Graphical abstract
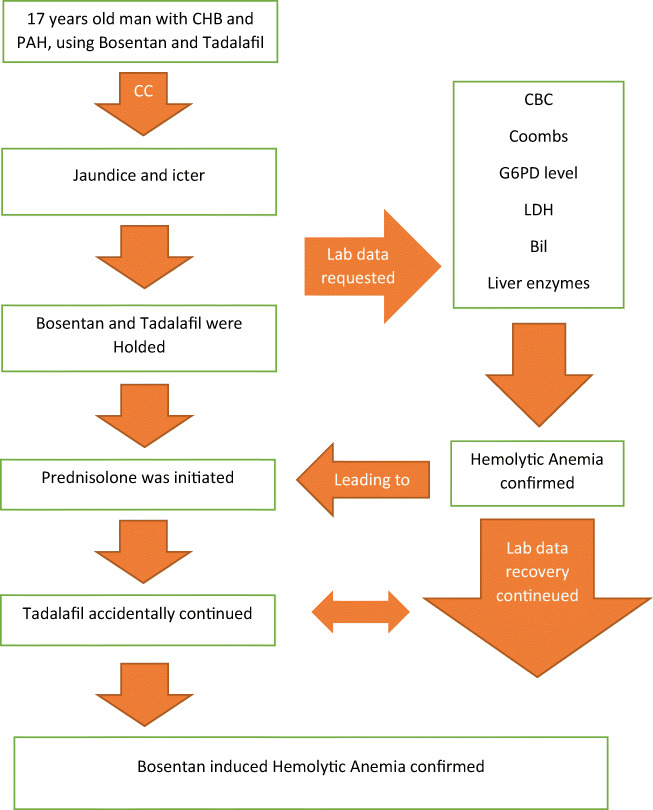
Hemolytic anemia is a very important immune-mediated reaction, which its late diagnosis can be fatal. Medications along with other causes can induce hemolytic anemia. Drug induced immune hemolytic anemia (DIIHA) is caused by the development of autoantibodies. Accordingly, DIIHA is rare and there is not enough data for its prevalence. Number of drugs that can cause DIIHA have increased in recent decades. A 17-year-old man who had congenital single ventricle heart (CHB) and pulmonary artery hypertension (PAH) was admitted at Imam Khomeini hospital complex affiliated to Tehran University of Medical Sciences, with chief complaint of jaundice and icter. Bosentan and Tadalafil were in the list of the drugs used by this patient. Although both drugs were recommended to be discontinued in the patient, in the course of hospitalization, the patient accidentally continued to take his Tadalafil. However, the patient’s recovery continued. Given that the patient’s Coombs test was positive, his hemolytic anemia mechanism was drug-induced immune-mediated hemolytic anemia. As a result, according to Naranjo score = 6, Bosentan was considered as the main possible culprit to induce DIIHA in this patient. Following the discontinuation of Bosentan and receiving Prednisolone, the patient’s clinical symptoms and laboratory parameters resolved and the patient was then discharged.
Keywords: Drug-induced immune hemolytic anemia (DIIHA), Pulmonary artery hypertension (PAH), Bosentan, Hemolytic anemia, Congenital heart block (CHB)
Introduction
HA is a very important immune-mediated reaction, which its late diagnosis can be fatal [1]. Medications along with other causes (e.g. infections, cancers, etc.) can induce HA [2]. DIHA has some mechanisms to occur such as immune mediated, oxidant injury, methemoglobinemia, and thrombotic microangiopathy [3].
DIIHA is caused by the development of autoantibodies. Its mechanism is associated with the immune destruction of RBCs that has some mechanisms. One of these mechanisms includes some types of antigen-antibody interactions such as binding antibodies to the RBCs leading to extravascular hemolysis, direct binding the drug to the RBCs surface (can be further subdivided to penicillin type, immune complex type, and passive absorption), and the alteration of RBC surface antigen. Direct binding of the drug to the RBC can lead to making IgG antibodies to the drug in patients, which can be attached to the drug-RBC complex, resulting in the invocation of macrophages and Fc-mediated extravascular RBC’s destruction. The complement component may be occasionally involved. These antibodies can be easily detected under in vitro by testing the patient’s serum or an eluate from the RBCs against drug-RBC complex [2, 4].
