Abstract
Over the past several decades, molecular imaging techniques to assess cellular processes in vivo have been integral in advancing our understanding of disease pathogenesis. 18F-fluorodeoxyglucose (18-FDG) positron emission tomography (PET) imaging in particular has shaped the field of atherosclerosis research by highlighting the importance of underlying inflammatory processes that are responsible for driving disease progression. The ability to assess physiology using molecular imaging, combining it with anatomic delineation using cardiac coronary angiography (CCTA) and magnetic resonance imaging (MRI) and lab-based techniques, provides a powerful combination to advance both research and ultimately clinical care. In this review, we demonstrate how molecular imaging studies, specifically using 18-FDG PET, have revealed that early vascular disease is a systemic process with multiple, concurrent biological mechanisms using inflammatory diseases as a basis to understand early atherosclerotic mechanisms in humans.
Keywords: Atherosclerosis, Inflammation, 18F- fluorodeoxyglucose (18-FDG), Immunology
Introduction
The use of radiolabeled imaging probes in molecular imaging offers the unique ability to assess molecular processes, evaluate organ function and probe underlying disease pathogenesis.1 Molecular imaging techniques are highly relevant in atherosclerosis, especially for early disease detection and understanding inflammation-driven disease progression related to coronary plaque disease activity.1 From early observations that increased deoxyglucose is trapped by macrophages in tumor cells2, 3 to determination that macrophage density is increased in atherosclerotic plaques,4, 5 18F- fluorodeoxyglucose (18-FDG) positron emission tomography (PET) was established as a useful methodology to investigate inflammatory plaques in both animal and human models.5–8 More recently, a second tracer, 18F- sodium fluoride (18F-NaF) PET, was employed for microcalcification imaging within coronary plaques to identify high risk coronary plaque disease activity and to detect the presence of ruptured plaques, which along with 18-FDG PET, may allow for identification of early- stage atherosclerosis.9–11 Such efforts in molecular imaging have advanced our understanding of the biology of atherosclerosis and aided in the design of translational studies to elucidate the effects of targeted interventions in inflammatory diseases. The purpose of this review is to highlight how the use of 18-FDG molecular imaging has shaped our understanding of atherosclerosis as an inflammatory disease, demonstrated multi-system effects of chronic inflammation and refined translational studies to dissect disease pathogenesis by combining immune cell- based laboratory studies and imaging-based clinical studies in humans.
Inflammation in the pathogenesis of atherosclerosis
Despite a wide range of contemporary treatment strategies, cardiovascular disease (CVD) remains the leading cause of death in the United States, with coronary artery disease (CAD) being the most common CVD and affecting an estimated 18.2 million American adults.12 Both clinical and pre-clinical studies demonstrate the importance of inflammation in the development and progression of atherosclerosis. Rather than a passive process of lipoprotein buildup, atherosclerosis pathogenesis is dynamic and complex with significant contributions from inflammation and immune effectors.13, 14 This immune response is initially triggered by cholesterol accumulation in the vessel wall.13 Local cellular response involving leukocyte adhesion molecules and chemokine expression further attract monocytes and mononuclear phagocytes resulting in low-grade, chronic inflammation and acceleration of atherosclerosis.14, 15 Activated T cells in plaques recruited via chemokine receptors illustrate the significant role of adaptive immunity.13, 14 Further differentiation of naive T- helper cells into separate T-helper phenotypes with immune- activating or protective effects13 demonstrates that immune regulation is nuanced and requires further careful investigation to understand specific cellular subsets, their etiology and function for development of potential therapeutic targets. The important role of immunology in early vascular disease including vascular inflammation has been previously reviewed in detail.16
Overview of 18-FDG PET imaging in vascular disease
Given their highly sensitive and non-invasive qualities, molecular imaging techniques are well- suited to advance our understanding of atherosclerosis, especially in the context of chronic inflammation. Owing to rich pre-clinical studies as well as contemporary molecular imaging techniques, the inflammatory process is recognized as central to atherosclerosis development16 from initial fatty streak formation to late plaque erosion and eventual rupture leading to myocardial infarction.17 Macrophages localized within atherosclerotic plaque consume glucose as an important respiratory substrate,18 and therefore take up detectable amounts of 18-FDG, allowing 18-FDG PET imaging to provide a non-invasive assessment of the inflammatory components of vascular disease activity (Figure 1). Given increased macrophage density in ruptured plaques as well as high levels of glucose transporter and hexokinase expression in activated macrophages,19 18-FDG PET imaging is a valuable tool to monitor atherosclerosis disease progression.
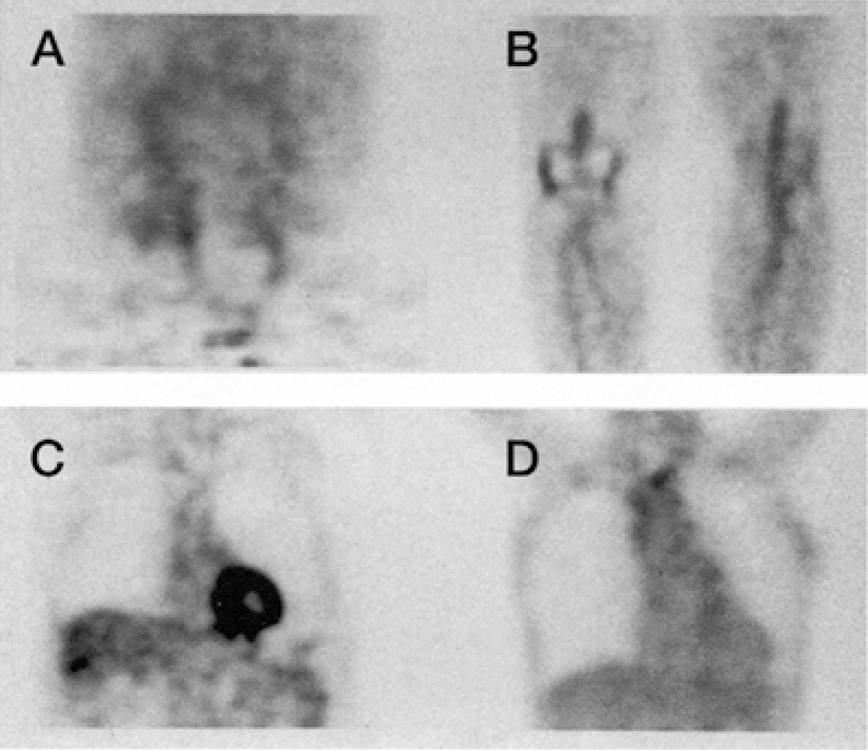
Figure 1:
18-FDG PET imaging for the evaluation of inflammatory activity of large and medium-sized arteries.
Representative PET images demonstrating 18-FDG uptake in the iliac and femoral arteries (A); popliteal arteries (B); abdominal aortic (C); aortic arch (D); in patient with psoriasis. The mean TBR was 1.26 (0.17) for the suprarenal abdominal aorta, 1.20 (0.16) for infrarenal abdominal aortic, and 1.30 (0.22) for the aortic arch.
TBR: tissue- to- background ratio
Initial diagnostic efforts in vascular inflammation focused on 18-FDG uptake within the carotid arteries. Rudd et. al observed an increase in 18-FDG uptake within culprit carotid lesions in patients with transient ischemic attack compared to plaques in asymptomatic patients.20 Utilization of this modality was then extended to imaging the aorta and peripheral arteries, allowing 18-FDG phenotyping of multiple vascular beds, an especially valuable tool for characterizing the systemic nature of inflammatory vascular disease.21–23 In particular, aortic vascular 18-FDG uptake was shown to be a reliable surrogate biomarker for stratifying patients at increased risk for CVD24 by identifying atherosclerotic plaque with high-risk morphological features,25,26 and predicting future cardiovascular events.24 Moreover, aortic vascular 18-FDG uptake correlated with coronary plaque burden as assessed by cardiac computed tomography angiography (CCTA) both in human immunodeficiency virus (HIV)26 and psoriasis.27 These findings suggest that elevated vascular uptake of 18-FDG, especially in the aortic arch, complements anatomic imaging techniques which delineate coronary plaque composition suggesting the importance of 18-FDG uptake in the aorta in identifying patients at high risk for coronary artery disease.
Not only is 18-FDG uptake a reliable diagnostic marker, it also varies in response to treatment, allowing for both disease diagnosis and treatment monitoring. Prior studies demonstrating attenuation of plaque inflammation in the thoracic aorta, carotid arteries and coronary arteries after statin threapy28–30 and lifestyle modification31 suggest that 18-FDG PET is a consistent method to monitor the effects of drug interventions on vascular disease through quantification of changes in early inflammatory disease burden which may precede changes in plaque morphology.32,22,23 Additionally, because inflammatory pathways and traditional lipid pathways both contribute to the pathogenesis of atherosclerosis, molecular imaging to assess inflammation may be useful to identify residual risk in patients already optimized on statin therapy.
