Abstract
Background
There is a paucity of data on the role of molecular methods in the diagnosis of osteoarticular tuberculosis. The present study was conducted to define the role of molecular (CBNAAT, LPA), phenotypic (AFB smear and culture) and histopathological evaluation in the diagnosis of osteoarticular TB.
Methods
Seventy-seven consecutive cases of osteoarticular tuberculosis were grouped into presumptive TB cases (group A) and presumptive drug-resistant cases (group B). Tissue samples obtained were submitted for CBNAAT, LPA, AFB smear, liquid culture and histological examinations. The diagnostic accuracy of each test was reported against histologically diagnosed cases and in all tests in tandem.
Results
Group A and group B had 65 and 12 cases, respectively. The diagnostic accuracy for tuberculosis was 84.62% by CBNAAT, 70.77% by LPA, 86.15% by molecular tests (combined), 47.69% by AFB smear, 50.77% by liquid culture and 87.69% by histology in group A, and 91.67% for CBNAAT, 83.33% for LPA, 91.67% for molecular tests (combined), 25% for AFB smear, 16.67% for liquid culture and 83.33% for histology in group B. The drug resistance detection rate was 4.62% on CBNAAT, 3.08% on LPA, 6.15% on molecular tests (combined) and 1.54% on DST in group A, while it was 33.33% on CBNAAT, 58.33% on LPA, 58.33% on molecular tests (combined) and 16.67% on DST among group B cases. Similar sensitivity rates for the various tests were obtained among both the groups on comparison with histology (taken as denominator). The addition of molecular methods increased the overall diagnostic accuracy (all tests in tandem) from 93.8 to 100% in group A and from 83.3 to 100% in group B cases.
Conclusion
No single tests could diagnose tuberculosis in all cases; hence, samples should be evaluated by molecular tests (CBNAAT and LPA), AFB smear, culture and histological examinations simultaneously. The molecular tests have better demonstration of drug resistance from mycobacterial culture.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43465-020-00326-w.
Keywords: Osteoarticular tuberculosis, Spinal tuberculosis, Molecular genotypic methods, Phenotypic methods, Presumptive tuberculosis, Presumptive drug-resistant tuberculosis, Cartridge-based nucleic acid amplification test, Line probe assay, Multidrug-resistant tuberculosis, Histopathology
Introduction
The etiological diagnosis of tuberculosis (TB) is made by demonstration of acid-fast bacilli (AFB) on smear in 30–50% cases [1], by mycobacterial culture in 10–30% instances [2] or by histology [1,2]. Mycobacterial culture, a gold standard for TB, is necessary for drug susceptibility testing (DST) [2].
Epithelioid cell granulomas, granular necrotic background including caseous necrosis, lymphocytic infiltration, scattered multinucleated and Langhans’ giant cells are observed on histology in TB. Histology report is consistent with tuberculosis (60%) if necrotizing granulomas with caseous necrosis are found while suggestive in the presence of non-necrotizing granulomas [3], with differential diagnosis of other chronic granulomatous diseases.
Molecular tests identify genetic makeup of live/dead bacteria [1] and do not require mycobacterial growth, hence substantially reducing time for diagnosis [2]. These tests are based on nucleic acid amplification of Mycobacterium tuberculosis bacilli (MTBC) using polymerase chain reaction (PCR) followed by its detection using certain markers in genetic material. They also demonstrate rifampicin and isoniazid resistance [1].
Mycobacterium is a fastidious slow-growing organism. Limited tissue procurement from deep seated extrapulmonary paucibacillary lesions makes bacteriological diagnosis difficult. No single test can ascertain diagnosis in all bone TB cases [4]. The demonstration of drug resistance is impossible if culture is negative, a common scenario with osteoarticular tuberculosis. The data on the use of molecular methods in osteoarticular tuberculosis are sparse as only five articles on use of molecular methods for diagnosis and two on detection of drug resistance were traced [2,5–10].
The diagnostic accuracy of cartridge-based nucleic acid amplification test (CBNAAT) and line probe assay (LPA) and its comparison with histology and liquid culture (Gold standard) in bone TB has never been reported. We report analysis of prospectively collected consecutive series of osteoarticular TB cases investigated by molecular (CBNAAT and LPA), phenotypic (AFB smear, culture) and histological methods. The objective of the study was to estimate the diagnostic accuracy of CBNAAT, LPA, AFB smear, culture and histology in the diagnosis of osteoarticular tuberculosis.
