Abstract
Purpose
Alzheimer’s disease (AD) is a multifaceted neurodegenerative disease. To target simultaneously multiple pathological processes involved in AD, natural-origin compounds with unique characteristics are promising scaffolds to develop novel multi-target compounds in the treatment of different neurodegenerative disease, especially AD. In this study, novel chromone-lipoic acid hybrids were prepared to find a new multifunctional lead structure for the treatment of AD.
Methods
Chromone-lipoic acid hybrids were prepared through click reaction and their neuroprotection and anticholinesterase activity were fully evaluated. The anti-amyloid aggregation, antioxidant and metal-chelation activities of the best compound were also investigated by standard methods to find a new multi-functional agent against AD.
Results
The primary biological screening demonstrated that all compounds had significant neuroprotection activity against H2O2-induced cell damage in PC12 cells. Compound 19 as the most potent butyrylcholinesterase (BuChE) inhibitor (IC50 = 7.55 μM) having significant neuroprotection activity as level as reference drug was selected for further biological evaluations. Docking and kinetic studies revealed non-competitive mixed-type inhibition of BuChE by compound 19. It could significantly reduce formation of the intracellular reactive oxygen species (ROS) and showed excellent reducing power (85.57 mM Fe+2), comparable with quercetin and lipoic acid. It could also moderately inhibit Aβ aggregation and selectively chelate with copper ions in 2:1 M ratio.
Conclusion
Compound 19 could be considered as a hopeful multifunctional agent for the further development gainst AD owing to the acceptable neuroprotective and anti-BuChE activity, moderate anti-Aβ aggregation activity, outstanding antioxidant activity as well as selective copper chelation ability.
Graphical abstract
A new chromone–lipoic acid hybrid was synthesized as anti-Alzheimer agent with BuChE inhibitory activity, anti-Aβ aggregation, metal-chelation and antioxidant properties.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40199-020-00378-1.
Keywords: Alzheimer’s disease, Lipoic acid, Chromone, Multifunctional agent, Antioxidant
Introduction
Alzheimer’s disease (AD), a multifaceted and irreversible neurodegenerative disease, is the major cause of dementia, and one of the biggest challenges of current pharmaceutical research [1]. During the past decades, despite substantial efforts to discover the pathogenic mechanisms of AD, the exact etiology of this disease is still complex in nature. The pathological factors playing remarkable role in development of AD are deficiency of brain cholinergic neurotransmitters level, accumulation of senile plaques particularly as extracellular β-amyloid (Aβ) deposits, hyperphosphorylated τ-protein aggregation [2], dyshomeostasis of biometals, oxidative stress, and neuroinflammation [3].
Notwithstanding all attempts performed to find innovative solution to control the hallmarks of the disease like gen and stem cell therapy, but these kind of therapies are still a prospective method for AD treatment and there are still many unsolved problems before they can be used in clinical applications [4, 5]. The currently available therapeutic method is mostly based on the increasing cholinergic neurotransmission, through acetylcholinesterase inhibitors (AChEI). Like AChE, butyrylcholinesterase (BuChE) as a coregulator of the ACh degradation, is an important enzyme involved in the neurotransmission [6]. The role of BuChE in AD pathology is unknown, but in vivo studies revealed that BuChE associated with Aβ plaques has key role in AD plaque maturation [7, 8]. To complete the symptomatic approaches, it is necessary to target simultaneously multiple pathological processes involved in AD [9, 10]. Currently, multi-target directed ligand (MTDL) strategy has been completely accepted as the main strategy for the drug design and discovery in AD, which is based on combination of appropriate pharmacophoric groups in one-molecule providing effective pharmacological responses for various potential receptors or enzymatic targets [11]. In recent years, many potential multifunctional agents have been designed based on MTDLs strategy against AD [12–16].
Moreover, quite an amount of natural-origin products is being used either as marketed pharmaceuticals or as bioactive molecular fragments in the development of hybrid drugs to combat the above-referred major risk factors involved in the pathogenesis of AD [17]. Chromones are ubiquitously found in plants and are known as the phytochemicals with a benzo-γ-pyrone structure, which possess a multiple range of pharmacological effects [18]. Recent studies revealed that chromone-based compounds were effective against dementia through free radicals scavenging and metal ions chelating [19–22]. Chromones have also showed anticholinesterase [23, 24], neuroprotective [25, 26], anti-inflammatory properties [27], and could effectively disrupt amyloid-β aggregation [28, 29]. Fernandez-Bachiller and co-workers introduced tacrine-chromone hybrids having improved ChE inhibitory as well as antioxidant activates compared to tacrine [30].
Lipoic acid (LA), as a naturally occurring antioxidant in animals, humans, and plants acts as an essential cofactor in many biochemical pathways [31]. Diverse range of pharmacological properties has been reported about this organosulfur. It can control the pathogenesis or progression of AD by increasing the level of acetylcholine as well as decreasing oxidative stress, inflammation, and Aβ plaque formation [32–34]. Lipocrine as a hybrid of LA and tacrine introduced by Rosini as an effective drug candidate against AD due to its multiple biological properties, such as AChE and BChE inhibition activity, inhibition of AChE-induced Aβ aggregation, and cell protectetion against ROS [35]. G. Nesi et al. combined rivastigmine with LA and chromone. They found that the combination led to the addition of anti-oxidants and anti-amyloid aggregating properties of LA and chromone scaffolds to the anticholinesterase activity of rivastigmine as the currently used drugs, and resulted to the formation of multifunctional compound to treat AD [28].
Based on the above findings, chromone scaffold is an important pharmacophore having great role in ChEs inhibition with antioxidant and metal chelation activities. LA is also the promising lead structure improving BuChE inhibition activity, neuroprotection potency, anti-amyloid aggregation, anti-oxidant and metal chelation activities (Fig. 1). These unique characteristics of chromone and LA have made them as promising scaffolds to develop novel multi-target compounds having improved therapeutic efficacy in the treatment of different neurodegenerative disorders, especially AD [36–42]. Considering the beneficial biological effects of chromone and LA scaffolds and the efficacy of triazole nucleus as an attractive pharmacophore to connecting various therapeutically active agents [43–45], in this work, we encouraged to conjugate these valuabe pharmacophores to each other and evaluate their efficacy against various targets. Two series of chromone derivatives conjugated with LA were synthesized via click reaction (Fig. 1, compounds 9–17 and 18–20) and the neuroprotective activity and ChEs inhibition activity of all the compounds were evaluated. The antioxidant, metal chelation and anti-amyloid activities of the selected compound was also evaluated. To the best of our knowledge, this is the first work reporting the synthesis of chromone-LA hybrids and their biological activities against AD.
Fig. 1.
Designed strategy for the preparation of the new chromone-LA hybrids
Experimental
Chemistry
Chemicals and solvents were obtained from commercial companies. Kofler hot stage apparatus was applied to determine melting points. The KBr disk method was used for FTIR analysis taken by Nicolet Magna FTIR-550 spectrometer. Brucker FT-500 was used for 1H and 13C NMR spectra. Elemental analysis of the compounds was performed on CHN-Rapid Heraeus Elemental Analyzer. The UV-Vis absorption spectra were recorded on a double beam GBC Cintra 101.
General method for the preparation of compounds 4a-d
2′-Hydroxyacetophenone derivative (1a-d, 1 equiv) was mixed with 4-methoxybenzaldehyde (2, 1 equiv) in the presence of pyrrolidine (0.5 equiv) and iodine (10 mol%) as catalyst in DMSO as solvent under reflux for 2 h. After finishing the reaction monitored by thin-layer chromatography (TLC), the reaction was worked up by ethylacetate and water. The organic part was wahsed with water, saturated sodium thiosulfate solution, brine, and finally dried by anhydrous sodium sulfate. The organic solvent was removed by rotary evaporator and the purified compound was obtained by column chromatography [46]. Then, the corresponding methoxyflavone (3a-d, 1 mmol) was dissolved in dry CH2Cl2 (15 mL) and temperature of the reaction decreased to −60 °C and BBr3 (3 mmol) was next added dropwise to the solution. The reaction was kept to be stirred at ambient temperature for 24 h until completion of the reaction. After that, ice water (50 mL) was added to the reaction and the product was extracted with ethylacetate (2 × 30 mL). The organic layer was then mixed with water, brine, and dried by anhydrous Na2SO4. The solvent was evaporated under vacuum and the product was used with no more purification [47].
General method for the preparation of 7-Hydroxy-4H-l-benzopyran-4-one (compound 5)
2,4-Dihydroxyacetophenone (1 mmol) in triethyl orthoformate (1 mL) was stirred, and 70% perchloric acid (0.1 mL) was next added dropwise. A dark solution was formed and the temperature of the mixture increased slightly. Anhydrous ethyl ether (3 mL) was next added to form a brown precipitate. The product was filtered, dissolved in hot water (2 mL), and refluxed for 5 min. The mixture was then cooled to ambient temperature overnight. A dark product was filtered and recrystallized from water/ethanol [48].
General method for the synthesis of bromoalkoxy intermediates 6a-i, and 8a-c
A mixture of hydroxylated chromone derivative (1 mmol), anhydrous K2CO3 (2 mmol) and appropriate dibromoalkane (10 mmol) was refluxed in acetone (5 mL). The precipitate was filtered off after 4 h and washed with acetone. Then, the acetone was removed under vacuum and the product was treated with petroleum ether to precipitate the pure product [49].