18-FDG PET application in chronic inflammatory diseases
The important contribution of chronic inflammation to CVD has been observed in patients with chronic inflammatory diseases. Compared to the general population, chronic inflammatory diseases have been associated with about a two-fold increased risk of developing CVD.33 Importantly, application of traditional risk assessment tools to patients with chronic inflammatory diseases such as psoriasis and rheumatoid arthritis has been shown to underpredict clinical events,34–38 prompting recommendations that chronic inflammatory disease be considered a risk- enhancing factor.39 Hence, capturing subclinical CVD using imaging in this population may improve risk prediction beyond traditional risk assessment, especially in intermediate- risk individuals.40 Indeed, 18-FDG PET studies in psoriasis, rheumatoid arthritis and human immunodeficiency virus (HIV) have shown heightened premature atherosclerosis and vascular 18-FDG uptake compared to healthy populations, thus offering a potential explanation for the increased risk of major adverse cardiac events observed in large epidemiological studies.34, 37 For example, in those with rheumatoid and psoriatic arthritis, joint inflammation severity as assessed by joint 18-FDG uptake correlated with vascular 18-FDG uptake.33, 41 In individuals with HIV infection, 18-FDG uptake in lymph nodes correlated with both nodal HIV activity and coronary atherosclerosis including high-risk coronary plaque.26, 42 Finally, 18-FDG uptake evaluation also improves long-term cardiovascular event prediction.24, 43 Taken together, these findings suggest a potential role for 18-FDG in molecular imaging-based evaluation of atherosclerosis in those with chronic inflammatory conditions whose cardiovascular risk may be under appreciated by conventional risk assessment.
18-FDG PET application in stress- mediated cardiovascular disease
Because maladaptive physiologic responses occur in multiple organ systems in chronic inflammatory diseases, simultaneous assessment of inflammation in several tissues better enables assessment of overall risk. Chronic psychosocial stress, independent of traditional cardiovascular risk factors, has been increasingly recognized as a potent contributor to the development of adverse cardiovascular outcomes ranging from hypertension to diabetes mellitus and CAD.44, 45,46 Although the exact mechanisms are poorly understood, complex neuro- immune- arterial axis involving dysregulation of the hypothalamic- pituitary- adrenocortical (HPA) axis, stress-induced immune dysregulation characterized by increased leukopoiesis from the bone marrow, and cytokine release propagating downstream endothelial and vascular dysfunction have all been proposed as mechanisms linking stress to CVD.45, 47 Because the limbic system and specifically the amygdala regulates the HPA axis,48, 49 the recent application of 18-FDG in quantifying perceived stress as represented by increased amygdalar 18-FDG uptake permits assessment of heighted neural biological activity to understand perceived stress on CV risk (Figure 2).45 Increased 18-FDG uptake in the amygdala, suggesting a higher stress response, was associated with worsening vascular 18-FDG uptake and greater risk for subsequent CV events.50 18-FDG uptake in the amygdala was also related to noise pollution exposure, which associated with vascular inflammation and major adverse cardiac events.51 Additional 18-FDG assessment within the bone- marrow provides information on leukopoietic activity (Figure 3) which was shown to be related to heighted amygdalar activation, vascular inflammation and increased future CVD events.50, 52
Figure 2:
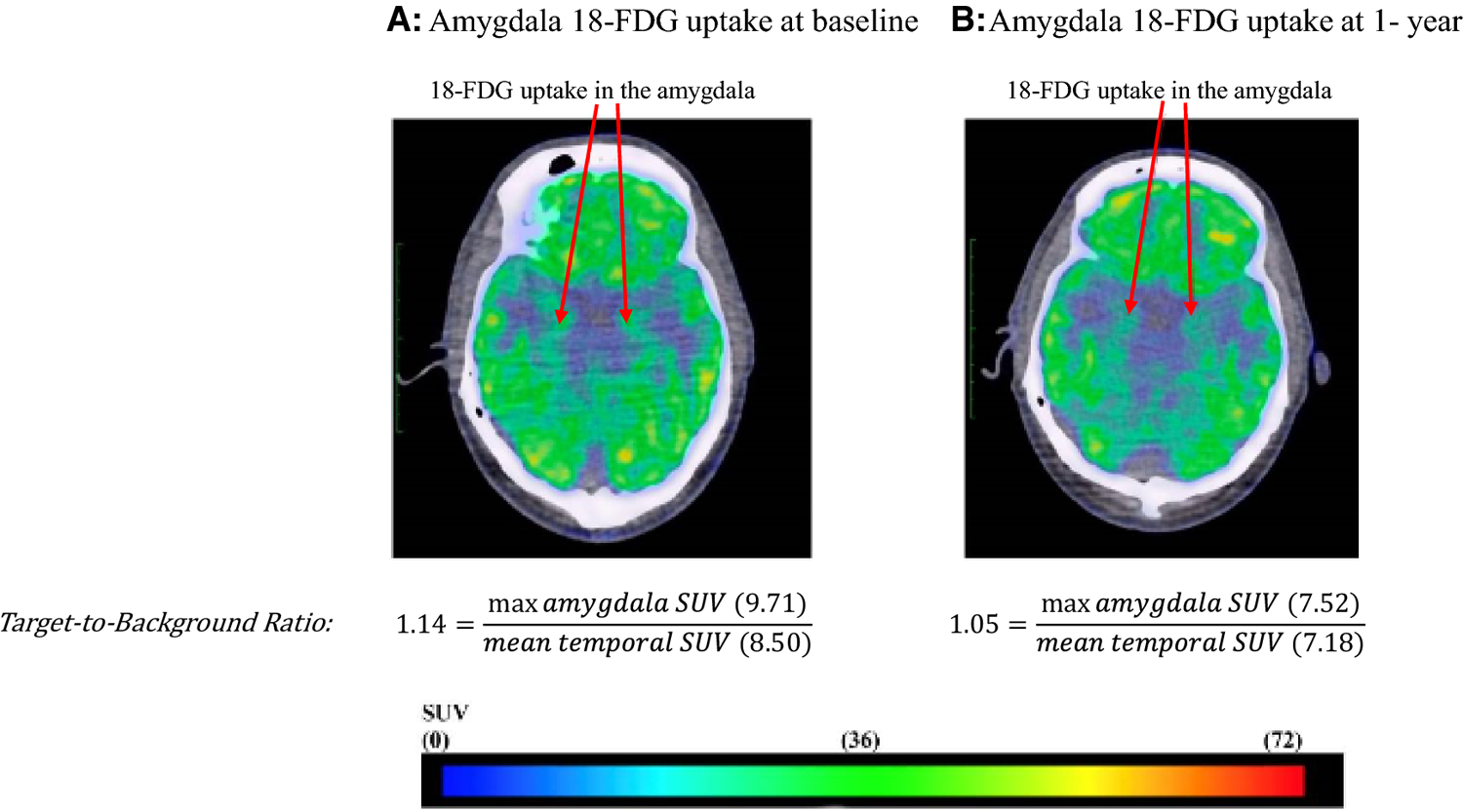
18-FDG PET/CT imaging for the evaluation of neural biological activity of the amygdala.
Representative fused PET/CT images from a patient with psoriasis who had reduction in psoriatic skin disease activity at baseline (A) and one year (B) showing decreased 18-FDG activity in the amygdala.
