Abstract
Background
The number of joint replacements in India is set to grow at the highest rate in the world from 2020 to 2026. It is high time for India to have an efficient and credible registry to help curtail the clinical impact of implant failure at a very early stage by prompt reporting.
Methods
Indian Joint Registry has been established by ISHKS with new data forms for reporting. These new detailed forms record, in addition to previous form, component-wise details of implants. Additional useful features include Linking with unique ID like PAN or Aadhaar, thromboprophylaxis, untoward intra-operative event, IJR consent and type of anaesthesia.
Results
There were 712 registered surgeons in IJR database till June 2020. Total TKRs being reported to registry increased from 1019 in 2006 to 27,000 in 2019. Majority of the patients (98.5%) were diagnosed with osteoarthritis knee. Company-wise distribution unveils that Johnson & Johnson DePuy represents the highest implant usage at over 37%. There has been increased utilisation of uncemented THR over cemented THR from 2006 to 2019. Dual-mobility THRs have gained ground as surgeon preference for the choice of implant.
Conclusion
Effective use of quality registries can lead to better health outcomes at a lower cost for the society. An effective, responsive and sustainable registry in India offers many benefits and should be considered as a key objective. Making the registry function in India successfully will undoubtedly require multi-pronged efforts, but can deliver many benefits both to the patient and to the nation as a whole.
Keywords: Indian Joint Registry, Indian Society of Hip and Knee Surgeons, Revision, Total hip arthroplasty, Total knee replacement
Introduction and Background
Innovation in the field of surgical orthopaedics especially joint replacement surgeries has shown a rapid development over the recent decades. Over the last few years, there has been an exponential rise in the number of joint replacement surgeries that are taking place in India. Also aiding in this surge have been numerous Government initiatives for the low economic strata as well as the introduction of the price capping which have led to people who earlier had no access to joint replacement surgeries also getting operated on a regular basis. There has been a concomitant rise in the varied types of prosthesis being used, some of them being indigenous implants for which there is paucity of long-term evidence. Parallel to it there has been an escalation in the number of court cases for medical negligence owing to growing dissatisfaction among the patients.
It has been painfully established by the extensive archives of joint replacement surgeries over the years that not all changes translate into progress. Few innovations worsen practice. Every now and then, new implants, techniques and practices are introduced worldwide which get translated into practices. The accountability of these implants and practices are suboptimally established in practical life.
The DePuy Articular Surface Replacement (ASR) implant from J&J recently made rippling waves across the countries for its supposedly flawed system. The metal on metal bearing cleared from US FDA in the year 2005 was implanted in 93,000 patients worldwide of which 4700 were reported in India till 2010. The Australian National Registry reported a cumulative revision rate of 10.9% for ASR compared to 4% for other resurfacing implants [1]. J&J in 2010 acknowledged the high failure rates of the system amidst lawsuits in US federal courts and consequently phased it out [2]. The company since then has an ASR reimbursement program dedicated to patients operated with the obsolete implant and recently, the Government of India proposed that the patients who had a faulty implant should receive a lump sum payment of ₹2 million (US$27,812). As of latest available figures from May 2019, of the total 4,700 hip implant surgeries conducted in India with DePuy’s ASR hip replacement system, only 882 patients (accounting for 1,056 implants) could be traced through the ASR helpline [3–5]. Had a national registry been in place in India during this period, it would have been possible to trace all patients affected by this device recall.
There were reports of Unusually High Rate of Early Failure of Tibial Component in ATTUNE Total Knee Arthroplasty System at Implant–Cement Interface. This was recognised at very early stage with corrective measures promptly taken and another disaster was averted by timely intervention. All this was possible thanks to pooled data collection and analysis [6]. This evidences the need for collecting a common set of data relating to all patients receiving joint implants.
The disturbing part of these implant failures were not the fact that they failed early, but how long it took to detect the failure. Prompt reporting and continuing data collection through registry could have curtailed the clinical, economic and social impact of these failures by reducing patient morbidity and mortality from their continued usage.
Sheer voluminous number of devices, need of long-term follow-up to evaluate effectiveness and safety, the need for data collection, evaluation, post-marketing surveillance and necessary funding are the factors which limit the establishment of accountability of the devices. This creates a unique problem for the surgeons, patients and companies in deciding which device is to be used.
Randomised studies of high level of evidence are rare and not simple to implement in many surgical and device trials. Thus, to bridge the gap in the existing evidence and meet the essential requirements, registries are best suited. As orthopaedic expertise is evolving rapidly, multiple products are offered for identical indications. Here registries are imperative in pursuance of the concept of evidence-based medicine and establishment of benchmarks.
