Danish Women. Obstet Gynecol Int. 2016;2016. doi: 10.1155/2016/3561675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowd NP, Karski JM, Cheng DC, et al. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology. 2002;97(2):390–399. doi:00000542-200208000-00016 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Boylan JF, Klinck JR, Sandler AN, Arellano R, Greig PD, Nierenberg H, Roger SL GM. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology. 1996;85(5):1043–1048. [DOI] [PubMed] [Google Scholar]
- 20.Hedlund PO. Antifibrinolytic therapy with cyklokapron in connection with prostatectomy. A double blind study. Scand J Urol Nephrol. 1969;3(3):177–182. [DOI] [PubMed] [Google Scholar]
- 21.Goobie SM, Meier PM, Sethna NF, et al. Population pharmacokinetics of tranexamic acid in paediatric patients undergoing craniosynostosis surgery. Clin Pharmacokinet. 2013;52(4):267–276. doi: 10.1007/s40262-013-0033-1 [DOI] [PubMed] [Google Scholar]
- 22.ACOG. Practice Bulletin 196: VTE Pregnancy. 2018;132(123):1–17. [Google Scholar]
- 23.Sentilhes L, Winer N, Azria E, et al. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379(8):731–742. doi: 10.1056/NEJMoa1800942 [DOI] [PubMed] [Google Scholar]
- 24.Sharma V, Katznelson R, Jerath A, Garrido-Olivares L, Carroll J, Rao V, Wasowicz MDG. The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia. 2014;69(2):124–130. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization (WHO). Updated WHO Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage: Highlights and Key Messages from the World Health Organization’s 2017 Global Recommendation. 2017;(October):5. [PubMed]
- 26.Grassin Delyle S, Abe E, Batisse A, et al. A validated assay for the quantitative analysis of tranexamic acid in human serum by liquid chromatography coupled with electrospray ionization mass spectrometry. Clin Chim Acta. 2010;411(5–6):438–443. doi: 10.1016/j.cca.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 27.Ausen K, Pleym H, Liu J, et al. Serum Concentrations and Pharmacokinetics of Tranexamic Acid after Two Means of Topical Administration in Massive Weight Loss Skin-Reducing Surgery. Plast Reconstr Surg. 2019;143(6):1169e–1178e. doi: 10.1097/PRS.0000000000005620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dirkmann D, Hanke AA, Görlinger K, Peters J. Perioperative use of modified thrombelastography in factor XI deficiency: A helpful method to assess drug effects. Acta Anaesthesiol Scand. 2007;51(5):640–643. doi: 10.1111/j.1399-6576.2007.01284.x [DOI] [PubMed] [Google Scholar]
- 29.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–521. doi: 10.1097/SHK.0000000000000161 [DOI] [PubMed] [Google Scholar]
- 30.Bolliger D, Seeberger MD, Tanaka KA. Principles and Practice of Thromboelastography in Clinical Coagulation Management and Transfusion Practice. Transfus Med Rev. 2012;26(1):1–13. doi: 10.1016/j.tmrv.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 31.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117(18):4953–4963. doi: 10.1182/blood-2010-11-316885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114(23):4886–4896. doi: 10.1182/blood-2009-06-228940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson L, Nilsoon IM, Colleen S, Granstrand JB, Melander B. Role of Urokinase and Tissue Activator in Sustaining Bleeding and the Management Thereof With Eaca and Amca. Ann N Y Acad Sci. 1968;146(2):642–656. doi: 10.1111/j.1749-6632.1968.tb20322.x [DOI] [PubMed] [Google Scholar]
- 34.Oudghiri M, Keïta H, Kouamou E, et al. Reference values for rotation thromboelastometry (ROTEM®) parameters following nonhaemorrhagic deliveries. correlations with standard haemostasis parameters. Thromb Haemost. 2011;106(1):176–178. doi: 10.1160/TH11-02-0058 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012;52(10):1601–1606. doi: 10.1177/0091270011422812 [DOI] [PubMed] [Google Scholar]
- 36.Grassin-Delyle S, Tremey B, Abe E, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013;111(6):916–924. doi: 10.1093/bja/aet255 [DOI] [PubMed] [Google Scholar]
- 37.Rackauckas C, Noack A, Dixit V, et al. Pumas: High Performance Pharmaceutical Modeling and Simulation.
- 38.Ordonez AA, Wang H, Magombedze G, et al. Dynamic imaging in patients with tuberculosis reveals heterogeneous drug exposures in pulmonary lesions. Nat Med. 2020. doi: 10.1038/s41591-020-0770-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madabushi R, Cox DS, Hossain M, et al. Pharmacokinetic and pharmacodynamic basis for effective argatroban dosing in pediatrics. J Clin Pharmacol. 2011;51(1):19–28. doi: 10.1177/0091270010365550 [DOI] [PubMed] [Google Scholar]
- 40.Ducloy-Bouthors AS, Duhamel A, Kipnis E, et al. Postpartum haemorrhage related early increase in D-dimers is inhibited by tranexamic acid: Haemostasis parameters of a randomized controlled open labelled trial. Br J Anaesth. 2016;116(5):641–648. doi: 10.1093/bja/aew021 [DOI] [PubMed] [Google Scholar]
- 41.Gilad O, Merlob P, Stahl B, Klinger G. Outcome following tranexamic acid exposure during breastfeeding. Breastfeed Med. 2014;9(8):407–410. doi: 10.1089/bfm.2014.0027 [DOI] [PubMed] [Google Scholar]
- 42.Ducloy-Bouthors AS, Jeanpierre E, Saidi I, et al. TRAnexamic acid in hemorrhagic CESarean section (TRACES) randomized placebo controlled dose-ranging pharmacobiological ancillary trial: Study protocol for a randomized controlled trial. Trials. 2018;19(1):1–16. doi: 10.1186/s13063-017-2421-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhunthan K, Balakumar S, Navaratnaraja TS, Premakrishna S, Arulkumaran S. Plasma Concentrations of Tranexamic Acid in Postpartum Women After Oral Administration. Obstet Gynecol. 2020;135(4):945–948. doi: 10.1097/aog.0000000000003750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ker K, Roberts I, Chaudhri R, et al. Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: Study protocol for an international, randomised, double-blind, placebo-controlled trial. Trials. 2018;19(1):1–19. doi: 10.1186/s13063-018-3081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassin-Delyle S, Semeraro M, Foissac F, et al. Tranexamic acid through intravenous, intramuscular and oral routes: an individual participant data meta-analysis of pharmacokinetic studies in healthy volunteers. Fundam Clin Pharmacol. 2019;33(6):670–678. doi: 10.1111/fcp.12474 [DOI] [PubMed] [Google Scholar]
- 46.Eke AC, Dooley KE, Sheffield JS. Pharmacologic research in pregnant women — Time to get it right. N Engl J Med. 2019;380(14):1293–1295. doi: 10.1056/NEJMp1815325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