DIIHA is rare and there is not enough data for its prevalence. Number of drugs that can cause DIIHA have increased in recent decades. The most common drug categories causing this reaction are Antibiotics; NSAIDs; Anticancer drugs; and some other drugs such as Hydrochlorothiazide and Insulin [2]. Clinical and laboratory presentations of DIIHA are fatigue; weakness; dyspnea; symptoms related to volume depletion; and hemolysis symptoms such as jaundice, dark or red urine due to hemoglobinuria [5]. Moreover, an increase in reticulocyte count, LDH, and indirect bilirubin as well as a decrease in haptoglobin can be seen in this disorder [6]. In this regard, one of the specific test for diagnosing DIIHA is the ‘Coombs’ test [7].
The primary approach to manage this condition is drug discontinuation. In severe cases, blood transfusion, dialysis, glucocorticoids and/or IVIG are also observed [3].
Bosentan is an endothelin receptor antagonist, acting as a vasodilator to treat PAH [8]. Based on our studies, there were no case reports of Bosentan or Tadalafil induced HA, so we reported a case of Bosentan induced HA in a 17-year-old man suffering from PAH and CHB.
Case presentation
A 17-year-old man who had congenital single ventricle heart, CHB, and PAH (PAP = 60 mmHg) was admitted at Imam Khomeini hospital complex affiliated to Tehran University of Medical Sciences, with chief complaint of jaundice and icter started about 3 weeks before his admission. In his drug history, Bosentan (125 mg per day, started 1 month before his admission, but the patient stopped the consumption of the drug for a week due to the lack of medication in his city drugstores, and he started taking it again after a week of discontinuation) and Tadalafil (20 mg per day, started 3 months before his admission), both of which were administered for the treatment of patient’s PAH. On the admission’s day, he was hemodynamically stable (BP = 112/51 mmHg, HR = 72 beat/min, RR = 20 beat/min), afebrile (Temperature = 37.5 °C oral), and icteric, and his lab data at the admission time are shown in Table 1 and his anti-ds DNA(8.3 IU/ml) and ANA(0.1 index) were in normal range. Moreover, his viral markers, including HBSAg, Anti-Hbc Ab, Anti-HCV, and HIV Ag/Ab were negative. He denied history of the consumption of any drugs/chemicals or foods allergies.
Table 1.
Patient’s lab data at the admission time
| Parameter | Value | Normal Valuea | Parameter | Value | Normal Valuea |
|---|---|---|---|---|---|
| WBC | 8.6 *1000/mm3 | 4.1–10.1 | Uric acid | 10.8 g/dl | 3.6–8.2 |
| Hgb | 10.4 g/dl | 12–16 | Coombs D/ID | +/+ | |
| MCV | 87.2 fl | 77–94 | Serum Cr | 0.8 mg/dl | 0.7–1.4 |
| RBC | 3.44 million/mm3 | 4.2–5.8 | Retic count | 9.2% | 0.5–1.5 |
| Plt | 274 *1000/mm3 | 150–400 | LDH | 1178 U/L | <480 |
| ALT | 32 U/L | <41 | Fe | 150 μg/dl | 65–175 |
| AST | 36 U/L | <37 | TIBC | 223 μg/dl | 250–450 |
| Bil T/D | 8.3/0.7 mg/dl | 0.1–1.2/<0.3 | G6PD | Sufficient | |
| ALP | 183 U/L | 70–306 | Haptoglobin | 1.38 g/L | 0.3–2 |
| ESR | 12 mm/h | <15 | CRP | 18 mg/L | <10 |
| Ferritin | 295 ng/ml | 25–350 |
aNormal range in our hospital’s Laboratory
To confirm lab data results: re-check of them was requested again 1 day after his admission and abnormal lab results were then confirmed.
