Abstract
In recent times, there is increased public interest and indeed strong movement against the use of Bisphenol A (4,4ʹ-(propane-2,2,-diphenol)) due to its endocrine disrupting properties. In the present study, biotransformation of Bisphenol A (BPA) was accomplished using Trametes versicolor laccase (E.C. 1.10.3.2) enzyme. The enzyme was entrapped in reverse micelles comprising of bis(2-ethylhexyl) sulfosuccinate sodium salt (AOT) and 2,2,4-trimethylpentane (isooctane) for non-aqueous catalysis considering hydrophobicity of BPA. Screening of various parameters that may affect micellar system was carried out using Plackett–Burman experimental design and central composite design (Design Expert 11). According to Design Expert actual concentration of different variables was 0.55, 150 (Wo 30), 0.0035 mM and 175 µg/ml for Mg+2ions, Hydration ratio (Wo), 2,6-dimethoxyphenol (2,6 DMP, substrate) and laccase, respectively, at 40 °C and pH 4.5. Under these conditions laccase activity in reverse micelles was increased two folds as compared to unoptimized micellar system. It was evident that the reverse micelles diameter was linearly proportionated to the amount of laccase enzyme incorporated. BPA bioremediation mediated by laccase in non-aqueous environment was found to be 84% in 8 h of treatment. Biotransformation of BPA was monitored using GC–MS. BPA degraded products, such as BPA-O-catechol and 4,4 (Ethane 2-oxy 2-ol) diphenol were identified indicating transformation by oxidation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02842-4.
Keywords: Bisphenol A, Bioremediation, Trametes versicolor, Reverse micelles, Non-aqueous catalysis
Introduction
Bisphenol A has been chemical of concern in last few years due to its acute toxicity towards wildlife and human health, several countries including US banned use of BPA in baby bottles and sippers due to its endocrine disrupting as well as teratogenic properties (Vom Saal et al. 2012; Rubin 2011). It was synthesized as synthetic estrogen but now it is widely used in the production of epoxy resin and polycarbonates (Srivastava and Godara 2013; Bae et al. 2002). These materials are extensively used in manufacturing of reusable bottles, baby bottles, dental sealants, various medical devices, thermal printing paper, CDs, containers for beverages and foods, etc. (Almeida et al. 2018; Mikolajewska et al. 2015). The global BPA consumption is estimated to be 10.6 million tons in 2022 having compound growth rate of 4.8%. Asia Pacific has largest BPA market (53%) (Industry experts 2016).
BPA is omnipresent contaminant which is found in lithosphere, biosphere, hydrosphere and atmosphere. Due to its presence in hydrosphere, aquatic animals get affected by this pollutant (Sangai et al. 2016). Humans are exposed to this chemical due to contaminated meat and fish preparation present in food cans (Repossi et al. 2016).
It is hazardous for humans and wildlife due to its endocrine disrupting properties. It imitates oestrogen hormone, interact and occupy hormone receptor and therefore interfere with natural hormone balance in body. Eventually it will result in various reproductive health related diseases including early puberty, breast cancer (Lozada and Keri 2011), ovarian cancer (Hui et al. 2018), endometriosis and Polycystic Ovarian Syndrome (PCOD) in females (Kandaraki et al. 2011; Fernandez et al. 2010). BPA is also responsible for reduction in male fertility because it decreases sperm count and quality, induces abnormality in penile/urethra development (Meeker et al. 2011; Li et al. 2010). It promotes certain lifestyle disorders including obesity and type 2 diabetes (Siddique et al. 2020; Nadal 2013; Vom Saal et al. 2012).
The removal of BPA from wastewater is important for the protection of ecological environment and human health. The problem faced during bioremediation of BPA is its hydrophobic nature which made it insoluble in water, but it is soluble in organic solvent. (Michizoe et al. 2001). Non-aqueous catalysis is emerging technology which offers advantages including higher solubility of hydrophobic substrate, increased enantioselectivity and elimination of unwanted water dependent side reactions (Wang and Chen 2009). Considering higher solubility of BPA in organic solvent non-aqueous catalysis can be employed using enzyme like laccase which are known for its bioremediation potential (Trivedi et al. 2021; Sun et al. 2020; Xu et al. 2020; Okazaki et al. 2002). Non-aqueous catalysis using laccase has not been much explored for the bioremediation of hazardous pollutants. To protect laccase from denaturing in organic solvent, it was immobilized in reverse micelles. Reverse micelles serve as nanosized reactors that carry out catalysis process which is important application of nanotechnology (Chaurasiya and Hebbar 2017). Nanotechnology also has been employed for the evaluation of ecotoxicological effects and bioaccumulation properties (Daglioglu and Ozturk 2018, 2019; Ozturk and Daglioglu 2018).
Laccases (EC Number 1.10.3.2) are multicopper containing enzymes produced by fungus, plants, insects, and bacteria. It catalyses oxidation of phenolic and other low redox potential compounds by one electron transfer reaction with reduction of molecular oxygen to water (Chhaya and Gupte 2010). Fungal laccases are preferred over bacterial laccases due to their high redox potential (Agrawal et al. 2018). Specifically, Pleurotus ostreatus, Agaricus bisporus, Phanerochaete chrysosporium and Trametes versicolor produces considerable amount of laccase (Arregui et al. 2019). The fungal laccases are able to utilize wide range of substrates including phenols, polyphenols and substituted phenol, aromatic amines and benzenethiols (More et al. 2011).
In present study, non-aqueous catalysis using Trametes versicolor laccase was carried out to remediate BPA using AOT-isooctane-water ternary system. In this system, sodium bis sulfosuccinate (AOT) was utilized as surfactant which is anionic in nature and spontaneously form micelles in aqueous phase, but when it gets suspended in non-polar solvent (here isooctane) its non-polar tails remain in non-polar solvent and polar heads are oriented towards little aqueous phase and forms reverse micelles (Michizoe et al. 2001). Thus, reverse micelles can be defined as tiny droplets of water surrounded by surfactant molecule in bulk of solvent system. BPA can be introduced in bulk solvent system and enzyme can remain in the cavity formed by surfactant. This ternary system was optimized using statistical methods. The stastical optimization of reverse micelles was done by Plackett–Burman design, which is widely accepted experimental method used for optimization purpose. It helps to identify major effective parameters from large number of factors (Abdel-Fattah et al. 2005). The optimum concentration of effective variables identified from Plackett–Burman design was determined from Response Surface Methodology. The optimized system was employed for BPA bioremediation and compared with aqueous laccase system to evaluate catalytic efficiency of laccase in non-aqueous system. BPA bioremediation products were evaluated using GC–MS analysis and its possible degradation pathway was also elucidated.
