Abstract
Aim
Due to lipases’ regio-selectivity and ability to catalyze different reactions such as hydrolysis, esterification, and transesterification, the enzyme is attractive in biotransformation technology. Besides, another technology, namely enzyme immobilization, has attracted scientists/technologists’ attention to employ immobilized lipase in such a field. Thus lipase of Candida rugosa was immobilized onto silica nanoparticles through adsorption. Furthermore, the immobilized biocatalyst was characterized and used to esterify ibuprofen enantioselectively.
Methods
To characterize immobilized lipase onto silica nanoparticles scanning electron microscopy (SEM) and dynamic light scattering (DLS) were used.
Results
The catalytic properties of both immobilized and free lipases such as optima pH and temperature were not different. According to the results, the immobilized lipase on silica nanoparticles showed 45% and 96% conversion (C) and enantioselectivity (ees), respectively. In comparison to free lipase, the immobilized enzyme came with better catalytic activity.
Conclusion
Silica nanoparticles as one of the most promising materials for the immobilization of lipase in enantioselective esterification of ibuprofen, were introduced in this work.
Graphical abstract

Keywords: Lipase, Characterization esterification, Enantiomeric resolution
Introduction
Lipase is one of the most promising biocatalysts that has recently attracted much attention because of its regio-selectivity in various types of reactions, such as trans-esterification, hydrolysis, and esterification [1, 2]. The enzyme’s ability to catalyzing different reactions has recently made it possible for investigators to develop novel resolution methods based on lipase catalytic activity and specificity. The resolution of racemic mixtures based on the enzymatic reaction is the most recent novel method with high efficiency and the ability to enter the commercial scales [3]. Principally, lipase’s specific molecular structure with an active center can discriminate between a racemic mixture’s enantiomers and the enantiomeric resolution [4]. According to close (inactive) and open (active) molecular structures of lipase and the effect of the nature of reaction medium on the enzyme structure, the amount of water in lipase-catalyzed media has a remarkable impact on the enzyme catalytic enantioselectivity. From the mechanistic view, the small number of water molecules can significantly affect the 3D molecular structure of lipase, molecular stability, and the active site’s polarity, all of which play vital roles in the catalyzing process [5, 6]. Many reports indicated that lipase could catalyze a broad range of reactions in organic media for industrial applications [7–10]. Using lipase, various types of enantiomeric resolutions have been reported. The enantiomeric resolution of the profen family of anti-inflammatory drugs has attracted much attention because of such drugs’ broad application [11–14]. Considering the presence of chiral carbon atom in profen molecular structure, the lipase-based enzymatic resolution has been recently proposed for the resolution of the (S)-enantiomer of ibuprofen. Pharmacologically, the activity of the (S)-enantiomer of ibuprofen is nearly 160 times more than (R)-ibuprofen. Moreover, the enantiomeric resolution of ibuprofen is important since the (R)-enantiomer can bring about serious side effects such as gastrointestinal abnormalities and pain [15–17], which can be related to the disruptive effect of the produced triglycerides on the lipid metabolism in gastric membranes [18–20]. Additionally, according to ibuprofen enantiomers’ opposite physiological effects, using pure (S)-enantiomer of ibuprofen makes it possible to decrease the effective required doses [21, 22]. The immobilization of lipase as a catalyst is currently being under development to improve the resolution process’s efficiency [23]. The immobilization process can enhance/alter the catalytic activity of lipase using various mechanisms. Reduction in the molecular movement can increase the probability of effective interaction between the active site of lipase and ibuprofen enantiomers as substrates. Moreover, the immobilization process can increase the lipase’s thermal stability and durability against the presence of water molecules that can be produced during the reaction and disrupt lipase’s catalytic activity. From the economic perspective, using immobilized lipase as a biocatalyst with high catalytic activity is not far from the expectation because of the economically available supports [24–26]. In recent years, significant efforts have been dedicated to the immobilization of lipase. Therefore, in this work, we considered the silica nanoparticles to support immobilization of lipase and the studied enzymatic resolution of racemic ibuprofen.
Materials and methods
Materials
Lipase of Candida rugosa, racemic ibuprofen, 4-nitrophenyl palmitate, and silicon dioxide nanoparticles was obtained from Sigma Aldrich. A chiral column from Phenomenex (USA) was used. Other reagents used were of analytical grade.