Materials and Methods
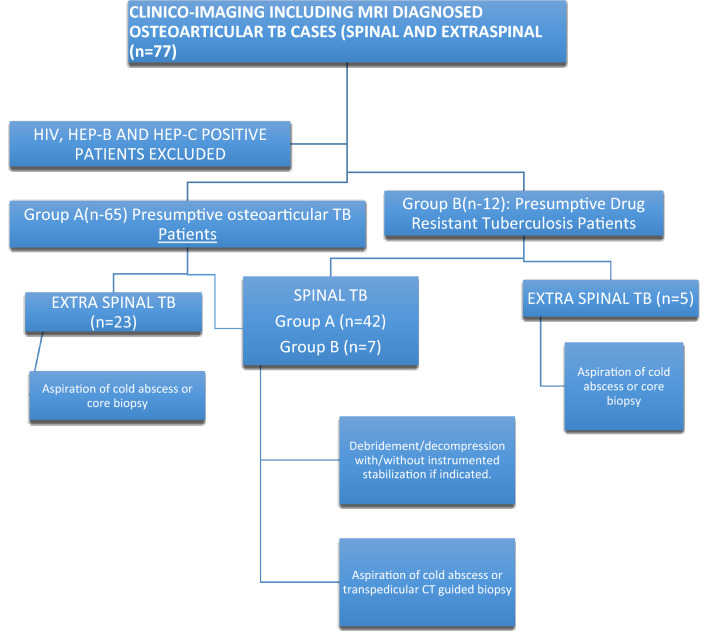
Using the STARD’s methodology, a sample size of 184 was obtained for the study. However, due to limited time and resource constraints, 77 consecutive patients of osteoarticular tuberculosis (spinal and extraspinal) diagnosed on clinico-imaging findings and on magnetic resonance imaging (MRI) observations were enrolled. HIV-, HBsAg- or HCV-positive patients were excluded. The cases were recruited from 1 November 2016 to 28 February 2018 and analysed by 30 April 2018. They were divided into two groups.
Group A (n-65) presumptive osteoarticular TB patients: These are recently diagnosed cases on clinico-imaging basis including MRI. Twenty-five cases were already on ATT (for less than 2 months) on presentation, and in the rest 40 cases, ATT was started by us.
Group B (n-12) presumptive drug-resistant tuberculosis patients: They presented with poor clinico-radiological response/appearance of a fresh lesion on 5 months or more ATT intake [11,12].
None of the patients included have been cited/included in any previous studies. All underwent aspiration of cold abscesses, core biopsy from extraspinal lesions or transpedicular CT guided biopsy from spinal lesions (Fig. 1). The spinal lesions were operated for debridement/decompression with/without instrumented stabilization, if indicated [13–17] (Fig. 2). Pus, fluid, cartilage, synovium, intervertebral disc material, granulation tissue, caseous tissue and bony tissue from the affected bone were sent for histopathology (at UCMS), and AFB smear, mycobacterial culture, CBNAAT and LPA at National Institute of Tuberculosis and Respiratory Diseases (NITRD), New Delhi [18,19]. All samples collected underwent all the above-mentioned tests. Standard operating procedures were also followed during specimen collection, transport and processing [18,19].
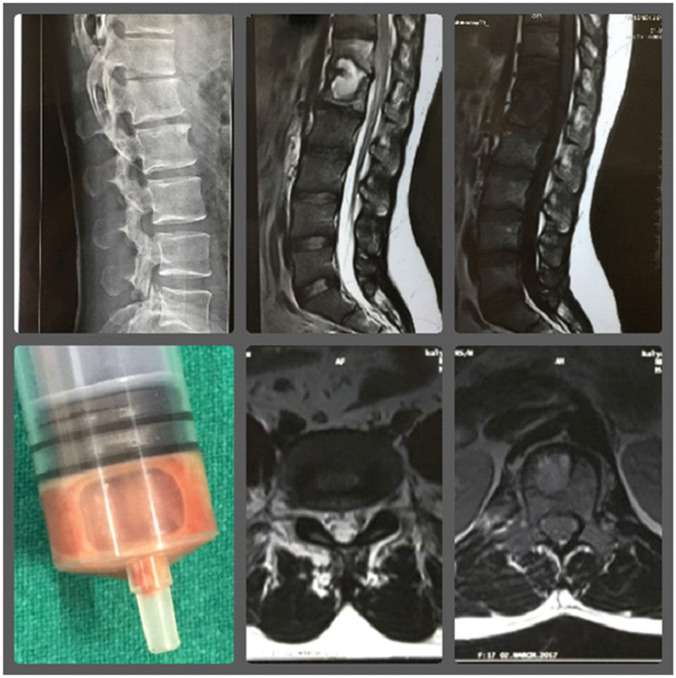
Fig. 1.
A 36-year-old male, a presumptive case (group A) of Pott’s spine D12-L1 with grade 1 paraplegia. Pt presented on 12/3/17 with low backache for 9 months. O/E-tenderness at D12, L1. Pt was elsewhere prescribed on Cat1 ATT since 7/3/17. CT-guided biopsy was done from D12 vertebrae on 16/3/17. CBNAAT and LPA TB positive, sensitive to R and H. Smear AFB positive, liquid culture negative, histology consistent for TB. PT was continued on Cat1 ATT
Fig. 2.