Many countries have recognised the need for a Joint Registry and many such registries exist around the world at various stages of maturity. Registries are often cited as evidence of the exponential growth in the field of arthroplasty surgery, to record the long-term benefits to patients and have been fundamental in upgrading the standards of arthroplasty surgeries. Not only that, registry studies also inform the surgeons of all latest developments occurring in the ever-expanding field of arthroplasty.
The capability of registry data to encourage constant qualitative improvement work and guide clinical research is based on the quality of its data. An effective registry requires that each and every surgery is recorded, reviewed and analysed.
According to a market survey, the numbers of joint replacement surgeries in India are increasing every year with the estimates for knee arthroplasty numbers in India to be around 2,00,000 in 2020 [7] and the hip arthroplasties are set to grow at the highest rate in the world from the period 2020–2026 [8]. The volume of companies providing implants has also increased drastically in the last decade. Both national and multinational companies are providing implants in India as of today. Since the population curve of India is bell shaped, it is expected that many more people are going to enter their 50s and 60s in the coming decade. It has been estimated that the total joint replacement burden after a decade would be multifold as compared to what it is today [8]. This is the ideal time to start the evaluation of the surgeries and the implants.
The first and foremost requirement for our country is the establishment of an effective joint registry at the national level. The biggest advantage would be an early recognition of any problem. Another crucial component is the adverse event reporting which would help to curtail the clinical impact of the failure of an implant at a very early stage by prompt reporting. It is high time for India to have an efficient and credible registry to cater to all the needs.
Where Does the World Stand?
The outcome data of the Swedish, UK, Australian and Canadian Registries have been gathered through their national health care programs. All of them have played a pivotal role in alerting to implant related failures. These reports directed well-timed interventions which prevented further detrimental effects to additional patients and helped to reduce arthroplasty revisions by as significant a number as 10% [9–11].
An effective registry collects and assimilates institutional, regional and national data. After prudent scientific analysis, it helps deduce conclusions with high statistical significance. These are conclusions regarding patient characteristics, surgical technique and implants that ultimately lead to good or poor outcomes. For example, decisive conclusions about outcomes and complications of arthroplasty in patients with specific disorders like AVN hip [12, 13], psoriasis [14] and previous patellectomy [15] were derived from registry data.
A large variety of pertinent issues in total hip replacement surgery have been positively impacted by significant inferences from over 40 plus years of registry data. Assessments of outcomes of implant designs [16, 17], instruments [18, 19], surgical techniques [20, 21], sepsis management [22, 23], mortality associated with hip replacement [24] and the first report of periprosthetic osteolysis in cementless hip arthroplasty [25, 26] all owe their roots of origin to the registry.
Also, enormous sample size of patients in the entire database helps analyse results of arthroplasty in rare conditions like osteogenesis imperfecta [27], Paget’s disease [28, 29], and osteopetrosis [30] which otherwise would have been difficult to be part of a case series.
Where Do We Stand Today?
The initiative of setting up a national joint registry in India has been taken up by ISHKS (Indian Society of Hip and Knee Surgeons), the fruits of which are bound to be seen in the years to come. Already the data from the ISHKS registry have revealed many findings which have led to myriad improvements in the arthroplasty practices in India and also would be useful in formulating future directives.
The joint registry in India headed by ISHKS was founded in the year 2005. The old ISHKS form for hip and knee arthroplasty surgeries only collected information about patient demographics, indication for surgery, implant details (the stickers for which had to be manually stuck and mailed physically to Ahmedabad) and in case of revision arthroplasty, the details of implants removed and the cause of failure of primary arthroplasty.
Now with the introduction of the IJR (Indian Joint Registry), a new detailed user-friendly form [31] has been adopted that in addition to the previously collected details also amalgamates component-wise details of companies selling implants in India with the help of a consolidated database. Additional useful features include linking with unique ID like PAN or Aadhaar (that will help in the identification of patient anytime in future and anywhere in the country), thromboprophylaxis regimes, noting of untoward intra-operative event, option for stage 1 of two-stage revision, introduction of IJR consent, and type of anaesthesia that has amplified the quality of data that will be recorded. The IJR hopes to come up with detailed analysis with respect to type of anaesthesia, postoperative modalities to reduce pain, venous thromboembolism prophylaxis, survivorship of the prosthesis, etc. in the future. The separate forms for revision surgery would help in the evaluation of the reason for revision and will definitely help in better understanding of the reasons for the failure. Analysis of already collected data gives us a sneak-peak into the current trends in arthroplasty in India and reveals certain important facts.
