Abstract
Ketamine is widely used in infants and children for anesthesia; both anesthetic and sub-anesthetic doses of ketamine have been reported to preferentially inhibit the GABAergic neurons. Medium spiny neurons (MSNs), the GABAergic projection neurons in the striatum, are vulnerable to anesthetic exposure in the newborn brain. Growth of dendrites requires a deacetylase to remove acetyl from tubulin in the growth cone to destabilize the tubulin. Histone deacetylase 6 (HDAC6) affects microtubule dynamics, which are involved in neurite elongation. In this study we used a human induced pluripotent stem cells (iPSCs)-derived striatal GABA neuron system to investigate the effects of ketamine on HDAC6 and the morphological development of MSNs. We showed that exposure to ketamine (1–500 μM) decreased dendritic growth, dendrite branches, and dendritic spine density in MSNs in a time- and concentration-dependent manner. We revealed that ketamine treatment concentration-dependently inhibited the expression of HDAC6 or aberrantly translocated HDAC6 into the nucleus. Ketamine inhibition on HDAC6 resulted in α-tubulin hyperacetylation, consequently increasing the stability of microtubules and delaying the dendritic growth of MSNs. Finally, we showed that the effects of a single-dose exposure on MSNs were reversible and lasted for at least 10 days. This study reveals a novel role of HDAC6 as a regulator for ketamine-induced deficits in the morphological development of MSNs and provides an innovative method for prevention and treatment with respect to ketamine clinical applications.
Keywords: ketamine, HDAC6, induced pluripotent stem cells (iPSCs), GABAergic projection neurons, medium spiny neuron, dendrite, dendritic spine
Introduction
Ketamine, a noncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonist, is widely used as an anesthetic in children and adults [1]. Emerging evidence indicates that ketamine exerts antidepressant [2], analgesic [3], and sedative effects [4] at sub-anesthetic doses. Ketamine regulates dendrites and their synapses, which are plastic anatomical structures in neurons, to affect neuronal activity [5, 6]. However, the extent to which ketamine exposure affects dendrites and dendritic spines in human neurons, especially inhibitory GABAergic neurons, is unknown.
Medium spiny neurons (MSNs), which make up a set of GABAergic projection neurons, have multiple extremely long dendrites and many spines in the striatum [7]. Anomalies in MSNs, including dendritic and spine abnormalities, can cause neuropsychiatric diseases or neurodevelopmental disorders, such as intellectual disability, schizophrenia, epilepsy, mental retardation, and autism [8]. In the newborn brain, these neurons are prone to death or malfunction upon anesthetic exposure [9]; neuronal malfunction refers to abnormalities in dendrites and spines [10, 11]. Excitotoxin exposure causes the disassembly of microtubules and alters cellular morphology and motility, tubulin stability, and dendritic development in neurons [12]. Microtubules, the cytoskeletal components of dendrites, are dynamic polymers comprised of α and β tubulin that support the function and structure of dendritic arborization [13]. Acetylation of α-tubulin, which forms stable microtubules, is absent in the neuronal growth cone [14]. The growth of dendrites requires a deacetylase to remove acetyl from tubulin to destabilize tubulin. Histone deacetylase 6 (HDAC6), a cytoplasmic deacetylase, deacetylates acetylated α-tubulin in microtubules, destabilizing microtubule assembly [15]. Loss of or a reduction in HDAC6 leads to hyperacetylation of α-tubulin, which impedes the dynamics of microtubules [16].
NMDA receptors, which mediate excitotoxic effects, are enriched in GABAergic projection neurons [17]. Injection of quinolinic acid, an NMDA receptor agonist, into the striatum leads to selective loss of striatal GABAergic projection neurons without significantly affecting somatostatin-positive and cholinergic interneurons [18]. NMDA receptor blockade also reduces the proliferation of GABAergic projection neurons in vivo and in vitro [19]. Administration of ketamine to Huntington patients has shown that at the median dose, ketamine causes a specific decline in memory and verbal fluency and that at higher sub-anesthetic doses, ketamine induces a significant increase in psychiatric symptoms and impairment of eye movements [20]. Collectively, these previous findings imply that GABAergic neurons might be selectively vulnerable to NMDA receptor blockade.
Here, we aimed to test the role of HDAC6 in the effects of ketamine on dendritic and spine growth in MSNs using a human induced pluripotent stem cell (iPSC)-derived neuron system that mimics neuronal development of the human brain [21, 22]. Our data revealed that ketamine exposure inhibits the growth of dendrites and dendritic spines of cultured MSNs. Furthermore, ketamine reversibly suppresses the expression of HDAC6 and aberrantly translocates HDAC6 to the nucleus. The failure of HDAC6 to regulate the deacetylation of α-tubulin may contribute to ketamine-induced disruption of dendrite and spine growth in MSNs.