Flow diagram
CBNAAT, if valid, is reported as MTB detected and sensitivity/resistance to rifampicin. Valid LPA is reported as MTB detected and sensitivity/resistance to rifampicin and isoniazid [18,19]. In case of indeterminate result, the same test was repeated in the specimen available. Otherwise, tests were done on the pure culture obtained from the specimen to resolve the issue.
The diagnosis of MTB was ascertained by various methods: molecular methods (CBNAAT, LPA), AFB smear, culture and histology. The drug resistance rates detected by molecular method and culture were calculated in both groups (Table 1). The diagnostic percentage was calculated including one modality with others one by one (Table 2). The sensitivity of various methods was estimated on comparison with histology (taken as denominator) (Tables 3, 4).
Table 1.
Drug resistance detection of various tests
| Tests with positive drug resistance report | Group A (n = 65) | Group B (n = 12) |
|---|---|---|
| Culture (DST) | 1/65 (1.54%) | 2/12 (16.67%) |
| Culture (DST) + CBNAAT | 3/65 (4.62%) | 4/12 (33.33%) |
| Culture (DST) + CBNAAT + LPA | 4/65 (6.15%) | 7/12 (58.33%) |
Table 2.
Diagnostic yield of various tests
| Tests done | Group A (n = 65) | Group B (n = 12) |
|---|---|---|
| Smear AFB positive | 31/65 (47.69%) | 3/12 (25%) |
| Smear AFB + culture positive | 46/65 (70.77%) | 3/12 (25%) |
| Smear AFB, culture + histology positive | 61/65 (93.85%) | 10/12 (83.33%) |
| Smear AFB, culture, histology + LPA positive | 63/65 (96.92%) | 11/12 (91.67%) |
| Smear AFB, culture, histology, LPA + CBNAAT positive | 65/65 (100%) | 12/12 (100%) |
| Total | 65 | 12 |
Table 3.
Sensitivity of various tests among histology-positive cases of osteoarticular tuberculosis
| Histology-positive patients (number) | CBNAAT positive | Sensitivity |
|---|---|---|
| Group A (n = 57) | 50 | 87.72% (50/57) |
| Group B (n = 10) | 9 | 90% (9/10) |
| LPA positive | Sensitivity | |
|---|---|---|
| Group A (n = 57) | 42 | 73.68% (42/57) |
| Group B (n = 10) | 9 | 90% (9/10) |
| Molecular tests (in tandem) positive | Sensitivity | |
|---|---|---|
| Group A (n = 57) | 50 | 87.72% (50/57) |
| Group B (n = 10) | 9 | 90% (9/10) |
| AFB smear positive | Sensitivity | |
|---|---|---|
| Group A (n = 57) | 29 | 50.88% (29/57) |
| Group B (n = 10) | 3 | 30% (3/10) |
| Liquid culture positive | Sensitivity | |
|---|---|---|
| Group A (n = 57) | 29 | 50.88% (29/57) |
| Group B (n = 10) | 2 | 20% (2/10) |
Table 4.
Sensitivity rates of various tests among histology-positive cases
| Type of patients with histology result (number) | Test with results (number) | Sensitivity (TP/TP + FN) | |
|---|---|---|---|
| CBNAAT positive (TP) | CBNAAT negative (FN) | ||
| Histology-positive primary cases (n = 57) | 50 | 7 | 87.72% (50/57) |
| Histology-positive presumptive drug-resistant cases (n = 10) | 9 | 1 | 90% (9/10) |
| LPA positive (TP) | LPA negative (FN) | Sensitivity | |
|---|---|---|---|
| Histology-positive primary cases (n = 57) | 42 | 15 | 73.68% (42/57) |
| Histology-positive presumptive drug-resistant cases (n = 10) | 9 | 1 | 90% (9/10) |
| Molecular tests (in tandem) positive (TP) | Molecular tests (in tandem) negative (FN) | Sensitivity | |
|---|---|---|---|
| Histology-positive primary cases (n = 57) | 50 | 7 | 87.72% (50/57) |
| Histology-positive presumptive drug-resistant cases (n = 10) | 9 | 1 | 90% (9/10) |
| AFB smear positive (TP) | AFB smear negative (FN) | Sensitivity | |
|---|---|---|---|
| Histology-positive primary cases (n = 57) | 29 | 28 | 50.88% (29/57) |
| Histology-positive presumptive drug-resistant cases (n = 10) | 3 | 7 | 30% (3/10) |
| Liquid culture positive (TP) | Liquid culture negative (FN) | Sensitivity | |
|---|---|---|---|
| Histology-positive primary cases (n = 57) | 29 | 28 | 50.88% (29/57) |
| Histology-positive presumptive drug-resistant cases (n = 10) | 2 | 8 | 20% (2/10) |
TP true positive, FN false negative, TP + FN total no of cases
Once diagnosis is ascertained, standard treatment for different sites of extrapulmonary (osteoarticular) tuberculosis was followed with appropriate ATT under directly observed treatment, short course (DOTS) alternate day regimen (2HRZE + 10HRE) [18] or appropriate regimen for proven drug resistance under the RNTCP guidelines [20].