Contributing surgeons
The number of contributing surgeons has increased from 14 in 2006 to 712 registered surgeons in IJR database as per latest records in June 2020. There have been multiple factors associated with this. The increased awareness, better publicity and enhanced motivation have led to this positive change (Figs. 1, 2).
Fig. 1.
Region-wise distribution of contributing surgeons. Surgeons from Gujarat have contributed the highest number of TKRs (21,283) and THRs (653) to the registry in the period from April 2016 to August 2017 and April 2019 till date whereas surgeons from Kerala have reported the least number of surgeries (6 TKRs and 1 THRs)
Fig. 2.
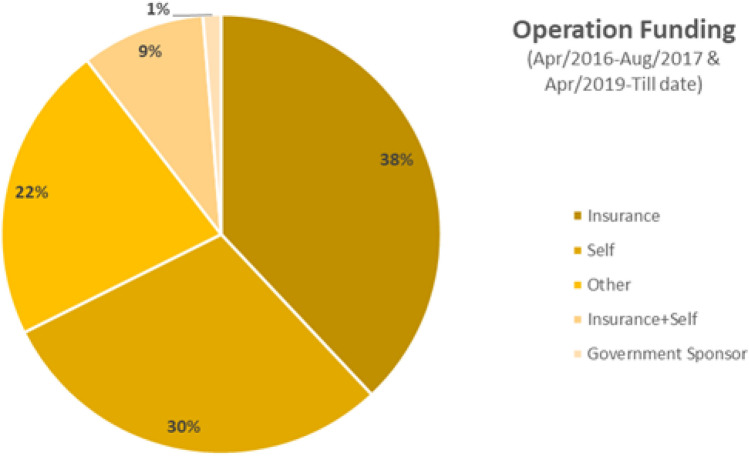
Operation funding of joint replacement surgeries reported to the IJR
Analysis of operation funding brings light to the fact that of the reported surgeries to the IJR in the period from April 2016 to August 2017 and April 2019 till date, majority (around 38%) of surgeries were covered under insurance while the Govt-sponsored surgeries are only about 1%. It may still be just an early observation and given the introduction of various government schemes in recent times, the figures in the future might be different.
-
(2)
Total number of TKRs
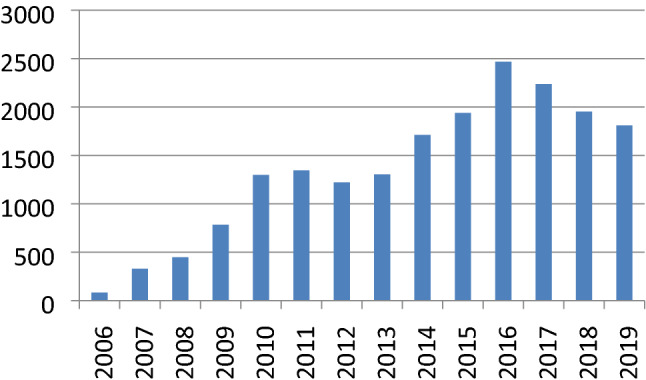
Analysis of data from the records of the existing ISHKS registry which has already been in place reveals that there has been a steady rise in the no of TKRs being reported to the registry from a mere 1019 in 2006 to around 27,000 in 2019 (Fig. 3).
Fig. 3.
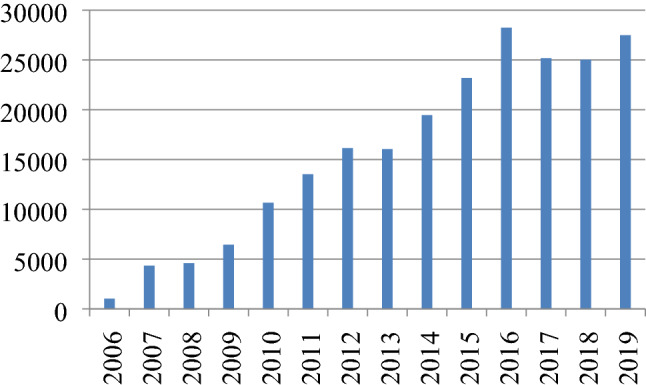
Total number of TKRs recorded on the registry in India year-wise
-
(3)
Diagnosis (TKR)
An overwhelming majority of the patients (approx. 98.5%) were diagnosed with osteoarthritis knee (Table 1).
Table 1.