Materials and methods
Chemicals
Purified Laccase (E.C. 1.10.3.2) from Trametes versicolor and Bis (2-ethylhexyl) sulfosuccinate sodium salt and 2, 6 dimethoxyphenol (2,6 DMP, M.W.154) was purchased from SIGMA life sciences, 4,4ʹ-(propane-2,2,-diphenol) (Bisphenol A) was procured from Loba Chemie (Mumbai). Organic solvent 2,2,4-Trimethylpentane (isooctane) used in this study were of analytical grade purchased from Merck Chemicals. All other chemicals used in this study were of analytical grade having highest possible purity.
Laccase activity determination in aqueous and reverse micelles system
The laccase activity was determined with the use of model substrate 2,6 dimethoxy phenol (2,6 DMP). Aqueous solution of Trametes versicolor (TV) laccase (5 mg/ml) and substrate 2,6 DMP (100 mM) was prepared in 100 mM citrate phosphate buffer (pH 4.5). Absorbance of resulting chromogenic product was determined after 3 min spectrophotometrically using ELICO SL 159 at 469 nm. Unit (one) activity of enzyme was defined as amount of enzyme that liberates product from one micromole of substrate 2,6 DMP per minute under assay conditions.
Preparation of TV laccase encapsulated reverse micellar system
Laccase hosted reverse micelles solution was prepared by spontaneous emulsification method in which 20 µl purified laccase solution prepared in 100 mM citrate phosphate buffer (pH 4.5) was directly injected in 150 mM of AOT prepared in 2 ml of isooctane. This resulting mixture was vortexed until it gets homogenised and optically transparent.
Statistical optimization of factors affecting TV laccase activity using Plackett—Burman design
Plackett–Burman design is very useful statistical tool to evaluate effect of different variables on response simultaneously in just n + 1 number of experiments where n is number of variables taken (Singh and Dikshit 2010). The dummy variables were taken into consideration to obtain estimate of error. In present study, 5 dummy variables were taken. The design consisting 20 runs was used to screen out parameters which may affect activity of enzyme hosted in reverse micelles system. In this design, 14 different parameters including pH, temperature, laccase enzyme and substrate (2,6 DMP) concentration, surfactant (AOT) concentration, hydration ratio (Wo) and effect of various metal ions (Co, Mn, Zn, Ca, Cu, Mg, Fe and Zr) were evaluated. Significance level (P value) was determined using student’s t-test. An effect is significant if it exceeds standard error.
Optimization of screened components using Response Surface Methodology (RSM)
Optimum concentration of the screened components from Plackett–Burman experiment was determined using Central Composite Design (CCD). The interaction between screened components and their effect on enzyme activity was studied by Response Surface Methodology using Design expert 11.0 software. A total of 28 experimental run with 4 variables [hydration ratio (Wo), Mg+2 ion, substrate (2,6-DMP) and laccase enzyme] at 5 coded level (5 different values) with α = 2 were carried out to develop mathematical correlation between screened parameters and response in terms of enzyme (laccase) activity (Y). The effect of each component on laccase activity was determined at five different levels, viz., − α, − 1, 0, + 1, + α. The behaviour of the system was explained by the following quadratic equation:
where Y denotes predicted response, is the offset term, is linear coefficient, is interaction effect and is the dimensionless coded value of Xi. The above given equation was solved with the help of design Expert (Version 11, State Ease Inc., USA).
Detection of TV laccase/RM system by Dynamic Light Scattering Spectroscopy
The size of Laccase/RM system was determined by Dynamic Light Scattering Spectroscopy (DLS-7000) using Malvern Panalytical Zeatasizer. The reverse micelles size was determined using laser light scattering due to Brownian motion of particles present in individual sample. In the first system, the size of AOT reverse micelles was determined in unoptimized system by adding 20 µl corresponding 100 µg/ml TV laccase suspended in 100 mM citrate phosphate buffer solution and in the second system diameter of the surfactant and laccase complex in solvent isooctane was measured by adding optimized quantity of enzyme from RSM which is 35 µl corresponding to 170 µg/ml.
BPA degradation studies mediated by aqueous laccase and laccase in reverse micelles system
The absorption maximum of BPA was determined using standard BPA (100 ppm) solution prepared in methanol and distilled water by UV–Visible Spectrophotometer (UV-1800, Shimadzu). The wavelength of maximum absorption λmax was in UV range at 276 nm. The optical path length was 1 cm and methanol and distilled water served as blank to obtain absorption maxima of BPA. Linear relationship between BPA concentration and its absorption was established using various concentration of BPA ranging from 30 to 200 ppm. Residual BPA concentration in reaction mixture was measured by comparing their absorbance to standard curve of BPA obtained. BPA degradation was carried out using optimized amount of laccase enzyme (175 µg/ml) in aqueous as well as reverse micelles system containing 100 ppm BPA concentration. The residual BPA concentration in each system was recorded at various time interval ranging from 120, 240, 480 min. BPA biotransformation efficiency of aqueous laccase and laccase in reverse micelles was determined at various time interval based on initial and residual BPA concentration using following equation:
Identification of BPA degradation intermediates by GC–MS
BPA biotransformation was investigated in laccase treated BPA samples obtained at time interval 240 and 480 min using GC–MS. Identification of BPA degradation products in its soluble form was done using gas chromatography-mass spectroscopy (GC–MS: GCMS- QC5050)- with 30 m fused silica column (HP-5 30 m × 0.53 mm; Agilent Technologies, Palo Alto, CA USA). The GC was programmed to raise oven temperature from 80 °C to 280 °C at 8 °C/min and MS was performed at 70 eV.