Immobilization of lipase on silica nanoparticles
For immobilization of Candida rugosa lipase on silica nanoparticles, 2 mg of lipase powder was dissolved in 10 mL phosphate buffer (0.2 M Na2HPO4 and 0.2 M NaH2PO4) at various pHs (6.0–9.5). The prepared lipase solution at different pHs was then added to a series of beakers, each containing 20 mg of silica nanoparticles, and was stirred at 4 °C for 30 min. Subsequently, the immobilized lipase on silica nanoparticles was separated using centrifugation (4 °C, 6000 rpm, 10 min). After all, the immobilized lipase was washed with phosphate buffer and ethanol, respectively. The total protein on the supernatant was measured by the Bradford method [27, 28].
Lipase activity
The enzyme activity was determined using p-nitrophenol palmitate (p-NPP) as a substrate. One mL of p-NPP (16.5 mM) in 2-propanol was added to 9 mL Tris-HCl (50 mM) buffer (pH 8) containing 0.4% (v/v) Triton X-100 and 0.1% (w/v) gum Arabica. Ten mg of lipase powder was weighed out and diluted with Tris-HCl buffer. Following this, 1.35 mL of the prepared substrate was incubated at 37 °C with 0.1 mL of the enzyme solution, and the absorbance was read at λ 410 nm within 3–5 min against blank devoid of the enzyme. One lipase activity unit was defined as 1 mL of enzyme solution that could liberate 1 μmol of p-nitrophenol (p-NP) as a product in one minute under assay condition [29].
Resolution of racemic ibuprofen
In non-aqueous media, the enantiomeric resolution of racemic ibuprofen was made using the esterification reaction. For initiation of the esterification reaction, 40 mg of immobilized lipase with 20 mL of isooctane containing 20 μL of water were added to ibuprofen and n-propanol (0.025 M). The reaction was performed at 37 °C under shaking. Conversion for esterification reaction (C) and the enantiomeric excess (ees) for products was calculated as per [30]. The analysis of racemic ibuprofen was studied through the Knauer HPLC system. The mobile phase contained n-hexane:2-propanol: acetic acid (99.2:0.6:0.2, v/v/v%) at a flow rate of 0.1 ml/min using Lux® 5 μm Cellulose-3, LC Column 250 × 4.6 mm, Ea.
Results and discussion
Adsorption of lipase on the silica nanoparticles
The maximum adsorption of the enzyme onto nanosilica was observed at alkaline pH (pH 8.0). At an optimum pH of adsorption, the time of the immobilization process was another factor to be considered. According to Fig. 1, 15 min was the optimum time for Candida rugosa lipase’s adsorption on the silica nanoparticles. After that, further adsorption did not occur. This could be due to silica nanoparticles’ pore size, which significantly limits the immobilization of lipase onto nanosilica [31].