Diagnosis of patients being operated for TKR
| Diagnosis | % (n = 165,000) |
|---|---|
| Osteoarthritis | 98.51% (n = 162,541) |
| Rheumatoid arthritis | 1.14% (n = 1881) |
| Previous trauma | 0.11% (n = 182) |
| Inflammatory trauma | 0.10% (n = 165) |
| Previous infection | 0.08% (n = 132) |
| Failed HTO | 0.06% (n = 99) |
-
(4)
Type of implant utilised
Further analysis sheds light on the fact that around 3/4ths of the TKRs being done in India are posterior stabilised as compared to around 20.61% of cruciate retaining type. Other including hinged knee and tumour prosthesis made up about 3% of all surgeries (Figs. 4, 5).
Fig. 4.
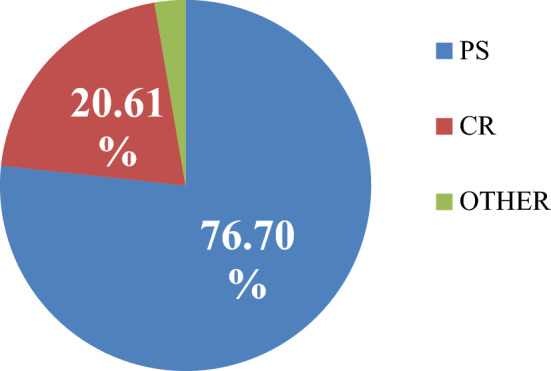
Type of implant utilised for TKR in the year 2019
Fig. 5.
Percentage of patella being resurfaced while doing TKRs
-
(5)
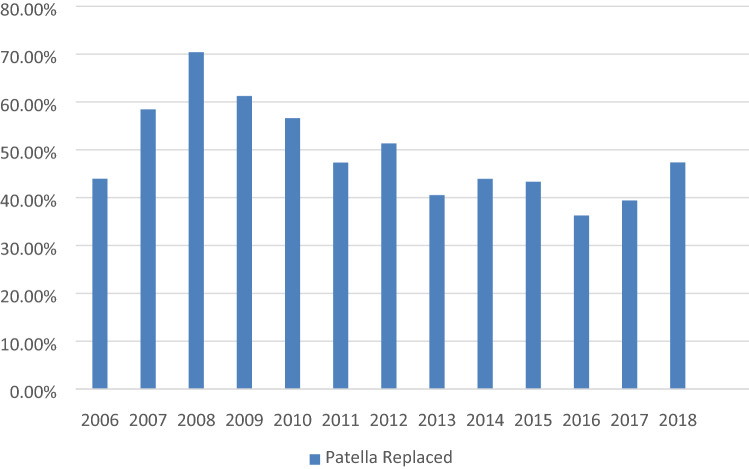
Patella resurfacing
With regard to patellar resurfacing, it is evident that over the period from 2006 to 2018 around 40–60% of surgeons prefer to resurface it except in the year 2008 when about 70% surgeons preferred to resurface the patella (Fig. 6).
Fig. 6.
Company-wise distribution of implants being utilised for TKR
-
(6)
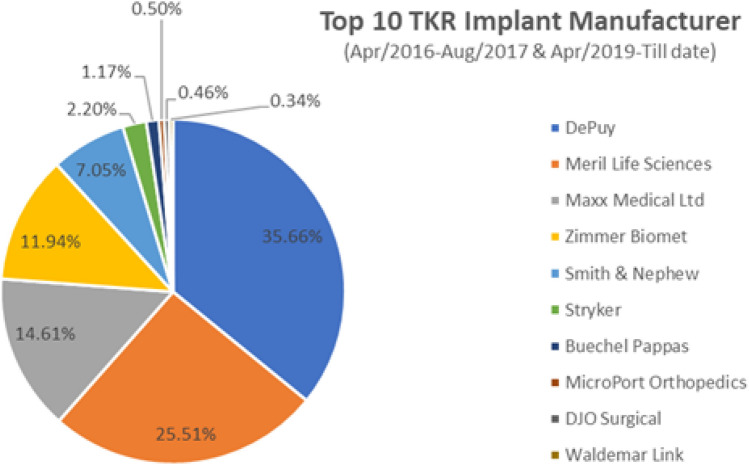
Company of implant
Company-wise distribution of registered cases in the period from April 2016 to August 2017 and April 2019 till date unveils the finding that Johnson & Johnson DePuy represents the highest recorded implant usage at over 35% of the surgeries and Meril Life Sciences stood as the second largest supplier. Combination of multitude of reasons like cost, many patients undergoing arthroplasty under various schemes such as PMJAY or MA card, variability in design, surgeon preference and/or training could be attributed to these findings.
-
(7)
No of revisions
There was an increase in the number of revision TKRs until 2016. Thereafter, the trend of decline in number of revision TKR surgeries has been noticed. This might be attributable to improvement in surgical techniques and availability of better implants (Fig. 7).