General method for the preparation of 1,2-dithiolane-3-pentanoic acid-N-propargylamide (compound 7)
LA (1 mmol), DMAP (1.2 mmol) and N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 1.2 mmol) was mixed in anhydrous CH2Cl2 (10 mL) at 0 °C. Then, propargylamine (1.2 mmol) in 2 mL dry CH2Cl2 was added dropwise and the reaction was performed at ambient temperature for 12 h. When the reaction was completed, the solvent was removed under vacuum and the crude product was obtained by column chromatography eluted by petroleum ether/ethyl acetate/(2:1) [50].
General method for the preparation of chromone–LA conjugates 9–20
A solution of the corresponding intermediate (6a-i, and 8a-c, 1 mmol), sodium azide (1 mmol), triethylamine (1 mmol) in t-BuOH (4 mL) and H2O (4 mL) was stirred at 70 °C for 0.5 h. After that, compound 7 (1 mmol), CuSO4.5H2O (0.2 mmol), and sodium ascorbate (0.2 mmol) were added to the reaction and it was stirred for 12 h. When the reaction was completed, water was added to the mixture and the reaction was stirred for next 0.5 h. Then, the mixture was filtered off and the product was purified by flash chromatography on silica gel eluted with petroleum ether / ethyl acetate (1:1) to give pure products 9–20 with 80–99% yields [51].
Characterization and structural determination of the synthesized products
5-(1,2-dithiolan-3-yl)-N-((1-(3-(4-(4-oxo-4H-chromen-2-yl)phenoxy)propyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (9) Off-white solid; mp 130–132 °C; IR (KBr, cm−1) νmax: 3300 (NH), 2930 (C-H), 1621 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.23 (t, 1H, J = 5.5 Hz, NH), 8.04 (d, 2H, J = 9.0 Hz, phenoxy), 8.04 (d, 1H, J = 7.5 Hz, H5), 7.93 (s, 1H, triazole), 7.80 (t, 1H, J = 7.5 Hz, H7), 7.76 (d, 1H, J = 7.5 Hz, H8), 7.49 (t, 1H, J = 7.5 Hz, H6), 7.10 (d, 2H, J = 9.0 Hz, phenoxy), 6.93 (s, 1H, H3), 4.53 (t, 2H, J = 7.0 Hz, CH2-N), 4.28 (d, 2H, J = 5.5 Hz, CH2-NH), 4.09 (t, 2H, J = 6.5 Hz, CH2-O), 3.57 (quintet, 1H, J = 6.0 Hz, CH-S), 3.17–3.08 (m, 2H, CH2-S), 2.38 (sextet, 1H, J = 6.7 Hz, S-CH2-CH2), 2.29 (quintet, 2H, aliphatic chain), 2.09 (t, 2H, J = 7.5 Hz, CH2-CO), 1.83 (sextet, 1H, J = 7.0 Hz, S-CH2-CH2), 1.65–1.48 (m, 4H, aliphatic chain), 1.33–1.30 (m, 2H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 176.7, 171.7, 162.5, 161.1, 155.5, 144.9, 133.9, 128.0, 125.2, 124.6, 123.3, 123.2, 122.7, 118.2, 114.9, 105.4, 64.8, 55.9, 46.2, 39.8, 39.7, 37.9, 34.9, 34.0, 33.9, 29.2, 28.1. Anal. Calcd for C29H32N4O4S2: C, 61.68; H, 5.71; N, 9.92. Found: C, 61.82; H, 5.43; N, 10.12.
N-((1-(3-(4-(6-bromo-4-oxo-4H-chromen-2-yl)phenoxy)propyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(1,2-dithiolan-3-yl)pentanamide (10) Off-white solid; mp 122–125 °C; IR (KBr, cm−1) νmax: 3306 (NH), 2927 (C-H), 1640 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.25 (t, 1H, J = 5.5 Hz, NH), 8.10 (s, 1H, triazole), 8.08 (d, J = 8.5 Hz, 2H, phenoxy), 7.98 (d, 1H, J = 8.5 Hz, H7), 7.93 (s, 1H, H5), 7.78 (d, 1H, J = 8.5 Hz, H8), 7.10 (d, 2H, J = 8.5 Hz, phenoxy), 7.00 (s, 1H, H3), 4.53 (t, 2H, J = 6.5 Hz, CH2-N), 4.28 (d, 2H, J = 5.5 Hz, CH2-NH), 4.09 (t, 2H, J = 5.5 Hz, CH2-O), 3.57–3.55 (m, 1H, CH-S), 3.17–3.09 (m, 2H, CH2-S), 2.39–2.34 (m, 1H, S-CH2-CH2), 2.31–2.27 (m, 2H, aliphatic chain), 2.08 (t, 2H, J = 7.0 Hz, CH2-CO), 1.84–1.80 (m, 1H, S-CH2-CH2), 1.65–1.59 (m, 1H, CH2-CH2-CO), 155–1.47 (m, 3H, aliphatic chain), 1.34–1.30 (m, 2H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.8, 171.8, 162.9, 161.3, 154.5, 145.0, 136.9, 128.3, 126.8, 124.8, 123.0, 122.8, 117.7, 115.0, 105.8, 65.2, 56.0, 46.2, 39.1, 34.9, 34.0, 29.3, 28.2, 24.8. Anal. Calcd for C29H31BrN4O4S2: C, 54.12; H, 4.86; N, 8.71. Found: C, 54.06; H, 4.55; N, 8.51.
5-(1,2-dithiolan-3-yl)-N-((1-(4-(4-(4-oxo-4H-chromen-2-yl)phenoxy)butyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (11) Off-white solid; mp 94–95 °C; IR (KBr, cm−1) νmax: 3298 (NH), 2920 (C-H), 1627 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.30 (t, J = 5.5 Hz, 1H, NH), 8.06 (d, J = 9.0 Hz, 2H, phenoxy), 8.03 (d, J = 7.5 Hz, 1H, H5), 7.93 (s, 1H, triazole), 7.82 (t, J = 7.5 Hz, 1H, H7), 7.77 (d, J = 7.5 Hz, 1H, H8), 7.49 (t, J = 7.5 Hz, 1H, H6), 7.10 (d, J = 9.0 Hz, 2H, phenoxy), 6.96 (s, 1H, H3), 4.41 (t, J = 7.0 Hz, 2H, CH2-N), 4.27 (d, J = 5.5 Hz, 2H, CH2-NH), 4.10 (t, J = 6.5 Hz, 2H, CH2-O), 3.57 (quintet, J = 6.0 Hz, 1H, CH-S), 3.16–3.08 (m, 2H, CH2-S), 2.39–2.32 (sextet, 1H, J = 6.7 Hz, S-CH2-CH2), 2.08 (t, 2H, J = 7.5 Hz, CH2-CO), 1.96 (quintet, 2H, aliphatic chain), 1.84 (sextet, 1H, J = 7.0 Hz, S-CH2-CH2), 1.73–1.70 (m, 2H, aliphatic chain), 1.65–1.60 (m, 1H, CH2-CH2-CO), 1.54–1.48 (m, 4H, aliphatic chain), 1.33–1.30 (m, 2H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 176.9, 171.8, 162.6, 161.4, 155.6, 145.0, 137.0, 128.2, 125.4, 124.7, 123.1, 122.7, 118.4, 115.0, 105.4, 67.1, 56.1, 48.9, 38.0, 34.9, 34.0, 28.3, 26.5, 25.5, 24.9. Anal. Calcd for C30H34N4O4S2: C, 62.26; H, 5.92; N, 9.68. Found: C, 62.59; H, 5. 61; N, 9.53.
N-((1-(4-(4-(6-bromo-4-oxo-4H-chromen-2-yl)phenoxy)butyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(1,2-dithiolan-3-yl)pentanamide (12) Off-white solid; mp 143–145 °C; IR (KBr, cm−1) νmax: 3303 (NH), 2929 (C-H), 1640 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.27 (bt, 1H, NH), 8.09 (s, 1H, triazole) 8.05 (d, 2H, J = 8.5 Hz, phenoxy), 7.97 (d, 1H, J = 9.0 Hz, H7), 7.91 (s, 1H, H5), 7.78 (d, 1H, J = 9.0 Hz, H8), 7.10 (d, 2H, J = 8.5 Hz, phenoxy), 7.00 (s, 1H, H3), 4.43 (t, 2H, J = 6.5 Hz, CH2-N), 4.28 (d, 2H, J = 5.5 Hz, CH2-NH), 4.10 (t, 2H, J = 6.0 Hz, CH2-O), 3.58–3.56 (m, 1H, CH-S), 3.19–3.08 (m, 2H, CH2-S), 2.40–2.37 (m, 1H, S-CH2-CH2), 2.09 (t, 2H, J = 7.0 Hz, CH2-CO), 1.97 (t, 2H, J = 7.0 Hz, CH2-CH2-N), 1.85–1.82 (m, 1H, CH2-CH2-S), 1.73–1.70 (m, 2H, O-CH2-CH2), 1.65–1.62 (m, 1H, CH2-CH2-CO), 1.51–1.50 (m, 3H, aliphatic chain), 1.33–1.30 (m, 2H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.6, 171.9, 163.0, 161.6, 154.5, 145.0, 136.7, 128.4, 128.3, 126.9, 124.8, 122.8, 122.7, 121.1, 117.7, 115.0, 67.2, 56.1, 48.9, 38.0, 34.9, 34.0, 28.2, 26.5, 25.5, 24.9. Anal. Calcd for C30H33BrN4O4S2: C, 54.79; H, 5.06; N, 8.52. Found: C, 55.09; H, 5.32; N, 8.43.