SUV: standard uptake value
Figure 3:
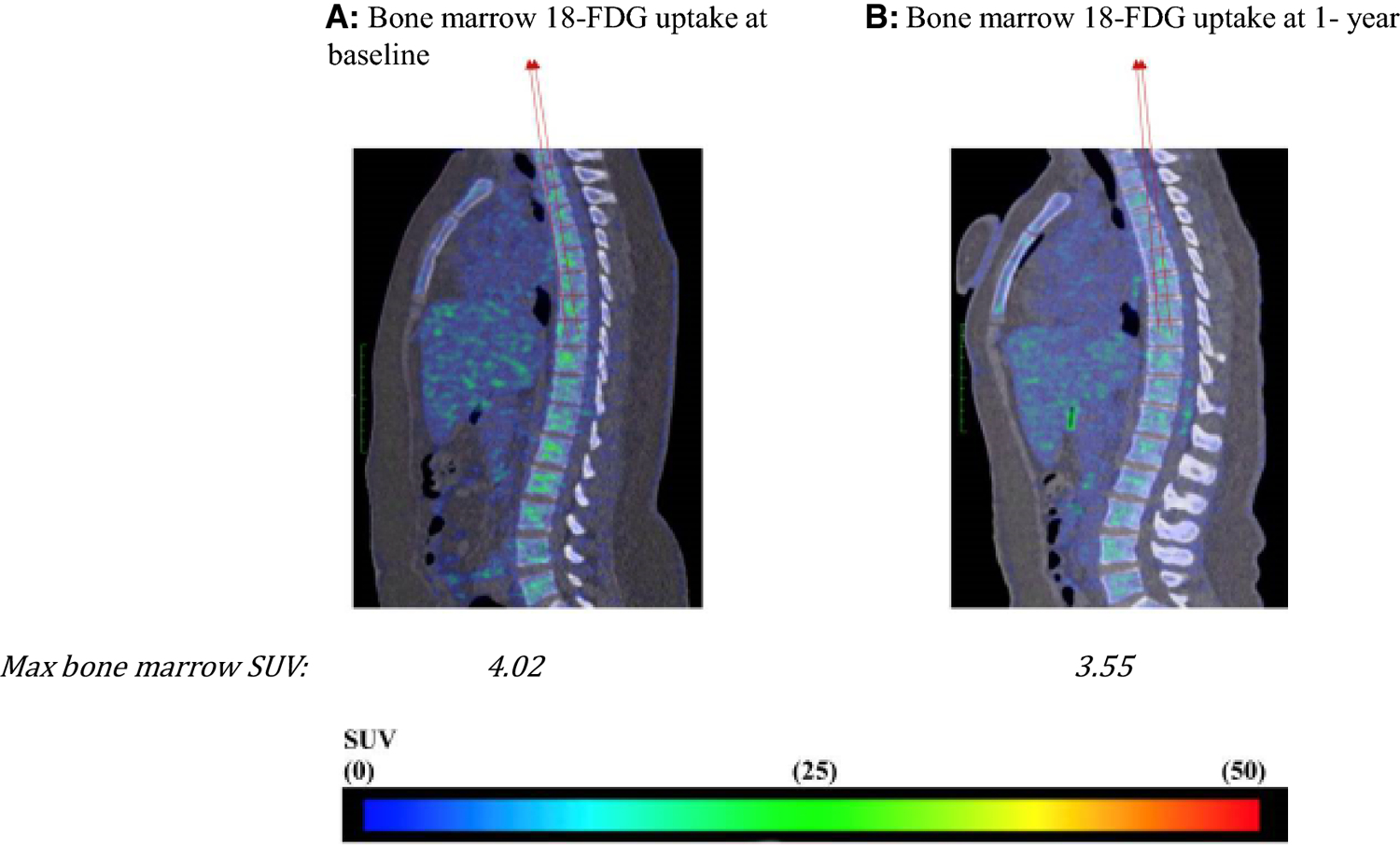
18-FDG PET/CT imaging for the evaluation of leukopoietic bone marrow activity.
Representative fused sagittal PET/CT images from a patient with psoriasis who had reduction in psoriatic skin disease activity at baseline (A) and one year (B) showing decreased 18- FDG activity in T1-L5 vertebrae.
SUV: standard uptake value
An example of 18-FDG PET application to advance translational science
Given that atherosclerosis development is accelerated in chronic inflammatory diseases, psoriasis, a chronic inflammatory, immune-mediated skin disease serves as a model to study the inflammatory contributions of vascular disease, especially since it impacts lipid handling and affects several key tisssues involved in early atherogenesis including skin, joints, liver, blood vessels (Figure 4). Psoriasis affects 2–3% of the adult US population and heightens the risk of developing early-onset atherosclerosis related to a variety of pathways leading to vascular dysfunction.35, 53, 54 Furthermore, patients with psoriasis have elevated high sensitivity C-reactive protein (hs-CRP) levels, heightened inflammatory biomarkers such as GlycA, and increased inflammatory cytokines in circulation.55, 56 18-FDG PET imaging is valuable in understanding the systemic nature of chronic inflammation by demonstrating that patients with psoriasis have increased skin inflammation as evidenced by increased uptake of 18-FDG in the skin (Figure 5), joints and liver compared to those without psoriasis (Figure 6). An important early observation was that aortic vascular inflammation as measured by 18-FDG PET was associated with skin disease severity independent of traditional cardiovascular risk factors (Figure 7) providing important evidence of external inflammation relating to internal inflammation. Those patients with more severe skin disease also had increased aortic wall thickness as measured by cardiac magnetic resonance imaging (CMR)57 (Figure 8) which was significantly associated with increased 18-FDG PET uptake in the aorta, suggesting that vascular inflammation by FDG captures early atherosclerotic disease.57 Most importantly was the fact that aortic vascular inflammation associated with non-calcified coronary plaque burden by CCTA, providing strong evidence that aortic vascular inflammation serves as a reliable surrogate marker of high-risk coronary plaque (Figure 9). 27, 35, 58, 59 Another important contribution facilitated by 18-FDG was proof of concept that treatment and withdrawl of inflammation impacted vascular disease. For example, when psoriasis was treated with biologic therapies, there was a 6% reduction in aortic vascular 18-FDG uptake after 1 year of therapy (Figure 10), a finding comparable to the effect of a low dose statin.60 This study’s findings provided preliminary evidence that psoriasis inflammation and its treatment can be used to test whether changing systemic inflammation in humans modifies coronary atherosclerosis.9, 10, 61, 62
Figure 4:
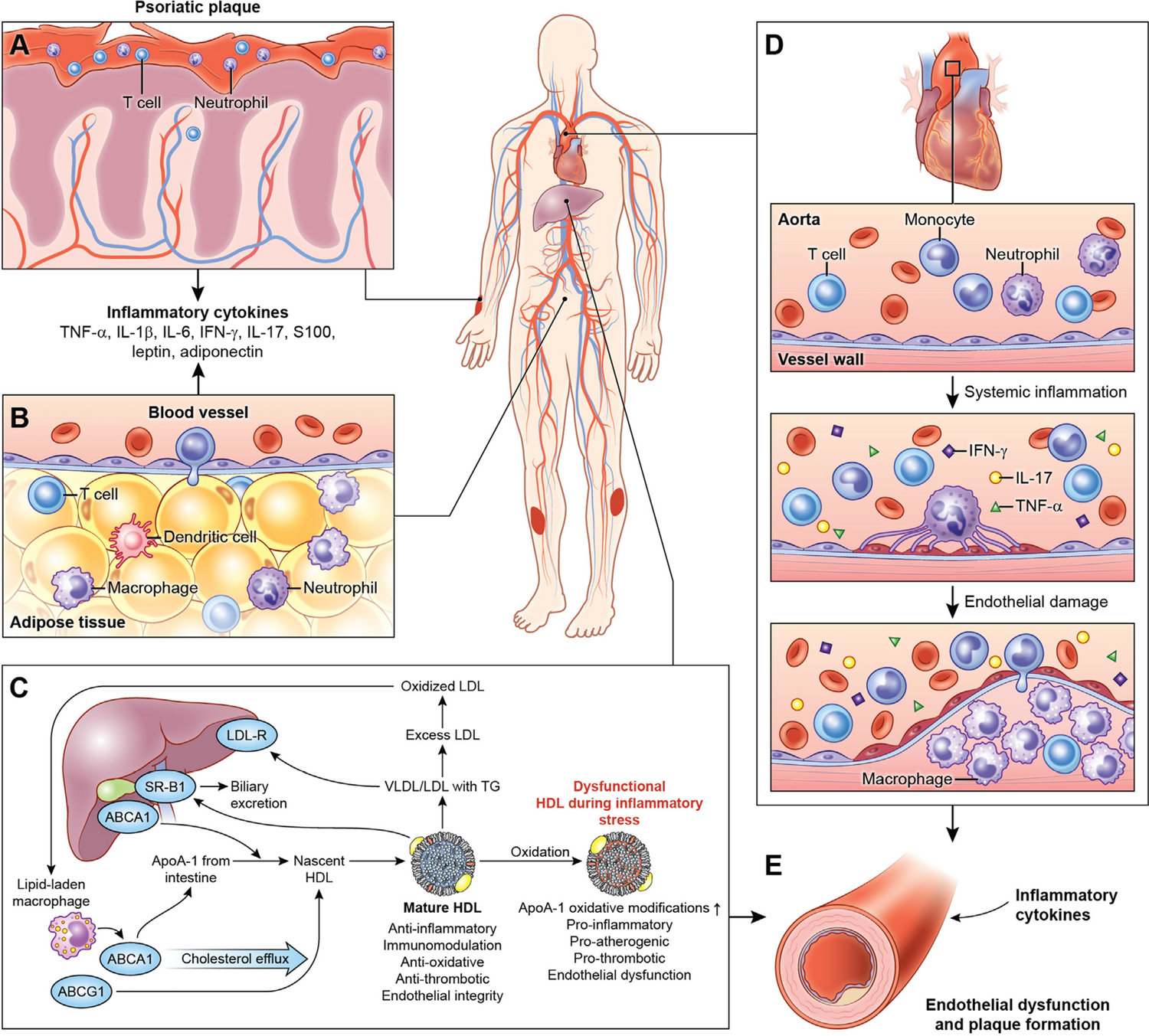
Psoriasis as a model to study inflammatory contributions of vascular disease.