Fig. 7.
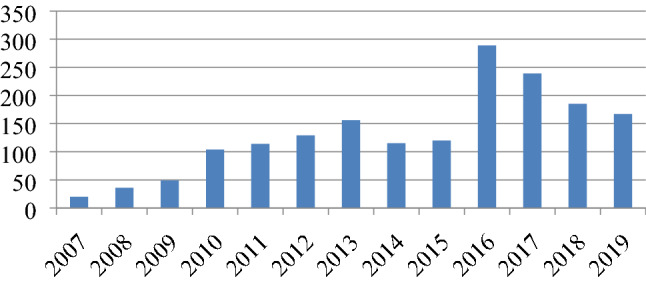
Total no. of revision TKRs being reported to the registry year-wise
-
(8)
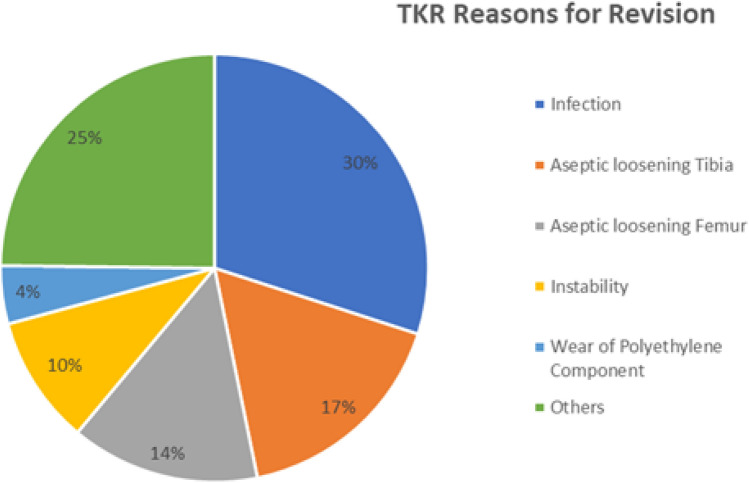
Reasons for revision TKRs
There were various reasons for revision of TKRs including infection (most common in 30% cases), aseptic loosening of tibia and/or femur, instability and polyethylene wear. In some cases, there were more than one reason responsible for revision (Fig. 8).
Fig. 8.
Reasons for revision TKR
-
(9)
THR year-wise
There has been a steady rise in the number of THRs being registered in our country from 2006 to 2016 after which there has been a decline. This might be attributed to lack of reporting and reinforces the need for a nationwide registry so that after realising the benefits of reporting, there can be maximum contribution to the registry (Fig. 9).
Fig. 9.
Total number of THRs being reported year-wise
-
(10)
Cemented vs. uncemented THR
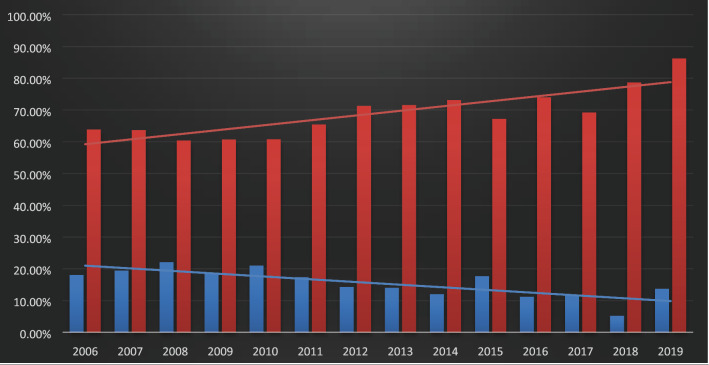
There seems to be a growing trend that has increasingly favoured the utilisation of uncemented THR over cemented THR over the period from 2006 to 2019 in India (Fig. 10).
Fig.10.
Uncemented (red bars) vs cemented THRs (blue bars) being reported year-wise
-
(11)
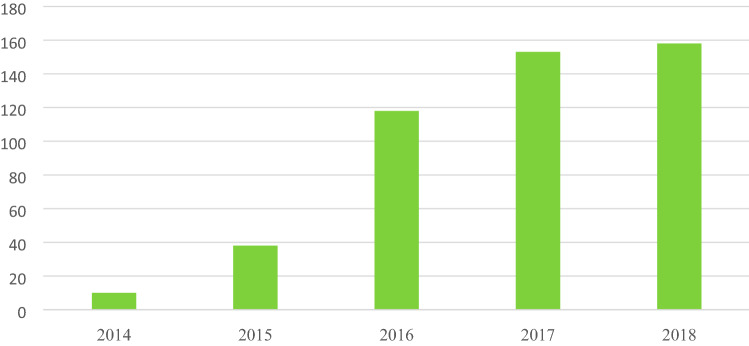
Use of dual mobility cup last 5 years (N = 477)
Dual-mobility THRs have now started gaining ground in terms of surgeon preference for the choice of implant. Literature suggests that the newer technology with dual mobility components offers enhanced stability and reduced risk of dislocation with excellent range of motion. The implant has been found out to be an attractive option for young, active patients and in revision scenarios [32] (Fig. 11).