Materials and methods
Cell culture
iPSCs between passages 15–35 and the H9 cell line were cultured as previously described with modifications [23]. Briefly, the cells were maintained on Matrigel or Vitronectin-coated (Corning Inc., Corning, NY, USA) plates in E8 medium (Gibco, Carlsbad, CA, USA) in an incubator at a constant temperature and humidity (37 °C/5% CO2).
Differentiation of iPSCs into GABAergic neurons
Striatal GABAergic neuron induction was performed according to a previously described protocol with minimal modifications [24]. In brief, our neural differentiation protocol followed a chemical recipe for the generation of committed striatal lateral ganglionic eminence-like progenitors and MSNs. In the first stage, iPSCs were digested using Dispase (1.0 U/mL) (Invitrogen, Carlsbad, CA, USA) for 3–5 min and then transferred to ultra-low-attachment dishes. The medium consisted of a 1:1 ratio of E8 and neural induction medium. After 24 h, the medium was changed to neural induction medium containing the SMAD inhibitors LDN193189 (100 nM, Stemgent, MA, USA) and SB431542 (10 μM, Amateksci, NY, USA). Next, embryoid bodies (EBs) were transferred to attachment dishes (Corning Inc., Corning, NY) containing neural induction medium on day 7. Then, the middle rosette cells were gently removed and transferred to ultra-low-attachment dishes containing neural differentiation medium on day 14. Sonic hedgehog (SHH, 200 ng/mL, Research and Development) or its small molecular agonist purmorphamine (PUR) (0.65 μM, Calbiochem, San Diego, CA, USA) was added on days 10–25 to induce differentiation into ventral GABAergic progenitors. Neural progenitor clusters were dissociated with Accutase (1 U/mL, Invitrogen, Carlsbad, CA, USA) at 37 °C for 5 min and then placed on polyornithine/laminin-coated coverslips in neural differentiation medium in the presence of a set of trophic factors on day 26.
Ketamine administration
Cultured neurons grown on coated coverslips or plates were exposed to increasing doses (1, 10, 50, 100, and 500 μM) of ketamine for 1, 8, and 24 h to examine the effects of ketamine on the dendritic growth of the neurons. The vehicle group was treated with DMSO or saline mixed with culture medium. The medium was replaced at the end of the treatment period, and the neurons were transferred to an incubator and cultured for 48 h before subsequent morphological analyses.
Immunofluorescence staining
After ketamine treatment, the cultured cells were fixed in 4% ice-cold paraformaldehyde for 15 min, rinsed with PBS, permeabilized with 0.2% Triton X-100 for 20 min, and incubated in blocking buffer (10% donkey serum) for 60 min at room temperature. Then, the cells were incubated overnight with the following primary antibodies: mouse anti-TUJ1 (1:1000, T8660, Sigma, MO, USA), rabbit anti-GABA (1:10000, A2052, Sigma, MO, USA), mouse anti-GABA (1:1000, A0310, Sigma, MO, USA), rabbit anti-GAD65/67 (1:1000, LV1422966, Millipore, MA, USA), rabbit anti-DARPP-32 (1:1000, AB1656, Chemicon, Japan), and rabbit anti-HDAC6 (1:500, 7558 s, Cell Signaling Technology, MA, USA) at 4 °C. Fluorescent-conjugated secondary antibodies were added to detect the primary antibodies for 60 min at room temperature. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:2000, D9542, Sigma, MO, USA). Images were obtained using a Leica SP8 confocal microscope (Leica Microsystems, Japan). Cell counts and morphological analyses were performed using Fiji (ImageJ) software. Morphological analysis was performed to assess the dendritic length, number of dendritic branches, and dendritic spine density of 30, 40, and 30 cells, respectively, obtained from three coverslips per treatment group. The simple dendrite tracer was later analyzed using a plugin in Fiji (ImageJ) software. Morphologically, axons were observed as thinner, elongated processes with a smooth surface. Dendrites were shorter processes with a rough surface due to spines or other contacts. During counting, we excluded thinner projections with smooth surfaces. Data were obtained from five fields for each culture condition from three independent experiments.
TUNEL assay
Cell death following ketamine treatment was detected using the TUNEL assay. According to the manufacturer’s instructions, the Click-iTTM Plus TUNEL Assay (Invitrogen, Carlsbad, CA, USA) was used to detect apoptotic cells in culture. Notably, the assay was performed after immunofluorescence staining. Briefly, coverslips were incubated with 50 μL TdT reaction mixture for 60 min at 37 °C and washed twice with 3% BSA in PBS. Click-iT™ Plus TUNEL reaction cocktail was added for an additional 30 min at 37 °C, and each coverslip was washed with 3% BSA in PBS. Finally, the coverslips were used for immunofluorescence staining. The data are shown in the Supplementary materials.