Psoriasis is a chronic systemic inflammatory disease associated with increased circulating pro-inflammatory cytokines and immune effectors (A), adipose tissue dysfunction (B), lipid profile derangement (C), cellular components, cholesterol crystals and lipoprotein accelerating atherosclerosis (D) and endothelial dysfunction (E).
Figure 5:
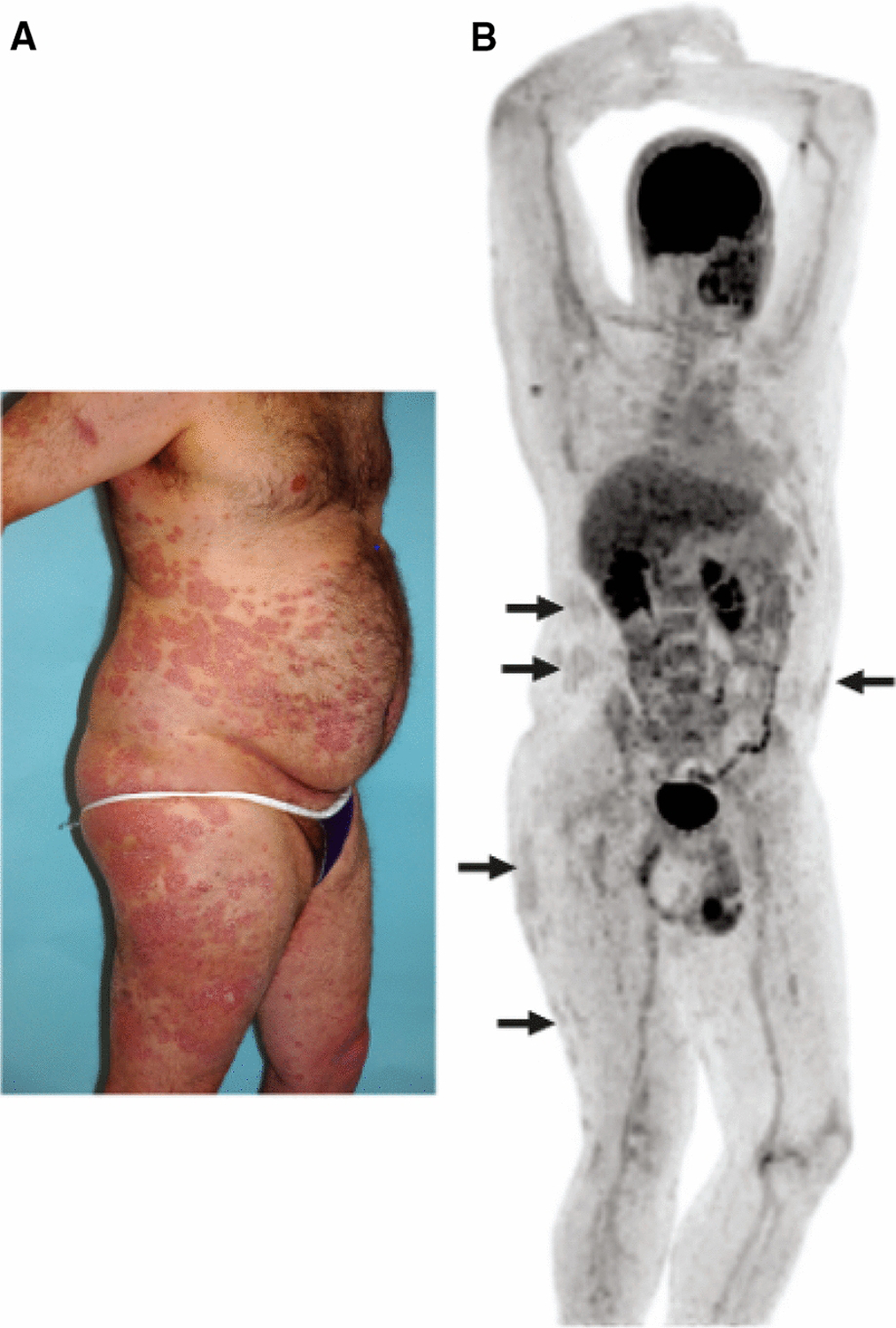
18-FDG PET imaging for the evaluation of skin inflammation in psoriasis.
Focal areas of extensive skin inflammation related to plaque psoriasis (A) corresponds to similar distribution of areas of 18-FDG uptake on PET (B).
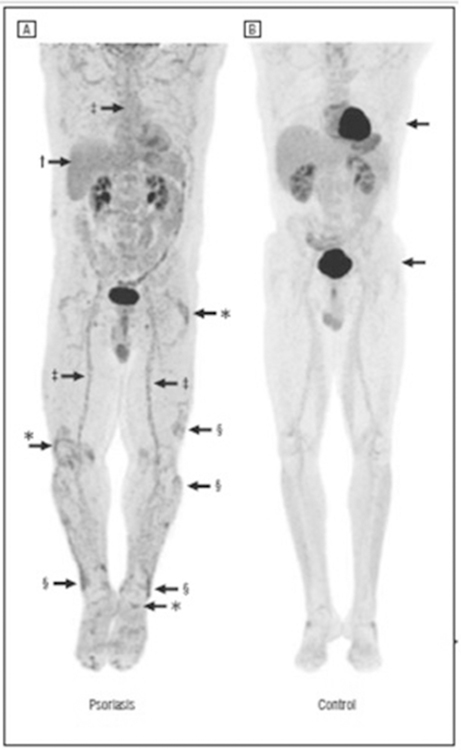
Figure 6:
18-FDG PET imaging demonstrates increased systemic inflammation in psoriasis compared to control.
Multifocal areas of increased 18-FDG uptake on PET are observed in a patient with psoriasis (A) compared to control patient (B). 18-FDG uptake is noted within the myocardium (top arrow) within the range of normal variation and also in the kidneys and bladder (bottom arrow), where 18-FDG is excreted. *FDG uptake in the right knee joint (standardized uptake value [SUV], 3.0) and distal right quadriceps tendon, left trochanteric bursa, and left ankle in asymptomatic patient with psoriasis. †Moderately diffusely increased FDG uptake throughout the liver (SUV, 1.64) consistent with increased hepatic inflammation. ‡Diffuse FDG uptake in the aortic wall (SUV, 1.29–1.72) and in the femoral arterial tree, consistent with vascular inflammation. §Focal areas of FDG uptake in skin consistent with inflammation in thick plaques in lower extremities. SUV: standard uptake value
Figure 7:
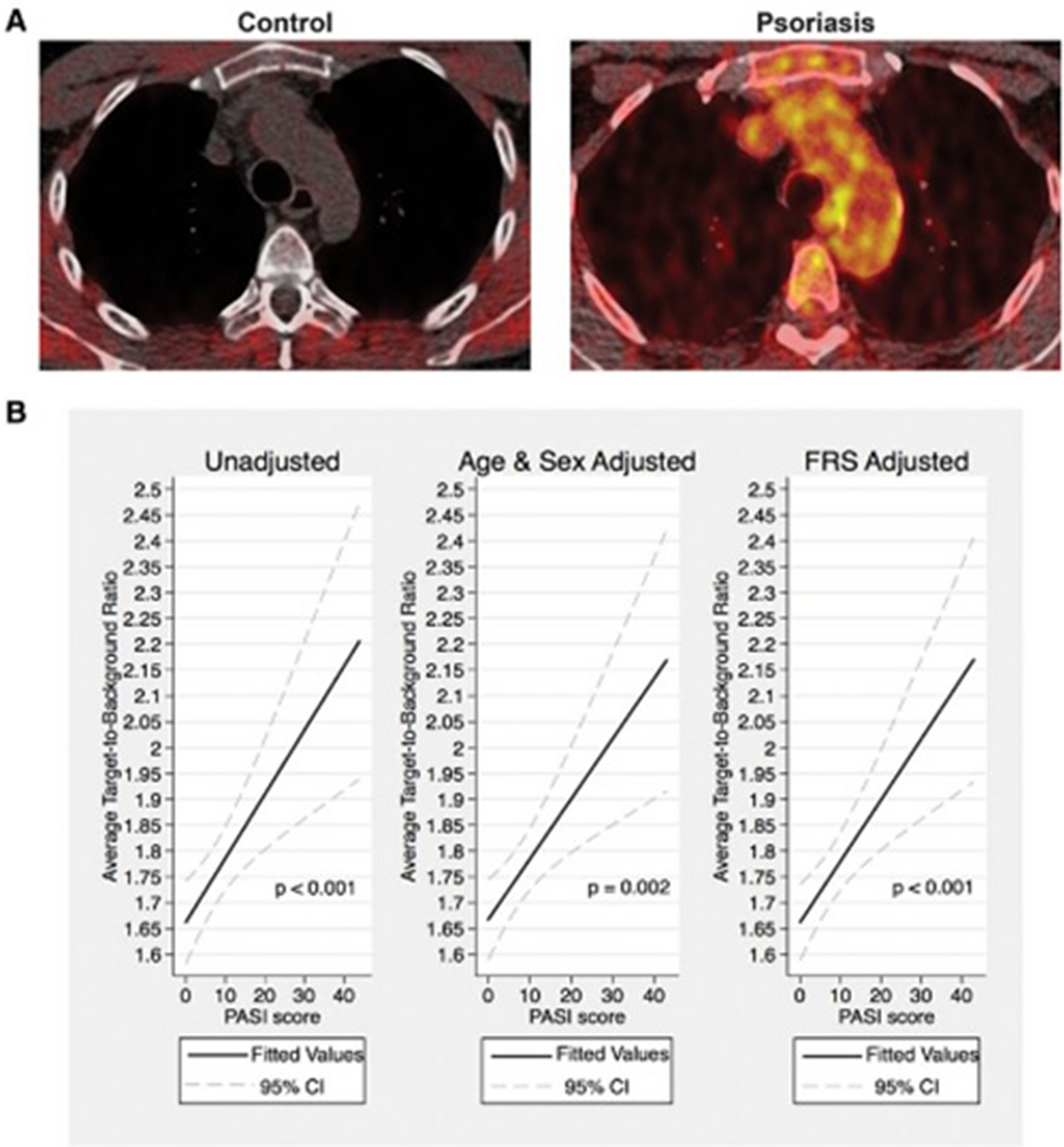
Vascular inflammation is associated with skin disease severity in psoriasis.