Fig. 11.
Total number of dual-mobility THRs being reported year-wise
-
(12)
Primary hip fixation
Analysis and further evaluation of data from other registries of the world have shown that in India there has been an overwhelming predominant preference for cementless type of fixation (93%). This finding has been replicated in the German and NJR UK registries whereas the Swedish orthopaedic surgeons prefer cemented arthroplasty for women and uncemented arthroplasty for men. According to Annual Report of Swedish Hip Arthroplasty Register, fracture as a diagnosis, osteoporosis, and high age are reasons why cemented arthroplasty is preferred [33]. The author acknowledges the limitation that data pertaining to the trend of usage of hybrid or reverse hybrid THR in India are not available thereby underlining the imminent need of wholesome participation of surgeons across the country to fill in the missing links of data and make it more exhaustive (Table 2).
Table 2.
Comparison of modality of fixation used for THRs in IJR vs other registries
| Fixation | IJR (%) | NJR (2016) (%) | Sweden (%) | Germany (%) |
|---|---|---|---|---|
| Cemented | 3 | 30 | 64 | 7 |
| Hybrid | 4 | 28 | 3 | 15 |
| Reverse hybrid | 0 | 3 | 11 | 1 |
| Cementless | 93 | 39 | 21 | 77 |
Looking Ahead
For the registry to function as a watchdog and provide meaningful as well as validated information, it needs to have at least 90% of the arthroplasty surgeons’ participation and assess the long-term outcomes of their surgeries [34]. A few concerns cited as the reason for the lack of willingness by surgeons for participation include apathy, fear of disclosure of confidential information and the sheer burden of submission of data. But the lack of credibility of the registry due to deficiency of participation of the arthroplasty surgeons needs to be handled pragmatically as it may well have reached a breaking point.
The burden on surgeons and hospitals submitting data to a registry remains a key challenge. The prospects for the participating surgeon to have prompt and hassle-free online feedback is the most critical factor to enhance compliance in a national level registry. They must be able to visualise the benefits of the registration process initially.
Of paramount importance is the need to ensure security and confidentiality of data. The registry must be run with assurance that there is no risk of loss or disclosure of patient data. On the one hand, no data pertaining to an individual surgeon will be shared publicly, but these data will be shared on a confidential basis with each centre by the IJR.
Previous experience with registries has shown that a strong motivation for continuation of data reporting by a medical centre was receipt of thorough data on the detailed evaluation of revision surgeries and complications pitted against the national average. This would help them to review the beneficial and detrimental practices and formulate future directives to improve outcomes of their surgeries. One of the pioneers in arthroplasty registries, Sweden, exemplifies the above-stated fact where due to the these conscious efforts, the incidence of critical complications and revision rates have shown a progressive decline so that now only around 10% of the hip arthroplasty cases come up for revision [10].
One of the most convenient ways in today’s world is the digital reporting of all cases. Not only does this ensure better compliance, but also helps easy maintenance and retrieval of records. Certain factors that would help to improve the compliance (and are being currently already applied by IJR) include consistent availability of dedicated online support staff, periodic training workshops for the local staff, statistical support for each centre and indeed the most pertinent being reliable and regular feedback to the surgeons.
Other means to collect registry data in the pipeline in the near future would include development and propagation of mobile apps for reporting of joint replacement surgeries. This offers a promising and expedient option of not missing out on data from even the remotest parts of the country.
Recognising the importance that registries can play within a regulatory framework, many existing registries are collaborating with their national or multinational (European Union) regulatory agencies, supporting and in many cases, strengthening regulatory decision-making. For any registry program to flourish, there is a compelling need for enhanced and better regulations. Close working of the society and the Government in concurrence would dramatically improve the quality and level of reporting in registries.
Ways to implement mandatory reporting as opposed to the current practice of voluntary reporting need to be thought of. The Food and Drug Authority can play an important part here. It is the responsibility of the Govt authorities to protect the public from the introduction of under-researched implants, for which there is inadequate data, or providing a means of collecting such data on patient follow-up.