Fig. 1.
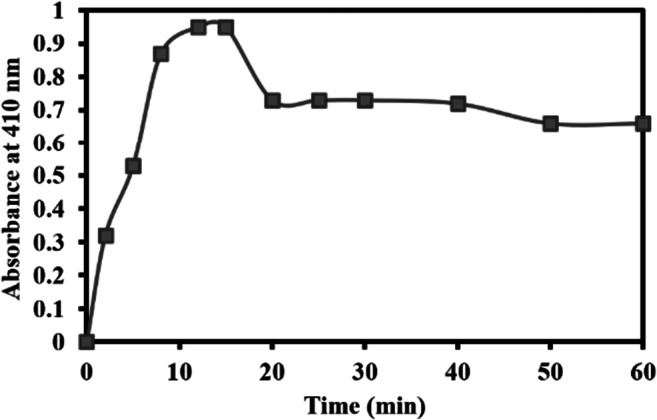
The adsorption of Candida rugosa lipase on the silica nanoparticles
Characterization of immobilized lipase
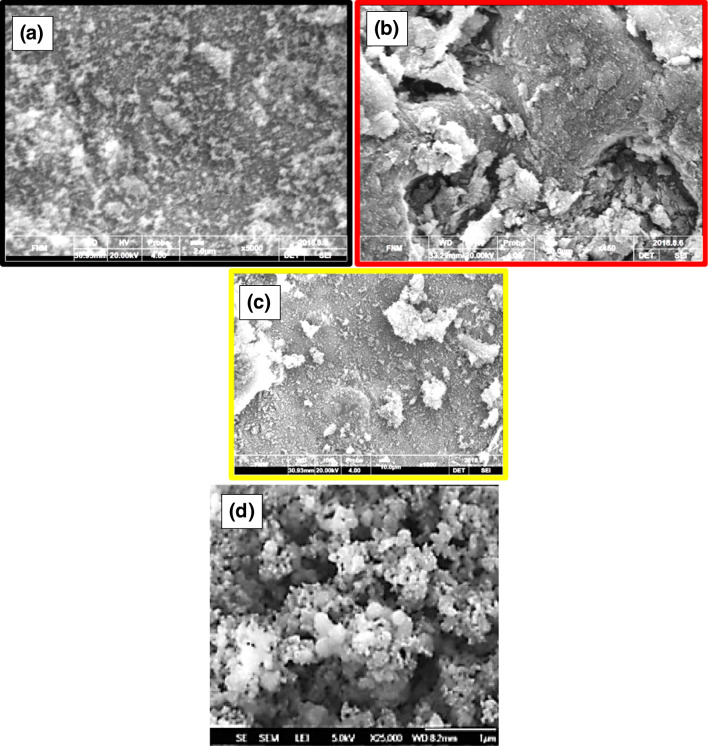
Scanning electron microscopy of immobilized silica nanoparticles showed that the lipase agglomeration on the silica nanoparticles formed and the mentioned hybrid structures were appropriate for ibuprofen’s enzymatic resolution. Figure 2A-C shows the 2, 10, and 20 μm scales of the immobilized silica nanoparticles.
Fig. 2.
The scanning electron microscopy images of lipase immobilized silica nanoparticles in (A) 2, (B) 10, and (C) 20 μm of resolution. Part D shows silica nanoparticles devoid of the enzyme
The size distribution analysis (DSL, Melvern, UK) of silica nanoparticles at room temperature revealed that the nanoparticles’ size distribution was in the range of 10 to 300 nm. The maximum amount of the particles were almost 100 nm in diameter. After the immobilization process, the immobilized silica nanoparticles’ size distribution was changed to be at the range of 20 to 500 nm. The immobilized particle distribution showed that the particles’ maximum amount was 200 nm in diameter (Fig. 3).
Fig. 3.
The size distribution for free lipase (left) and immobilized lipase (right)
The optimization of pH of both free and immobilized lipase was carried out at the range of 6 to 9.5, as shown in (Fig. 4). Both forms of the enzyme showed optimum activity at pH 8.5. Compared to the free lipase, in the optimum pH of the immobilized lipase, no changes were occurred, indicating that the adsorption of lipase onto silica nanoparticles did not cause any changes in the conformation of lipase. The optima pH of free and immobilized Rhizopus oryazae lipase (ROL) was determined to be in the range of 7.0 to 9.0 [32]. While the optimum pH of ROL immobilized onto graphene oxide was found to be 8.0. Candida rugosa lipase was immobilized onto three different supports, and the immobilized lipases were characterized. No significant changes in the optimal pH for the activity of the immobilized lipases were observed [33].
Fig. 4.
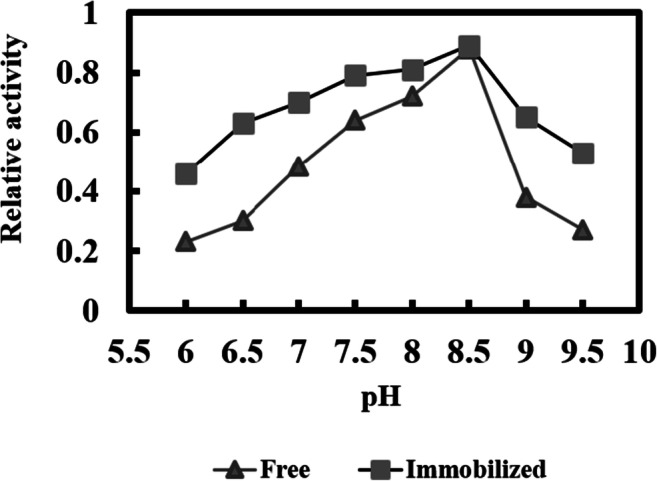
Optimal pHs of free and immobilized lipase
Further, the optimum temperature for the immobilized and free lipase catalytic activity was performed at temperatures ranging from 10 to 50 °C at the interval of 5 °C. The optimal temperature for both forms was determined to be 30 °C (Fig. 5). Aspergilus niger lipase was immobilized onto Cellite through adsorption. The optimal temperature for both immobilized and free enzyme were 30 and 40 °C, respectively [34].