Tomographic fused 18-FDG PET/ CT image of the aortic arch from a patient with severe skin disease and control patient (A). Regression plots for multivariable regression analysis of vascular inflammation as measured by target-to-background (TBR) with skin disease severity as measured by psoriasis area and severity (PASI) score. CI indicates confidence interval; and FRS indicates Framingham risk score.
The median TBR was 1.6±0.1 for controls and 1.8±0.3 (p<.001). CI indicates confidence interval; and FRS indicates Framingham risk score.
TBR: tissue- to- background ratio
Figure 8:
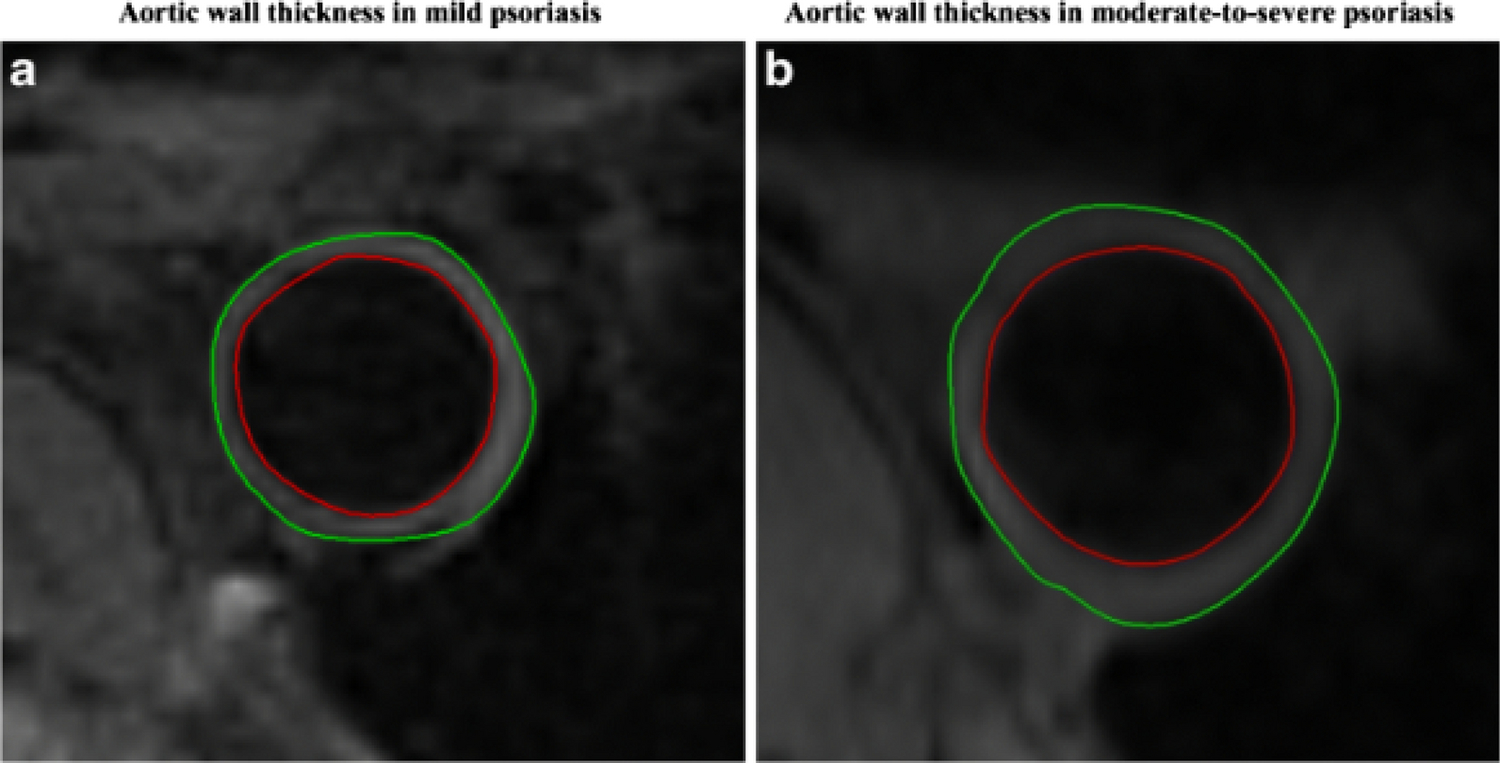
Aortic wall thickness is associated with skin disease severity in psoriasis.
Transverse magnetic resonance imaging slices of a patient with mild psoriasis (A) at the level of the descending aorta depicting lower aortic wall thickness when compared with patient with moderate to severe psoriasis (B). The green and the red contours represent the outer and inner border of the aortic wall respectively.
Figure 9:
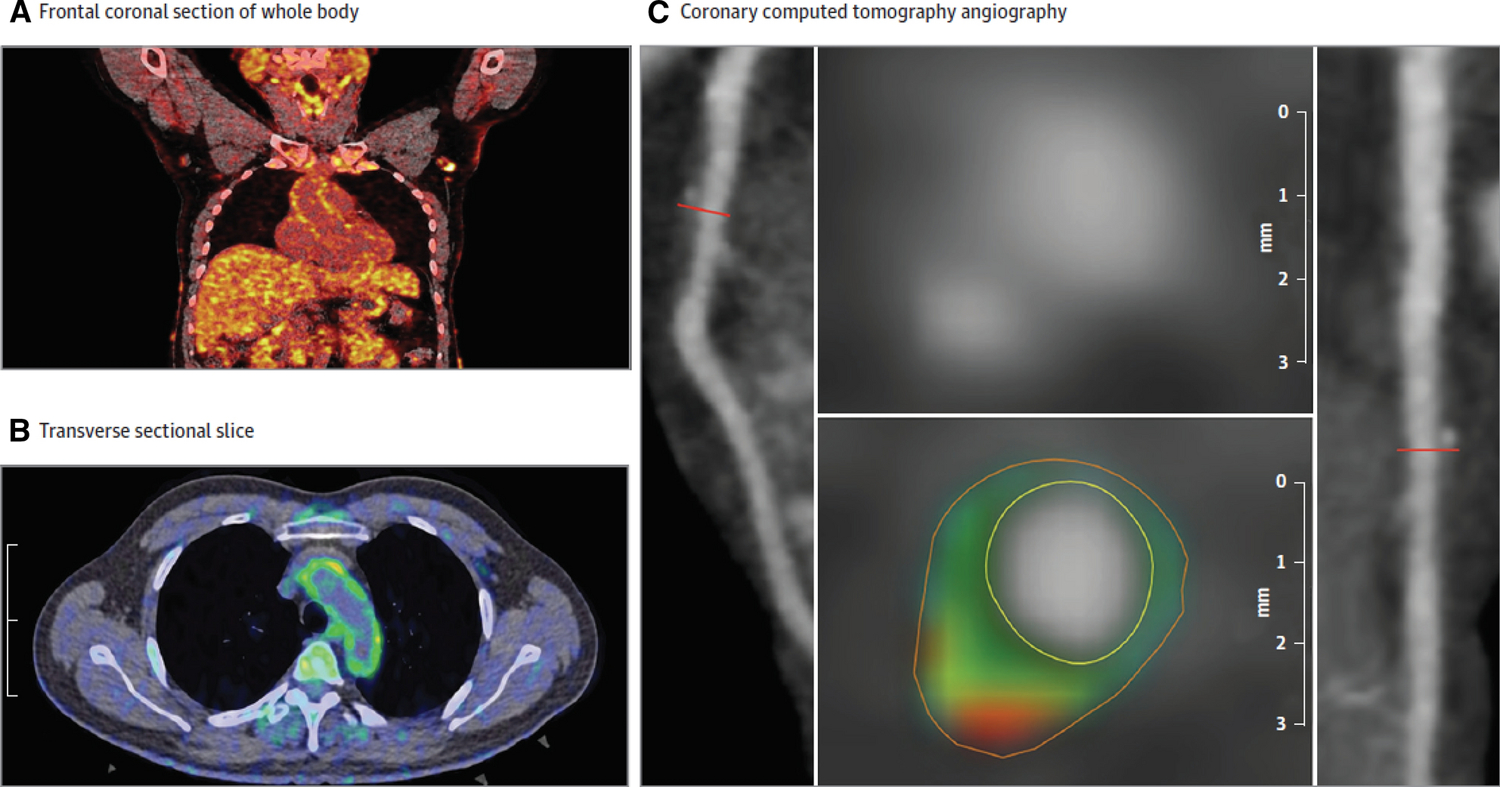
Aortic vascular inflammation by 18-FDG PET/CT and coronary artery characterization by cardiac computed tomography angiography (CCTA).
