Abstract
Background
Beginning in 1994, Vancouver experienced an explosive outbreak of HIV infection among injection drug users (IDUs). The objectives of this study were to measure the prevalence and incidence of hepatitis C virus (HCV) infection in this context and to examine factors associated with HCV seroconversion among IDUs.
Methods
IDUs recruited through a study site and street outreach completed interviewer-administered questionnaires covering subjects' characteristics, behaviour, health status and service utilization and underwent serologic testing for HIV and HCV at baseline and semiannually thereafter. A Cox proportional hazards model was used to identify independent correlates of HCV seroconversion.
Results
As of Nov. 30, 1999, 1345 subjects had been recruited into the study cohort. The prevalence of anti-HCV antibodies was 81.6% (95% confidence interval [CI] 79.6% to 83.6%) at enrolment. Sixty-two HCV seroconversions occurred among 155 IDUs who were initially HCV negative and who returned for follow-up, for an overall incidence density rate of 29.1 per 100 person-years (95% CI 22.3 to 37.3). The HCV incidence remained above 16 per 100 person-years over 3 years of observation (December 1996 to November 1999), whereas HIV incidence declined from more than 19 to less than 5 per 100 person-years. Independent correlates of HCV seroconversion included female sex, cocaine use, injecting at least daily and frequent attendance at a needle exchange program.
Interpretation
Because of high transmissibility of HCV among those injecting frequently and using cocaine, the harm reduction initiatives deployed in Vancouver during the study period proved insufficient to eliminate hepatitis C transmission in this population.
Beginning in 1994, Vancouver experienced an explosive outbreak of HIV infection among injection drug users (IDUs).1,2 HIV incidence was estimated in 1997 to be 18.6 per 100 person-years.2 HIV seroconversion was associated with lower levels of education, unstable housing, commercial sex work, borrowing syringes, a longer history of injection drug use and frequent attendance at a needle exchange program.2,3
Of almost as much concern as these high rates of HIV transmission was the high rate of infection with hepatitis C virus (HCV) among IDUs.2 Elsewhere, the prevalence of HCV infection among IDUs has been reported at 30% to 98%,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18 whereas incidence has ranged from 4.2 to 22.0 per 100 person-years.4,6,12,16,19,20,21,22 Among populations receiving treatment for drug abuse, prevalence and incidence of HCV infection are lower.16 At a population level, the use of injection drugs is the single most important risk factor for acquiring HCV infection in developed countries.23
The objectives of this study were to measure the prevalence and incidence of HCV infection among a cohort of IDUs in Vancouver and to identify predictors of seroconversion. The study of HCV in parallel with HIV allowed comparison of incidence trends and of sociodemographic and behavioural factors associated with each virus during an outbreak of HIV.
Methods
The Vancouver Injection Drug User Study (VIDUS) is an open cohort study that began recruitment in May 1996.2 IDUs residing in the greater Vancouver area who had injected at least once in the previous month were enrolled. Baseline and semiannual visits included serological screening for anti-HCV and anti-HIV antibodies and a questionnaire administered by a trained interviewer blinded to HIV and HCV serostatus. The questionnaire covered information on subjects' characteristics, injection and non-injection drug use, borrowing and lending of syringes and other paraphernalia, incarceration, housing, health care utilization, sexual behaviour, and drug and alcohol treatment.
At each visit, counselling was provided before and after the serological testing, and medical and treatment referrals were made as requested. These referrals included sending subjects to clinics that provide immunization against hepatitis A and B.
HCV antibody was assayed by enzyme-linked immunosorbent assay (ELISA) at the University of British Columbia Virology Laboratory according to a modified testing algorithm.24 Briefly, a third-generation synthetic peptide-based ELISA (HCV enzyme immunoassay (EIA) 4.0, United Biomedical Inc., Haupagge, NY) was used to screen all sera. Samples that tested negative underwent no further testing. Those testing positive were retested with a third-generation ELISA containing recombinant antigens (HCV 3.0, Ortho Diagnostic Systems, Rochester, NY). Samples that tested positive in both assays were classified as positive and underwent no further testing. Samples that gave discordant results were tested by immunoblot assay (RIBA III, Ortho Diagnostic Systems). No indeterminate results were encountered in the immunoblot assay. Therefore, all sera were classified definitively with this algorithm.
Participants who were seropositive for HCV at baseline were compared with those who were seronegative at baseline by means of univariate techniques, including contingency table analysis for categorical variables and Wilcoxon's rank-sum test for continuous variables.