Surgeon and implant manufacturer alike must take responsibility to ensure that all cases are documented. Buy-in of all stakeholders is essential for the success of a registry. These stakeholders include patients, surgeons, policy makers, implant companies, and the registry provider. Alongside securing buy-in from across the stakeholder community, it is important to establish a fair and sustainable funding model for the registry. Ideally, funding should come from multiple sources including companies as well as policymakers, each of which benefits from the success of a registry.
Emulating the model followed by NJR UK, the IJR has associated with an independent organisation for data handling. This alliance brings numerous advantages. It can avoid any potential conflicts of interest that may arise should one interested party take the lead in collecting data on behalf of others. The details would be fed into the software and would help in the analysis of outcomes. This would help answer the all-important question about whether early revision is caused by technique or technology, or indeed both. The availability of high-quality registry data would provide surgeons with a better high-level scientific evidence upon which their practice can be improved.
National level registries with wide coverage and exhaustive completeness possess huge unutilized potential to be utilised as an instrument pertaining to health economics. The whole idea of a registry focuses on decreasing the revision burden on the state in turn culminating into benefit of the patient. Effective use of quality registries can lead to better health outcomes at a lower cost for the society. An effective, responsive and sustainable registry function in India offers many benefits and should be considered as a key objective. Making the registry function in India successfully will undoubtedly require multi-pronged efforts, but can deliver many benefits both to the patient, and to the nation as a whole.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shrinand V. Vaidya, Email: drsvv1@yahoo.com
Abhinav D. Jogani, Email: drabhinavdjogani@gmail.com
Jahavir A. Pachore, Email: japachore@rediffmail.com
Richard Armstrong, Email: richard.armstrong@northgateps.com.
Chintan S. Vaidya, Email: csvaidya1993@gmail.com
References
- 1.de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthopaedica. 2013;84(4):348–352. doi: 10.3109/17453674.2013.831320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ASR Hip System recall. (2020). In: J & J Private limited India [Internet].https://www.jnj.in/div-class-cms-textalign-center-b-asr-hip-system-recall-b-div. Accessed 4 June 2020
- 3.https://www.thehindu.com/sci-tech/health/out-of-joint/article24949401.ece. Accessed 4 June 2020
- 4.https://www.business-standard.com/article/companies/j-j-to-pay-rs-25-lakh-each-to-67-victims-of-faulty-asr-hip-implants-119053001186_1.html. Accessed 4 June 2020
- 5.https://timesofindia.indiatimes.com/blogs/toi-editorials/joint-pains-johnson-johnson-must-compensate-patients-for-faulty-hip-implants/. Accessed 4 June 2020
- 6.Bonutti P, Khlopas A, Chughtai M, Cole C, Gwam C, Harwin S, Whited B, Omiyi D, Drumm J. Unusually high rate of early failure of tibial component in ATTUNE total knee arthroplasty system at implant-cement interface. The Journal of Knee Surgery. 2017 doi: 10.1055/s-0037-1603756. [DOI] [PubMed] [Google Scholar]
- 7.https://www.market-scope.com/pages/reports/orthopedic?page=1. Accessed 4 June 2020
- 8.https://axiommrc.com/product/1735-joint-replacement-market-report. Accessed 4 June 2020
- 9.Herberts P, Malchau H. Long term registration has improved the quality of hip replacement: A review of the Swedish THR Register comparing 160,000 cases. Acta Orthopaedica Scandinavica. 2000;71:111–121. doi: 10.1080/000164700317413067. [DOI] [PubMed] [Google Scholar]
- 10.Herberts P, Malchau H. How outcome studies have changed total hip arthroplasty practices in Sweden. Clinical Orthopaedics and Related Research. 1997;344:44–60. doi: 10.1097/00003086-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Orthopaedic Surgeons. (2020). American Joint Replacement Registry project. https://www.aaos.org/registry. Accessed 4 June 2020
- 12.Ortiguera CJ, Pulliam IT, Cabanela ME. Total hip arthroplasty for osteonecrosis: Matched-pair analysis of 188 hips with long-term follow-up. Journal of Arthroplasty. 1999;14(1):21–28. doi: 10.1016/S0883-5403(99)90197-3. [DOI] [PubMed] [Google Scholar]
- 13.Cabanela ME. Bipolar versus total hip arthroplasty for avascular necrosis of the femoral head. A comparison. Clinical Orthopaedics. 1990;261:59–62. [PubMed] [Google Scholar]