Frontal coronal section of whole- body 18- FDG PET/CT demonstrating 18- FDG uptake in the aortic wall (A). Transverse section of 18-FDG PET/CT demonstrating vascular inflammation in the aortic wall (B). A panel of reconstructed images from the CCTA demonstrating path of left anterior descending coronary artery (left), depicting noncalcified coronary burden and transverse section of the left descending coronary artery (right). The planar reconstruction (middle) reveals low- attenuation lipid- rich plaque (green and red). The mean TBR was 1.70 [0.26].
TBR: tissue- to- background ratio
Figure 10:
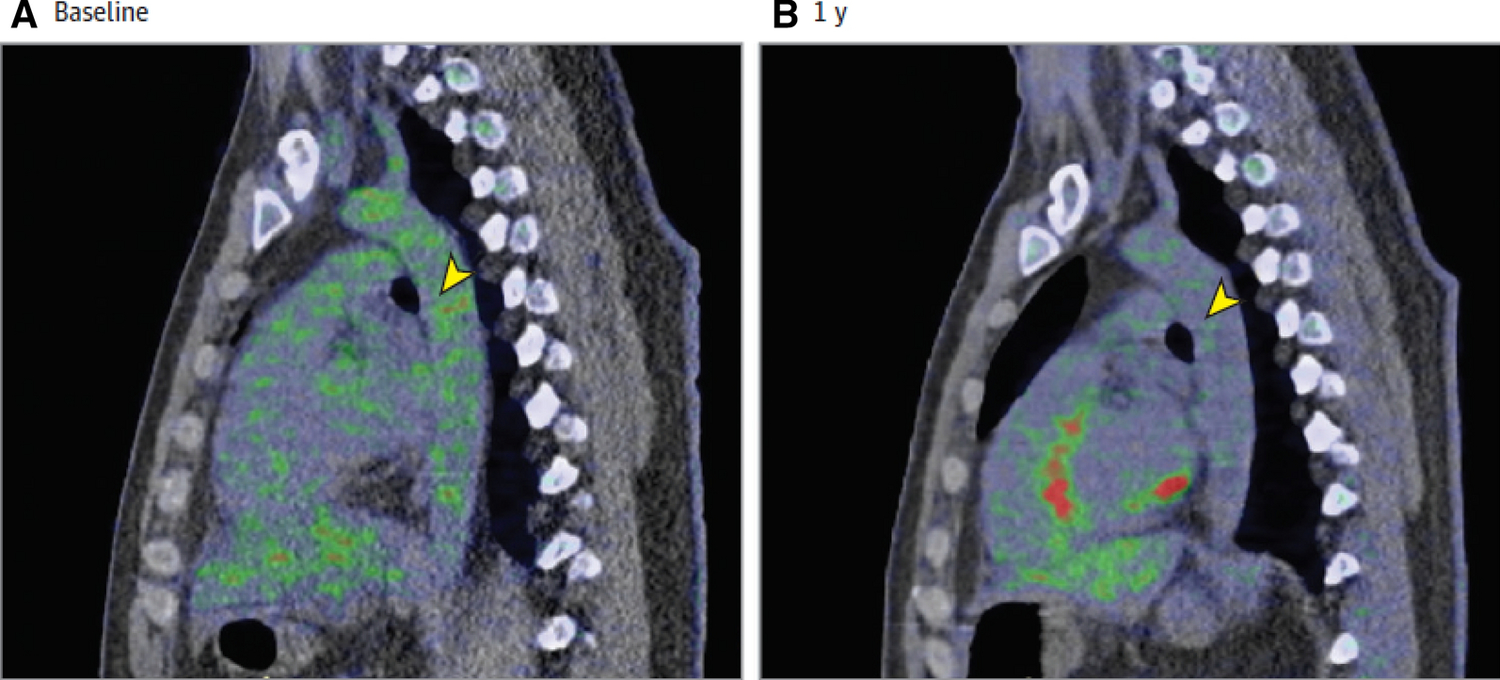
Treatment with biologic therapy for psoriasis is associated with reduction in aortic vascular inflammation.
The images show a sagittal section of the level of the mid aorta at baseline (A) and at 1 year after treatment with biologic therapy for psoriasis (B). 18-FDG uptake in the aorta is higher at baseline compared to 1 year after treatment (yellow arrowheads). The mean TBR at baseline was 1.91 [0.29] vs 1.79 [0.22] at 1 year follow up (p<0.001).
TBR: tissue- to- background ratio
In addition to being used for assessment of vascular inflammation, 18-FDG PET also enables simultaneous assessment of the neuro- immune- arterial axis as a mechanism for stress induced CVD as previously mentioned. Patient with psoriasis demonstrated higher 18-FDG amygdalar uptake, increased bone marrow 18-FDG uptake and higher coronary disease burden on CCTA.63 A reduction in skin disease severity by treating psoriasis resulted in a reduction in amygdalar 18-FDG uptake, bone- marrow FDG uptake, and aortic vascular inflammation.64 Recently, amygdalar activity assessed by18-FDG was shown to associate with an allostatic load score (physiologic effects of stress on cardiovascular, metabolic and inflammatory indices),65 underscoring the need to further investigate the effects of stress- induced physiological dysregulation on CVD.
Bone marrow 18-FDG uptake and understanding myeloid cells
The observation of a reduction of bone marrow 18-FDG uptake following primary skin disease therapy in psoriasis spawned a series of lab- based experiments. These studies provide an illustratation of how biologic understanding can be augmented by synergistic use of lab-based experiments and image-based studies. Myeloid cells are critical mediators of CVD with both macrophages and neutrophils detected in coronary plaques and present at the onset of plaque development. In psoriasis, neutrophils are abundant in circulation and maintain an activated state. 55 Furthermore, the pathogenic neutrophil subset, low-density neutrophils, are elevated in psoriasis and associate with early onset CAD and the severity of skin disease.66 Murine studies of atherosclerosis have highlighted biological pathways interlinking myeloid cells and lipid homeostasis, which are shown to converge in the bone marrow.67 Cholesterol accumulation in the bone marrow upregulates myelopoiesis, resulting in accelerated release of myeloid cells into the blood stream, exacerbating atherosclerotic plaque development. In psoriasis, 18-FDG uptake in the bone marrow is a surrogate for increased bone marrow activity as previously discussed. When bone marrow 18-FDG uptake was examined against the total frequency of low-density neutrophils, classical monocytes and platelets in circulation, there was a strong relationship between these indices by flow cytometry and 18-FDG uptake in the bone marrow.68 This total myeloid score, termed the atherogenic myeloid score, associated with psoriasis severity, bone marrow 18-FDG uptake as well as non-calcified coronary burden in psoriasis.68 These studies provide evidence as to how PET molecular imaging can be coupled with lab-based investigations to accelerate understanding of biologic relationships. The studies also support further investigation using molecular imaging to enhance translational research in humans utilizing this multi-disclipinary approach.
Limitations of 18-FDG PET Imaging
The ability to diagnose inflammatory vascular disease, monitor therapeutic intervention, provide cardiovascular risk stratification69, 70 and guide lab- based efforts makes 18-FDG PET imaging application promising for clinical translational investigation. However, studies using 18-FDG PET must be considered in light of several biologic and technical limitations. Although there is a strong correlation between macrophage density and 18-FDG uptake in endarterectomy specimens,8, 25 non- macrophage cells have also been observed to take up 18- FDG,71 leading to uncertainty about whether macrophages are the only cell type within vessel walls to take up 18-FDG. Indeed, activated smooth muscle cells within the blood vessel wall also take up 18-FDG.72 Additionally, spatial resolution limitations of <4mm, combined with the small caliber of the coronary arteries, make identification of the exact location of coronary plaque challenging, although application of this technique has been successfully applied to larger, peripheral arteries.11 Furthermore, areas of 18- FDG uptake do not directly correlate with coronary calcification on CT, suggesting that the detected metabolic activity assessed as vascular inflammation by 18- FDG detects more ‘active’ and inflamed plaque compared to more mature, calcified disease observed late in atherosclerosis.11, 73 Because 18- FDG is more likely to be detected in younger patients with less calcification, 18- FDG uptake may represent earlier, predominantly inflammatory components of disease development and progression11 however dedicated prospective studies are needed to better understand the significance of this.