HCV incidence was calculated by the incidence density approach and is expressed here in terms of person-years of observation. All 95% confidence intervals (CIs) were based on the Poisson distribution.
To identify factors associated with HCV seroconversion, persistently seronegative subjects were frequency matched for the duration of follow-up with subjects who had seroincident HCV infection. These 2 groups were compared by means of univariate techniques, as described above. Factors independently correlated with seroconversion to anti-HCV antibody positive status were assessed by Cox proportional hazards modelling. Variables measuring exposures or behaviour in the 6 months before a newly positive test result for HCV (and that were significant at the 5% level in univariate analyses) were entered into the multivariate models. All possible 2-way interactions were evaluated in the final model.
Results
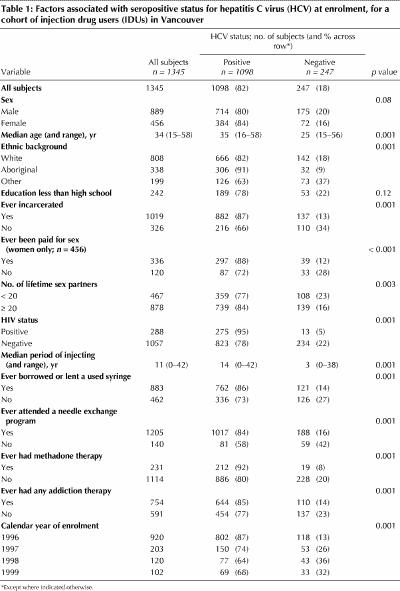
As of Nov. 30, 1999, 1345 subjects had been recruited into the study cohort. The prevalence of anti-HCV antibodies at enrolment was 81.6% (95% CI 79.6% to 83.6%). HCV-seropositive IDUs were older than HCV-seronegative participants (median age 35 v. 25 years). IDUs who had been incarcerated, who reported 20 or more sexual partners in their lifetime or who were HIV positive were significantly more likely to be HCV seropositive (Table 1). HCV-seropositive subjects reported injecting for a median of 14 years, whereas seronegative subjects had been injecting for a median of only 3 years. Female IDUs who had been paid for sex had significantly higher rates of HCV infection at enrolment than those who had not been paid for sex. Similarly, IDUs who had borrowed or lent used syringes, who had attended needle exchange programs, who had been enrolled in a methadone maintenance program or who had accessed some other form of addiction therapy had significantly higher rates of HCV infection at enrolment.
Table 1
Sixty-two HCV seroconversions were documented among the 155 subjects who were initially HCV seronegative and who returned for follow-up visits. The mean interval to follow-up was 16.1 months, and the overall incidence density rate was 29.1 per 100 person-years (95% CI 22.3 to 37.3). The incidence of HIV infection was high at the beginning of the study (19.4 per 100 person-years) but declined to between 2 and 5 per 100 person-years after the first 2 semesters of the study (Fig. 1). The incidence of HCV was also highest during the earlier follow-up periods, yet it remained above 16 per 100 person-years for the duration of the study (Fig. 1).
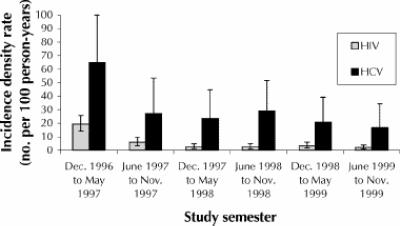
Fig. 1: Incidence density rate (and 95% confidence interval) for hepatitis C virus and HIV over 3 years of the Vancouver Injection Drug User Study.