5-(1,2-Dithiolan-3-yl)-N-((1-(4-(4-(7-fluoro-4-oxo-4H-chromen-2-yl)phenoxy)butyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (13) Off-white solid; mp 152–154 °C; IR (KBr, cm−1) νmax: 3309 (NH), 2928 (C-H), 1640 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.27 (bs, 1H, NH), 8.10 (d, 1H, J = 8.0 Hz, H5), 8.06 (d, 2H, J = 8.0 Hz, phenoxy), 7.92 (s, 1H, triazole), 7.73 (d, 1H, J = 9.0 Hz, H8), 7.38 (t, 1H, J = 8.0 Hz, H6), 7.10 (d, 2H, J = 8.0 Hz, phenoxy), 6.95 (s, 1H, H3), 4.41 (t, 2H, J = 6.5 Hz, CH2-N), 4.27 (d, 2H, J = 5.5 Hz, CH2-NH), 4.10 (t, 2H, J = 6.5 Hz, CH2-O), 3.60–3.55 (m, 1H, CH-S), 3.18–3.09 (m, 2H, CH2-S), 2.41–2.36 (m, 1H, S-CH2-CH2), 2.09 (t, 2H, J = 7.0 Hz, CH2-CO), 1.99–1.96 (m, 2H, aliphatic chain), 1.85–1.81 (m, 1H, S-CH2-CH2), 1.73–1.72 (m, 2H, aliphatic chain), 1.66–1.62 (m, 1H, CH2-CH2-CO), 1.51–1.30 (m, 5H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 176.1, 172.1, 163.3, 161.5, 154.2, 144.9, 128.2, 128.1, 122.8, 114.9, 107.5, 105.5, 67.2, 56.0, 48.8, 34.9, 34.0, 28.2, 26.5, 25.5, 24.9. Anal. Calcd for C30H33FN4O4S2: C, 60.38; H, 5.57; N, 9.39. Found: C, 60.18; H, 5. 31; N, 9.28.
N-((1-(4-(4-(6-chloro-4-oxo-4H-chromen-2-yl)phenoxy)butyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(1,2-dithiolan-3-yl)pentanamide (14) Off-white solid; mp 142–144 °C; IR (KBr, cm−1) νmax: 3307 (NH), 2929 (C-H), 1630 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.28 (t, 1H, J = 5.5 Hz, NH), 8.07 (d, 2H, J = 8.5 Hz, phenoxy), 7.96 (s, 1H, triazole), 7.92–7.86 (m, 3H, H5, H7 and H8), 7.10 (d, 2H, J = 8.5 Hz, phenoxy), 7.00 (s, 1H, H3), 4.41 (t, 2H, J = 6.5 Hz, CH2-N), 4.28 (d, 2H, J = 5.5 Hz, CH2-NH), 4.10 (t, 2H, J = 6.5 Hz, CH2-O), 3.59–3.56 (m, 1H, CH-S), 3.19–3.07 (m, 2H, CH2-S), 2.40–2.36 (m, 1H, S-CH2-CH2), 2.09 (t, 2H, J = 7.0 Hz, CH2-CO), 1.99–1.95 (m, 2H, CH2-CH2-N), 1.85 (sextet, 1H, CH2-CH2-S), 1.73–1.69 (m, 2H, CH2-CH2-O), 1.65–1.62 (m, 1H, CH2-CH2-CO), 1.53–1.50 (m, 3H, aliphatic chain), 1.35–1.30 (m, 2H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.7, 171.8, 162.9, 161.6, 154.1, 150.4, 144.9, 133.9, 129.7, 128.4, 128.2, 124.4, 123.6, 122.8, 120.9, 115.0, 67.2, 56.1, 48.9, 38.0, 34.9, 34.0, 28.2, 26.5, 25.5, 24.9. Anal. Calcd for C30H33ClN4O4S2: C, 58.76; H, 5.42; N, 9.14. Found: C, 58.61; H, 5.39; N, 9.36.
5-(1,2-Dithiolan-3-yl)-N-((1-(5-(4-(4-oxo-4H-chromen-2-yl)phenoxy)pentyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (15) Off-white solid; mp 108–110 °C; IR (KBr, cm−1) νmax: 3301 (NH), 2928 (C-H), 1628 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.24 (s, 1H, NH), 8.03 (m, 2H, phenoxy and H5), 7.89 (s, 1H, triazole), 7.81 (t, 1H, J = 8.0 Hz, H7), 7.76 (d, 1H, J = 8.0 Hz, H8), 7.48 (t, 1H, J = 8.0 Hz, H6), 7.08 (d, 2H, J = 9.0 Hz, phenoxy), 6.91(s, 1H, H3), 4.36 (t, 2H, J = 7.0 Hz, CH2-N), 4.28 (d, 2H, J = 5.5 Hz, CH2-NH), 4.06 (t, 2H, J = 6.5 Hz, CH2-O), 3.57 (quintet, J = 6.0 Hz, 1H, CH-S), 3.19–3.07 (m, 2H, S-CH2), 2.39 (sextet, 1H, J = 6.7 Hz, S-CH2-CH2), 2.10 (t, 2H, J = 7.5 Hz, CH2-CO), 1.88–1.76 (m, 5H, aliphatic chain), 1.65–1.60 (m, 1H, CH2-CH2-CO), 1.54–1.50 (m, 3H, aliphatic chain), 1.42–1.31 (m, 4H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 176.7, 171.7, 162.5, 161.4, 155.5, 144.8, 133.9, 128.0, 125.2, 424.6, 123.2, 123.0, 122.4, 118.2, 114.9, 105.3, 67.5, 56.0, 49.0, 39.7, 37.9, 34.9, 33.9, 29.3, 28.1, 27.7, 24.8. Anal. Calcd for C31H36N4O4S2: C, 62.81; H, 6.12; N, 9.45. Found: C, 62.57; H, 5.86; N, 9.56.
N-((1-(5-(4-(6-bromo-4-oxo-4H-chromen-2-yl)phenoxy)pentyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(1,2-dithiolan-3-yl)pentanamide (16) Off-white solid; mp 105–107 °C; IR (KBr, cm−1) νmax: 3310 (NH), 2927 (C-H), 1631 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.26 (bs, 1H, NH), 8.09 (s, 1H, triazole), 8.05 (d, 2H, J = 8.0 Hz, phenoxy), 7.77 (d, 1H, J = 9.0 Hz, H7), 7.88 (s, 1H, H5), 7.77 (d, 1H, J = 9.0 Hz, H8), 7.10 (d, 2H, J = 8.0 Hz, phenoxy), 6.99 (s, 1H, H3), 4.35 (t, 2H, J = 6.5 Hz, CH2-N), 4.28 (d, 2H, J = 5.5 Hz, CH2-NH), 4.06 (t, 2H, J = 6.5 Hz, CH2-O), 3.58–3.55 (m, 1H, CH-S), 3.19–3.09 (m, 2H, 2H, CH2-S), 2.40–2.37 (m, 1H, S-CH2-CH2), 2.08 (bs, 1H, CH2-CO), 1.88–1.76 (m, 5H, aliphatic chain), 1.65–1.59 (m, 1H, CH2-CH2-CO), 1.55–1.32 (m, 7H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.6, 171.8, 162.9, 161.6, 156.2, 154.9, 144.9, 136.7, 130.4, 126.7, 128.3, 122.7, 121.1, 117.7, 114.9, 67.6, 56.1, 49.0, 34.9, 34.0, 29.4, 28.2, 27.8, 24.9, 22.0. Anal. Calcd for C31H36N4O4S2: C, 55.43; H, 5.25; N, 8.34. Found: C, 55.23; H, 5.13; N, 8.04.
N-((1-(5-(4-(6-chloro-4-oxo-4H-chromen-2-yl)phenoxy)pentyl)-1H-1,2,3-triazol-4-yl)methyl)-5-(1,2-dithiolan-3-yl)pentanamide (17) Off-white solid; mp 140–142 °C; IR (KBr, cm−1) νmax: 3297 (NH), 2920 (C-H), 1630 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.26 (bs, 1H, NH), 8.05 (d, 2H, J = 8.0 Hz, phenoxy), 7.96 (s, 1H, triazole), 7.89–7.85 (m, 3H, H5, H7 and H8), 7.10 (d, 2H, J = 8.0 Hz, phenoxy), 6.99 (s, 1H, H3), 4.35 (t, 2H, J = 6.5 Hz, CH2-N), 4.28 (d, 2H, J = 5.0 Hz, CH2-NH), 4.06 (t, 2H, J = 6.0 Hz, CH2-O), 3.59–3.56 (m, 1H, CH-S), 3.18–3.08 (m, 2H, CH2-S), 2.40–2.36 (m, 1H, S-CH2-CH2), 2.09 (t, 2H, J = 7.0 Hz, CH2-CO), 1.89–1.76 (m, 5H, aliphatic chain), 1.64–1.62 (m, 1H, CH2-CH2-CO), 1.51–1.32 (m, 7H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.7, 171.8, 161.6, 163.2, 157.2, 144.2, 129.7, 128.3, 128.2, 123.7, 122.7, 122.2, 114.9, 67.6, 56.3, 49.0, 38.1, 34.9, 34.0, 29.4, 28.2, 27.8, 24.9, 22.4. Anal. Calcd for C31H35ClN4O4S2: C, 59.36; H, 5.62; N, 8.93. Found: C, 59.12; H, 5.29; N, 9.18.