Result and discussion
Screening of parameters affecting activity of reverse micelle encapsulated TV laccase
In this study, Plackett–Burman design was applied for the screening of important parameters affecting encapsulated laccase. The parameters taken into consideration and their respective higher and lower concentration are presented in Table 1. The PBD matrix of 20 runs and their respective laccase activity in the form of U/ml are presented in Table 2. In this 20 run design variables, X1–X14 are parameters affecting enzyme activity and D1–D5 represents dummy variable.
Table 1.
Parameters for screening using Plackett–Burman design
| Parameter | + value | − value |
|---|---|---|
| Temperature | 60 | 40 |
| pH | 6 | 4 |
| 2,6 DMP (substrate) | 100 mM | 10 mM |
| AOT | 250 mM | 50 mM |
| Wo | 17.78 | 88.89 |
| CoCl2.6H2O | 1 mM | 0.1 mM |
| MnSO4.7H2O | 1 mM | 0.1 mM |
| ZnSO4.7H2O | 1 mM | 0.1 mM |
| CaCl2.2H2O | 1 mM | 0.1 mM |
| CuSO4.5H2O | 1 mM | 0.1 mM |
| MgSO4.7H2O | 1 mM | 0.1 mM |
| FeSO4.7H2O | 1 mM | 0.1 mM |
| Zr(NO3)4 | 1 mM | 0.1 mM |
| Enzyme (protein) | 20 ug/ml | 2 ug/ml |
Table 2.
Plackett–Burman design matrix of 14 variables (X1–X14) and 5 dummy variables (D1–D5) along with observed response (laccase activity U/ml)
| Run no | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | X12 | X13 | X14 | D1 | D2 | D3 | D4 | D5 | Laccase activity (U/ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | − | − | + | + | + | + | − | + | − | + | − | − | − | − | + | + | − | 372 |
| 2 | − | + | + | − | − | + | + | + | + | − | + | − | + | − | − | − | − | + | + | 163 |
| 3 | + | − | + | + | − | − | + | + | + | + | − | + | − | + | − | − | − | − | + | 91 |
| 4 | + | + | − | + | + | − | − | + | + | + | + | − | + | − | + | − | − | − | − | 318 |
| 5 | − | + | + | − | + | + | − | − | + | + | + | + | − | + | − | + | − | − | − | 48 |
| 6 | − | − | + | + | − | + | + | − | − | + | + | + | + | − | + | − | + | − | − | 1190 |
| 7 | − | − | − | + | + | − | + | + | − | − | + | + | + | + | − | + | − | + | − | 47 |
| 8 | − | − | − | − | + | + | − | + | + | − | − | + | + | + | + | − | + | − | + | 11 |
| 9 | + | − | − | − | − | + | + | − | + | + | − | − | + | + | + | + | − | + | − | 30 |
| 10 | − | + | − | − | − | − | + | + | − | + | + | − | − | + | + | + | + | − | + | 447 |
| 11 | + | − | + | − | − | − | − | + | + | − | + | + | − | − | + | + | + | + | − | 827 |
| 12 | − | + | − | + | − | − | − | − | + | + | − | + | + | − | − | + | + | + | + | 481 |
| 13 | + | − | + | − | + | − | − | − | − | + | + | − | + | + | − | − | + | + | + | 1032 |
| 14 | + | + | − | + | − | + | − | − | − | − | + | + | − | + | + | − | − | + | + | 92 |
| 15 | + | + | + | − | + | − | + | − | − | − | − | + | + | − | + | + | − | − | + | 981 |
| 16 | + | + | + | + | − | + | − | + | − | − | − | − | + | + | − | + | + | − | − | 861 |
| 17 | − | + | + | + | + | − | + | − | + | − | − | − | − | + | + | − | + | + | − | 974 |
| 18 | − | − | + | + | + | + | − | + | − | + | − | − | − | − | + | + | − | + | + | 763 |
| 19 | + | − | − | + | + | + | + | − | + | − | + | − | − | − | − | + | + | − | + | 418 |
| 20 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 554 |
The PBD matrix of 20 runs and their respective laccase activity in the form of U/ml are presented in Table 2. In this 20 run design variables, X1–X14 are parameters affecting enzyme activity and D1–D5 represents dummy variable.
Effect of individual parameter, standard error, t(xi) value, P value and confidence level are presented in Table 3.
Table 3.
Statistical analysis of parameter’s effect on laccase activity in according to Plackett–Burman design
| Variables | Parameter | Effect E(xi) | Standard Error | t value | P value | Confidence (%) |
|---|---|---|---|---|---|---|
| X1 | Temperature | 3445 | 8794 | 0.391744 | 0.711386 | 29% |
| X2 | pH | 7701 | 8794 | 0.875711 | 0.421573 | 58% |
| X3 | 2,6 DMP (substrate) | − 2264 | 8794 | 4.73152 | 0.007413 | 99% |
| X4 | AOT | 2263 | 8794 | − 2.04969 | 0.095680 | 91% |
| X5 | Wo | − 24,335 | 8794 | − 2.76732 | 0.039494 | 97% |
| X6 | CoCl2.6H2O | − 2735 | 8794 | 0.27007 | 0.797898 | 21% |
| X7 | MnSO4.7H2O | − 14,191 | 8794 | − 0.1766 | 0.866753 | 14% |
| X8 | ZnSO4.7H2O | − 1553 | 8794 | − 0.61349 | 0.566384 | 44% |
| X9 | CaCl2.2H2O | − 29,793 | 8794 | − 1.61371 | 0.167510 | 84% |
| X10 | CuSO4.5H2O | − 18,025 | 8794 | 0.60302 | 0.572798 | 43% |
| X11 | MgSO4.7H2O | − 19,011 | 8794 | − 3.38788 | 0.019506 | 99% |
| X12 | FeSO4.7H2O | − 5395 | 8794 | 0.257335 | 0.807175 | 20% |
| X13 | Zr(NO3)4 | 5303 | 8794 | 2.161371 | 0.167510 | 84% |
| X14 | Enzyme (protein) | 41,609 | 8794 | 4.731521 | 0.005189 | 99% |
The components were screened at 95% confidence interval based on their effect. Parameters showing confidence level greater than 95% indicate that they positively affect encapsulated enzyme activity. These parameters were 2,6-DMP (substrate), MgSO4 and laccase enzyme (protein) having confidence level 99%, while hydration ratio (Wo) showed 97% confidence level. A positive effect of parameter (enzyme concentration) indicates that to attain higher activity of encapsulated laccase concentration of these component should be increased, and concentration of parameters (2,6 DMP, Wo and Mg+2) should be decreased because they are showing negative effect. Parameters showing confidence level lower than 95% were considered non-significant.