Western blot analysis
The cultured cells were subjected to Western blot analysis after exposure to ketamine. The cells were lysed using ice-cold RIPA buffer and a mixture of protein phosphatase and proteinase inhibitors and centrifuged at 4 °C. Cytoplasmic and nuclear proteins were separated using the Tissue Protein Extraction Kit (Boster Biological Technology, CA, USA) according to the manufacturer’s instructions. Equal amounts of proteins were subjected to 8% or 12% SDS-PAGE, and the following steps were performed according to the protocol as described in our previous studies. The following primary antibodies were used at the indicated concentrations: acetyl-α-tubulin (1:1000, T6793, Sigma, MO, USA), α-tubulin (1:10,000, 3837T, Cell Signaling Technology, MA, USA), β-actin (1:5000, BM0627, Boster Biological Technology, CA, USA), HDAC6 (1:1000, 7558s, Cell Signaling Technology, MA, USA) and H3 (1:2000, 4499s, Cell Signaling Technology, MA, USA). The immunoreactive bands were analyzed using plugins in Fiji (ImageJ) software. Representative Western blotting bands from three independent experiments are shown.
Pharmacological manipulation
Cells were treated with tubastatin A, a highly selective HDAC6 inhibitor, or ACY-1215, an HDAC6 inhibitor, dissolved in DMSO. The cells were treated with 0.1 μM tubastatin A (ApexBio Technology, USA) or ACY-1215 (ApexBio Technology, USA). The health of cells treated with tubastatin A or ACY-1215 was not significantly different from that of the vehicle-treated control cells, as determined using the TUNEL assay.
Quantitative real-time PCR
Total RNA was extracted from iPSC-derived striatal GABAergic neurons using the RNeasy Micro Kit (Qiagen, Germany) according to the manufacturer’s instructions. The quantity and purity of the RNA were assessed using a Nanodrop spectrophotometer. The RNA was reverse-transcribed using a First-Strand cDNA synthesis kit (Thermo Scientific, Carlsbad, CA, USA), and quantitative (q)PCR was performed using Fast SYBR Green PCR Master Mix. The primers used in this study were as follows: HDAC6: 5′-CGGAGGGTCCTTATCGTAGA-3′ and 5′-GTAGCGGTGGATGGAGAAAT-3′; β-actin: 5′-AGCAGAAGTGCGAAGAGGAGGTC-3′ and 5′-GGAAAGAAAGTGCGTTGTGCGGTAG-3′. The ratio of the expression of the gene of interest and the expression of β-actin as a housekeeping gene was calculated using the ΔΔCT method.
Statistical analysis
All statistical analyses were performed and all graphical representations were produced using GraphPad Prism 7.0 software. The data are expressed as the mean ± SEM unless otherwise specified. Structural plasticity parameter data (dendrite length, dendrite branches, and dendritic spine) and immunofluorescence data were tested for normality, both as crude data. Differences between vehicle and ketamine exposure conditions were analyzed using one-way or two-way analysis of variance followed by Bonferroni’s post hoc test for multiple comparisons. Each measurement was assessed at least in triplicate. P < 0.05 was considered significant.
Results
Characterization of iPSC-derived MSNs
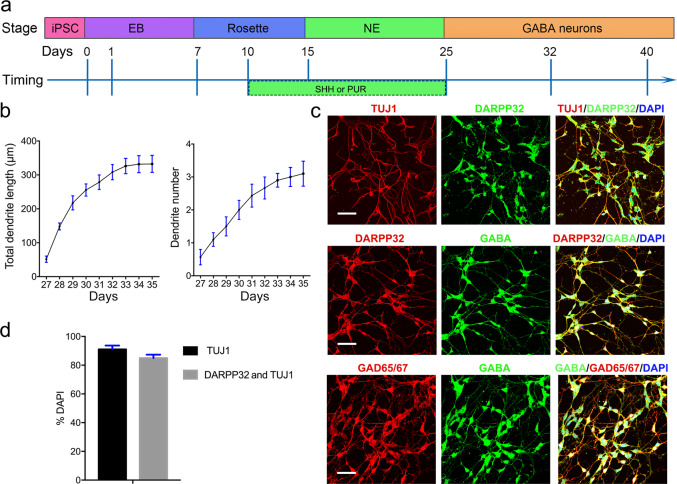
Using an iPSC line derived from the fibroblasts of a healthy male, we obtained enriched striatal GABA neurons by combining PUR with SHH. These neurons passed through the four developmental stages of brain development (Fig. 1a). We measured dendrite outgrowth and observed that the dendrites of the neurons rapidly expanded during development (Fig. 1b). Costaining for DARPP32 and TUJ1 (Fig. 1c) and for MSN and pan-neuronal markers revealed that TUJ1+ cells accounted for 90% of the total cell population, while DARPP32+ and TUJ1+ cells accounted for 85% of TUJ1+ cells (Fig. 1d). Mature MSNs secrete inhibitory neurotransmitters (GABA). To determine whether the DARPP32+ and TUJ1+ cells expressed GABA, we stained these cells with DARPP32, GAD65/67, and GABA antibodies and observed that DARPP32+ and GAD65/67+ neurons both expressed GABA (Fig. 1c). These findings illustrate that this system is a suitable tool for studying the effects of ketamine on GABAergic neurons.