Clinical translational perspective
The power of molecular imaging lies in its ability to improve the understanding of disease pathophysiology at the molecular and cellular levels within an in vivo system. We have illustrated how studies using 18-FDG PET imaging techniques have helped to establish the role of inflammation in atherosclerotic disease development and progression and further serve as a reliable non- invasive method for monitoring effectiveness of disease treatment. Furthermore, 18-FDG PET imaging measures vascular inflammation, detects early vessel wall abnormalities and assesses bone marrow activity and stress-related neural activity. Given continued technical advances, including improvements in scanner technology and incorporation of magnetic resonance imaging, future studies targeting a broader array of subclinical molecular processes will enable better understanding of biology to broaden discovery across the spectrum of cardiovascular diseases to improve diagnosis, treatment and prevention of disease in humans.
Supplementary Material
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Chen IY and Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation. 2011;123:425–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota R, Kubota K, Yamada S, Tada M, Ido T and Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994;35:104–12. [PubMed] [Google Scholar]
- 3.Vallabhajosula S and Fuster V. Atherosclerosis: imaging techniques and the evolving role of nuclear medicine. J Nucl Med. 1997;38:1788–96. [PubMed] [Google Scholar]
- 4.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J and Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–9. [PubMed] [Google Scholar]
- 5.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, Kudomi N, Shiomi M, Magata Y, Iida H and Saji H. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–50. [PubMed] [Google Scholar]
- 6.Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, Muller JE, Brady TJ and Fischman AJ. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. [DOI] [PubMed] [Google Scholar]
- 7.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD and Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. [DOI] [PubMed] [Google Scholar]
- 8.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ and Fischman AJ. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24. [DOI] [PubMed] [Google Scholar]
- 9.Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJR, Flapan AD, Uren NG, Behan MWH, Cruden NLM, Mills NL, Fox KAA, Rudd JHF, Dweck MR and Newby DE. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. The Lancet. 2014;383:705–713. [DOI] [PubMed] [Google Scholar]
- 10.Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJ, Boon NA, Rudd JH and Newby DE. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–48. [DOI] [PubMed] [Google Scholar]
- 11.McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, Salavati A, Sturek JM, Territo PR, Weaver C, Werner TJ, Hoilund-Carlsen PF, Sturek M and Alavi A. (18)F-NaF and (18)F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging. 2018;45:2190–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 13.Wolf D and Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P, Lichtman AH and Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mestas J and Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teague HL, Ahlman MA, Alavi A, Wagner DD, Lichtman AH, Nahrendorf M, Swirski FK, Nestle F, Gelfand JM, Kaplan MJ, Grinspoon S, Ridker PM, Newby DE, Tawakol A, Fayad ZA and Mehta NN. Unraveling Vascular Inflammation: From Immunology to Imaging. J Am Coll Cardiol. 2017;70:1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby P Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–13. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK and Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. [DOI] [PubMed] [Google Scholar]
- 19.Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W Jr., Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD and et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1992;12:120–34. [DOI] [PubMed] [Google Scholar]
- 20.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnstrom P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD and Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. [DOI] [PubMed] [Google Scholar]
- 21.Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V and Fayad ZA. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–8. [DOI] [PubMed] [Google Scholar]
- 22.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V and Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–6. [DOI] [PubMed] [Google Scholar]
- 23.Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, Tahara N, Fuster V, Warburton EA, Fayad ZA and Tawakol AA. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55:2527–35. [DOI] [PubMed] [Google Scholar]
- 24.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U and Tawakol A. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–9. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, Hoffmann U, Brady TJ and Tawakol A. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5:69–77. [DOI] [PubMed] [Google Scholar]
- 26.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, Wai B, Hoffmann U, Abbara S and Grinspoon S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, Rodante JA, Aberra TM, Chung J, Salahuddin T, Natarajan B, Dave J, Goyal A, Groenendyk JW, Rivers JP, Baumer Y, Teague HL, Playford MP, Bluemke DA, Ahlman MA, Chen MY, Gelfand JM and Mehta NN. Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis. JAMA Cardiol. 2018;3:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N and Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–31. [DOI] [PubMed] [Google Scholar]
- 29.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR and Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–17. [DOI] [PubMed] [Google Scholar]
- 30.Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM, Abdelbaky A, Alon A, Shankar SS, Rudd JH, Fayad ZA, Hoffmann U and Tawakol A. Coronary Plaque Morphology and the Anti-Inflammatory Impact of Atorvastatin: A Multicenter 18F-Fluorodeoxyglucose Positron Emission Tomographic/Computed Tomographic Study. Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT and Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49:1277–82. [DOI] [PubMed] [Google Scholar]
- 32.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A and dal PI. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, Werner TJ, Thomas P, Vanvoorhees A, Alavi A, Torigian DA, Gelfand JM and Mehta NN. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB and Gelfand JM. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB and Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM and Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 37.Mehta NN, Krishnamoorthy P, Yu Y, Khan O, Raper A, Van Voorhees A, Troxel AB and Gelfand JM. The impact of psoriasis on 10-year Framingham risk. J Am Acad Dermatol. 2012;67:796–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvaro Gonzalez-Cantero ASR, Amit K. Dey,, Jorge Gonzalez-Cantero EM, Justin Rodante, Ana I. Sanchez-Moya,, Cristina Perez-Hortet JLG-C, Martin P. Playford,, María G Barderas AnB, Natalia Jimenez-Gomez, Pedro Jaén, and Marcus Y Chen JMG, Nehal N. Mehta. Underperformance of clinical risk scores in identifying imaging-based high cardiovascular risk in psoriasis: results from two observational cohorts. European Journal of Preventive Cardiology. In Press. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr., Sperling LS, Virani SS and Blumenthal RS. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Circulation. 2019;139:e1162–e1177. [DOI] [PubMed] [Google Scholar]
- 40.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P and Herrington DM. Comparison of Novel Risk Markers for Improvement in Cardiovascular Risk Assessment in Intermediate-Risk Individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose S, Dave J, Millo C, Naik HB, Siegel EL and Mehta NN. Psoriatic arthritis and sacroiliitis are associated with increased vascular inflammation by 18-fluorodeoxyglucose positron emission tomography computed tomography: baseline report from the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative. Arthritis Res Ther. 2014;16:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tawakol A, Ishai A, Li D, Takx RA, Hur S, Kaiser Y, Pampaloni M, Rupert A, Hsu D, Sereti I, Fromentin R, Chomont N, Ganz P, Deeks SG and Hsue PY. Association of Arterial and Lymph Node Inflammation With Distinct Inflammatory Pathways in Human Immunodeficiency Virus Infection. JAMA cardiology. 2017;2:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emami H and Tawakol A. Noninvasive imaging of arterial inflammation using FDG-PET/CT. Curr Opin Lipidol. 2014;25:431–7. [DOI] [PubMed] [Google Scholar]
- 44.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J and Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 45.Osborne MT, Shin LM, Mehta NN, Pitman RK, Fayad ZA and Tawakol A. Disentangling the Links Between Psychosocial Stress and Cardiovascular Disease. Circ Cardiovasc Imaging. 2020;13:e010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steptoe A and Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360–370. [DOI] [PubMed] [Google Scholar]