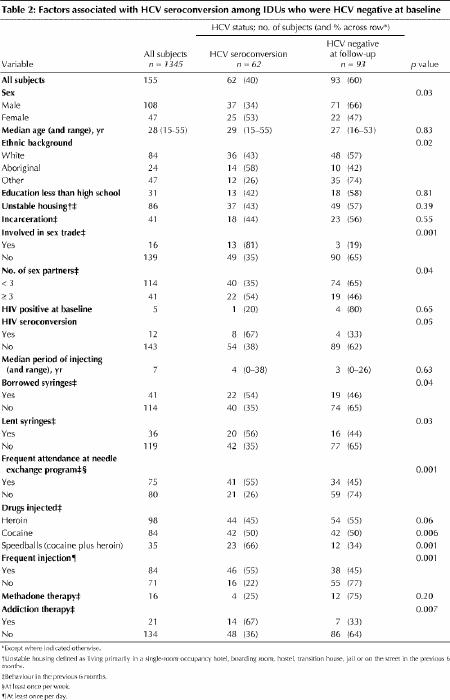
On univariate analysis, HCV seroconversion occurred more frequently among females than among males (53% v. 34%, p = 0.03, Table 2) and among Aboriginals and white people than among those with other ethnic backgrounds (58%, 43% and 26% respectively, p = 0.02). Subjects who participated in the sex trade, borrowed or lent syringes, attended needle exchange programs frequently, injected cocaine or “speedballs” (a combination of cocaine and heroin), or accessed some form of addiction therapy (all within the previous 6 months) were significantly more likely to experience HCV seroconversion, as were those who injected frequently (at least once daily). HIV seroconversion was marginally higher among those who experienced HCV seroconversion.
Table 2
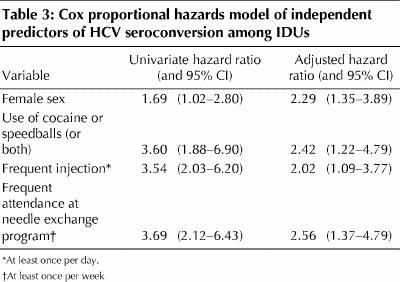
Independent predictors of HCV seroconversion, according to the Cox proportional hazards model, were female sex, injection of cocaine alone or as a component of speedballs, frequent injection and frequent attendance at a needle exchange program (Table 3). Interaction terms did not remain significant when entered in models containing these variables, and sex-specific modelling did not significantly affect the variables associated with HCV seroconversion.
Table 3
Interpretation
We documented a sustained high incidence of HCV among IDUs and found that seroincident infection was independently associated with female sex, cocaine use, frequent injection and frequent attendance at a needle exchange program.
The relation between HIV and HCV seropositivity among IDUs is not always consistent, but it has been hypothesized that positive associations are likely where the prevalence of both pathogens is high.25 This dual high-prevalence condition is satisfied in the VIDUS cohort, in which there was a clear association between prevalences of HIV and HCV infection at baseline. However, the study documents interesting comparative trends in the rates of HIV and HCV seroincidence among IDUs. Although HIV incidence was high at study inception, it subsequently declined, as had been predicted through mathematical modelling.26 In contrast, despite a prevalence of 81.6% at baseline, HCV incidence remained above 16 per 100 person-years over the 3-year period of observation. High rates of incidence for HCV are well documented among young and new IDUs,15,27 such that 80% become infected within 4 years of initiating injection drug use.28
High rates of HCV transmission are explained by a combination of high risk of infection for each syringe- sharing contact and high infectivity throughout much of the natural history of an infection (and consequent high prevalence among syringe lenders), as well as the frequency of syringe-sharing contacts in a given community.29 The infection rate following needlestick exposure to HCV appears to be at least an order of magnitude greater than that following similar exposure to HIV.30
Factors associated with HCV seroconversion in this study resemble those previously shown to predict HIV infection in the same cohort2 and include frequent injection and use of cocaine either alone or combined with heroin as a speedball. Higher frequency of injection and more chaotic drug use have been associated with cocaine use in a number of cohort studies. Although borrowing used syringes is the most likely operative mechanism of infection and a widely reported predictor of HCV infection among IDUs,13 socially desirable responses may explain why it was not a more significant direct measure of risk in this study. Measures of high frequency of injection may be a better, albeit surrogate, measure of this core risk behaviour. Conversely, other researchers have reported that practices such as sharing other injection paraphernalia (e.g., cookers, cotton or water) and “front-end loading” (syringe-mediated drug sharing) may contribute to transmission above and beyond that associated with simple syringe sharing.14,20,31 This finding suggests that prevention messages aimed at IDUs must go beyond simply advising against sharing needles and should discourage sharing of any injection equipment.