Therapeutic interventions and treatments
Tadalafil and Bosentan were discontinued to confirm HA, a consultation with a hematologist was also requested. Additionally, hematologist ordered checking direct and indirect Coombs test, G6PD level, reticulocyte count, haptoglobin, serum creatinine, PBS, ANA, and Anti ds- DNA.
Based on the patient’s lab data and his normal PBS and positive Coombs test, the first diagnosis was DIIHA for the patient. So, treatment with tablet Prednisolone 60 mg (1 mg/kg) daily and Folic acid 5 mg daily for 1 month were started. Moreover, for more evaluation of the DIHA, pharmacotherapy consultation was performed.
Pharmacotherapy counseling stated that based on patient’s lab data, ruling out of other diagnoses of HA, any history of contact with suspicious substances, and any history of drugs or foods allergy and Naranjo [9] score (Table 2), this complication is probably related to one of these two drugs. To confirm the diagnosis, the consumption of both drugs should be discontinued without re-challenging;
Table 2.
Naranjoa scale score for Bosentan
| Question | Yes | No | Do Not Know | Score of our case |
|---|---|---|---|---|
| 1.Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | 0 |
| 2. Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 | 2 |
| 3. Did the adverse event improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | 1 |
| 4. Did the adverse event reappear when the drug was re-administered? | +2 | −1 | 0 | 0 |
| 5. Are there alternative causes that could on their own have caused the reaction? | −1 | +2 | 0 | 2 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 | 0 |
| 7. Was the drug detected in blood or other fluids in concentrations known to be toxic? | +1 | 0 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | +1 | 0 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | 0 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 | 1 |
| Total Score:6 | ||||
a Naranjo Scale score is a questionnaire designed by Naranjo et al., in order to assess if adverse drug reaction is actually resulted from the drug and not the other causes. Probability is determined by terms such as definite, probable, possible or doubtful. This scale consists of 10 questions that can be answered by yes, no or “I do not know”, and different point values ranged from −1 to 2 are assigned to each answer [9]
So, according to the diagnosis of DIIHA, by drugs’ discontinuation and starting treatment with Prednisolone, the patient has recovered and his lab data improved (Table 3).
Table 3.
Patient’s lab data after the improvement (by passing 20 days from his admission)
| Parameter | Value | Normal Valuea |
|---|---|---|
| WBC | 8.2 *1000/mm3 | 4.1–10.1 |
| Hgb | 12.1 g/dl | 12–16 |
| MCV | 91.9 fl | 77–94 |
| RBC | 4.3 million/mm3 | 4.2–5.8 |
| PLT | 240 *1000/mm3 | 150–400 |
| Ferritin | 360 ng/ml | 25–350 |
| LDH | 476 U/L | <480 |
| Retic count | 4.2% | 0.5–1.5 |
| ALT | 21 U/L | <41 |
| AST | 25 U/L | <37 |
| ALP | 149 U/L | 70–306 |
| Bil T/D | 1.7/0.7 mg/dl | 0.1–1.2/<0.3 |
aNormal range in our hospital’s Laboratory
After his stabilization, he was transferred to cardiac surgery ward and a heart pace maker was inserted because of his CHB.
Other patient’s drugs during his hospitalization after pace maker insertion were as follows: Cefazoline ampule 1 g three times daily, Pantoprazole ampule 40 mg once daily, Allopurinol tablet 100 mg once daily, Folic acid tablet 5 mg once daily, Heparin ampule 5000 IU SC twice daily, Vitamin B12 ampule 1000 mg daily, Tadalafil tablet 20 mg daily, Prednisolone tablet 60 mg daily, Diclofenac suppository 100 mg, Acetaminophen ampule 1 g, and Morphine sulphate ampule 2 mg as needed SC.
Furthermore, the other patient’s medications at discharge were as follows: Allopurinol tablet 100 mg daily, Pantoprazole tablet 40 mg daily, Prednisolone tablet 60 mg daily for 1 month, and Sildenafil tablet 25 mg twice daily (Due to the lack of Tadalafil tablets in the hospital, it was changed to Sildenafil).