Fig. 5.

The optimal temperature of immobilized and free lipase
Furthermore, the effect of substrate concentration on the free and immobilized lipase activity was studied at various concentrations. As shown in Fig. 6, by increasing the substrate concentration, both forms of lipase activity decreased.
Fig. 6.

The activity of immobilized lipase versus different substrate concentrations
Enzymatic resolution of ibuprofen
The enantioselective resolution of ibuprofen in organic media was made in the presence of the free and immobilized lipase. The esterification process of ibuprofen is a known method for the enantioselective resolution criterion, reflecting the resolution efficiency. As mentioned above, lipase of Candida rugosa can selectively esterify the (S) enantiomer of ibuprofen, which has more medical and clinical importance [35–38]. The presence of water molecules can control the enzyme molecular flexibility. Therefore, the amount of water can affect the esterification reaction. On the one hand, water in organic solvents can influence the enzyme’s molecular structure. Also, the production of water molecules during the esterification reaction can be absorbed on the lipase’s hydrophilic part, which can further cause the enzyme aggregation in the organic solvent. Thus, more water production during the esterification reaction can eliminate efficient mass production in a reaction batch. In the present work, using silica nanoparticles to support the lipase immobilization, successful enantioselective resolution of the racemic ibuprofen was achieved. The results indicated the remarkable ees and C for the immobilized lipase compared to the free one. The ees and C of the immobilized lipase was 29.2% and 49%, respectively. But as for free lipase, ees and C was 13% and 30% for 36 h of reaction, respectively. This increase in ibuprofen’s enantioselective resolution can be due to the silica nanoparticles’ effects on the lipase enzymatic activity and remarkable stability. Immobilization of Burkholderia cepacia lipase onto functionalized magnetic nanoparticles was more stable with higher ees than free lipase [39]. E. Swetha, C. Vijitha, and C. Veeresham studied the effect of immobilization of lipases of different sources on the enantio-selective conversion of racemic sotalol toR(−)-sotalol. They observed enantioselectivities of lipases of various sources were improved upon immobilization [40]. To understand how the time of resolution can affect ees and C, the indicated parameter was evaluated at different times, and the results are shown in Fig. 7.
Fig. 7.
C and ees in various resolution times for free (up) and immobilized (down) lipase
As shown in Fig. 7, both the immobilized CRL and free CRL reached equilibrium after 35 min of reaction. The effect of reaction time on enantioselectivity (ees) of (R,S)-1-phenyl ethanol by Burkholderia cepacia Lipase (BCL) has been studied. During the catalytic resolution, the conversion and ees increased with the extension of reaction time and showed a consistent tendency. Nevertheless, the immobilized BCL showed a significantly higher initial reaction rate than the free form [41].
pH of the reaction can affect ees of esterification reaction of ibuprofen catalyzed by lipase. As seen in Fig. 8, the highest ees value could obtain at pH 9 for both the enzyme. The effect of pHs in enantioselectivity of naproxen by Candida rougosa lipase immobilized onto three different supports like Amberlit XRD7, chitosan beads, and chitosan beads activated by glutaraldehyde were studied. It was found that the higher enantioselectivity was obtained at neutral pH for all types of supports [42].
Fig. 8.
The ees of esterification of ibuprofen in the presence of free (left) and (right) immobilized lipase on silica nanoparticles
As mentioned, silica nanoparticles as one of the most promising materials for the immobilization of lipase in enantioselective esterification of ibuprofen were introduced in this work. The high stability, great enantioselective efficiency, appropriate ees for esterification reaction, and the availability of silica nanoparticles are the main advantages that can make them promising for ibuprofen’s enzymatic resolution of the most useful anti-inflammatory drugs.