5-(1,2-Dithiolan-3-yl)-N-((1-(3-((4-oxo-4H-chromen-7-yl)oxy)propyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (18) Off-white solid; mp 89–91 °C; IR (KBr, cm−1) νmax: 3295 (NH), 2928 (C-H), 1640 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.29 (t, 1H, J = 5.5 Hz, NH), 8.22 (d, 1H, J = 6.0 Hz, H5), 7.94–7.92 (m, 2H, triazole and H2), 7.11 (d, 1H, J = 2.0 Hz, H8), 7.03 (dd, 1H, J = 9.0 Hz, J = 2.5 Hz, H6), 6.26 (d, 1H, J = 6.0 Hz, H3), 4.51 (t, 2H, J = 7.0 Hz, CH2-N), 4.26 (d, 2H, J = 5.5 Hz, CH2-NH), 4.11 (t, 2H, J = 6.0 Hz, CH2-O), 3.60–3.54 (m, 1H, CH-S), 3.16–3.10 (m, 2H, CH2-S), 2.41–2.36 (m, 1H, S-CH2-CH2), 2.32–2.27 (m, 2H, aliphatic chain), 2.07 (t, 2H, J = 7.5 Hz, CH2-CO), 1.85–1.82 (m, 1H, S-CH2-CH2), 1.65–1.59 (m, 1H, CH2-CH2-CO), 1.51–1.49 (m, 3H, aliphatic chain), 1.33–1.29 (m, 2H, aliphatic chain).13C NMR (DMSO-d6, 125 MHz) δ: 175.6, 171.8, 162.7, 157.7, 145.1, 126.2, 118.1, 114.9, 114.8, 112.1, 101.3, 65.5, 56.1, 46.3, 38.1, 35.0, 34.1, 29.2, 28.3, 24.9. Anal. Calcd for C23H28N4O4S2: C, 56.54; H, 5.78; N, 11.47. Found: C, 56.29; H, 6. 01; N, 11.55.
5-(1,2-Dithiolan-3-yl)-N-((1-(4-((4-oxo-4H-chromen-7-yl)oxy)butyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (19) Off-white solid; mp 128–130 °C; IR (KBr, cm−1) νmax: 3296 (NH), 2927 (C-H), 1644 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.30 (t, 1H, J = 6.0 Hz, NH), 8.10 (d, 1H, J = 6.0 Hz, H5), 7.93–7.91 (m, 2H, triazole and H2), 7.12 (d, 1H, J = 2.5 Hz, H8), 7.04 (dd, 1H, J = 8.5 Hz, J = 2.5 Hz, H6), 6.26 (d, 1H, J = 6.0 Hz, H3), 4.40 (t, 2H, J = 7.0 Hz, CH2-N), 4.26 (d, 2H, J = 4.5 Hz, CH2-NH), 4.12 (t, 2H, J = 6.5 Hz, CH2-O), 3.60–3.55 (m, 1H, CH-S), 3.18–3.08 (m, 2H, CH2-S), 2.41–2.34 (m, 1H, S-CH2-CH2), 2.08 (t, 2H, J = 7.5 Hz, CH2-CO), 1.98–1.93 (m, 2H, aliphatic chain), 1.85–1.79 (m, 1H, S-CH2-CH2), 1.74–1.68 (m, 2H, aliphatic chain), 1.66–1.60 (m, 1H, CH2-CH2-CO), 1.55–1.47 (m, 3H, aliphatic chain), 1.34–1.29 (m, 2H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.6, 171.9, 163.0, 156.5, 145.0, 122.1, 118.0, 114.9, 112.1, 101.4, 67.7, 56.1, 48.8, 38.1, 35.0, 34.1, 28.3, 26.5, 25.4, 24.9. Anal. Calcd for C24H30N4O4S2: C, 57.35; H, 6.02; N, 11.15. Found: C, 57.28; H, 6. 33; N, 11.37.
5-(1,2-Dithiolan-3-yl)-N-((1-(5-((4-oxo-4H-chromen-7-yl)oxy)pentyl)-1H-1,2,3-triazol-4-yl)methyl)pentanamide (20) Off-white solid; mp 89–90 °C; IR (KBr, cm−1) νmax: 3296 (NH), 2929 (C-H), 1645 (C=O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.29 (t, 1H, J = 5.5 Hz, NH), 8.21 (d, 1H, J = 6.0 Hz, H5), 7.92–7.89 (m, 2H, triazole and H2), 7.11 (d, 1H, J = 2.0 Hz, H8), 7.02 (dd, 1H, J = 8.8 Hz, J = 2.0 Hz, H6), 6.25 (d, 1H, J = 6.0 Hz, H3), 4.34 (t, 2H, J = 7.0 Hz, CH2-N), 4.26 (d, 2H, J = 5.5 Hz, CH2-NH), 4.08 (t, 2H, J = 6.2 Hz, CH2-O), 3.58–3.55 (m, 1H, CH-S), 3.18–3.08 (m, 2H, CH2-S), 2.39–2.35 (m, 1H, S-CH2-CH2), 2.08 (t, 2H, J = 7.5 Hz, CH2-CO), 1.89–1.74 (m, 5H, aliphatic chain), 1.63–1.61 (m, 1H, CH2-CH2-CO), 1.54–1.47 (m, 3H, aliphatic chain), 1.41–1.29 (m, 4H, aliphatic chain). 13C NMR (DMSO-d6, 125 MHz) δ: 175.6, 1718, 163.0, 147.7, 156.4, 144.9, 126.3, 122.3, 117.9, 114.9, 112.1, 101.2, 68.2, 56.1, 49.1, 38.0, 34.9, 34.0, 29.4, 28.3, 27.7, 24.9, 22.4. Anal. Calcd for C25H32N4O4S2: C, 58.12; H, 6.24; N, 10.84. Found: C, 57.96; H, 6. 07; N, 10.53.
Biological assays
Neuroprotective activity of the compounds
The cell viability was evaluated with the colorimetric assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) [52]. PC12 cell line was obtained from the Iranian Biological Resource Center, IBRC. The seeded cells in 96-well plates (1 × 104 cells/well) were incubated for 24 h at 37 °C under a humidified air containing 5% CO2. The cells were next treated with the tested compound (1–50 μM) and incubated for 3 h. Then, the cells were exposed with H2O2 (150 μM) and incubated for another 2 h. The medium was then replaced with 20 μL of MTT solution (5 mg/mL) and the cells were next incubated for another 4 h. The MTT solution was next removed and the crystals of formazan were solubilized using 100 μL of DMSO. A multi-mode plate reader (Biotek, Winooski, VT) was employed to record the appropriate absorbance at 570 nm. The results were reported as the percentage of untreated control cells and were the mean of three times determinations.
AChE/BuChE inhibition activity of the compounds
The Ellman method was used for ChE inhibition assay [53]. Donepezil was used as reference compound. The assay solution contained of phosphate buffer (0.1 M, pH 8.0), 5,5-dithiobis(2-nitrobenzoicacid) (0.01 M), eelAChE or eqBuChE (5 IU/mL, Sigma Chemical), various concentration of the tested compound solution and acetyl- or butyrylthiocholine iodide (0.05 M). The assay solutions in the presence or absence of the inhibitor were pre-incubated at 37 °C for 3 min after addition of the substrate. Each experiment was performed in triplicate, and log concentration versus inhibition curve was applied to achieve the IC50 values graphically.
Determination of the inhibitory potency of the compound against Aβ1–42 self-aggregation
Phosphate-buffered saline (PBS, pH 7.4, HyClone Thermo Scientific) having 1% ammonium hydroxide was utilized to solve Aβ1–42 (50 μM, Sigma A9810). The solution was shaked for 72 h at 37 °C. Aliquot of Aβ1–42 (10 μL) was incubated in PBS (0.05 M, pH 7.4) at 37 °C for 48 h with or without the tested inhibitor (100 μM). To investigate the Aβ1–42 self-aggregation, the thioflavin T (ThT) fluorescence method was employed [54]. After incubation, the mixture was treated with thioflavin T (50 μL, 200 μM, in 50 mM glycine-NaOH buffer, pH = 8.5). The amyloid fibril formation was screened by Microplate Reader (Spectra Max) at λexc = 448 nm and λem = 490 nm. Donepezil and rifampicin were applied as standard compounds. The inhibitory activity of the compound against Aβ-self-aggregation was calculated by the following equation: [(IFi/IFo) × 100] where IFi refers to the fluorescence intensity of Aβ1–42 with inhibitor and IFo is related to the fluorescence intensity achieved for Aβ1–42 in the absence of inhibitor.
Determination of intracellular ROS formation in PC12 cells induced by H2O2
The antioxidant potency of the selected compound was determined by measuring the amount of ROS evoked after PC12 cells exposure with H2O2. 2′,7′-dichloro-fluorescein diacetate (DCFH-DA) was employed as a ROS-sensitive dye to measure intracellular ROS formation [55]. PC12 cells seeded in 96-well plates were incubated at 37 °C in humidified CO2 atmosphere (5%) for 24 h (1 × 104 cells/well in growth medium). The cells were then exposed with the tested compound (1–50 μM) for 6 h. After cell washing with PBS, the cells were incubated with 150 μM of H2O2 for 18 h. After washing the cells with PBS, they were incubated with 10 μM of DCFH-DA for 30 min at 37 °C under dark. The fluorescence of the DCF in the cells was calculated using ELISA spectrofluorometer (BioTek, excitation/emission at 485 nm/528 nm) after removal of DCFH-DA and washing the cells with PBS. The results are the percentage of increase in intracellular ROS compared to the control (untreated cells) and calculated by the formula [(Ft – Fnt)/Fnt × 100], where Ft = fluorescence of the treated cells and Fnt = fluorescence of untreated cells.