Data were analysed using SPSS software v23.0, and Fisher’s exact test of independence was used to compare the nominal variables.
Results
Seventy-seven patients, 49 (63.64%) spinal and 28 (36.36%) extraspinal tuberculosis, were enrolled with a mean age of 30.42 years (range 4.6–72 years). Fifteen (19.48%) were children, while 62 (80.52%) adults. Thirty-nine patients (50.65%) were females, while 38 (49.35%) were males.
Group A: Presumptive Osteoarticular Tuberculosis Cases (n = 65)
Forty-two patients (64.62%) were spinal and 23 (35.38%) extraspinal TB. The diagnostic accuracy of AFB smear was 47.69% (31/65) (Table 5). On histology 40 patients (61.54%) were labelled consistent with TB, while 17 patients (26.15%) were suggestive of TB. These 17 cases were also diagnosed TB by other methods. Thus, diagnostic accuracy of histopathology was 87.69% (57/65) (Table 5).
Table 5.
Diagnostic accuracy of various tests among group A cases (n = 65)
| Test done | Diagnostic accuracy (95% CI) |
|---|---|
| CBNAAT | 84.62% (77.55–91.69%) |
| LPA | 70.77% (61.85–79.68%) |
| Molecular tests in tandem (CBNAAT + LPA) | 86.15% (79.38–92.92%) |
| AFB smear | 47.69% (37.90–57.47%) |
| Liquid culture | 50.77% (40.97–60.57%) |
| Histopathology | 87.69% (81.25–4.13%) |
The diagnostic accuracy of liquid culture was 50.77% (33/65), of CBNAAT was 84.62% (55/65) and of LPA was 70.77% (46/65). The diagnostic accuracy of CBNAAT and LPA (in tandem) was 86.15% (56/65) (Table 5).
Drug resistance was detected by culture/DST in 1/65 (1.54%). CBNAAT demonstrated rifampicin resistance in 3/65 (4.62%) (including the above case). LPA demonstrated drug resistance in 2/65 (3.08%) patients out of which 1 had isoniazid monoresistance, while the other had multidrug-resistant (MDR) TB (rifampicin and isoniazid resistance) [18]. Thus, drug resistance was demonstrated by CBNAAT and LPA in tandem in 4/65 (6.15%) patients (Table 1).
Group B: Presumptive Drug-Resistant Tuberculosis (n = 12) [11,12]
Seven patients (58.33%) and 5 (41.67%) were of spinal and extraspinal TB, respectively. The diagnostic accuracy by AFB smear was 25% (3/12) and of liquid culture was 16.67% (2/12). The diagnostic accuracy of histopathology was 83.33% (10/12), with 8/12 (66.67%) reported as consistent and 2/12 (16.67%) as suggestive. The diagnostic accuracy of CBNAAT was 91.67% (11/12), of LPA 83.33% (10/12) and in tandem 91.67% (11/12) (Table 6).
Table 6.
Diagnostic accuracy of various tests among group B cases (n = 12)
| Test done | Diagnostic accuracy (95% CI) |
|---|---|
| CBNAAT | 91.67% (86.25–97.09%) |
| LPA | 83.33% (76.02–90.64%) |
| Molecular tests in tandem (CBNAAT + LPA) | 91.67% (86.25–97.09%) |
| AFB smear | 25% (16.51–33.49%) |
| Liquid culture | 16.67% (9.36–23.98%) |
| Histopathology | 83.33% (76.02–90.64%) |
On DST, only 2/12 demonstrated resistance to rifampicin and isoniazid, while CBNAAT demonstrated rifampicin resistance in 4/12 (33.33%) including above 2. LPA demonstrated rifampicin and/or isoniazid resistance rate in 58.33% (7/12) patients with isoniazid monoresistance in two. Thus, in tandem, the drug (rifampicin and/or isoniazid) resistance detection rate was 58.33% (7/12). Overall, 5/12 (41.66%) culture-negative cases also demonstrated drug resistance by molecular methods (Table 1).
Diagnostic accuracy of all tests in tandem: The diagnostic accuracy of each test is variable. Hence, we tried to use all the methods in tandem.
Group A: AFB smear was diagnostic in 47.69% (31/65) cases. The addition of culture increased diagnostic accuracy to 70.77% (46/65) and histology increased it to 93.85% (61/65). In the remaining 4 cases, the diagnosis was made by LPA (2/4) and CBNAAT (4/4) (Table 2). The diagnosis could be ascertained in all cases when all tests were performed simultaneously.