Fig. 1. Differentiation and characterization of striatal GABA neurons.
a Schematic overview of differentiation strategies involving a four developmental stage protocol used to obtain striatal MSNs. b Quantitative assessment of dendritic development in terms of total dendritic length and dendrite number after cell plating (n = 10). c Immunofluorescence images of markers of various stages of differentiation. TUJ1, DARPP32, GABA, and GAD65/67 were used to stain striatal GABA neurons, and DAPI was used to stain the nucleus. Scale bar = 50 μm. d Quantification of the expression of markers of various differentiation stages. Efficiencies are presented as the percentage of positive cells ± SEM in all fields counted (n = 3). EB embryoid body, NE neurosphere, SHH sonic hedgehog, PUR purmorphamine, MSNs medium spiny neurons.
Ketamine exposure inhibits dendrite extension and branching of MSNs
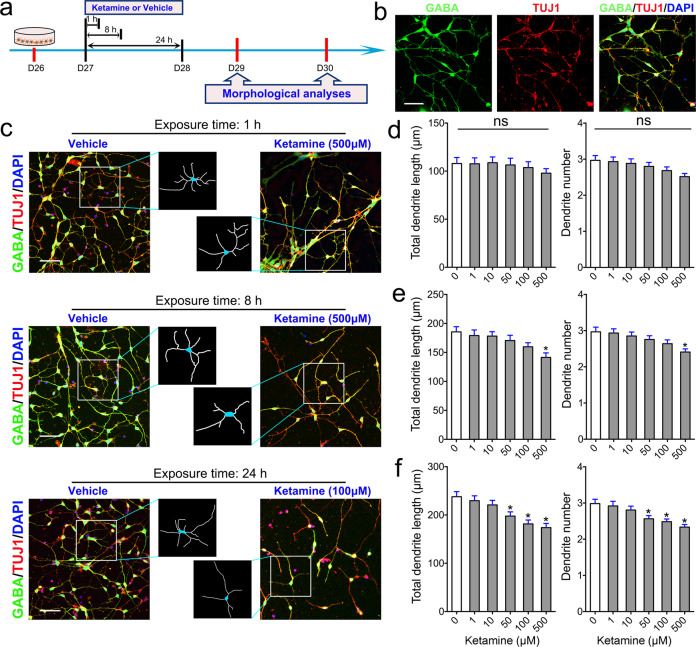
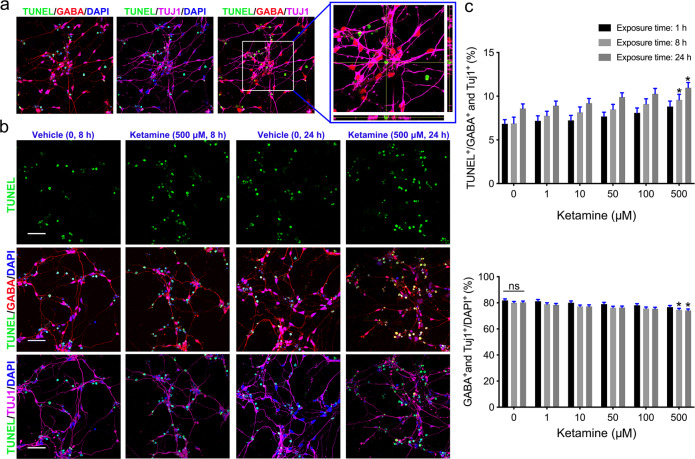
To test the effects of ketamine on striatal GABA neurons, neurons were plated in a dish for 24 h and exposed to various doses of ketamine ranging from 1 to 500 μM for 1, 8, or 24 h (Fig. 2a). After exposure to ketamine, the culture medium containing ketamine was replaced with ketamine-free medium. We cultured ketamine-exposed neurons in ketamine-free medium for an additional 48 h and fixed and stained the cells to assess dendrites and spines. Neurons from both the vehicle and ketamine groups exhibited radially extended dendrites and formed complex, branching dendritic structures (Fig. 2b). We observed that ketamine affected the growth and branching of the dendrites of GABA+ and TUJ1+ MSNs in a dose- and time-dependent manner (Fig. 2c). Exposure to various concentrations of ketamine for a short period did not affect dendritic growth (Fig. 2d). However, dendritic length and dendritic number were significantly reduced in the group exposed to 500 μM ketamine for 8 h compared to the vehicle group (Fig. 2e). Exposure to 50, 100, or 500 μM ketamine for 24 h also significantly decreased dendritic growth (Fig. 2f). Consistent with the data from iPSC-derived MSNs, 24 h of exposure to 100 μM ketamine, but not 10 μM ketamine, also reduced dendritic growth of H9-derived MSNs (Supplementary Fig. S1a, b). Ketamine exposure decreased the percentage of MSNs in a time- and dose-dependent manner (Fig. 2c). Thus, we hypothesized that ketamine exposure might specifically induce apoptosis of MSNs. To assess apoptosis of MSNs, the cells were subjected to TUNEL staining before immunostaining with GABA+ and TUJ1+ antibodies (Fig. 3a). We found that apoptosis of MSNs was significantly higher in the group exposed to 500 μM ketamine for 8 h or 24 h than in the vehicle group (Fig. 3b), while exposure to 50 or 100 μM ketamine for different times (1, 8, or 24 h) did not specifically reduce the percentage of MSNs (Fig. 3c). Overall, these data indicate that ketamine exposure affects the dendritic growth and branching of MSNs but does not specifically induce MSN death.