FRAP assay
Previously reported method with slight modifications was used to determine the total antioxidant power of the compound [56]. A 240 μL of FRAP reagent (a mixture of three solutions containing 20 mM FeCl3, 10 mM TPTZ (2,4,6-tripyridyl-s-triazine), and 0.3 M acetate buffer having pH of 3.6) was added to the selected compound (10 μL, 10 μM) and the obtained solution was incubated for 15 min. The changes in the absorbance was studied by a microplate reader (BioTek Synergy HT) at 593 nm and the proportion of Fe3+ reduced to Fe2+ concentration was then calculated according to the plotted standard curve of Fe2+.
Metal chelating ability
The metal chelation activity of the selected compound was studied in the presence and absence of bio-metal ions (FeSO4, CuCl2, MgCl2, ZnCl2, CaCl2, and AlCl3). The tested compound was incubated with various concentrations metal ions for 0.5 h at ambient temperature and the appropriate spectrum was taken by a dual-beam (GBC Cintra 101) spectrophotometer in the range of wavelength from 200 to 500 nm. The stoichiometry of the complex was determined using the molar ratio method. UV-vis spectra of metal solution (2.5 mL, 80 μM) at increasing ligand concentrations were recorded [57].
Docking simulation
The Autodock vina software 1-1-2 and the AChE and BuChE crystal structure (PDB cod: 1EVE and 4BDS, respectively) was used for docking study. The conversion of the ligand to pdbqt format was conducted by Open babel (2.3.1) [58]. The active site dimensions were set as box size: 20 × 20 × 20 Å. Molecular visualization was carried out by DS visualizer molecular graphics system [59].
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM) of the experiments (at least three independent measurements). One-way ANOVA with Dunnett post hoc test was used for statistical analysis and a p value less than 0.05 was considered statistically significant.
Results and discussion
Chemistry
As illustrated in Scheme 1, compound 3 was initially synthesized via reaction of substituted 2′-hydroxyacetophenone with 4-methoxy benzaldehyde through a domino aldol-Michael-oxidation reaction catalyzed by pyrrolidine and iodine in dimethyl sulfoxide (DMSO). Compound 3 was converted to the corresponding 2-(4-hydroxyphenyl)-4H-chromen-4-one derivatives (4a-d) by BBr3 in dichloromethane. Compound 5 was also prepared in one step from the reaction of 2,4-dihydroxyacetophenone and triethyl orthoformate in the present of 70% perchloric acid leading to the formation of perchlorate salt which was then hydrolyzed to reach target compound (5). The bromoalkoxy intermediates 6a-i, and 8a-c were then prepared via the reaction of 4a-d or 5 with appropriate amount of dibromoalkanes in acetone solution of anhydrous K2CO3 under refluxing condition for 4 h. Compound 7 was separately synthesized through the amidation reaction between LA and propargylamine in the presence of 4-dimethylaminopyridine (DMAP) and N,N′-dicyclohexylcarbodimide (EDCI). Target compounds 9–20 were finally prepared through a one-pot three-component reaction between compounds 6a-i or 8a-c, sodium azide and compound 7 catalyzed by copper (II).
Scheme 1.
Synthetic routes to compounds 9–20. Reagents and conditions: (a) pyrrolidine, DMSO, I2; (b) BBr3, DCM; (c) HC(OEt)3, 70% HClO4; (d) H2O, 100 °C; (e) Br(CH)nBr (n = 3–5), anhydrous K2CO3, acetone, reflux, 4 h; (f) propargylamine, EDCI, DMAP, CH2Cl2, rt., 12 h; (g) NaN3, CuSO4, sodium ascorbate, t-Butanol/H2O, 70 °C, 12 h
Biological assay
Primarily, neuroprotection and cholinesterase inhibitory activities of all target compounds 9–20 were evaluated to find the best potent compound for further studies.
Neuroprotection potency against PC12 cell damaged induced by H2O2
Oxidative damage and neurotoxicity created by H2O2 are considered as most important factor controlling the progress of neurodegenerative disorder [60]. Therefore, neuroprotection activity of the prepared chromone–LA conjugates 9–20 were screened at different concentrations of 1, 5, 10, 20 and 50 μM using MTT assay. All compounds, in all concentrations, could significantly increase the cell viability of PC12 cells dose dependently, even at low concentration of 1 μM (Table 1, p < 0.001). Interestingly, compounds 15, 16, and 17 having five carbon chain length (n = 5) exhibited higher neuroprotective activity than that of quercetin as reference drug in all concentrations. By comparison between compounds 9, 11, and 15 bearing simple 2-phenyl-4H-chromen-4-one moiety and different carbon chain length (n = 3, 4 and 5 for compounds 9, 11, and 15, respectively), it could be implied that increasing the size of cross-linker could increase the neuroprotection activity of the target compounds (Table 1). The same behaviour was also seen for compounds 18–20 as 4H-chromen-4-one derivatives substituted at 7 position. Notably, the unsubstituted chromen derivatives had less neuroprotective activity on the H2O2-induced cell death than halo-substituted analogues (compare compound 9 with compound 10, compound 11 with compounds 12–14 and compound 15 with compounds 16 and even 17 at especially high concentrations).
Table 1.
The protective effect of compounds 9–20 against H2O2-induced injury in PC12 cell line at different concentrations
| Compound | n | R | PC12 cells viability (% of control)a | |||||
|---|---|---|---|---|---|---|---|---|
| H2O2 | 1 μM | 5 μM | 10 μM | 20 μM | 50 μM | |||
| 9 | 3 | H | 22.2 ± 2.1 | 28.1 ± 1.6*** | 32.4 ± 2.6*** | 36.0 ± 1.8*** | 37.2 ± 1.5*** | 38.5 ± 1.7*** |
| 10 | 3 | 6-Br | 20.1 ± 1.2 | 31.3 ± 1.0*** | 33.5 ± 0.9*** | 40.5 ± 0.9*** | 43.8 ± 1.0*** | 48.1 ± 1.1*** |
| 11 | 4 | H | 25.0 ± 1.4 | 33.0 ± 1.2*** | 39.3 ± 0.8*** | 41.3 ± 1.2*** | 44.2 ± 0.8*** | 46..5 ± 1.2*** |
| 12 | 4 | 6-Br | 26.6 ± 0.5 | 38.3 ± 1.0*** | 48.9 ± 1.2*** | 52.8 ± 0.5*** | 59.3 ± 0.6*** | 64.3 ± 1.4*** |
| 13 | 4 | 7-F | 24.7 ± 1.4 | 37.2 ± 0.6*** | 41.0 ± 1.0*** | 48.3 ± 2.0*** | 53.0 ± 2.6*** | 59.7 ± 2.2*** |
| 14 | 4 | 6-Cl | 24.6 ± 0.2 | 38.8 ± 0.8*** | 44.7 ± 0.5*** | 50.5 ± 0.6*** | 60.9 ± 0.6*** | 66.4 ± 1.0*** |
| 15 | 5 | H | 25.8 ± 1.1 | 44.9 ± 1.2*** | 52.0 ± 1.1*** | 57.2 ± 0.7*** | 61.6 ± 1.1*** | 65.2 ± 0.2*** |
| 16 | 5 | 6-Br | 25.9 ± 1.3 | 47.7 ± 1.0*** | 59.0 ± 0.8*** | 67.8 ± 0.6*** | 71.6 ± 1.6*** | 81.8 ± 1.2*** |
| 17 | 5 | 6-Cl | 25.9 ± 0.9 | 44.9 ± 0.6*** | 52.8 ± 0.7*** | 60.8 ± 1.1*** | 69.3 ± 0.7*** | 75.3 ± 1.4*** |
| 18 | 3 | – | 22.8 ± 1.2 | 28.3 ± 0.9*** | 31.0 ± 2.1*** | 36.7 ± 1.1*** | 40.1 ± 0.9*** | 43.4 ± 0.5*** |
| 19 | 4 | – | 27.5 ± 1.0 | 35.6 ± 0.3*** | 42.8 ± 0.7*** | 45.4 ± 1.2*** | 50.2 ± 0.6*** | 53.9 ± 0.1*** |
| 20 | 5 | – | 28.0 ± 0.4 | 41.0 ± 0.3*** | 47.2 ± 0.5*** | 52.3 ± 0.5*** | 57.4 ± 0.5*** | 62.5 ± 0.8*** |
| Quercetin | – | – | 28.6 ± 0.8 | 40.7 ± 0.4*** | 47.3 ± 0.9*** | 53.7 ± 1.2*** | 56.9 ± 0.5*** | 58.8 ± 0.1*** |
a)***Cell viability was determined using MTT assay protocol. Data are expressed as the mean ± SEM of three independent replicates. The significant (p < 0.001) values with respect to the H2O2 group
Cholinesterase inhibitory activity
AChE and BuChE inhibitory activity of all chromone–LA conjugates 9–20 were investigated. Table 2 shows the IC50 values of the compounds compared with standard drug donepezil. No significant effect was observed against AChE at 100 μM for most of the compounds, except 7-fluoro derivative (13) with moderate activity against AChE (IC50 = 56.50 μM). The results revealed that the activity of the 7-position substituted derivatives (18–20) against BuChE was better than the 2-position modified derivatives (9–17). Among the 7-substituted derivatives, only compound 18 and 19 with 3 or 4 atom chain lengths showed appropriate anti-BuChE activity (IC50 = 15.32 and 7.55 μM, respectively). Compound 20 with 5 carbone spacer (n = 5) revealed no activity against BuChE confirming the great effect of the cross-linker and size of the molecule to occupy the enzyme active site. Therefore, the extension of the linker has no positive effect on the BuChE inhibitory potency.
Table 2.