DST demonstrated drug resistance in 1 case (1/65, 1.54%), while on addition of molecular tests, the demonstration of drug resistance increased to 4 (4/65, 6.15%) (Table 1).
Group B: AFB smear and addition of culture established the diagnosis in 3/12 (25%) cases. Histology increased the diagnostic accuracy for TB to 83.33% (10/12). The remaining 2 cases (16.67%) were diagnosed by LPA(1/2) and CBNAAT (2/2) (Table 2). The drug resistance was detected in 2/12 (16.67%) on DST, while molecular tests increased the number to 7/12 (58.33%) (Table 1). In the remaining 5 (41.66%) culture-negative cases, drug resistance could not be established by molecular methods.
Combining all tests, the diagnostic accuracy touched 100% (Table 2). No single test was diagnostic in all cases of osteoarticular TB, and hence, no single gold standard diagnostic modality could be defined for statistical comparison. We included presumptive osteoarticular TB cases [18] in the series and thus had no true negatives. The diagnosis of TB was ascertained in all cases; hence, there were no false positive cases. Thus, the specificity, positive predictive value (PPV) and the negative predictive value (NPV) parameters could not be calculated. The molecular tests, AFB smear and liquid culture were compared among the histopathology-positive cases, and sensitivity was calculated (taking a positive result as true positive cases and a negative result as false negative cases). On comparison with histopathology, the sensitivity of CBNAAT was 87.72% in group A and 90% in group B. The sensitivity of LPA was 73.68% in group A and 90% in group B. The sensitivity of the molecular tests (in tandem) was 87.72% and 90% in group A and group B, respectively. The sensitivity of AFB smear was 50.88% and 30% in group A and group B, respectively. The sensitivity of liquid culture was 50.88% and 20% in group A and group B, respectively (Tables 3, 4). No adverse events were reported during any of the procedures or tests.
Discussion
Etiological diagnosis of paucibacillary osteoarticular TB is established by demonstration of AFB smear/culture or by histology [1,2]. Wang et al. [21] reported a diagnostic accuracy of 51.72% for culture in spinal TB cases. The diagnosis in culture-negative cases is established on histology with findings consistent with tuberculosis (60%) and suggestive in the remaining [3]. The emerging multidrug-resistant strains [18] are a threat to cure due to poor mycobacterial isolation and inability to demonstrate drug resistance by DST.
Since mycobacterium growth is not required by molecular methods, the time duration for diagnosis is substantially reduced [2]. No single test is diagnostic for bone TB in all cases [4]. All enrolled cases were clinically and MRI-diagnosed cases of osteoarticular TB, and the tissue was subjected for AFB smear/histology/culture and molecular tests. Since in all enrolled cases, the diagnosis was confirmed by one or the other methods (Table 2), we compared the sensitivities of various tests among histology-positive patients (Tables 3, 4).
Diagnostic Accuracy of CBNAAT and LPA
Sample processing and PCR amplification/detection are integrated into a single self-enclosed test unit in CBNAAT, thus reducing time needed for diagnosis, and it is species specific [1].The diagnostic accuracy of CBNAAT in group A and group B was 84.62% (55/65) and 91.67% (11/12), respectively. Jain et al. [2] reported 98% diagnostic accuracy for PCR among clinico-radiologically diagnosed cases (n = 50). PCR detected 16srRNA as target sequence, which is universally present ruling out false negative results [2]. However, the target sequence used was genus specific and thus present in both typical and atypical mycobacteria, increasing chances of false positive result [2]. The diagnostic accuracy of 70% (65/93) was reported by in-house nested PCR which detected IS6110 DNA gene sequence in another study [8]. PCR has a limit of detection of 1–10 colony-forming units (CFU)/ml of amplified sample, while for CBNAAT it is 131 CFU/ml in non-amplified sample, contributing to the higher diagnostic accuracy of CBNAAT [1]. Rest all studies done in the past have also reported sensitivity of CBNAAT when compared to histopathology [6,10,22]. Overall sensitivity of 88.5% for CBNAAT was reported from India among 1000 samples of presumptive TB (pulmonary and extrapulmonary) with culture as gold standard [23].
The diagnostic accuracy of LPA in our series was 70.77% (46/65) and 83.33% (10/12) among presumptive TB cases and presumptive drug-resistant cases, respectively, which has not been reported for osteoarticular samples. Since we obtained moderate sensitivity with LPA, it alone is not an effective diagnostic test. This could be explained by the fact that LPA detects only purified DNA extracted from the sample which requires at least 103–104 bacilli per ml for the minimum amount of DNA to be extracted for a positive test [1].