Optimization of TV laccase reverse micellar system using Response Surface Methodology (RSM)
Response surface methodology is mathematical and statistical tool that is widely used to optimize the response which is affected by various variables. After screening potential variables through Plackett–Burman Design (PBD), their optimum response region in terms of enzyme catalysis were identified using Central Component Design (CCD). The coded values of different variables obtained through CCD and their response in terms of laccase activity U/ml is shown in Table 4. The quadratic model was used to explain the mathematical relationship between the independent variables and the dependent response.
Table 4.
Central composite design matrix with coded values and actual values for laccase activity
| Run | Factor A: Mg+2 ion, mM | Factor B: Hydration ratio | Factor C: DMP concentration, mM | Factor D: Protein concentration, µg/ml | Actual value | Predicted value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Actual value | Coded value | Actual value | Coded value | Actual value | Coded value | Actual value | Coded value | |||
| 1 | 0.1 | − 1 | 100 | − 1 | 0.004 | 1 | 40 | 1 | 861 | 1135.58 |
| 2 | 0.55 | 0 | 150 | 0 | 0.0035 | 0 | 35 | 0 | 1740 | 1686.37 |
| 3 | 1 | 1 | 100 | − 1 | 0.003 | − 1 | 30 | − 1 | 512 | 424.91 |
| 4 | 1 | 1 | 200 | 1 | 0.003 | − 1 | 40 | 1 | 891 | 831.25 |
| 5 | 0.1 | − 1 | 100 | − 1 | 0.004 | 1 | 30 | − 1 | 927 | 954.91 |
| 6 | 1 | 1 | 200 | 1 | 0.004 | 1 | 40 | 1 | 861 | 909.58 |
| 7 | 1 | 1 | 200 | 1 | 0.003 | − 1 | 30 | − 1 | 754 | 569.58 |
| 8 | 0.1 | − 1 | 200 | 1 | 0.003 | − 1 | 40 | 1 | 674 | 883.91 |
| 9 | 0.1 | − 1 | 100 | − 1 | 0.003 | − 1 | 40 | 1 | 981 | 1058.75 |
| 10 | 0.1 | − 1 | 200 | 1 | 0.003 | − 1 | 30 | − 1 | 500 | 605.75 |
| 11 | 0.1 | − 1 | 200 | 1 | 0.004 | 1 | 30 | − 1 | 894 | 991.58 |
| 12 | 1 | 1 | 100 | − 1 | 0.003 | − 1 | 40 | 1 | 821 | 813.58 |
| 13 | 1 | 1 | 200 | 1 | 0.004 | 1 | 30 | − 1 | 982 | 872.41 |
| 14 | 1 | 1 | 100 | − 1 | 0.004 | 1 | 30 | − 1 | 763 | 643.25 |
| 15 | 0.55 | 0 | 150 | 0 | 0.0035 | 0 | 35 | 0 | 1866 | 1686.37 |
| 16 | 1 | 1 | 100 | − 1 | 0.004 | 1 | 40 | 1 | 945 | 807.41 |
| 17 | 0.1 | − 1 | 200 | 1 | 0.004 | 1 | 40 | 1 | 990 | 1045.25 |
| 18 | 0.1 | − 1 | 100 | − 1 | 0.003 | − 1 | 30 | − 1 | 612 | 653.58 |
| 19 | 0.55 | 0 | 250 | 2 | 0.0035 | 0 | 35 | 0 | 641 | 588.51 |
| 20 | 0.55 | 0 | 150 | 0 | 0.0035 | 0 | 35 | 0 | 1940 | 2033.13 |
| 21 | − 0.35 | − 2 | 150 | 0 | 0.0035 | 0 | 35 | 0 | 1894 | 1478.01 |
| 22 | 0.55 | 0 | 150 | 0 | 0.0025 | − 2 | 35 | 0 | 1241 | 1222.01 |
| 23 | 0.55 | 0 | 150 | 0 | 0.0035 | 0 | 25 | − 2 | 1054 | 1197.18 |
| 24 | 0.55 | 0 | 150 | 0 | 0.0045 | 2 | 35 | 0 | 1641 | 1601.68 |
| 25 | 0.55 | 0 | 150 | 0 | 0.0035 | 0 | 35 | 0 | 1893 | 2033.13 |
| 26 | 0.55 | 0 | 150 | 0 | 0.0035 | 0 | 45 | 2 | 1841 | 1639.51 |
| 27 | 0.55 | 0 | 50 | − 2 | 0.0035 | 0 | 35 | 0 | 540 | 534.18 |
| 28 | 1.45 | 2 | 150 | 0 | 0.0035 | 0 | 35 | 0 | 756 | 1113.68 |
The behaviour of the system was explained by the following quadratic model coded equation.
Where Y is predicted laccase activity, A: Mg+2 ion concentration (mM), B: hydration ratio (Wo) C: 2,6- DMP (substrate) concentration (mM) and D: protein (laccase) concentration (µg/ml).
Statistical adequateness of this quadratic model was evaluated using Analysis of Variance (ANOVA), as shown in Table 5. The F value of current model was found to be 20.47 which indicate that this model is significant. Lack of fit value 4.07 and value of P > F value < 0.0001 suggested high significance of the model which means the variables are showing significant effect on enzyme activity. The response in terms of enzyme activity is largely dependent upon two variables which are 2,6-DMP concentration and enzyme concentration as their P value is less than 0.05. The model’s goodness of fit was checked using determination coefficient (R2). The Predicted R2 of 0.7409 is in reasonable agreement with the Adjusted R2 of 0.9129. Here, ratio of 15.452 indicates an adequate signal. Thus, this model can be used to navigate the design space.
Table 5.