Inhibitory activity of the synthesized compounds 9–20 against AChE and BuChE
| Compound | n | R | AChEa IC50 (μM) | BuChEa IC50 (μM) |
|---|---|---|---|---|
| 9 | 3 | H | >100 | >100 |
| 10 | 3 | 6-Br | >100 | >100 |
| 11 | 4 | H | >100 | >100 |
| 12 | 4 | 6-Br | >100 | >100 |
| 13 | 4 | 7-F | 56.50 ± 3.91 | >100 |
| 14 | 4 | 6-Cl | >100 | >100 |
| 15 | 5 | H | >100 | >100 |
| 16 | 5 | 6-Br | >100 | >100 |
| 17 | 5 | 6-Cl | >100 | >100 |
| 18 | 3 | – | >100 | 15.32 ± 1.21 |
| 19 | 4 | – | >100 | 7.55 ± 0.78 |
| 20 | 5 | – | >100 | >100 |
| Donepezil | – | – | 0.027 ± 0.004 | 4.01 ± 0.56 |
aInhibitor concentration (mean ± SEM of three experiments) required for 50% inactivation of ChE
When tacrin bearing halogen substituent was conjugated to LA by 3 carbone spacer, the AChE and BuChE inhibition activities, especially AChEI activity, were improved in comparison with tacrin [35]. The study confirmed the important role of the length of the cross-linker on ChEs inhibition activity of the target compounds. Previous study also revealed that hybridization of tacrin with chromone scaffold improves BuChE inhibition activity more than that of AChEI which is in good agreement with the achievement of our results [30]. The same result was also observed when other well-known pharmacophores combine with each other. Attachment of LA and/or chromone to rivastigmine resulted in a higher BuChE inhibition activity than AChE inhibition [28]. It seems that LA can improve the BuChE inhibition activity of the mother scaffold more than AChE inhibition activity. Based on the results, it seems that electron donating groups on chromone scaffold and also using appropriate spacer to prevent molecular folding may facilitate proper interactions with the enzyme transforming target compounds into more potent inhibitors.
Other biological evaluation for the selected compound (19)
The neuroprotection assay revealed that all compounds could significantly protect PC12 cells against oxidative damage. However, among all chromone–LA conjugates, only compounds 13, 18 and 19 showed moderate anti-ChE activity. Also, compound 19 exhibited more potent neuroprotective activity (comparable with quercetin as reference drug) and the highest anti-BuChE activity. Therefore, all other studies were performed for compound 19.
Kinetic analysis of BuChE inhibition
The kinetic study of the most active anti-BuChE compound 19 was evaluated at different concentrations of the substrate. Lineweaver-Burk plots confirmed mixed-type of inhibition against BuChE (Fig. 2a) suggesting the ability of compound 19 to interact with both catalytic active site (CAS) and peripheral anionic site (PAS) of the enzyme. The inhibition constant Ki was calculated (Ki = 7.97 μM) and a non-competitive inhibition was obtained from secondary plots of the slope versus concentration of compound 19 (Fig. 2b).
Fig. 2.
a Lineweaver-Burk plot for the inhibition of BuChE by compound 19 at different concentrations of BuTCh, (b) Secondary plot for calculation of steady-state inhibition constant of compound 19, Ki = 7.97 μM
Docking studies
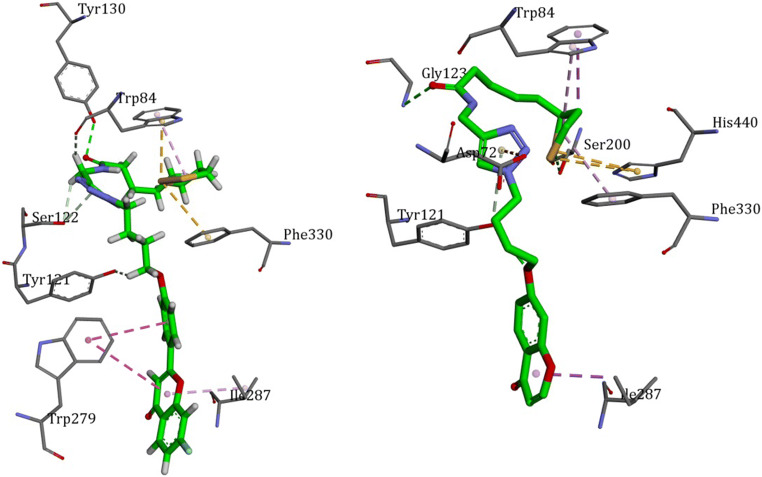
The binding modes of the most active compounds against AChE (13) and BuChE (19) in the active site of the enzymes were defined by docking study. According to the docking scores, the binding energies of −10.60 Kcal/mol and −9.32 Kcal/mol were calculated for 13 and 19 in the active site of AChE, respectively. The lower affinity of compound 19 in the AChE active site could be attributed to the lack of suitable interaction with PAS of the enzyme. In contrast, two stabilizing π-π stacking with Trp 279 could be occurred for compound 13. As depicted in Fig. 3, both molecules have folded to accommodate in the enzymes active site. The π-sulfur interaction between 1,2-dithiolane and central triazole ring stabilized the conformation. Moreover, an extra π-sulfur interaction between 1,2-dithiolane and Trp 84 and Phe 330 were observed. In this orientation, the more lipophilic part of the molecule tends to bind with PAS. The folding of the molecule has closed up the benzopyranone ring to the PAS. In such conformation, π-π stacking could be formed with Trp 279. This π-π stacking is crucial to discriminate between 13 and 19 to bind more tightly to the active site.
Fig. 3.
The binding mode of compound 13 (left) and compound 19 (right) in the gorge of AChE
In the case of BuChE, compounds 13 and 19 expressed different modes of binding in such a way that the folding of the molecules was quite different. In both molecules the triazole ring served as hinge to help molecule forming U-shape. As can be seen in Fig. 4, compound 19 folded outside the gorge; while, compound 13 folded inside. The binding energies were −8.89 and −8.43 for compounds 19 and 13, respectively. The less binding energy of compound 13 could be explained by more conformational energy and steric hindrance with the receptor.
Fig. 4.
The binding mode of compound 13 (left) and compound 19 (right) in the gorge of BuChE
Anti-amyloid aggregation activity
According to the role of BuChE in Aβ formation [5], the ability of compound 19, as most active BuChEI, against amyloid-beta aggregation was determined using the thioflavin T (ThT) analysis and compared with standard drugs (donepezil and rifampicin). The results in Table 3 show that compound 19 display moderate potency (13% inhibition, at 100 μM concentration), in comparison to donepezil (22% at 100 μM concentration) to inhibit Aβ aggregation. This reduced inhibitory ability compared to our previous coumarin-LA adducts with 62.4% and 51.2% inhibition activity [14], could be probably due to the absence of electron-donating functional groups (specially methoxy group) on compound 19 and also lack of appropriate hydrophobic and aromatic interactions between Aβ probes and inhibitor [61].
Table 3.
Inhibition of Aβ self-aggregation by the compound 19
| Compound | Inhibition of Aβ Self-aggregationa (%) |
|---|---|
| 19 | 13.1 ± 3.2 |
| Rifampicin | 27.5 ± 4.3 |
| Donepezil | 22 ± 5.4 |
aAβ1–42 (10 μM) aggregation in presence of the tested compound (100 μM)
Intracellular ROS inhibition activity of the selected compound
DCFH-DA (Dichloro-dihydro-fluorescein diacetate) assay was applied to investigate the antioxidant activity of compound 19 against intracellular ROS formation in PC12 cells at concentrations having no effect on the cell viability (1, 5, 10 and 50 μM). The results revealed that ROS production significantly decreased in a concentration-independent manner (Fig. 5, Table S1). In concentration of 10 μM, ROS formation was significantly lower than the H2O2 group, while LA showed similar inhibition activity at the higher concentration (50 μM). Moreover, the endogenous ROS significantly decreased in the treated cells with compound 19 compared with untreated control cells. Beside antioxidant effects of LA part, the presence of an α,β-unsaturated carbonyl group in chromone scaffold could have a complementary effect on the antioxidant activity. Based on previous reports, it seems that the presence of electron donating groups like hydroxyl and methoxy group can improve the antioxidant activity of the target compounds [36].
Fig. 5.
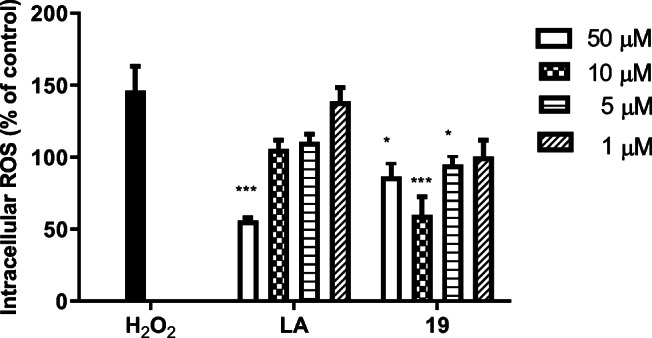
The effect of compound 19 and LA on intracellular ROS formation in neuronal PC12 cells. Values are the mean ± SEM of three independent experiments (*P < 0.05, ***P < 0.001) vs. H2O2 group (See supporting information for details, Table S1)
Total antioxidant assay
The FRAP (ferric reducing antioxidant power) of the compound 19 was compared with LA, quercetin, and ascorbic acid. The results indicated that compound 19 have excellent reducing power (Fe+2, 85.57 mM), comparable with quercetin and LA (Table 4) and could be considered as strong antioxidant.
Table 4.