The diagnostic accuracy of the CBNAAT and LPA in tandem among presumptive TB (group A) cases was 86.15% (56/65), while 91.67% (11/12) among the presumptive drug-resistant cases. The above data suggested the addition of LPA to CBNAAT did not increase the diagnostic accuracy. The sensitivity of LPA compared to liquid culture among sputum smear-negative pulmonary TB samples was reported to be 68.4% [24].
Comparison of diagnostic accuracy of various tests: This is the first of its kind study where diagnostic accuracy of multiple tests is analysed and defined.
AFB smear: Among the 34 (34/65, 52.31%) and 9 (9/12, 75%) smear-negative cases in group A and B, 27 (27/34, 79.41%) and 9 (9/9, 100%) cases were diagnosed TB on molecular tests, suggesting paucibacillary nature of disease. Thus, molecular tests are useful in detecting TB in smear-negative cases. Even liquid culture was positive in only 15 (44.12%) of group A cases, suggesting low bacillary load of live bacilli and histology was diagnostic in most patients.
Liquid culture: 32/65 (49.23%) cases in group A and 10/12 (83.33%) cases in group B were culture negative. The molecular tests established a diagnosis in 26/32 (81.25%) and 9/10 (90%) cases, respectively. This could be attributed to the presence of non-viable bacilli in the samples. However, histology also established the diagnosis in 28/32 (87.5%) group A and 8/10 (80%) group B cases, respectively.
Histology: 8/65 (12.31%) group A and 2/12 (16.67%) group B cases were histology negative. The diagnosis could be established in 5/8 (62.5%) group A and 2/2 (100%) group B cases by molecular tests. 2/8 (25%) of remaining group A cases were smear and culture positive, thus ascertaining the diagnosis in all cases.
Molecular tests: Among the 9/65 (13.85%) molecular test-negative group A cases, histopathology was diagnostic in 7 cases, AFB smear in 3 and culture in 4 cases. 1/12 (8.33%) molecular test-negative group B case was diagnosed on histology. This could be attributed to the bacillary load lower than the limit of detection on molecular tests in active diseases. An AFB smear and culture positivity in the three new patients could be attributed to either a sample sent for molecular tests containing a relatively low bacillary load or rarely, the presence of non-tuberculous AFB contaminants and tubercle bacilli dual growth in the samples, with the MTBC bacillary load being lower than the limit of detection for the molecular tests. PCR, which has a limit of detection of 1–10 bacilli per ml, was not done on the tests to confirm the low bacillary load [1].
The p value on comparing the diagnostic accuracy of various tests in both the groups (Table 7) was done using Fisher’s exact test of independence. There was no significant difference in the diagnostic accuracy of all the tests among the two groups.
Table 7.
Comparison of the results obtained from the various tests among group A and group B using Fisher’s exact test of independence
| Test used | P value | Statistical difference |
|---|---|---|
| CBNAAT | 1 | Not significant (P > 0.05) |
| LPA | 0.494 | Not significant (P > 0.05) |
| Molecular tests (in tandem) | 1.000 | Not significant (P > 0.05) |
| AFB smear | 0.209 | Not significant (P > 0.05) |
| Liquid culture | 0.0551 | Not significant (P > 0.05) |
| Histopathology | 0.650 | Not significant (P > 0.05) |
Demonstration of Drug Resistance by CBNAAT and LPA
Rifampicin resistance (RR) by CBNAAT among group A and group B was 4.62% (3/65) and 33.33% (4/12), respectively, while by LPA it was 3.08% (2/65) and 58.33% (7/12), respectively, which is comparable to the reported RR rate of 5.8% on CBNAAT (n = 69) [5]. Culture DST demonstrated MDR TB in 1/65 (1.54%) group A case and 2/12 (16.67%) group B cases only. This suggested that molecular tests could be more reliable in detecting drug resistance among culture-negative cases.
Drug resistance detection by CBNAAT and LPA in tandem among group A and B cases was 6.15% (4/65) and 58.33% (7/12), respectively. LPA demonstrated isoniazid monoresistance in 1 (1.54%) group A case and 2 (16.67%) group B cases. The high incidence of INH monoresistance suggested the role of CBNAAT and LPA in tandem in detecting drug resistance. The group B has only 12 cases, and a similar study with more number of cases is required for establishing role of molecular methods in evaluating presumptive drug-resistant cases as previously published studies reported sensitivity and specificity of detection of drug resistance by molecular methods in already proven pulmonary and extrapulmonary TB cases.