The patient was followed-up after discharge for 3 months and he reported no problems.
Discussion
A 17-year-old man who had congenital single ventricle heart, CHB, and PAH was admitted to the hospital with chief complaint of jaundice and icter started about 3 weeks before his admission. Based on his clinical and lab data, HA was diagnosed for him.
In this patient, there were several reasons for HA such as G6PD deficiency, hematologic malignancies, hepatic and autoimmune disorders, congenital HA, DIC, DIHA, and transfusion-related hemolysis. According to the diagnostic work ups (clinical and laboratory data and past drug history), as mentioned earlier in the case presentation section, DIHA was diagnosed for this patient.
Considering that the patient had no history of contact with suspicious substances, which can lead to HA and any history of drugs or foods allergy, as well as his newly started drugs as Bosentan and Tadalafil and his positive Coombs’ test, so the possibility of DIIHA has increased for the patient. Since DIIHA is an IgG mediated reaction, IgG can be used to assess this allergic reaction. However, this test can only be performed in specialized laboratories, and the turnaround time is approximately 1 week. Thus, the detection of IgG does not alter immediate management appreciably, as long as the responsible drug has been stopped. However, the detection of IgG can be used to avoid future drug-induced hemolytic anemia [10]. In this case, antibody detection test was also done, but no results were recorded in the patient’s document until the patient’s discharge.
Based on our research, Tadalafil or Bosentan induced HA were not reported yet (by searching in PubMed and google scholar). However, there were some reports on Bosentan-induced anemia. Nevertheless, this type of anemia is not caused by hemolysis or bone marrow toxicity [11–15]. Of course, rare reports of angioedema and anaphylactic shock that can be related to immunologic reaction, thrombocytopenia, neutropenia, and leukopenia have been reported with Bosentan [16]. Based on some clinical trials, Bosentan induced anemia chronically occurs, which is generally mild and stable over the course of treatment and does not require the drug’s discontinuation [17].
Due to the fact that DIIHA can occur even in the case of chronic use of the drugs [2], differentiating whether this complication is caused by Bosentan or Tadalafil (considering the fact that the duration of Tadalafil usage was 3 months and Bosentan was 1 month) is not possible with 100% accuracy. So, the consumption of both drugs were temporarily discontinued in the patient. However, by the recent administration of Bosentan in this patient, the possibility of this complication was higher with Bosentan compared to Tadalafil. In the course of hospitalization, although the patient accidentally continued to take his Tadalafil, the patient’s recovery (bilirubin correction, retic count, and LDH level) continued.
Scale scores of Naranjo adverse drug reaction probability as 9 or 10 indicate that the adverse drug reaction was “definite”, scores of 5–8 indicate “probable”, scores of 1–4 indicate “possible”, and scores of less than 1 indicate “doubtful [9].
As a result, according to Naranjo score = 6 for Bosentan that is higher than the Naranjo score = 3 for Tadalafil, Bosentan was considered as the main possible culprit to induce DIIHA in this patient.
As stated in Naranjo, one way to confirm a drug’s adverse reaction is to re-challenge the drug, but re-challenging is not recommended for all drugs because of some adverse drug reactions that can be life threatening (including angioedema, abnormal liver tests or low blood cells). Therefore, re-challenge was not performed [18]. Moreover, the patient was also advised to never take this drug for any reason in the future.
Conclusion
In this research, we reported a case of Bosentan induced HA who was a 17-year-old man suffering from PAH came to hospital with chief complaint of icter.
Given that the patient’s Coombs’ test was positive, so his HA mechanism was drug-induced immune-mediated HA.