Antioxidant activity of compound 19 determined by FRAP assay
| Compound | FRAP (mM)a |
|---|---|
| 19 | 85.57 ± 0.61 |
| Lipoic acid | 84.23 ± 0.79 |
| Ascorbic acid | 81.16 ± 0.50 |
| Quercetin | 84.77 ± 1.18 |
a)The data are expressed as Mean ± SEM of three experiments
Metal-chelating property
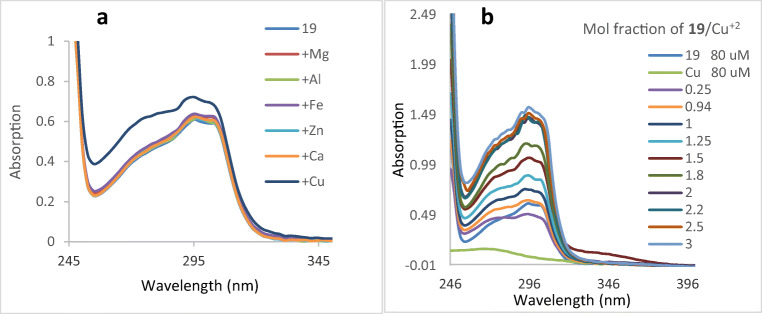
The chelating ability of compound 19 towards biologically relevant metal ions (Cu+2, Fe+2, Ca+2, Mg+2, Zn+2, and Al+3) was investigated by UV-vis spectrometry (Fig. 6a). In the absence of metal ions, the spectrum of compound 19 showed the maximum absorption at 303, and 297 nm. The curve intensity increased when CuCl2 was added suggesting the formation of 19-Cu2+ complex. However, no significant change was observed in the UV spectrum after adding FeSO4, CaCl2, MgCl2, ZnCl2 or AlCl3. The ability of compound 19 to chelate Cu2+ as bio-metal was studied by using mole ratio method. In this method, the total molar concentration of the tested ligand was continuously varied, while the molar concentration of Cu(II) was kept constant. As indicated in Fig. 6b, the addition of compound 19 to Cu+2 leads to an increase in the maximum absorbance which was then plateaued at a mole ratio of 2, indicating a 2:1 stoichiometry for complex of 19-copper ions. The presence of several chelating sites including amid and disulfide bonds on the target compounds is one of the reasons why compound 19 turn into great antioxidant.
Fig. 6.
a The UV spectra of compound 19 (final concentration, 80 μM in methanol) alone or in the presence of MgCl2, AlCl3, FeSO4, ZnCl2, CaCl2, and CuCl2 (final concentration, 80 μM in methanol). b Determination of the stoichiometry of Cu+2-19 complex using molar ratio method through titrating the methanol solution of CuCl2 (80 μM) with ascending amounts of compound 19 (1000 μM in methanol, titration step 0.01 mL)
Conclusion
In summary, a new series of chromone-LA hybrids were introduced as multifunctional agents against AD. All target compounds were potent as neuroprotective agents against H2O2-induced cell death. Especially, compounds 15, 16, and 17 were more active than quercetin (reference drug) in all range of concentrations. The anti-ChE assay showed that only 7-fluoro-flavone derivative 13 has moderate activity against AChE. In the case of BuChE inhibition, 7-position substituted derivatives showed more inhibitory activity than 2-position modified compounds. Among the 7-position substituted congeners, only compounds 18 and 19 showed proper BuChE inhibitory activity (IC50 = 15.32 and 7.55 μM, respectively). It could be eventually concluded that the chromone-LA hybrids showed significant neuroprotective activity in all concentrations, especially in halo- or methoxy-substituted derivatives. In the case of anti-ChE activity, most of the compounds were weak inhibitors of AChE and BChE, suggesting that further studies are needed on the chromone substitution and the length of the cross-linker. It seems that changing the substituents on the chromone scaffold may improve the BuChE inhibition activity of the target compound through well matching with the active site of the enzymes. However, the main aim of this work was to make a balanced in multi-target profile of the target compound rather than finding high potent ChE inhibitor. In modern medicinal chemistry, “one-molecule, one-target” paradigm has been replaced with MTDL approach leading to simplify the drug discovery process and generation of novel and efficient multi-target small molecules against AD. Some studies suggested that MTDLs with the mild activity against one or several targets may result in better in vivo outcomes compared to the one-target compounds having high affinity. Since week connections mostly control cellular networks, low-affinity MTDLs may be enough to create the significant results.
The novel chromone-LA hybrid (compound 19) introduced in this work, could be considered as potent neuroprotective agent having selective copper chelation ability with good BuChE inhibition activity and moderate anti-amyloid aggregation potency. More study on the effect of the substituent on chromone scaffold and the type and length of the cross-linker would be the future objective of the work.
Supplementary Information
(DOC 7351 kb)
Acknowledgments
This work was supported by a grant from The National Institute for Medical Research Development (NIMAD, grant number: 971370).
Author’s contributions
Leili Jalili-Baleh synthesized and characterized the compounds and participated in the writing of the manuscript. Hamid Nadri evaluated ChEs inhibition activity of the compounds, docking and kinetic studies. Hamid Forootanfar evaluated neuroprotective activity of the compounds. Tuba Tüylü Küçükkılınç and Beyza Ayazgök participated in intracellular ROS inhibition activity of the compound. Mahban Rahimifard and Maryam Baeeri performed FRAP assay. Mohammad Sharifzadeh, Mohammad Abdollahi and Alireza Foroumadi contributed to the characterization of the compounds, data analysis and revision of the manuscript. Mehdi Khoobi conceived the main idea of the study, organized the work and contributed to the overall analysis of the results. All the authors reviewed and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Querfurth HW, Laferla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Ekavali AS. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang FQ, Jiang JL, Zhang JT, Niu H, Fu XQ, Zeng LL. Current status and future prospects of stem cell therapy in Alzheimer’s disease. Neural Regen Res. 2020;15:242–250. doi: 10.4103/1673-5374.265544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson P, Iwata N, Muramatsu S, Tjernberg LO, Winblad B, Saido TC. Gene therapy in Alzheimer’s disease-potential for disease modification. J Cell Mol Med. 2010;14:741–757. doi: 10.1111/j.1582-4934.2010.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mushtaq G, Greig NH, Khan JA, Kamal MA. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:1432–1439. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darvesh S, Cash MK, Reid GA, Martin E, Mitnitski A, Geula C. Butyrylcholinesterase is associated with β-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:2–14. doi: 10.1097/NEN.0b013e31823cc7a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podoly E, Hanin G, Soreq H. Alanine-to-threonine substitutions and amyloid diseases: butyrylcholinesterase as a case study. Chem Biol Interact. 2010;187:64–71. doi: 10.1016/j.cbi.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Zhang A, Sun H, Han Y, Kong L, Wang X. Two decades of new drug discovery and development for Alzheimer’s disease. RSC Adv. 2017;7:6046–6058. [Google Scholar]
- 10.Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 11.Jesus Oset-Gasque M, Marco-Contelles J. Alzheimer’s disease, the “One-Molecule, One-Target” paradigm, and the multitarget directed ligand approach. ACS Chem Neurosci. 2018;9:401–403. doi: 10.1021/acschemneuro.8b00069. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel TJ, Klegeris A. Novel multi-target directed ligand-based strategies for reducing neuroinflammation in Alzheimer’s disease. Life Sci. 2018;15:314–322. doi: 10.1016/j.lfs.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Blaikie L, Kay G, Thoo Lin PK. Current and emerging therapeutic targets of alzheimer’s disease for the design of multi-target directed ligands. Med Chem Commun. 2019;10:2052–2072. doi: 10.1039/c9md00337a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim MM, Gabr MT. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen Res. 2019;14(3):437–440. doi: 10.4103/1673-5374.245463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalili-Baleh L, Nadri H, Forootanfar H, Samzadeh-Kermani A, Tüylü Küçükkılınç T, Ayazgok B, Rahimifard M, Baeeri M, Doostmohammadi M, Firoozpour L, et al. Novel 3-phenylcoumarin–lipoic acid conjugates as multi-functional agents for potential treatment of Alzheimer’s disease. Bioorg Chem. 2018;79:223–234. doi: 10.1016/j.bioorg.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Jalili-Baleh L, Forootanfar H, Tüylü Küçükkılınç T, Nadri H, Abdolahi Z, Ameri A, Jafari M, Ayazgok B, Baeeri M, Rahimifard M, SNA B, et al. Design, synthesis and evaluation of novel multi-target-directed ligands for treatment of Alzheimer’s disease based on coumarin and lipoic acid scaffolds. Eur J Med Chem. 2018b;152:600–14. [DOI] [PubMed]
- 17.de Freitas SM, Dias KST, Gontijo VS, Ortiz CJC, Viegas C., Jr Multi-target directed drugs as a modern approach for drug design towards Alzheimer’s disease: an update. Curr Med Chem. 2018;25:3491–3525. doi: 10.2174/0929867325666180111101843. [DOI] [PubMed] [Google Scholar]
- 18.Keri RS, Quintanova C, Chaves S, Silva DF, Cardoso SM, Santos MA. New tacrine hybrids with natural-based cysteine derivatives as multi targeted drugs for potential treatment of Alzheimer’s disease. Chem Boil Drug Des. 2016;87:101–111. doi: 10.1111/cbdd.12633. [DOI] [PubMed] [Google Scholar]
- 19.Choudhary S, Kumar Singh P, Verma H, Singh H, Silakari O. Success stories of natural product-based hybrid molecules for multifactorial diseases. Eur J Med Chem. 2018;151:62–97. doi: 10.1016/j.ejmech.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Reis J, Gaspar A, Milhazes N, Borges F. Chromone as a privileged scaffold in drug discovery: recent advances. J Med Chem. 2017;60(19):7941–7957. doi: 10.1021/acs.jmedchem.6b01720. [DOI] [PubMed] [Google Scholar]