Fig. 2. Effects of ketamine on MSN dendritic growth.
a Schematic and timeline of the experiments. b Representative images of selective markers of striatal MSNs. Color legend: green, GABA; red, TUJ1; blue, DAPI. Scale bar = 50 μm. c Comparison of the morphology of MSNs in the vehicle and ketamine groups after 1, 8, and 24 h of exposure. The box shows magnified and traced neurons in each group. Scale bar = 50 μm. d–f Morphometric assessment of MSNs 48 h after ketamine (1–500 μM) or vehicle treatment for 1 h (d), 8 h (e), or 24 h (f). Two structural plasticity parameters were measured, namely, total dendritic length and dendrite number. In all panels, the data are expressed as the mean ± SEM; ns not significant; *P < 0.05 vs. the vehicle group.
Fig. 3. Assessment of MSN apoptosis after ketamine exposure.
a Immunofluorescence images of TUNEL staining. The box show TUNEL-positive cells costained with GABA, TUJ1, and DAPI. b Comparison of MSN apoptosis between the vehicle group and ketamine groups after 8 and 24 h of exposure. Scale bar = 50 μm. c Quantification of apoptosis of GABA neurons and the percentage of GABA- and TUJ1-positive neurons in each group (n = 10). The data are expressed as the mean ± SEM; ns not significant; *P < 0.05 vs. the vehicle group.
Ketamine exposure delays maturation of the dendritic spines of MSNs
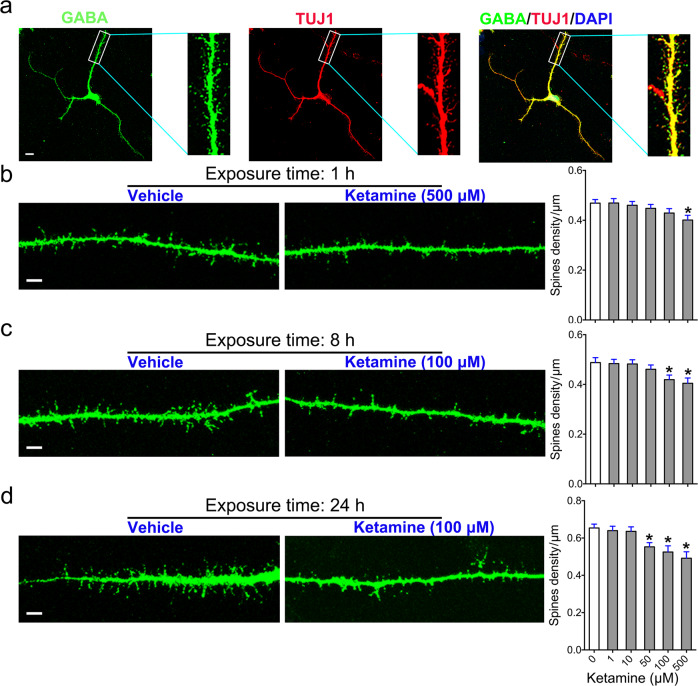
Dendritic spines, bulbous protrusions that form synapses with other neurons, are the primary indicators or neuronal maturation. To investigate the impact of ketamine on the maturation of MSNs, we analyzed the dendritic spine density of MSNs following ketamine exposure. We exposed neurons to 1–500 μM ketamine for 1, 8, or 24 h and then cultured them in fresh ketamine-free media culture for an additional 48 h. After fixation and staining with GABA and TUJ1 antibodies, we examined dendritic spines by high-resolution scanning microscopy and counted the shafts of the dendritic protrusions (Fig. 4a). Our data revealed that the spine density of the group exposed to 500 μM ketamine for 1 h was lower than that of the vehicle group (Fig. 4b). The number of dendritic spines was significantly decreased in the groups exposed to 100 and 500 μM ketamine for 8 h compared to the vehicle group (Fig. 4c). Furthermore, exposure to 50, 100, and 500 μM ketamine for 24 h dramatically decreased dendritic spine density (Fig. 4d). Consistent with the data from iPSC-derived neurons, 24 h of exposure to 100 μM ketamine, but not 10 μM ketamine, also reduced the spine density of immature H9-derived MSNs (Supplementary Fig. S1c, d). Taken together, these results demonstrate that ketamine treatment blocks or delays dendritic maturation in MSNs.