Conclusion
In the present study, the silica nanoparticles as the most promising support for Candida rugosa lipase’s immobilization were applied. The immobilized lipase was used as an enantioselective catalyst for ibuprofen’s resolution during the esterification process using isooctane as an organic solvent. The results indicated that lipase’s immobilization on silica nanoparticles can enhance the optimum catalytic condition of enantioselective lipase resolution and increase conversion efficiency and enantiomeric excess (S) enantiomer of ibuprofen during the esterification reaction under the above-mentioned conditions. Both time and pH of reactions affect the ees and C. Therefore, as compared to free lipase, the immobilized lipase showed better catalytic properties.
Acknowledgments
The authors acknowledge the technical support of the Department of Biochemistry, Tehran University of Medical Sciences.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xie Y-C, Liu H-Z, Chen J-Y. Candida rugosa lipase catalyzed esterification of racemic ibuprofen with butanol: racemization of R-ibuprofen and chemical hydrolysis of S-ester formed. Biotechnology Letters. 1998;20:455–458. doi: 10.1023/A:1005460808406. [DOI] [Google Scholar]
- 2.Shoda SI, Uyama H, Kadokawa JI, Kimura S, Kobayashi S. Enzymes as green catalysts for precision macromolecular synthesis. Chemical Reviews. 2016;116:2307–2413. doi: 10.1021/acs.chemrev.5b00472. [DOI] [PubMed] [Google Scholar]
- 3.Sie Yon L, Gonawan FN, Kamaruddin AH, Uzir MH. Enzymatic deracemization of (R, S)-ibuprofen ester via lipase-catalyzed membrane reactor. Ind Eng Chem Res. 2013;52:9441–9453. doi: 10.1021/ie400795j. [DOI] [Google Scholar]
- 4.Muralidhar RV, Marchant R, Nigam P. Lipases in racemic resolutions. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology. 2001;76:3–8. doi: 10.1002/1097-4660(200101)76:1<3::AID-JCTB336>3.0.CO;2-8. [DOI] [Google Scholar]
- 5.Bayramoğlu G, Arıca MY. Preparation of poly (glycidylmethacrylate–methylmethacrylate) magnetic beads: application in lipase immobilization. J Mol Catal B Enzym. 2008;55:76–83. doi: 10.1016/j.molcatb.2008.01.012. [DOI] [Google Scholar]
- 6.Yahya AR, Anderson WA, Moo-Young M. Ester synthesis in lipase-catalyzed reactions. Enzyme and Microbial Technology. 1998;23:438–450. doi: 10.1016/S0141-0229(98)00065-9. [DOI] [Google Scholar]
- 7.Carvalho PDO, Contesini FJ, Ikegaki M. Enzymatic resolution of (R, S)-ibuprofen and (R, S)-ketoprofen by microbial lipases from native and commercial sources. Brazilian Journal of Microbiology. 2006;37:329–337. doi: 10.1590/S1517-83822006000300024. [DOI] [Google Scholar]
- 8.Ghanem A. Direct enantioselective HPLC monitoring of lipase-catalyzed kinetic resolution of flurbiprofen. Chirality. 2010;22:597–603. doi: 10.1002/chir.20798. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wang F, Tan T. Effects of alcohol and solvent on the performance of lipase from Candida sp. in enantioselective esterification of racemic ibuprofen. J Mol Catal B Enzym. 2009;56:126–130. doi: 10.1016/j.molcatb.2008.03.003. [DOI] [Google Scholar]
- 10.Burney PR, Pfaendtner J. Structural and dynamic features of Candida rugosa lipase 1 in water, octane, toluene, and ionic liquids BMIM-PF6 and BMIM-NO3. J Phys Chem B. 2013;117:2662–2670. doi: 10.1021/jp312299d. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez A, Valero F, Lafuente J, Solà C. Highly enantioselective esterification of racemic ibuprofen in a packed bed reactor using immobilised Rhizomucor miehei lipase. Enzyme and Microbial Technology. 2000;27:157–166. doi: 10.1016/S0141-0229(00)00207-6. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Gong Y, Saito T, Kimoto H. Efficient preparation of optically active ketoprofen by Mucor javanicus lipase immobilized on an inorganic support. J Biosci Bioeng. 2000;90:332–334. doi: 10.1016/S1389-1723(00)80090-0. [DOI] [PubMed] [Google Scholar]