- 21.Pachón-Angona I, Refouvelet B, Andrýs R, Martin H, Luzet V, Iriepa I, Moraleda I, Diez-Iriepa D, Oset-Gasque MJ, Marco-Contelles J, Musilek K, Ismaili L. Donepezil+chromone+melatonin hybrids as promising agents for Alzheimer’s disease therapy. J Enzy Inhib Med Chem. 2019;34(1):479–489. doi: 10.1080/14756366.2018.1545766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Compl Altern Med. 2013;13:120. doi: 10.1186/1472-6882-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Pandey AK. Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Int J Plant Res. 2013;26:301–307. [Google Scholar]
- 24.Leopoldini M, Russo N, Chiodo S, Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agricul Food Chem. 2006;54:6343–6351. doi: 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- 25.Reis J, Cagide F, Valencia ME, Teixeira J, Bagetta D, Pérez C, Uriarte E, Oliveira PJ, Ortuso F, Alcaro S, Rodríguez-Franco MI, Borges F. Multi-target-directed ligands for Alzheimer’s disease: discovery of chromone-based monoamine oxidase/cholinesterase inhibitors. Eur J Med Chem. 2018;158:781–800. doi: 10.1016/j.ejmech.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 26.Cruz I, Puthongking P, Cravo S, et al. Xanthone and flavone derivatives as dual agents with acetylcholinesterase inhibition and antioxidant activity as potential anti-alzheimer agents. J Chem. 2017;2017:e8587260. [Google Scholar]
- 27.Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE. The neuroprotective potential of flavonoids: a multiplicity of effects. Gene Nutr. 2008;3:115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabavi SF, Braidy N, Habtemariam S, Orhan IE, Daglia M, Manayi A, Gortzi O, Nabavi SM. Neuroprotective effects of chrysin: from chemistry to medicine. Neurochem Int. 2015;90:224–231. doi: 10.1016/j.neuint.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Gautam R, Jachak SM, Kumar V, Mohan CG. Synthesis, biological evaluation and molecular docking studies of stellatin derivatives a cyclooxygenase (COX-1, COX-2) inhibitors and anti-inflammatory agents. Bioorg Med Chem Lett. 2011;21:1612–1616. doi: 10.1016/j.bmcl.2011.01.116. [DOI] [PubMed] [Google Scholar]
- 30.Nesi G, Chen Q, Sestito S, Digiacomo M, Yang X, Wang S, Pi R, Rapposelli S. Nature-based molecules combined with rivastigmine: a symbiotic approach for the synthesis of new agents against Alzheimer’s disease. Eur J Med Chem. 2017;141:232–239. doi: 10.1016/j.ejmech.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Iida A, Usui T, Zar Kalai F, Han J, Isoda H, Nagumo Y. Protective effects of Nitraria retusa extract and its constituent isorhamnetin against amyloid binduced cytotoxicity and amyloid b aggregation. Biosci Biotechnol Biochem. 2015;79:1548–1551. doi: 10.1080/09168451.2015.1027655. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Bachiller MI, Perez C, Monjas L, Rademann J, Rodriguez-Franco MI. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J Med Chem. 2012;55:1303–1317. doi: 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- 33.Moini H, Packer L, Saris NEL. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 34.Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Münch G. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol Ther. 2007;113:154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Münch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Ad Drug Deliv Rev. 2008;60:1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RW., Jr Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28(2):213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Rosini M, Andrisano V, Bartolini M, Bolognesi ML, Hrelia P, Minarini A, Tarozzi A, Melchiorre C. Rational approach to discover multipotent anti-Alzheimer drugs. J Med Chem. 2005;48:360–363. doi: 10.1021/jm049112h. [DOI] [PubMed] [Google Scholar]
- 38.Sanga Z, Lia Y, Qiang X, Xiao G, Liu Q, Tan Z, Deng Y. Multifunctional scutellarin–rivastigmine hybrids with cholinergic, antioxidant, biometal chelating and neuroprotective properties for the treatment of Alzheimer’s disease. Bioorg Med Chem. 2015;23:668–680. doi: 10.1016/j.bmc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Airoldi C, La Ferla B, D’Orazio G, Ciaramelli C. Palmioli A flavonoids in the treatment of Alzheimer’s and other neurodegenerative diseases. Curr Med Chem. 2018;25:3228–3246. doi: 10.2174/0929867325666180209132125. [DOI] [PubMed] [Google Scholar]
- 40.Jalili-Baleh L, Babaei E, Abdpour S, Bukhari SNA, Foroumadi A, Ramazani A, Sharifzadeh M, Abdollahi M, Khoobi M. A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer’s disease. Eur J Med Chem. 2018;152:570–589. doi: 10.1016/j.ejmech.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Melagraki G, Afantitis A, Igglessi-Markopoulou O, Detsi A, Koufaki M, Kontogiorgis C, Hadjipavlou-Litina DJ. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem. 2009;44:3020–3026. doi: 10.1016/j.ejmech.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 42.Hager K, Marahrens A, Kenklies M, Riederer P, Munch G. R-Lipoic acid as a new treatment option for Alzheimer type dementia. Arch Gerontol Geriatr. 2001;32:275–282. doi: 10.1016/s0167-4943(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 43.Sang Z, Wang K, Shi J, Liu W, Cheng X, Zhu G, Wang Y, Zhao Y, Qiao Z, Wu A, Tan Z. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur J Med Chem. 2020;192:112180. doi: 10.1016/j.ejmech.2020.112180. [DOI] [PubMed] [Google Scholar]
- 44.Bolognesi ML, Rosini M, Andrisano V, Bartolini M, Minarini A, Tumiatti V, Melchiorre C. MTDL design strategy in the context of Alzheimer’s disease: from lipocrine to memoquin and beyond. Curr Pharm Des. 2009;15:601–613. doi: 10.2174/138161209787315585. [DOI] [PubMed] [Google Scholar]
- 45.Agalave SG, Maujan SR, Pore VS. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem Asian J. 2011;6:2696–2718. doi: 10.1002/asia.201100432. [DOI] [PubMed] [Google Scholar]
- 46.Xu M, Peng Y, Zhu L, Wang S, Ji J, Rakesh KP. Triazole derivatives as inhibitors of Alzheimer’s disease: current developments and structure-activity relationships. Eur J Med Chem. 2019;180:656–672. doi: 10.1016/j.ejmech.2019.07.059. [DOI] [PubMed] [Google Scholar]
- 47.Rastegari A, Nadri H, Mahdavi M, Moradi A, Mirfazli SS, Edraki N, Moghadam FH, Larijani B, Akbarzadeh T, Saeedi M. Design, synthesis and anti-Alzheimer’s activity of novel 1,2,3-triazole-chromenone carboxamide derivatives. Bioorg Chem. 2019;83:391–401. doi: 10.1016/j.bioorg.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 48.Naik MM, Tilve SG. Pyrrolidine and iodine catalyzed domino aldol-Michael-dehydrogenative synthesis of flavones. Tetrahedron Lett. 2014;55:3340–3343. [Google Scholar]
- 49.Sagrera G, Bertucci A, Vazquez A, Seoane G. Synthesis and antifungal activities of natural and synthetic bioflavonoids. Bioorg Med Chem. 2011;19:3060–3073. doi: 10.1016/j.bmc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Jaen JC, Wise LD, Heffner TG, Pugsley TA, Meltzer LT. Dopamine auto receptor agonists as potential antipsychotics. 2. (Aminoalkoxy)-4H-1-benzopyran-4-ones. J Med Chem. 1991;34:248–256. doi: 10.1021/jm00105a039. [DOI] [PubMed] [Google Scholar]
- 51.Luo W, Su YB, Hong C, Tian RG, Su LP, Wang YQ, Li Y, Yue JJ, Wang CJ. Design, synthesis and evaluation of novel 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Bioorg Med Chem. 2013;21:7275–7282. doi: 10.1016/j.bmc.2013.09.061. [DOI] [PubMed] [Google Scholar]
- 52.Wu T, Zhang Q, Hu J, Zhang G, Liu S. Composite silica nanospheres covalently anchored with gold nanoparticles at the outer periphery of thermoresponsive polymer brushes. J Mater Chem. 2012;22:5155–5163. [Google Scholar]
- 53.Garin D, Oukhatar F, Mahon AB, Try AC, Dubois-Dauphin M, Laferla FM, Demeunynck M, Sallanon MM, Chierici S. Proflavine derivatives as fluorescent imaging agents of amyloid deposits. Bioorg Med Chem Lett. 2011;21:2203–2206. doi: 10.1016/j.bmcl.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Zsolt D, Juhász A, Gálfi M, Soós K, Papp R, Zádori D, Penke B. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma. Cells Brain Res Bull. 2003;62:223–229. doi: 10.1016/j.brainresbull.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 56.Levine H. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptide: detection of amyloid aggregation in solution. Protein Sci. 1993;20:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baeeri M, Momtaz S, Navaei-Nigjeh M, Niaz K, Rahimifard M, GhasemiNiri SF, Sanadgol N, Hodjat M, Sharifzadeh M, Abdollahi M. Molecular evidence on the protective effect of ellagic acid on phosaloneinduced senescence in rat embryonic fibroblast cells. Food Chem Toxicol. 2017;100:8–23. doi: 10.1016/j.fct.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 59.Pazik A, Skwierawska A. Synthesis and spectroscopic properties of new bis-tetrazoles. J Incl Phenom Macrocycl Chem. 2013;77:83–94. doi: 10.1007/s10847-012-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dassault Systèmes BIOVIA . Discovery studio modeling, release, 4. San Diego: Dassault Systemes; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 7351 kb)