Fig. 4. Effects of ketamine on dendritic spine maturation in MSNs.
a Representative images and enlarged insets showing isolated dendritic branches from GABA- and TUJ1-positive neurons used to analyze dendritic spines. Scale bar = 10 μm. b–d Comparison of dendritic spine morphology in the vehicle group and the groups treated with various doses of ketamine or for 1 h (b), 8 h (c), and 24 h (d). Scale bar = 5 μm. Morphometric assessment of the number of dendritic spines observed in each group. The data are expressed as the mean ± SEM; *P < 0.05 vs. the vehicle group.
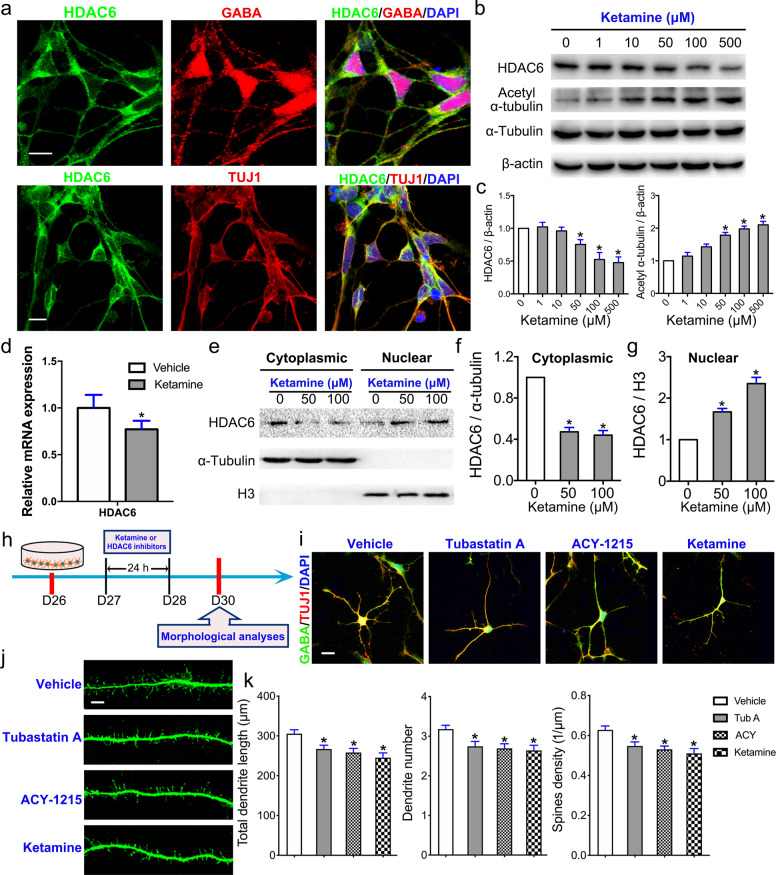
Ketamine induces HDAC6 inhibition, mediating aberrant dendritic maturation in MSNs
HDAC6, a cytoplasmic deacetylase enzyme, deacetylates α-tubulin, stabilizing assembled microtubules during dendritic extension [25]. Immunostaining of MSNs with an HDAC6 antibody showed that HDAC6 localized to the cytoplasm, but not the nuclei, of GABA+ and TUJ1+ MSNs (Fig. 5a). To detect the transcription and protein expression of HDAC6 and the level of acetylated α-tubulin after exposure to ketamine, we detected mRNA levels by RT-PCR and protein expression by Western blotting. The expression of HDAC6 was dose-dependently decreased, while the acetylation of α-tubulin was increased, in response to ketamine exposure for 24 h (Fig. 5b, c). RT-PCR analysis showed that 100 μM ketamine exposure mediated significant decreases in the mRNA levels of HDAC6 (Fig. 5d). HDAC6 shuttles between the nucleus and cytoplasm under different stimuli [26]. We assessed HDAC6 levels in the cytoplasm and nucleus by Western blotting after isolation of cytoplasmic and nuclear protein fractions (Fig. 5e) and observed that cytoplasmic HDAC6 expression was significantly decreased in the ketamine-treated group compared to the vehicle group (Fig. 5f). In contrast, nuclear HDAC6 expression in the ketamine-exposed group was significantly higher than that in the control group (Fig. 5g).