Following the discontinuation of Bosentan and receiving Prednisolone, the patient’s clinical symptoms and laboratory parameters resolved and the patient was then discharged. So, the main defendant of this condition based on Naranjo score = 6, was recognized as Bosentan.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BIL
Bilirubin
- BP
Blood pressure
- CHB
Congenital heart block
- Cr
Creatinine
- CRP
C-Reactive Protein
- DIC
Disseminated intravascular coagulation
- D/ID
Direct and indirect
- DIHA
Drug induced hemolytic anemia
- DIIHA
Drug induced immune hemolytic anemia
- EF
Ejection fraction
- ESR
Erythrocyte sedimentation rate
- G6PD
Glucose-6-phosphate dehydrogenase
- HA
Hemolytic anemia
- Hgb
Hemoglobin
- HR
Heart rate
- IVIG
Intravenous immunoglobulin
- LDH
Lactate dehydrogenase
- Mg
Milligram
- MCV
Mean cell volume
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PAH
Pulmonary artery hypertension
- PAP
Pulmonary artery pressure
- PBS
Peripheral blood smear
- RBC
Red blood cell
- RR
Respiratory rate
- SC
Sub-cutaneous
- TIBC
Total iron binding capacity
- WBC
White blood cell count
Authors’ contributions
The author(s) read and approved the final manuscript.
Funding
The author(s) received no financial support for the research, author-ship, and/or the publication of this article.
Compliance with ethical standards
Conflict of interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Afra, Email: fatemehafraus@gmail.com.
Marjan Mehri, Email: m_mehri57@yahoo.com.
Soha Namazi, Email: namazisoha@yahoo.com.
References
- 1.Kumar S, Bansal R, Bansal P, et al. Ceftriaxone-induced hemolytic Anemia: a rare case report. Perm J. 2020;24:19.088. doi: 10.7812/TPP/19.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2009;1:73–79. doi: 10.1182/asheducation-2009.1.73. [DOI] [PubMed] [Google Scholar]
- 3.Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol. 2019;102:157–162. doi: 10.1111/ejh.13187. [DOI] [PubMed] [Google Scholar]
- 4.Garbe E, Andersohn F, Bronder E, Klimpel A, Thomae M, Schrezenmeier H, Hildebrandt M, Späth-Schwalbe E, Grüneisen A, Mayer B, Salama A, Kurtal H. Drug induced immune haemolytic anaemia in the Berlin case-control surveillance study. Br J Haematol. 2011;154:644–653. doi: 10.1111/j.1365-2141.2011.08784.x. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky RA. Warm autoimmune hemolytic Anemia. N Engl J Med. 2019;381:647–654. doi: 10.1056/NEJMcp1900554. [DOI] [PubMed] [Google Scholar]
- 6.Stahl WM. Acute phase protein response to tissue injury. Crit Care Med. 1987;15:545–550. doi: 10.1097/00003246-198706000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Snapper I, Marks D, Schwartz L, et al. Hemolytic anemia secondary to Mesantoin. Ann Intern Med. 1953;39:619–623. doi: 10.7326/0003-4819-39-3-619. [DOI] [PubMed] [Google Scholar]
- 8.Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve-Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019;155(3):565–586. doi: 10.1016/j.chest.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Elissa M, et al. Diagnosing and managing drug allergy. CMAJ. 2018;190:532–538. doi: 10.1503/cmaj.171315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valerio C. Bosentan in the treatment of pulmonary arterial hypertension with the focus on the mildly symptomatic patient. Vasc Health Risk Manag. 2009;5:607–619. doi: 10.2147/VHRM.S4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, Naeije R, Sood N, Swerdlow PS, Hildesheim M, Gladwin MT, on behalf of the ASSET study group Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149:426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007;30:338–344. doi: 10.1183/09031936.00138706. [DOI] [PubMed] [Google Scholar]
- 15.Gabbay E, Fraser J, McNeil K. Review of bosentan in the management of pulmonary arterial hypertension. Vasc Health Risk Manag. 2007;3:887–900. [PMC free article] [PubMed] [Google Scholar]
- 16.Mylan Pharmaceuticals ULC . Product Mmonograph PrMYLAN-BOSENTAN. 2016. [Google Scholar]
- 17.Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143–150. doi: 10.1016/j.blre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Meyboom R. Intentional Rechallenge and the clinical Management of Drug-Related Problems. Drug Saf. 2013;36:163–165. doi: 10.1007/s40264-013-0023-0. [DOI] [PubMed] [Google Scholar]