- 13.Morrone R, D’Antona N, Lambusta D, Nicolosi G. Biocatalyzed irreversible esterification in the preparation of S-naproxen. Journal of molecular catalysis B: Enzymatic. 2010;65:49–51. doi: 10.1016/j.molcatb.2010.01.014. [DOI] [Google Scholar]
- 14.Pinnen F, Sozio P, Cacciatore I, Cornacchia C, Mollica A, et al. Ibuprofen and glutathione conjugate as a potential therapeutic agent for treating Alzheimer's disease. Archiv der Pharmazie. 2011;344:139–148. doi: 10.1002/ardp.201000209. [DOI] [PubMed] [Google Scholar]
- 15.Madhav MV, Ching CB. Study on the enzymatic hydrolysis of racemic methyl ibuprofen ester. Journal of Chemical Technology & Biotechnology. 2001;76:941–948. doi: 10.1002/jctb.466. [DOI] [Google Scholar]
- 16.Zhao X-G, Wei D-Z, Song Q-X. A facile enzymatic process for the preparation of ibuprofen ester prodrug in organic media. Journal of Molecular Catalysis B: Enzymatic. 2005;36:47–53. doi: 10.1016/j.molcatb.2005.08.005. [DOI] [Google Scholar]
- 17.Chen JC, Tsai SW. Enantioselective synthesis of (S)-ibuprofen ester prodrug in cyclohexane by Candida rugosa lipase immobilized on Accurel MP1000. Biotechnology progress. 2000;16:986–992. doi: 10.1021/bp0000961. [DOI] [PubMed] [Google Scholar]
- 18.Long WS, Kow PC, Kamaruddin AH, Bhatia S. Comparison of kinetic resolution between two racemic ibuprofen esters in an enzymic membrane reactor. Process Biochemistry. 2005;40:2417–2425. doi: 10.1016/j.procbio.2004.09.014. [DOI] [Google Scholar]
- 19.Tsai SW, Lin JJ, Chang CS, Chen JP. Enzymatic synthesis of (S)-ibuprofen ester prodrug from racemic ibuprofen by lipase in organic solvents. Biotechnology Progress. 1997;13:82–88. doi: 10.1021/bp9600952. [DOI] [Google Scholar]
- 20.Mazaleuskaya LL, Theken KN, Gong L, Thorn CF, FitzGerald GA, Altman RB, Klein TE. PharmGKB summary: ibuprofen pathways. Pharmacogenet Genomics. 2015;25(2):96–106. doi: 10.1097/FPC.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fazlena H, Kamaruddin A, Zulkali M. Dynamic kinetic resolution: alternative approach in optimizing S-ibuprofen production. Bioprocess and Biosystems Engineering. 2006;28:227–233. doi: 10.1007/s00449-005-0024-1. [DOI] [PubMed] [Google Scholar]
- 22.Foresti ML, Galle M, Ferreira ML, Briand LE. Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: experimental and molecular modeling aspects. Journal of Chemical Technology & Biotechnology. 2009;84:1461–1473. doi: 10.1002/jctb.2200. [DOI] [Google Scholar]
- 23.Cao S-L, Huang Y-M, Li X-H, et al. Preparation and characterization of immobilized lipase from Pseudomonas cepacia onto magnetic cellulose nanocrystals. Scientific reports. 2016;6:20420. doi: 10.1038/srep20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva VCF, Contesini FJ, de Oliveira Carvalho P. Enantioselective behavior of lipases from Aspergillus niger immobilized in different supports. Journal of Industrial Microbiology & Biotechnology. 2009;36:949–954. doi: 10.1007/s10295-009-0573-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Guan Y, Shen R, Liu H. Immobilization of lipase onto micron-size magnetic beads. J Chromatogr B. 2005;822:91–97. doi: 10.1016/j.jchromb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Guo C, Xia H, Mahmood I, Liu C, Liu H. Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. Journal of Molecular Catalysis B: Enzymatic. 2009;58:103–109. doi: 10.1016/j.molcatb.2008.12.001. [DOI] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Kruger NJ. The Bradford method for protein quantitation, The protein protocols handbook, Springer. 2009; pp. 17–24.