Fig. 5. HDAC6 dysfunction mediates aberrant dendritic maturation in MSNs.
a Immunofluorescence labeling of HDAC6 in GABA+ and TUJ1+ MSNs. Scale bar = 10 μm. b Cultured MSNs treated with various doses of ketamine or vehicle for 24 h were subjected to Western blotting with antibodies against total HDAC6, acetyl-α-tubulin and α-tubulin. β-Actin was used as an internal reference. c The expression of total HDAC6 and acetyl-α-tubulin was calculated (n = 3). d Quantitative real-time PCR (RT-PCR) analysis of HDAC6 expression in the 100 μM ketamine-treated and vehicle groups (n = 3). e Cultured MSNs treated with 50 or 100 μM ketamine or vehicle for 24 h were subjected to Western blotting with antibodies against HDAC6. α-Tubulin was used as a control for cytoplasmic proteins, while H3 was used as a control for nuclear proteins. The expression of HDAC6 in the cytoplasmic (f) and nuclear (g) fractions was calculated. h Schematic and timeline of the experiments. i Comparison of MSN morphology between the vehicle group, HDAC6 inhibitor (tubastatin A and ACY-1215)-treated groups, and ketamine (100 μM)-treated group. The concentrations of tubastatin A and ACY-1215 were 0.1 μM. Scale bar = 10 μm. j Comparison of MSN spines in each group. Scale bar = 5 μm. k Morphometric assessment of dendritic length, dendrite number, and dendritic spine density. The data are expressed as the mean ± SEM; ns not significant; *P < 0.05 vs. the vehicle group.
To evaluate the role of HDAC6 in dendritic and spine growth in iPSC-derived MSNs, we exposed these neurons to tubastatin A, a highly selective HDAC6 inhibitor, or ACY-1215, an HDAC6 inhibitor, for 24 h (Fig. 5h). We analyzed dendritic and spine growth by costaining with GABA and TUJ1 antibodies (Fig. 5i). Total dendritic length, the number of dendritic branches, and spine density in immature MSNs were significant reduced in the tubastatin A and ACY-1215 groups compared to the vehicle group (Fig. 5j, k). The effects of tubastatin A and ACY-1215 on the dendrites and spines of MSNs were nearly identical to those of ketamine (Fig. 5k). Collectively, these results reveal that ketamine inhibits and induces the translocation of HDAC6 from the cytoplasm to the nucleus, which is mediated by hyperacetylation of α-tubulin, eventually reducing the dynamic flexibility of microtubules and impairing the dendritic growth of MSNs.
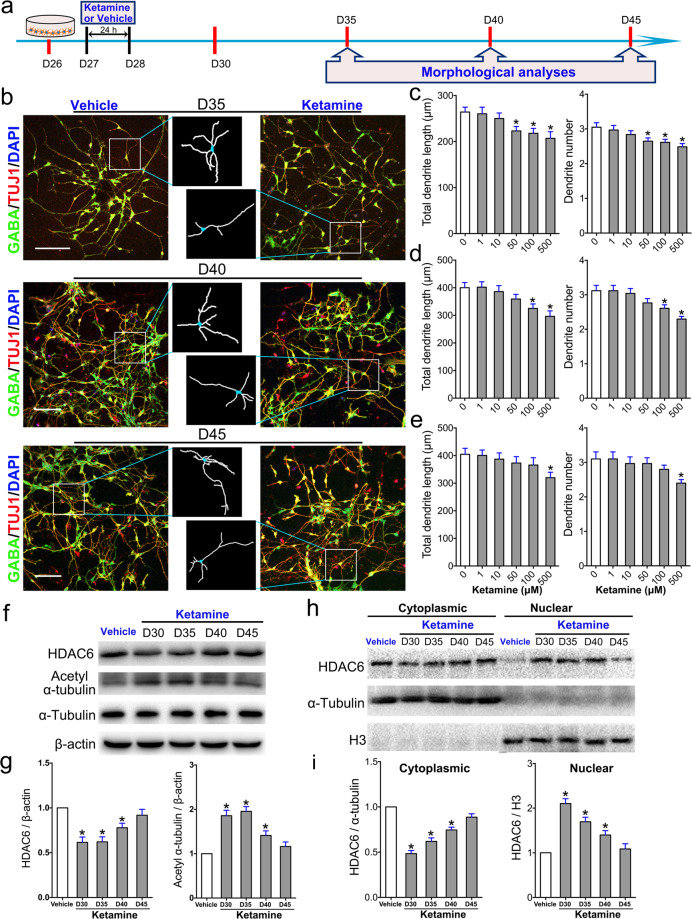
The effect of a single dose of ketamine on the maturation of MSNs is transiently and reversibly mediated via HDAC6
Ketamine exposure delays or inhibits dendritic growth and spine maturation in MSNs. However, whether a single dose of ketamine exerts a long-term effect on human MSNs is unknown. To answer this question, we assessed dendritic growth in GABA+ and TUJ1+ MSNs 35, 40, and 45 days after a single exposure to ketamine (Fig. 6a, b). Our data revealed that exposure to an apoptotic dose (500 μM) of ketamine had long-term effects on MSNs that lasted for 15 days and that the total dendritic length and number of primary dendrites in 500 μM ketamine-treated MSNs were still lower than those in the control group at day 45 (Fig. 6c–e). However, the effect of exposure to nonapoptotic doses (50 or 100 μM) of ketamine on MSNs was diminished by 45 days (Fig. 6e). The lower the dose of ketamine, the shorter its effect on MSNs. These data indicate that the effect of exposure to a low dose of ketamine on MSNs is both transient and reversible.