Analysis of variance (ANNOVA) for the quadratic model
| Source | Sum of squares | df | Mean Square | F value | P value | |
|---|---|---|---|---|---|---|
| Block | 6.829E + 05 | 1 | 6.829E + 05 | |||
| Model | 4.614E + 06 | 14 | 3.296E + 05 | 20.47 | < 0.0001 | Significant |
| A-Mg+2 ion | 2360.17 | 1 | 2360.17 | 0.1466 | 0.7085 | |
| B-hydration ratio | 4428.17 | 1 | 4428.17 | 0.2750 | 0.6095 | |
| C-2,6 DMP concentration | 2.162E + 05 | 1 | 2.162E + 05 | 13.43 | 0.0032 | |
| D-protein concentration | 2.935E + 05 | 1 | 2.935E + 05 | 18.23 | 0.0011 | |
| AB | 37,056.25 | 1 | 37,056.25 | 2.30 | 0.1551 | |
| AC | 6889.00 | 1 | 6889.00 | 0.4279 | 0.5254 | |
| AD | 272.25 | 1 | 272.25 | 0.0169 | 0.8987 | |
| BC | 7140.25 | 1 | 7140.25 | 0.4435 | 0.5181 | |
| BD | 16,129.00 | 1 | 16,129.00 | 1.00 | 0.3366 | |
| CD | 50,400.25 | 1 | 50,400.25 | 3.13 | 0.1022 | |
| A2 | 2.006E + 06 | 1 | 2.006E + 06 | 124.58 | < 0.0001 | |
| B2 | 2.953E + 06 | 1 | 2.953E + 06 | 183.41 | < 0.0001 | |
| C2 | 4.627E + 05 | 1 | 4.627E + 05 | 28.74 | 0.0002 | |
| D2 | 4.519E + 05 | 1 | 4.519E + 05 | 28.07 | 0.0002 | |
| Residual | 1.932E + 05 | 12 | 16,101.06 | |||
| Lack of Fit | 1.842E + 05 | 10 | 18,417.02 | 4.07 | 0.2131 | Not significant |
| Pure error | 9042.50 | 2 | 4521.25 | |||
| Cor total | 5.490E + 06 | 27 |
The graphical representations of the above equation called the response surface, and the contour plots were obtained using the Design Expert and are presented in Figs 1–6
Fig. 1.
A Two-dimensional plot showing the effect of Mg+2 ion and hydration ratio on laccase activity. B Three-dimensional plot showing the effect of Mg+2 ion and hydration ratio on laccase activity
Fig. 6.
A Two-dimensional plot showing the effect of 2,6-DMP concentration and protein concentration on laccase activity. B Three-dimensional plot showing the effect of 2,6-DMP concentration and protein concentration on laccase activity
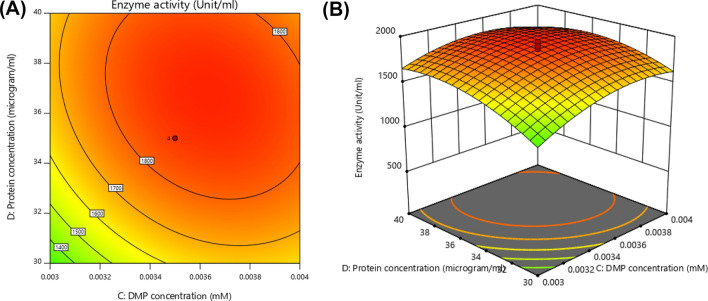
Interaction between hydration ratio and Mg+2 ion concentration is shown in Fig. 1A and B. It is evident from the contour plots that laccase activity increases with increase in hydration ratio and Mg+2 ion concentration, but up to certain limit and stabilizes afterwards and there is decrease in activity at higher concentration of given variables. In Fig. 2A and B interaction between 2,6 DMP concentration and Mg+2 ion concentration is shown, from the contour plot it is visible that there is slight increase in response in terms of activity which gets stabilized. Figure 3A and B represents interaction between Mg+2 ion concentration and protein concentration, here also slight increase in activity is seen which stabilized at further increasing concentration of given variable. In Fig. 4 (A) and (B) contour plots demonstrate interaction between 2,6 DMP concentration and hydration ratio, there is slight increase in enzyme activity with increasing concentration of given variables, and slight decline at higher concentration of substrate 2,6 DMP and AOT concentration (hydration ratio) in between stabilized response was obtained. The contour plot shows that at lower AOT concentration (< 150 mM), fewer reverse micelles are formed which encapsulate laccase enzyme as well as low substrate concentration resulted in less enzyme activity, as the AOT concentration increases more amount of enzyme is incorporated in reverse micelles and higher substrate concentration is also responsible for the increases in the enzyme activity due to improved enzyme substate interaction at the periphery of the hydrated reverse micelles. The increasing surfactant (AOT) and substrate (2,6- DMP) concentration showed slight decline in enzyme activity that may be due to enzyme inactivation by surfactant molecules and unavailability of free laccase enzyme. Similar trends were obtained for protein concentration and hydration ratio which are shown in Fig. 5A and B. In Fig. 6A and B, interaction between 2,6 DMP concentration and enzyme concentration is shown. With the increase in both variable’s value, slight increase in response is there which gets stabilized there is no further significant decrease in enzyme activity is seen which suggest that optimum concentration of enzyme and substrate has been achieved for this reverse micellar system.
Fig. 2.
A Two-dimensional plot showing the effect of Mg+2 ion and 2,6-DMP concentration on laccase activity. B Three-dimensional plot showing the effect of Mg+2 ion and 2,6-DMP concentration on laccase activity
Fig. 3.
A Two-dimensional plot showing the effect of Mg+2 ion and protein concentration on laccase activity. B Three-dimensional plot showing the effect of Mg+2 ion and protein concentration on laccase activity
Fig. 4.
A Two-dimensional plot showing the effect of hydration ratio and 2,6-DMP concentration on laccase activity. B Three-dimensional plot showing the effect of hydration ratio and 2,6-DMP concentration on laccase activity
Fig. 5.