The association of HCV seroincidence with female sex suggests a higher vulnerability to parenteral exposure, an important role for sexual exposure or both. Romanowski and associates13 also reported associations between high prevalence of HCV infection and the sex trade, but these associations did not remain significant on multivariate analysis. Sexual transmission of HCV has been documented but appears relatively inefficient.32,33,34 In studies of HCV transmission, associations with numbers of partners, condom use and commercial sex have been less consistently demonstrated in populations of IDUs than in other affected populations.8,35
We and others have previously reported an association between HIV infection and frequent attendance at needle exchange programs.2,21,36 We now report a similar association for HCV infection, an association that may result from unmeasured confounders — factors promoting transmission that are common among those frequently attending needle exchange programs. However, such findings have also been interpreted as suggesting that needle exchange programs may actually promote HIV transmission.37,38 It should be noted that a close analysis of the association between HIV and frequency of attendance at needle exchange programs for the Vancouver IDU cohort did not support a hypothesis of causality.3
High and increasing rates of HCV infection have been documented in Canadian prison populations.39 Our study illustrates that IDUs who have been incarcerated are especially likely to be infected with HCV.
This study had several limitations. Because of high HCV prevalence at enrolment and limited follow-up to date, the statistical power to determine incidence and associated risk factors was low. Participants who were initially HCV seronegative returned for follow-up less frequently than did study subjects in general. It is plausible that some HCV-negative IDUs were not highly street entrenched (i.e., were not involved in a chronic and intractable way with the street scene) and were therefore less likely to return for a follow-up visit. Indeed, some of the participants who were negative for HCV at baseline were deported from Canada during the follow-up period. Also, the study instrument did not address the practices of tattooing and body piercing, which have been associated elsewhere with HCV infection.35,40,41,42 Observations in this study were made over the course of a documented outbreak of HIV. This and the unique characteristics of the Vancouver injection drug use scene may limit the generalizability of the results.
HCV infection represents a major cause of morbidity and stands as a sensitive marker for activities that may transmit HIV parenterally. Because this and other studies indicate extreme pressure toward HCV transmission among seronegative IDUs, resulting from both high prevalence and high infectivity, it appears that the prevention efforts must be expanded yet further.
Mathematical models suggest that even in populations with a high prevalence of HCV infection, there is hope that a drop in syringe sharing will reduce the incidence of such infection.43 However, such reductions have been hard to realize. Because of high transmissibility of HCV among those injecting frequently and using cocaine, we conclude that the harm reduction initiatives deployed in Vancouver during the study period proved insufficient to eliminate hepatitis C transmission in this population. In addition to promoting behavioural change and harm reduction among established users, researchers and prevention workers urgently need to focus on primary prevention of injection drug use, early intervention with and treatment of non-injection and injection drug users, and altering the social determinants that predispose people to engage in these activities.
Footnotes
This article has been peer reviewed.
Acknowledgements: We acknowledge the staff of the Vancouver Injection Drug User Study (VIDUS), the study's community advisory board, and the study's participants for their involvement in VIDUS. We are grateful for the assistance of the University of British Columbia Diagnostic Virology and Reference Laboratory and the Virology Section of the Provincial Laboratory, British Columbia Centre for Disease Control, in testing the samples, as well as to Dorothy Rachar and Rashpal Toor for administrative assistance in preparing the manuscript.
Competing interests: None declared.
Correspondence to: Dr. David M. Patrick, University of British Columbia Centre for Disease Control, 655 W 12th Ave., Room 2104, Vancouver BC V5Z 4R4; fax 604 660-0197; david.patrick@bccdc.hnet.bc.ca
References
- 1.Patrick DM, Strathdee SA, Archibald CP, Ofner M, Craib KJ, Cornelisse PG, et al. Determinants of HIV seroconversion in injection drug users during a period of rising prevalence in Vancouver. Int J STD AIDS 1997;8(7):437-45. [DOI] [PubMed]