Vadwai et al. [25] analysed 40 MDR TB cases from 547 extrapulmonary TB samples by MGIT (liquid culture) DST. Thirty-nine of these demonstrated RR on CBNAAT (sensitivity 98%). However, this study did not report drug resistance among the 507 culture-negative cases. Pandey et al. [26] reported 98.6% sensitivity and 100% specificity in detecting RR by CBNAAT compared with solid culture DST among 83 culture-positive pulmonary and 2 culture-positive extrapulmonary tuberculosis samples. Gu et al. [6] reported sensitivity of 100% for CBNAAT and 83.33% and 85.71% for rifampicin and isoniazid resistance detection, respectively, on LPA among 24 culture-positive cases. The specificity was 100% for both methods. These three studies did not consider culture-negative cases. We did not calculate sensitivity and specificity in detection of drug resistance, although our three MDR cases on DST (one in group A and two in group B) were rifampicin resistant on CBNAAT and both rifampicin and isoniazid resistant on LPA. We also identified an additional 3/65 group A and 5/12 group B cases which were drug resistant on the molecular methods and culture negative, making it an useful investigation to establish drug resistance in culture-negative bone TB. Studies in the past for sensitivity of molecular tests in drug resistance rate cannot be compared to our study as they have only taken culture-positive drug-resistant cases and did not consider culture-negative cases [5,7,26].
To the best of our knowledge, no study has compared the drug resistance detection accuracy of molecular tests in tandem among clinico-radiologically diagnosed cases of osteoarticular tuberculosis. The above data suggested that the use of molecular tests in tandem could provide an advantage in terms of increased drug resistance detection among osteoarticular tuberculosis patients.
To conclude, no single test could ascertain the diagnosis of osteoarticular TB in all cases as diagnosis could be established using either one of the above-mentioned methods. The diagnostic accuracy of the molecular tests was much higher than AFB smear and culture. Molecular tests could detect drug resistance in AFB smear- and culture-negative cases. LPA is useful to detect isoniazid monoresistance. Histology continued to play a major role in the diagnosis of all cases of osteoarticular tuberculosis. Adequate tissue should be obtained and evaluated using molecular tests (CBNAAT and LPA), AFB smear, culture and histopathology, as no single test could diagnose osteoarticular tuberculosis in all the cases.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The present study was conducted from within regular running expenditure available to the Government Institution with no extra institutional financial grant or funding availed.
Compliance with Ethical Standards
Ethical statement
The authors declare that the above manuscript is in compliance with ethical standards for research in human participants, and have no potential sources of conflict of interest associated with its publication. All participants involved were recruited only from UCMS and GTB hospital, Delhi, after getting an appropriate ethical clearance from the institutional board and after taking an informed consent. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors also declare that we have nothing to disclose and have no direct or indirect commercial and or financial incentive associated with this research.
Informed consent
The authors also certify that informed consent was obtained from all the human participants in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
S. Abhimanyu, Email: abhimanyu3000@yahoo.com
Anil K. Jain, Email: principal@ucms.ac.in, Email: profakjain@gmail.com, Email: akjain@ucms.ac.in
V. P. Myneedu, Email: v.myneedu@nitrd.nic.in, Email: vpm_myn@yahoo.com
Vinod K. Arora, Email: vinodkumarora@hotmail.com
Manish Chadha, Email: mchadha@hotmail.com.
Rohit Sarin, Email: r.sarin@nitrd.nic.in.
References
- 1.Oommen S, Banaji N. Laboratory diagnosis of tuberculosis: Advances in technology and drug susceptibility testing. Indian Journal of Medical Microbiology. 2017;35(3):323. doi: 10.4103/ijmm.IJMM_16_204. [DOI] [PubMed] [Google Scholar]
- 2.Jain AK, Jena SK, Singh MP, Dhammi IK, Ramachadran VG, Dev G. Evaluation of clinico-radiological, bacteriological, serological, molecular and histological diagnosis of osteoarticular tuberculosis. Indian Journal of Orthopaedics. 2008;42(2):173–177. doi: 10.4103/0019-5413.40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg RK, Somvanshi DS. Spinal tuberculosis: A review. Journal of Spinal Cord Medicine. 2011;34(5):440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhanpal VP, Tuli SM, Singh H, Sen PC. The value of histology, culture and guinea pig inoculation examination in osteo-articular tuberculosis. ActaOrthopaedicaScandinavica. 1974;45(1):36–42. doi: 10.3109/17453677408989119. [DOI] [PubMed] [Google Scholar]