A Two-dimensional plot showing the effect of hydration ratio and protein concentration on laccase activity. B Three-dimensional plot showing the effect of hydration ratio and protein concentration on laccase activity
From all these response surface graphs, it was observed that hydration ratio plays an important part in the functionality of encapsulated laccase enzyme. Hydration ratio of reverse micelles can be defined as water pool available surrounding entrapped enzyme confined in boundaries of surfactant molecules. At higher concentration of surfactant AOT (> 150 mM, Wo 30), there is decline noted in resulting laccase activity. It may be due to denaturation of laccase by AOT (> 150 mM), negatively charged surfactant might be denaturing laccase by electrostatic repulsion (Chang et al. 2000). Lower concentration of surfactant AOT leads to a situation where there is higher water pool surrounding enzyme laccase which result into lowering down its catalytic efficiency towards its substrate (Muniz-Mouro et al. 2117).
According to various diagnostic plots and point prediction, actual concentration of different variables was 0.55, 150 (Wo 30), 0.0035 mM and 175 µg/ml for Mg+2 ions, Hydration ratio (Wo), 2,6-dimethoxyphenol (2,6 DMP, substrate) and laccase (protein), respectively, at 40 °C and pH 4.5. Laccase activity was measured using these optimized concentrations of variables and it was found to be 1890 U/ml, there is almost two-fold increase in laccase activity in comparison with previous unoptimized reverse micellar system.
Detection of TV laccase/RM system by Dynamic Light Scattering Spectroscopy (DLS)
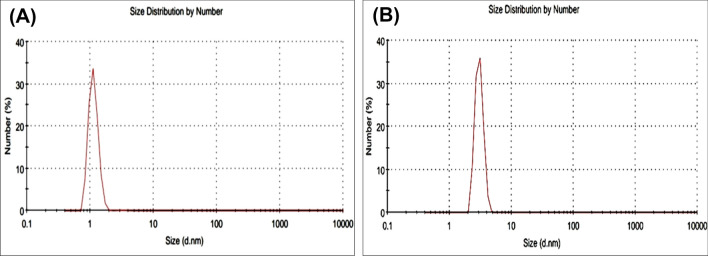
In the present study, formation of reverse micelles was confirmed, and measurement of their size was done using dynamic light scattering spectroscopy. The size of reverse micelles was investigated having unoptimized and optimized laccase enzyme (protein) concentration. The average micellar diameter was found to be increasing in the optimized system having higher protein concentration (170 µg/ml) as compared to unoptimized system comprising less protein concentration (100 µg/ml). Size of micelle in unoptimized system was 1.1 nm whereas size of reverse micelles in optimized system was 3 nm (Fig. 7). The size distribution by number (%) indicated that 100% reverse micelles were having size of 3 nm in optimized sample, and 99.6% reverse micelles were having diameter of 1 nm in unoptimized sample. The size of reverse micelles increases as more water is incorporated maintaining same surfactant concentration. To incorporate increased water pool more surfactant molecule will aggregate and as a result there is an increase in reverse micelles diameter which is calculated using translational diffusion coefficient (velocity of Brownian motion) of each reverse micelle. The average intensity of scattered laser light increases in samples with high amount of water incorporated (Chhaya and Ingale 2016). The incorporated water pool here contains hydrophilic enzyme. As the inner cavity of reverse micelles increases and matches to the size of enzyme molecule, enzyme can fit into micelles in appropriate configuration which leads to increase in catalytic activity. (Martinek et al. 1989; Pileni et al. 1985).
Fig. 7.
DLS analysis of (A) unoptimized and B optimized reverse micelles system
BPA degradation studies mediated by aqueous laccase and laccase in reverse micelles system
The ability of free laccase to remove BPA from reaction mixture was found to be 21, 39 and 54% at 120, 240 and 480 min of interval. Whereas in reverse micelles system BPA removal was increased and found to be 40, 52, and 86% at 120, 240, and 480 min of interval (Fig. 8). Biotransformation efficiency in non-aqueous system was higher in comparison with aqueous system, laccase in reverse micelles system was able to remove 86% of BPA in 8 h of treatment. Catalysis process by oxidation was elevated in non-aqueous environment as compared to aqueous environment. This proves reverse micelles provides environment where enzyme is immobilized and protected from external environment. The increased bioavailability of BPA in non-aqueous environment also contributed to achieve higher catalytic efficiency.
Fig. 8.
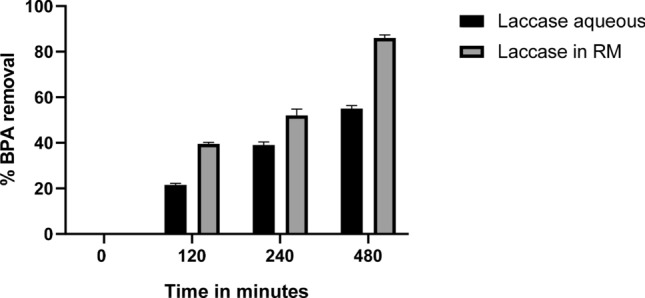
Comparative study of BPA removal mediated by aqueous laccase and laccase entrapped in reverse micelles system
To identify degradation products of BPA, GC–MS analysis was carried out for various isooctane soluble fraction obtained after TV laccase-reverse micelles mediated catalysis. Based on m/z ratio and fragmentation pattern of GC–MS spectrum, (Fig. 9A–C) two degradation products named BPA-O-catechol with m/z ratio 244.11 (100%), 245.11 (16.2%), 246.12 (1.2%) and 4,4 (Ethane 2-oxy 2-ol) diphenol with m/z ratio 212.08 (100.0%), 213.09 (15.1%), 214.09 (1.1%) were identified. The molecular weight, retention time log P value and name of BPA along with the identified compound are presented in Table 6.
Fig. 9.
A GC–MS chromatogram of BPA. B TV laccase-RM catalysed BPA products at time interval 360 min. C TV laccase-RM catalysed BPA products at time interval 480 min
Table 6.
Characteristics of Bisphenol A and its degradation products
The oxidation of BPA begins at nucleophilic carbon present on its aromatic ring, which leads to formation of BPA-O-catechol. Similar BPA degradation reaction was reported by Li et al. 2016, in which oxidation of BPA via porous FexCo3–xO4 nanocages resulted in formation of BPA-O-catechol. Oxidation reaction may also occur at the quaternary alpha carbon of BPA which could lead to formation of 4,4 (Ethane 2-oxy 2-ol) diphenol. Furthermore, subsequent oxidation may result into aromatic ring opening and mineralization afterwards. Possible BPA degradation pathway depicted in Fig. 10. The transformed products obtained after oxidation shown negative log P value as compared to its parent molecule BPA. Reduction in log P value suggest higher affinity in aqueous phase, so it is difficult for this molecule to cross hydrophobic membrane and penetrate cells and thus their endocrine disrupting activity may reduce.