- 2.Strathdee SA, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS 1997;11(8):F59-65. [DOI] [PubMed]
- 3.Schechter MT, Strathdee SA, Cornelisse PG, Currie S, Patrick DM, Rekart ML, et al. Do needle exchange programmes increase the spread of HIV among injection drug users? An investigation of the Vancouver outbreak. AIDS 1999;13(6):F45-51. [DOI] [PubMed]
- 4.Van Ameijden EJ, Van den Hoek JA, Mientjes GH, Coutinho RA. A longitudinal study on the incidence and transmission patterns of HIV, HBV and HCV infection among drug users in Amsterdam. Eur J Epidemiol 1993; 9 (3): 255-62. [DOI] [PubMed]
- 5.McCruden EA, Hillan KJ, McKay IC, Cassidy MT, Clark JC. Hepatitis virus infection and liver disease in injecting drug users who died suddenly. J Clin Pathol 1996;49(7):552-5. [DOI] [PMC free article] [PubMed]
- 6.Galeazzi B, Tufano A, Barbierato E, Bortolotti F. Hepatitis C virus infection in Italian intravenous drug users: epidemiological and clinical aspects. Liver 1995;15(4):209-12. [DOI] [PubMed]
- 7.Krook A, Albert J, Andersson S, Biberfeld G, Blomberg J, Eklund I, et al. Prevalence and risk factors for HTLV-II infection in 913 injecting drug users in Stockholm, 1994. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 15 (5): 381-6. [DOI] [PubMed]
- 8.Coppola RC, Masia G, di Martino ML, Carboni G, Muggianu E, Piro R, et al. Sexual behaviour and multiple infections in drug abusers. Eur J Epidemiol 1996; 2(5):429-35. [DOI] [PubMed]
- 9.Alter MJ, Hadler SC, Judson FN, Mares A, Alexander WJ, Hu PY, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 990;264(17):2231-5. [PubMed]
- 10.McHutchison JG, Kuo G, Houghton M, Choo QL, Redeker AG. Hepatitis C virus antibodies in acute icteric and chronic non-A, non-B hepatitis. Gastroenterology 1991;101(4):1117-9. [DOI] [PubMed]
- 11.Donahue JG, Nelson KE, Munoz A, Vlahov D, Rennie LL, Taylor EL, et al. Antibody to hepatitis C virus among cardiac surgery patients, homosexual men, and intravenous drug users in Baltimore, Maryland. Am J Epidemiol 1991;134(10):1206-11. [DOI] [PubMed]
- 12.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1998;18(Suppl 1):S11-9. [DOI] [PubMed]
- 13.Romanowski B, Campbell PJ, Preiksaitis JK, Fonseca K. Human immunodeficiency virus seroprevalence and risk behaviors in patients attending sexually transmitted disease clinics in Alberta. Sex Transm Dis 1997;24(8):487-94. [DOI] [PubMed]
- 14.Denis B, Dedobbeleer M, Collet T, Petit J, Jamoulle M, Hayani A, et al. High prevalence of hepatitis C virus infection in Belgian intravenous drug users and potential role of the “cotton-filter” in transmission: the GEMT Study. Acta Gastroenterol Belg 2000;63(2):147-53. [PubMed]
- 15.Chang CJ, Lin CH, Lee CT, Chang SJ, Ko YC, Liu HW. Hepatitis C virus infection among short-term intravenous drug users in southern Taiwan. Eur J Epidemiol 1999;15(7):597-601. [DOI] [PubMed]
- 16.Broers B, Junet C, Bourquin M, Deglon JJ, Perrin L, Hirschel B. Prevalence and incidence rate of HIV, hepatitis B and C among drug users on methadone maintenance treatment in Geneva between 1988 and 1995. AIDS 1998;12(15):2059-66. [DOI] [PubMed]
- 17.Santana Rodriguez OE, Male Gil ML, HernandezSantana JF, Liminana Canal JM, Martin Sanchez AM. Prevalence of serologic markers of HBV, HDV, HCV and HIV in non-injection drug users compared to injection drug users in Gran Canaria, Spain. Eur J Epidemiol 1998;14(6):555-61. [DOI] [PubMed]
- 18.Kemp R, Miller J, Lungley S, Baker M. Injecting behaviours and prevalence of hepatitis B, C and D markers in New Zealand injecting drug user populations. N Z Med J 1998;111(1060):50-3. [PubMed]
- 19.Crofts N, Nigro L, Oman K, Stevenson E, Sherman J. Methadone maintenance and hepatitis C virus infection among injecting drug users. Addiction 1997;92(8):999-1005. [PubMed]
- 20.Thorpe L, Ouellet L, Hershow R, Bailey S, Williams I, Monerosso E. The multiperson use of non-syringe injection equipment and risk of hepatitis C infection in a cohort of young adult injection drug users, Chicago 1997–1999. Ann Epidemiol 2000;10(7):472-3. [DOI] [PubMed]
- 21.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol 1999;149(3):203-13. [DOI] [PubMed]
- 22.Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol 1997;35(12):3274-7. [DOI] [PMC free article] [PubMed]
- 23.Alter MJ, Moyer LA. The importance of preventing hepatitis C virus infection among injection drug users in the United States. J Acquir Immune Defic Syndr Hum Retrovirol 1998;18(Suppl 1):S6-10. [DOI] [PubMed]
- 24.Cook D, Sherlock C, Mak A, Littlewood R, Karakas L, Middleton P. Comparison of 5 commercial EIA kits and 2 immunoblot kits for detection of hepatitis C antibody [oral presentation]. Conjoint meeting on infectious diseases; 1994 Nov 21; Montreal.