- 14.Beyer CA, et al. Primary total knee arthroplasty in patients with psoriasis. The Journal of Bone and Joint Surgery. 1991;73B(2):258–259. doi: 10.1302/0301-620X.73B2.2005150. [DOI] [PubMed] [Google Scholar]
- 15.Chang MA, Rand JA, Trousdale RT. Patellectomy after total knee arthroplasty. Clin Orthop. 2005;440:175–177. doi: 10.1097/01.blo.0000188559.74796.69. [DOI] [PubMed] [Google Scholar]
- 16.Goetz DE, Smith EJ, Harris W. The prevalence of femoral osteolysis associated with components inserted with or without cement in total hip replacements A retrospective matched-pair series. The Journal of Bone and Joint Surgery. 1994;76:1121–1129. doi: 10.2106/00004623-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Harris W, Penenberg B. Further follow-up on socket fixation using a metal-backed acetabular component for total hip replacement. A minimum ten-year follow-up study. The Journal of Bone and Joint Surgery. 1987;69A:1140–1143. doi: 10.2106/00004623-198769080-00005. [DOI] [PubMed] [Google Scholar]
- 18.Harris W, Oh I. A new power tool for removal of methylmethacrylate from the femur. Clinical Orthopaedics. 1978;132:53–54. [PubMed] [Google Scholar]
- 19.Oh I, Harris W. A cement fixation system for total hip arthroplasty. Clinical Orthopaedics. 1982;164:221–229. [PubMed] [Google Scholar]
- 20.Harris W. A new approach to total hip replacement without osteotomy of the greater trochanter. Clinical Orthopaedics. 1975;106:19–26. doi: 10.1097/00003086-197501000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Harris WH, et al. A new technique for removal of broken femoral stems in total hip replacement. A technical note. Journal of Bone and Joint Surgery. 1981;63(5):843–845. doi: 10.2106/00004623-198163050-00028. [DOI] [PubMed] [Google Scholar]
- 22.Patel D, Karchmer A, Harris W. The role of preoperative aspiration of the hip prior to total hip replacement. In: Evarts C, editor. The Hip, Proceedings of the Fourth Open Scientific Meeting of the Hip Society. St Louis: C.V. Mosby Inc; 1976. pp. 219–223. [Google Scholar]
- 23.Schutzer SF, Harris WH. Deep-wound infection after total hip replacement under contemporary aseptic conditions. Journal of Bone and Joint Surgery. 1988;70(5):724–727. doi: 10.2106/00004623-198870050-00013. [DOI] [PubMed] [Google Scholar]
- 24.Dearborn JT, Harris WH. Postoperative mortality after total hip arthroplasty. An analysis of deaths after two thousand seven hundred and thirty-six procedures. Journal of Bone and Joint Surgery. 1998;80(9):1291–1294. doi: 10.2106/00004623-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Goldring SR, et al. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. Journal of Bone and Joint Surgery. 1983;65(5):575–584. doi: 10.2106/00004623-198365050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Harris W. Osteolysis and particle disease in hip replacement: A review. Acta Orthopaedica Scandinavica. 1994;65:113–123. doi: 10.3109/17453679408993734. [DOI] [PubMed] [Google Scholar]
- 27.Papagelopoulos PJ, Morrey BF. Hip and knee replacement in osteogenesis imperfecta. The Journal of Bone and Joint Surgery. 1993;75A(4):572–580. doi: 10.2106/00004623-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Parvizi J, et al. Outcome of uncemented hip arthroplasty components in patients with Paget's disease. Clinical Orthopaedics. 2002;403:127–134. doi: 10.1097/00003086-200210000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Lewallen DG. Hip arthroplasty in patients with Paget's disease. Clinical Orthopaedics. 1999;369:243–250. doi: 10.1097/00003086-199912000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Strickland JP, Berry DJ. Total joint arthroplasty in patients with osteopetrosis: A report of 5 cases and review of the literature. Journal of Arthroplasty. 2005;20(6):815–820. doi: 10.1016/j.arth.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 31.www.indianjointregistry.in. Accessed 4 June 2020
- 32.Blakeney WG, Jean-Alain Epinette JA, Vendittoli PA. Dual mobility total hip arthroplasty: Should everyone get one? EFORT Open Reviews. 2019;4:541–547. doi: 10.1302/2058-5241.4.180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kärrholm, J., Mohaddes, M., Odin, D., Vinblad, J., Rogmark, C., & Rolfson, O. (2018). Swedish Hip Arthroplasty Register Annual Report 2017. 10.18158/BkOffx7U4.
- 34.Pachore JA, Vaidya SV, Thakkar CJ, Bhalodia HP, Wakankar HM. ISHKS joint registry: A preliminary report. Indian Journal of Orthopaedics. 2013;47:505–509. doi: 10.4103/0019-5413.118208. [DOI] [PMC free article] [PubMed] [Google Scholar]