Fig. 10.
Proposed pathway for Bisphenol A degradation by TV laccase/RM system
In organic media, BPA is freely soluble and better bioavailability of substrate may be the reason for higher percentage of BPA removal in reverse micelles system. Michizoe et al. (2001) obtained complete removal of BPA in 3-h treatment by laccase/RM system. Chhaya and Gupte (2013) reported 91.43% BPA removal in 75 min by Laccase from Fusarium incarnatum UC-14 in reverse micelles system. These results agree with our findings.
Non-aqueous catalysis of BPA has been reported in some of the published literature. Non-aqueous catalysis mediated by laccase-RM system exhibited a high and stable enzymatic activity, and better efficiency of catalysis compared to aqueous media (Ingale et al, 2015). Reverse micelles system comprising of surfactant AOT and 4-isopropylphenol and 4-isopropenylphenol were able to oxidize BPA effectively but AOT-laccase complexes in water saturated isooctane were not as effective as reverse micelles systems for BPA oxidation (Michizoe et al. 2001). In another study, laccase from mutant strain of litter dwelling fungi Fusarium incarnatum UC-14 was successfully utilized for BPA degradation in isooctane: AOT reverse micelles. This system was able to degrade up to 94% of 200 ppm BPA in 75 min at 50 °C and pH 6.0 (Chhaya and Gupte 2013). All these observations are in the agreement with our findings.
Conclusion
Laccase obtained from Trametes versicolor encapsulated in reverse micelles successfully carried out oxidation reaction of Bisphenol A. Laccase activity in unoptimized and statistically optimized non-aqueous system was 998 and 1890 U/ml, respectively. There was almost two-fold increase in catalytic efficiency of laccase. BPA bioremediation by laccase in aqueous and non-aqueous system after 8 h was 54% and 84%, respectively. Considering hydrophobicity of BPA, non-aqueous catalysis is advantageous over aqueous laccase system. BPA biotransformation in by TV laccase-RM under optimized condition gives oxidized products of BPA named BPA-O-catechol and 4,4 (Ethane 2-oxy 2-ol) diphenol identified by GC–MS analysis. The oxidized products obtained after BPA bioremediation are structurally different and showing decrease in their log P value, reduction in log P value suggest its increased solubility in aqueous environment and better bioavailability aid in its complete degradation. BPA bioremediation in non-aqueous environment using TV laccase proven to be efficient and better alternative over aqueous laccase system due to high and stable catalytic efficiency.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Janki Trivedi, Email: jankitrivedi6@gmail.com.
Urvish Chhaya, Email: urvish@nvpas.edu.in.
References
- Abdel-Fattah YR, Saeed HM, Gohar YM, El-Baz MA. Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem. 2005;40(5):1707–1714. doi: 10.1016/j.procbio.2004.06.048. [DOI] [Google Scholar]
- Agrawal K, Chaturvedi V, Verma P. Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess. 2018;5:4. doi: 10.1186/s40643-018-0190-z. [DOI] [Google Scholar]
- Almeida S, Raposo A, Almeida-Gonzalez CC. Bisphenol A: food exposure and impact on human health. Compr Rev Food Sci Food Saf. 2018;17:1503–1517. doi: 10.1111/1541-4337.12388. [DOI] [PubMed] [Google Scholar]
- Arregui L, Ayala M, Gomez-Gil X, et al. Laccases: structure, function, and potential application in water bioremediation. Microb Cell Fact. 2019;18:200. doi: 10.1186/s12934-019-1248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae B, Jeong JH, Lee SJ. The quantification and characterization of endocrine disruptor bisphenol-A leaching from epoxy resin. Water Sci Technol. 2002;46(11–12):381–387. doi: 10.2166/wst.2002.0766. [DOI] [PubMed] [Google Scholar]
- Chang, GG, Huang TM, Hung HC (2000) Reverse micelles as life-mimicking systems. Proceedings of the National Science Council, Republic of China, Part B, Life sciences 24(3):89–100. http://europepmc.org/article/med/10943941 [PubMed]
- Chaurasiya RS, Hebbar HU. Reverse micelles for nanoparticle synthesis and biomolecule separation. In: Ranjan S, Dasgupta N, Lichtfouse E, editors. Nanoscience in food and agriculture 4. Sustainable agriculture reviews. Cham: Springer; 2017. pp. 181–211. [Google Scholar]
- Chhaya U, Gupte A. Optimization of media components for laccase production by litter dwelling fungal isolate Fusarium incarnatum LD-3. J Basic Microbiol. 2010;50(1):43–51. doi: 10.1002/jobm.200900203. [DOI] [PubMed] [Google Scholar]
- Chhaya U, Gupte A. Possible role of laccase from Fusarium incarnatum UC-14 in bioremediation of Bisphenol A using reverse micelles system. J Hazard Mater. 2013;254–255(1):149–156. doi: 10.1016/j.jhazmat.2013.03.054. [DOI] [PubMed] [Google Scholar]
- Chhaya U, Ingale S. Micellar enzymology- chemistry and applications. Open Biotechnol J. 2016;10(Suppl-2, M5):326–334. doi: 10.2174/1874070701610010326. [DOI] [Google Scholar]
- Daglioglu Y, Ozturk BY. Effect of concentration and exposure time of ZnO-TiO2 nanocomposite on photosynthetic pigment contents, ROS production ability, and bioaccumulation of freshwater algae (Desmodesmus multivariabilis) Caryologia. 2018;71(1):13–23. doi: 10.1080/00087114.2017.1400262. [DOI] [Google Scholar]
- Daglioglu Y, Ozturk BY. A novel intracellular synthesis of silver nanoparticles using Desmodesmus sp. (Scenedesmaceae): different methods of pigment change. Rendiconti Lincei Scienze Fisiche e Naturali. 2019;30(3):611–621. doi: 10.1007/s12210-019-00822-8. [DOI] [Google Scholar]
- Experts I (2016) Bisphenol-A research report | BPA market research report. https://industry-experts.com/verticals/chemicals-and-materials/bisphenol-a-a-global-market-overview. Accessed 31 Jan 2021
- Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol A and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Persp. 2010;118(9):1217–1222. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Li H, Lu G, Chen Z, Sun W, Shi Y, Fu Z, Huang B, Zhu X, Lu W, et al. Low dose of bisphenol a modulates ovarian cancer gene expression profile and promotes epithelial to mesenchymal transition via canonical wnt pathway. Toxicol Sci. 2018;164:527–538. doi: 10.1093/toxsci/kfy107. [DOI] [PubMed] [Google Scholar]
- Ingale SS, Joshi R, Chhaya U (2015) Optimization of Tremetes versicolor laccase reverse micelles system for the removal of phenolic environmental pollutant Bisphenol A. Int J Curr Microbiol Appl Sci 4(5):39–49. http://www.ijcmas.com
- Kandaraki E, Chatzigeorgiou A, Livadas S, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of Bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96(3):E480–E484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- Li D, Zhou Z, Qing D, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010;25(2):519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Z, Zhang B, et al. FexCo3-xO4 nanocages derived from nanoscale metal-organic frameworks for removal of Bisphenol A by activation of peroxymonosulfate. Appl Catal B Environ. 2016;181:788–799. doi: 10.1016/j.apcatb.2015.08.050. [DOI] [Google Scholar]
- Lozada KW, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod. 2011;85:490–497. doi: 10.1095/biolreprod.110.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek K, Klyachko NL, Kabanov AV, Khmelnitsky YL, Levashov AV. Micellar enzymology: its relation to membranology. Biochim Biophys Acta-Biomembr. 1989;981(2):161–172. doi: 10.1016/0005-2736(89)90024-2. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Persp. 2011;119(2):252–257. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michizoe J, Goto M, Furusaki S. Catalytic activity of laccase hosted in reversed micelles. J Biosci Bioeng. 2001;92(1):67–71. doi: 10.1016/s1389-1723(01)80201-2. [DOI] [PubMed] [Google Scholar]
- Mikolajewska K, Stragierowicz J, Gromadzinska J. Bisphenol A—application, sources of exposure and potential risks in infants, children and pregnant women. Int J Occup Med Environ Health. 2015;28(2):209–241. doi: 10.13075/ijomeh.1896.00343. [DOI] [PubMed] [Google Scholar]
- More S, Renuka PS, Pruthvi K, Swetha M, Malini S, Veena SM. Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res. 2011 doi: 10.4061/2011/248735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz-Mouro A, Oliveira IM, Gullon B, et al. Comprehensive investigation of the enzymatic oligomerization of esculin by laccase in ethanol: water mixtures. RSC Adv. 2117;7(61):38424–38433. doi: 10.1039/c7ra06972c. [DOI] [Google Scholar]
- Nadal A. Obesity: fat from plastics? Linking Bisphenol A exposure and obesity. Nat Rev Endocrinol. 2013;9(1):9–10. doi: 10.1038/nrendo.2012.205. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Michizoe J, Goto M, Furusaki S, Wariishi H, Tanaka H. Oxidation of bisphenol A catalyzed by laccase hosted in reversed micelles in organic media. Enzyme Microb Technol. 2002;16(4):583–588. doi: 10.1016/S0141-0229(02)00104-7. [DOI] [Google Scholar]
- Ozturk BY, Daglioglu Y. The ecotoxicological effects of ZnO-TiO nanocomposite in Chodatodesmus mucranulatus. Feb-Fresenius Environ Bull. 2018;27(5):2951–2962. [Google Scholar]
- Pileni MP, Zemb T, Petit C. Solubilization by reverse micelles: solute localization and structure perturbation. Chem Phys Lett. 1985;118(4):414–420. doi: 10.1016/0009-2614(85)85402-6. [DOI] [Google Scholar]
- Repossi A, Farabegoli F, Gazzotti T, et al. Bisphenol A in edible part of seafood. Ital J Food Saf. 2016;5(2):98–105. doi: 10.4081/ijfs.2016.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1–2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Sangai N, Pandya HA, Singh R. Critical review on Bisphenol A: invisible pollution. Int J Pharma Res Heal Sci. 2016;4(2):1043–1049. [Google Scholar]
- Siddique S, Zhang G, Kubwabo C. Exposure to Bisphenol A and risk of developing type 2 diabetes: a mini review. Emerging Contaminants. 2020;6:274–282. doi: 10.1016/j.emcon.2020.07.005. [DOI] [Google Scholar]
- Singh SS, Dikshit AK. Optimization of the parameters for decolourization by Aspergillus niger of anaerobically digested distillery spentwash pretreated with polyaluminium chloride. J Hazard Mater. 2010;176(1–3):864–869. doi: 10.1016/j.jhazmat.2009.11.116. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Godara S. Use of polycarbonate plastic products and human health. Int J Basic Clin Pharmacol. 2013;2(1):12–17. doi: 10.5455/2319-2003.ijbcp2013010. [DOI] [Google Scholar]
- Sun J, Wang L, Ding S, Sun X, Xu L. Solubility behavior and thermodynamic analysis of Bisphenol A in 14 different pure solvents. J Chem Eng Data. 2020;65(5):2846–2858. doi: 10.1021/acs.jced.0c00166. [DOI] [Google Scholar]
- Trivedi J, Chhaya U, Patel Y, Rudakiya D. Nonaqueous Catalysis: a way forward for the intermediation of phenolic environmental pollutant Bisphenol A. In: Panpatte DG, Jhala YK, editors. Microbial rejuvenation of polluted environment. Microorganisms for sustainability. Singapore: Springer; 2021. pp. 291–316. [Google Scholar]
- Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354(1–2):74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen Y. Enzymatic catalysis in non-aqueous solvents. Sheng Wu Gong Cheng Xue Bao. 2009;25(12):1789–1794. [PubMed] [Google Scholar]
- Xu P, Du H, Peng X, et al. Degradation of several polycyclic aromatic hydrocarbons by laccase in reverse micelle system. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2019.13497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.