- 5.Held M, Laubscher M, Zar HJ, Dunn RN. GeneXpert polymerase chain reaction for spinal tuberculosis: An accurate and rapid diagnostic test. Bone and Joint Journal. 2014;96-B(10):1366–1369. doi: 10.1302/0301-620X.96B10.34048. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y, Wang G, Dong W, Li Y, Ma Y, Shang Y, Qin S, Huang H. Xpert MTB/RIF and GenoTypeMTBDRplus assays for the rapid diagnosis of bone and joint tuberculosis. International Journal of Infectious Diseases. 2015;36:27–30. doi: 10.1016/j.ijid.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Chhabra HS, Mahajan R, Chabra T, Batra S. Magnetic resonance imaging and GeneXpert: A rapid and accurate diagnostic tool for the management of tuberculosis of the spine. Asian Spine Journal. 2016;10(5):850–856. doi: 10.4184/asj.2016.10.5.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agashe V, Shenai S, Mohrir G, Deshmukh M, Bhaduri A, Deshpande R, et al. Osteoarticular tuberculosis—diagnostic solutions in a disease endemic region. Journal of Infection in Developing Countries. 2009;3(7):511–516. doi: 10.3855/jidc.469. [DOI] [PubMed] [Google Scholar]
- 9.Wei G, Mu J, Wang G, Huo F, Dong L, Li Y, et al. The reliability analysis of Xpert-positive result for smear-negative and culture-negative specimen collected from bone and joint tuberculosis suspects. Journal of Thoracic Disease. 2016;8(6):1205–1209. doi: 10.21037/jtd.2016.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Held M, Laubscher M, Mears S, Dix-Peek S, Workman L, Zar H, et al. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary tuberculosis in children with musculoskeletal infections. Pediatric Infectious Disease Journal. 2016;35(11):1165–1168. doi: 10.1097/INF.0000000000001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuli SM. Challenge of therapeutically refractory and multidrug resistant tuberculosis in orthopaedic practice. Indian Journal of Orthopaedics. 2002;36(4):211. [Google Scholar]
- 12.Jain AK, Jaggi KR, Bhayana H, Saha R. Drug-resistant spinal tuberculosis. Indian Journal of Orthopaedics [Internet] 2019;2018:100–107. doi: 10.4103/ortho.IJOrtho_306_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuli SM. Results of treatment of spinal tuberculosis by“ middle-path” regime. Journal of Bone and Joint Surgery British Volume. 1975;57(1):13–23. doi: 10.1302/0301-620X.57B1.13. [DOI] [PubMed] [Google Scholar]
- 14.Tuli S. Tuberculosis of the skeletal system. 4. New Delhi: Jaypee Brothers; 2004. [Google Scholar]
- 15.Jain AK. Tuberculosis of the spine a fresh look at an old disease. Journal of Bone and Joint Surgery British Volume. 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 16.Moon M-S. Tuberculosis of spine: Current views in diagnosis and management. Asian Spine Journal. 2014;8(1):97. doi: 10.4184/asj.2014.8.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain AK. Tuberculosis of the spine. Clinical Orthopaedics. 2007;460:2–3. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 18.Technical and Operational Guidelines for TB Control in India 2016. Central TB Division [Internet]. [cited 2019 July 20]. https://tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=4573&lid=3177. Accessed 10 July 2019.
- 19.Hain Lifescience. GenoType MTBDRplus Ver 2.0. Instructions for use (IFU-304A). https://www.ghdonline.org/uploads/MTBDRplusV2_0212_304A-02-02.pdf. Accessed 13 Aug 2020.
- 20.Central TB Division. (2017). Guidelines for the programmatic management of drug-resistant tuberculosis. https://www.tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=4780&lid=3306. Accessed 14 Aug 2020.
- 21.Wang G, Dong W, Lan T, Fan J, Tang K, Li Y, et al. Diagnostic accuracy evaluation of the conventional and molecular tests for spinal tuberculosis in a cohort, head-to-head study. Emerging Microbes and Infections. 2018;7(1):109. doi: 10.1038/s41426-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polepole P, Kabwe M, Kasonde M, Tembo J, Shibemba A, O’Grady J, et al. Performance of the Xpert MTB/RIF assay in the diagnosis of tuberculosis in formalin-fixed, paraffin-embedded tissues. International Journal of Mycobacteriology. 2017;6(1):87–93. doi: 10.4103/2212-5531.201892. [DOI] [PubMed] [Google Scholar]
- 23.Prakash AK, Datta B, Tripathy JP, Kumar N, Chatterjee P, Jaiswal A. The clinical utility of cycle of threshold value of GeneXpert MTB/RIF (CBNAAT) and its diagnostic accuracy in pulmonary and extra-pulmonary samples at a tertiary care center in India. Indian Journal of Tuberculosis. 2018;65(4):296–302. doi: 10.1016/j.ijtb.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Singh BK, Sharma SK, Sharma R, Sreenivas V, Myneedu VP, Kohli M, et al. Diagnostic utility of a line probe assay for multidrug resistant-TB in smear-negative pulmonary tuberculosis. PLoS ONE. 2017;12(8):e0182988. doi: 10.1371/journal.pone.0182988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: A new pillar in diagnosis of extrapulmonary tuberculosis? Journal of Clinical Microbiology. 2011;49(7):2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey P, Pant ND, Rijal KR, Shrestha B, Kattel S, Banjara MR, et al. Diagnostic accuracy of GeneXpert MTB/RIF assay in comparison to conventional drug susceptibility testing method for the diagnosis of multidrug-resistant tuberculosis. PLoS ONE. 2017;12(1):e0169798. doi: 10.1371/journal.pone.0169798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.