- 25.Hagan H. Hepatitis C virus transmission dynamics in injection drug users. Subst Use Misuse 1998;33(5):1197-212. [DOI] [PubMed]
- 26.Raboud J, Thorne A, Strathdee SA, O'Shaughnessy MV, Schechter MT. Explosive HIV epidemics in injection drug users — What are the causes and controls? [abstract]. In: Proceedings of the Fifth Conference on Retroviruses and Opportunistic Infections; 1998 Feb 1; Chicago. Abstr. no. 133. p. 104.
- 27.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T- lymphotropic viruses. Am J Public Health 1996;86(5):655-61. [DOI] [PMC free article] [PubMed]
- 28.Thomas DL, Vlahov D, Solomon L, Cohn S, Taylor EL, Garfein RS, et al. Correlates of hepatitis C virus infection drug users. Medicine 1995;74(4):212-20. [DOI] [PubMed]
- 29.Crofts N, Aitken CK, Kaldor JM. The force of numbers: why hepatitis C is spreading among Australian injecting drug users while HIV is not. Med J Aust 1999;170(5):220-1. [PubMed]
- 30.Heintges T, Wands JR. Hepatitis C virus: epidemiology and transmission. Hepatology 1997;26(3):521-6. [DOI] [PubMed]
- 31.Stark K, Muller R, Bienzle U, Guggenmoos-Holzmann I. Frontloading: a risk factor for HIV and hepatitis C virus infection among injecting drug users in Berlin. AIDS 1996;10(3):311-7. [PubMed]
- 32.Gordon SC, Patel AH, Kulesza GW, Barnes RE, Silverman AL. Lack of evidence for the heterosexual transmission of hepatitis C. Am J Gastroenterol 1992;87(12):1849-51. [PubMed]
- 33.Soto B, Rodrigo L, Garcia-Bengoechea M, Sanchez-Quijano A, Riestra S, Arenas JI, et al. Heterosexual transmission of hepatitis C virus and the possible role of coexistent human immunodeficiency virus infection in the index case. A multicentre study of 423 pairings. J Intern Med 1994;236(5):515-9. [DOI] [PubMed]
- 34.Meisel H, Reip A, Faltus B, Lu M, Porst H, Wiese M, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet 1995;345(8959):1209-11. [DOI] [PubMed]
- 35.Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996;334 (26): 1691-6. [DOI] [PubMed]
- 36.Bruneau J, Lamothe F, Franco E, Lachance N, Desy M, Soto J, et al. High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am J Epidemiol 1997;146 (12):994-1002. [DOI] [PubMed]
- 37.Department of Labour, Health and Human Services, and Education, and related agencies. Appropriations Act, 1998. PH7218. Washington: House of Representatives; 1997 Sep 1.
- 38.The Needle Exchange Programs Prohibition Act of 1998 — statements on introduced bills and joint resolutions. Task force report on a site visit to Vancouver (Senate, 1998 Apr 21). Washington: Office of the National Drug Control Policy, Executive Office of the President. p. S3356.
- 39.Ford PM, Pearson M, Sankar-Mistry P, Stevenson T, Bell D, Austin J. HIV, hepatitis C and risk behaviour in a Canadian medium-security federal penitentiary. Queen's University HIV Prison Study Group. Queen's J Med 2000; 93 (2):113-9. [DOI] [PubMed]
- 40.Shev S, Widell A, Foberg U, Fryden A, Hermodsson S, Lindh G, et al. HCV genotypes in Swedish blood donors as correlated to epidemiology, liver disease and hepatitis C virus antibody profile. Infection 1995;23(5):253-7. [DOI] [PubMed]
- 41.Sherman KE, Lewey SM, Goodman ZD. Talc in the liver of patients with chronic hepatitis C infection. Am J Gastroenterol 1995;90(12):2164-6. [PubMed]
- 42.Simonian PT, Gilbert M, Trumble TE. Incidence of hepatitis C in patients requiring orthopaedic surgery. J Bone Joint Surg Br 1995;77(6):971-4. [PubMed]
- 43.Mather D, Crofts N. A computer model of the spread of hepatitis C virus among injecting drug users. Eur J Epidemiol 1999;15(1):5-10. [DOI] [PubMed]