- 29.Guo J, Chen C-P, Wang S-G, Huang X-J. A convenient test for lipase activity in aqueous-based solutions. Enzyme Microb Technol. 2015;71:8–12. [DOI] [PubMed]
- 30.López N, Pernas MA, Pastrana LM, Sánchez A, Valero F, Rúa ML. Reactivity of pure Candida rugosa lipase isoenzymes (Lip1, Lip2, and Lip3) in aqueous and organic media. Influence of the isoenzymatic profile on the lipase performance in organic media. Biotechnology Progress. 2004;20:65–73. doi: 10.1021/bp034188c. [DOI] [PubMed] [Google Scholar]
- 31.Serra E, Mayoral Á, Sakamoto Y, Blanco RM, Díaz I. Immobilization of lipase in ordered mesoporous materials: Effect of textural and structural parameters. Microporous and Mesoporous Materials. 2008;114:201–213. doi: 10.1016/j.micromeso.2008.01.005. [DOI] [Google Scholar]
- 32.Hermanova S, Zarevucka M, Bousa D, Pumera M, Sofer Z. Graphene oxide immobilized enzymes show high thermal and solvent stability. Nanoscale. 2015;7:5852–5858. doi: 10.1039/C5NR00438A. [DOI] [PubMed] [Google Scholar]
- 33.Sagiroglu A, Klinnc A, Telefoncu A. Preparation and properties of lipases immobilized on different supports. Artificial cells and blood substitute. Biotechnology. 2004;32(4):625–636. doi: 10.1081/bio-200039656. [DOI] [PubMed] [Google Scholar]
- 34.Dasilva VF, Contesini FJ, de O. Carvalho P. Characterization and catalytic activity of free and immobilized lipase from Aspergilus niger :a comparitive study. J. Braz. Chem. Soc. 2008;1998:1468–1474. [Google Scholar]
- 35.Wu SH, Guo ZW, Sih CJ. Enhancing the enantioselectivity of Candida lipase-catalyzed ester hydrolysis via noncovalent enzyme modification. Journal of American Chemical Society. 1990;112:1990–1995. doi: 10.1021/ja00161a052. [DOI] [Google Scholar]
- 36.Mustranta A. Use of lipases in the resolution of racemic ibuprofen. Applied Microbiology and Biotechnology. 1992;38:61–66. doi: 10.1007/BF00169420. [DOI] [PubMed] [Google Scholar]
- 37.Goto M, Kamiya N, Miyata M, Nakashio F. Enzymatic esterification by surfactant-coated lipase in organic media. Biotechnology Progress. 1994;10:263–268. doi: 10.1021/bp00027a005. [DOI] [Google Scholar]
- 38.Hongwei Y, Jinchuan W, Chi Bun C. Kinetic resolution of ibuprofen catalyzed by Candida rugosa lipase in ionic liquids. Chirality. 2005;17:16–21. doi: 10.1002/chir.20078. [DOI] [PubMed] [Google Scholar]
- 39.Li K, Wang J, He Y, Cui G, Abdulrazaq MA, Yan Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine-glutaraldehyde dendrimer modified magnetic nanoparticles. Chemical Engineering Journal. 2018;351:258–268. doi: 10.1016/j.cej.2018.06.086. [DOI] [Google Scholar]
- 40.Swetha E, Vijitha C, Veeresham C. Enantioselective conversion of racemic Sotalol to R(−)-Sotalol by lipase AP6. Indian Journal of Pharmaceutical Sciences. 2018;80(4):676–685. doi: 10.4172/pharmaceutical-sciences.1000407. [DOI] [Google Scholar]
- 41.Xu L, Cui G, Ke C, Fan Y, Yan Y. Immobilized Burkholderia cepacia Lipase on pH-Responsive Pullulan Derivatives with Improved Enantioselectivity in Chiral Resolution. Catalysts. 2018;8(1):13. doi: 10.3390/catal8010013. [DOI] [Google Scholar]
- 42.Gilani SL, Najafpour GD, Heydarzadeh HD, Moghadamnia A. Enantioselective synthesis of (S)-naproxen using immobilizedlipase on chitosan beads. Chirality. 2017;29:304–314. doi: 10.1002/chir.22689. [DOI] [PubMed] [Google Scholar]