- 47.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J and Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganesh SK, Tragante V, Guo W, Guo Y, Lanktree MB, Smith EN, Johnson T, Castillo BA, Barnard J, Baumert J, Chang YP, Elbers CC, Farrall M, Fischer ME, Franceschini N, Gaunt TR, Gho JM, Gieger C, Gong Y, Isaacs A, Kleber ME, Mateo Leach I, McDonough CW, Meijs MF, Mellander O, Molony CM, Nolte IM, Padmanabhan S, Price TS, Rajagopalan R, Shaffer J, Shah S, Shen H, Soranzo N, van der Most PJ, Van Iperen EP, Van Setten J, Vonk JM, Zhang L, Beitelshees AL, Berenson GS, Bhatt DL, Boer JM, Boerwinkle E, Burkley B, Burt A, Chakravarti A, Chen W, Cooper-Dehoff RM, Curtis SP, Dreisbach A, Duggan D, Ehret GB, Fabsitz RR, Fornage M, Fox E, Furlong CE, Gansevoort RT, Hofker MH, Hovingh GK, Kirkland SA, Kottke-Marchant K, Kutlar A, Lacroix AZ, Langaee TY, Li YR, Lin H, Liu K, Maiwald S, Malik R, Cardiogram M, Murugesan G, Newton-Cheh C, O’Connell JR, Onland-Moret NC, Ouwehand WH, Palmas W, Penninx BW, Pepine CJ, Pettinger M, Polak JF, Ramachandran VS, Ranchalis J, Redline S, Ridker PM, Rose LM, Scharnag H, Schork NJ, Shimbo D, Shuldiner AR, Srinivasan SR, Stolk RP, Taylor HA, Thorand B, Trip MD, van Duijn CM, Verschuren WM, Wijmenga C, Winkelmann BR, Wyatt S, Young JH, Boehm BO, Caulfield MJ, Chasman DI, Davidson KW, Doevendans PA, Fitzgerald GA, Gums JG, Hakonarson H, Hillege HL, Illig T, Jarvik GP, Johnson JA, Kastelein JJ, Koenig W, LifeLines Cohort S, Marz W, Mitchell BD, Murray SS, Oldehinkel AJ, Rader DJ, Reilly MP, Reiner AP, Schadt EE, Silverstein RL, Snieder H, Stanton AV, Uitterlinden AG, van der Harst P, van der Schouw YT, Samani NJ, Johnson AD, Munroe PB, de Bakker PI, Zhu X, Levy D, Keating BJ and Asselbergs FW. Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum Mol Genet. 2013;22:1663–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jankord R and Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tawakol A, Ishai A, Takx RAP, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJE, Calcagno C, Mani V, Tang CY, Mulder WJM, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA and Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. The Lancet. 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, Shin LM, Fayad ZA, Koenen KC, Rajagopalan S, Pitman RK and Tawakol A. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. European Heart Journal. 2019;41:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiechter M, Haider A, Bengs S, Maredziak M, Burger IA, Roggo A, Portmann A, Schade K, Warnock GI, Treyer V, Messerli M, Fuchs TA, Pazhenkottil AP, Buechel RR, Kaufmann PA and Gebhard C. Sex-dependent association between inflammation, neural stress responses, and impaired myocardial function. Eur J Nucl Med Mol Imaging. 2020;47:2010–2015. [DOI] [PubMed] [Google Scholar]
- 53.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L and Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–3. [DOI] [PubMed] [Google Scholar]
- 54.Rachakonda TD, Schupp CW and Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–6. [DOI] [PubMed] [Google Scholar]
- 55.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, Ng Q, Joshi AA, Krishnamoorthy P, Dave J, Rose SM, Doveikis J, Playford MP, Prussick RB, Ehrlich A, Kaplan MJ, Lockshin BN, Gelfand JM and Mehta NN. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35:2667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, Lockshin BN, Ahlman MA, Chen MY, Rader DJ, Reilly MP, Remaley AT, Bluemke DA, Playford MP, Gelfand JM and Mehta NN. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. 2016;119:1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groenendyk JW, Shukla P, Dey AK, Elnabawi YA, Aksentijevich M, Choi H, Genovese LD, Harrington CL, Natarajan B, Goyal A, Reddy AS, Rodante J, Kabbany MT, Sadek A, Al Najafi M, Playford MP, Joshi AA, Ahlman MA, Gelfand JM, Bluemke DA and Mehta NN. Association of aortic vascular uptake of (18)FDG by PET/CT and aortic wall thickness by MRI in psoriasis: a prospective observational study. Eur J Nucl Med Mol Imaging. 2019;46:2488–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brauchli YB, Jick SS, Miret M and Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol. 2009;160:1048–56. [DOI] [PubMed] [Google Scholar]
- 59.Lerman JB, Joshi AA, Chaturvedi A, Aberra TM, Dey AK, Rodante JA, Salahuddin T, Chung JH, Rana A, Teague HL, Wu JJ, Playford MP, Lockshin BA, Chen MY, Sandfort V, Bluemke DA and Mehta NN. Coronary Plaque Characterization in Psoriasis Reveals High-Risk Features That Improve After Treatment in a Prospective Observational Study. Circulation. 2017;136:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, Teague HL, Harrington CL, Rivers JP, Chung JH, Kabbany MT, Natarajan B, Silverman JI, Ng Q, Sanda GE, Sorokin AV, Baumer Y, Gerson E, Prussick RB, Ehrlich A, Green LJ, Lockshin BN, Ahlman MA, Playford MP, Gelfand JM and Mehta NN. Association Between Skin and Aortic Vascular Inflammation in Patients With Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol. 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elnabawi Y Immunomodulatory therapy reduces atherosclerotic plaque burden by coronary computed tomography angiography in psoriasis at one-year. Catheterization and Cardiovascular Interventions 2018;91:S5–S6. [Google Scholar]
- 62.Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, Rodante J, Harrington CL, Teague HL, Baumer Y, Keel A, Playford MP, Sandfort V, Chen MY, Lockshin B, Gelfand JM, Bluemke DA and Mehta NN. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovascular research. 2019;115:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, Belur AD, Groenendyk JW, Lerman JB, Rivers JP, Rodante JA, Harrington CL, Varghese NJ, Sanda GE, Baumer Y, Sorokin AV, Teague HL, Genovese LD, Natarajan B, Joshi AA, Playford MP, Bluemke DA, Chen MY, Alavi A, Pitman RK, Powell-Wiley TM, Tawakol A, Gelfand JM and Mehta NN. Chronic Stress-Related Neural Activity Associates With Subclinical Cardiovascular Disease in Psoriasis: A Prospective Cohort Study. JACC: Cardiovascular Imaging. 2018. [DOI] [PMC free article] [PubMed]
- 64.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, Belur AD, Groenendyk JW, Lerman JB, Rivers JP, Rodante JA, Harrington CL, Varghese NJ, Sanda GE, Baumer Y, Sorokin AV, Teague HL, Genovese LD, Natarajan B, Joshi AA, Playford MP, Bluemke DA, Chen MY, Alavi A, Pitman RK, Powell-Wiley TM, Tawakol A, Gelfand JM and Mehta NN. Chronic Stress-Related Neural Activity Associates With Subclinical Cardiovascular Disease in Psoriasis: A Prospective Cohort Study. JACC Cardiovasc Imaging. 2018. [DOI] [PMC free article] [PubMed]
- 65.Lateef SS, Al Najafi M, Dey AK, Batool M, Abdelrahman KM, Uceda DE, Reddy AS, Svirydava MD, Nanda N, Ortiz JE, Prakash N, Rodante JA, Keel A, Zhou W, Chen MY, Playford MP, Teague HL, Tawakol AA, Gelfand JM, Powell-Wiley TM and Mehta NN. Relationship between chronic stress-related neural activity, physiological dysregulation and coronary artery disease in psoriasis: Findings from a longitudinal observational cohort study. Atherosclerosis. 2020;310:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teague HL, Varghese NJ, Tsoi LC, Dey AK, Garshick MS, Silverman JI, Baumer Y, Harrington CL, Stempinski E, Elnabawi YA, Dagur PK, Cui K, Tunc I, Seifuddin F, Joshi AA, Stansky E, Purmalek MM, Rodante JA, Keel A, Aridi TZ, Carmona-Rivera C, Sanda GE, Chen MY, Pirooznia M, McCoy JP Jr., Gelfand JM, Zhao K, Gudjonsson JE, Playford MP, Kaplan MJ, Berger JS and Mehta NN. Neutrophil Subsets, Platelets, and Vascular Disease in Psoriasis. JACC Basic Transl Sci. 2019;4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tall AR, Yvan-Charvet L, Westerterp M and Murphy AJ. Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler Thromb Vasc Biol. 2012;32:2547–52. [DOI] [PubMed] [Google Scholar]
- 68.Teague HL, Aksentijevich M, Stansky E, Silverman JI, Varghese NJ, Dey AK, Elnabawi Y, Goyal A, Dagur PK, Chen MY, McCoy JP, Playford MP, Hourigan C, Gelfand JM and Mehta NN. Cells of Myeloid Origin Partly Mediate the Association between Psoriasis Severity and Coronary Plaque. J Invest Dermatol. 2019. [DOI] [PMC free article] [PubMed]
- 69.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P and Hacker M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–20. [DOI] [PubMed] [Google Scholar]
- 70.Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy JP, Maunoury C, Hugonnet F, Sauvaget E, Trinquart L and Faraggi M. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008;15:209–17. [DOI] [PubMed] [Google Scholar]
- 71.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF and Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58:603–14. [DOI] [PubMed] [Google Scholar]
- 72.Pahk K, Joung C, Jung SM, Young Song H, Yong Park J, Woo Byun J, Lee YA-O, Chul Paeng J, Kim C, Kim S and Kim WK. Visualization of Synthetic Vascular Smooth Muscle Cells in Atherosclerotic Carotid Rat Arteries by F-18 FDG PET. [DOI] [PMC free article] [PubMed]
- 73.Tatsumi M, Cohade C, Nakamoto Y and Wahl RL. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology. 2003;229:831–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.