Fig. 6. Lasting effect of a single dose of ketamine on dendritic growth in MSNs.
a Schematic and timeline of the experiments. Morphological analyses were performed on days 35, 40, and 45 after exposure to ketamine. b Comparison of MSN morphology between the vehicle- and ketamine-treated groups. The box shows magnified and traced neurons in each group. Scale bar = 50 μm. c–e Morphometric assessment of MSNs was performed on day 35 (c), day 40 (d), and day 45 (e) after ketamine treatment. Two structural plasticity parameters were measured, namely, total dendritic length, and dendrite number. f Cultured MSNs treated with 100 μM ketamine or vehicle for 24 h were subjected to Western blotting with antibodies against total HDAC6, acetyl-α-tubulin and α-tubulin at different time points. β-Actin was used as an internal reference. g The expression of total HDAC6 and acetyl-α-tubulin was calculated (n = 3). h Cultured MSNs treated with 100 μM ketamine or vehicle for 24 h were subjected to Western blotting with antibodies against HDAC6. α-Tubulin was used as a control for cytoplasmic proteins, while H3 was used as a control for nuclear proteins. i The expression of HDAC6 in the cytosol and nucleus was calculated. The data are expressed as the mean ± SEM (n = 3); *P < 0.05 vs. the vehicle group.
To further examine whether exposure to a single dose of ketamine exerts long-term effects on HDAC6 and α-tubulin acetylation in MSNs, we detected the levels of HDAC6 and α-tubulin acetylation in both the total and cytoplasmic/nuclear protein fractions by Western blotting (Fig. 6f). The results showed that exposure to a single dose (100 μM) of ketamine significantly decreased the total expression of HDAC6 and that this decrease lasted for ~5 days, at which point the expression of HDAC6 increased. Changes in acetylated tubulin levels were inversely correlated with changes in HDAC6 expression in MSNs (Fig. 6g). Cytoplasmic levels of HDAC6 in MSNs were also decreased in a time-dependent manner after exposure to a single dose of ketamine, while nuclear HDAC6 expression increased until day 45 (Fig. 6h, i). In summary, the effects of a single dose of ketamine on MSNs are reversible and lasts for at least 10 days, and these effects may depend on a decrease in HDAC6 or translocation of HDAC6 into the nucleus, leading to decreased deacetylation of α-tubulin in MSNs.
Discussion
Using in vitro models of human iPSC-derived striatal MSNs, we demonstrated that a single exposure to ketamine time- and dose-dependently impaired morphological development, as determined by reductions in dendritic growth, dendrite number, and dendritic spine density. Decreases in HDAC6 expression or mislocalization of HDAC6 induced by ketamine resulted in hyperacetylated α-tubulin, leading to increased stability of microtubules and delayed dendritic growth in MSNs. Furthermore, the effect of a single exposure of ketamine on MSNs was reversible and lasted for a limited period, and these effects may have been dependent on decreases in HDAC6 levels or translocation of HDAC6, which consequently increased the stability of microtubules and delayed dendritic growth in MSNs. The current study investigated the role of HDAC6 and ketamine’s impact on the morphological development of MSNs, and our results suggest that failure of HDAC6 to regulate deacetylation of α-tubulin may contribute to ketamine-induced impairment of dendritic growth in MSNs.
Ketamine is a desirable anesthetic drug that is commonly used in infants and young children [27]. Clinically, ketamine levels in the cerebrospinal fluid reach ~100 μM for anesthesia induction and 15–20 μM for maintenance of anesthesia [28–30]. Neural toxicity of ketamine has been reported in both human and mouse neurons following exposure to concentrations of 100–500 μM for 6–24 h [31–33]. Recent studies reported that the total dose of propofol can be reduced by the addition of ketamine, leading to stable hemodynamics and faster postanesthetic recovery following both short and long surgeries in pediatric patients [34, 35]. Moreover, ketamine provides effective analgesia [36] and safe sedation [37] in children with acute life-threatening injuries. These studies indicate that ketamine can be used at higher doses for longer durations in children. Our results show that only high concentrations of ketamine or long exposure to the drug increases apoptosis of MSNs, suggesting that ketamine may be relatively safe for routine clinical use. However, single exposure to 100 μM ketamine for 8 or 24 h delayed dendritic outgrowth, indicating that it may damage the developing brain upon overdose or long-term use in children.
Previous studies have demonstrated that GABAergic neurons are vulnerable to ketamine exposure due to their higher frequency of firing than pyramidal neurons [38]. A single dose of 0.01 μg/mL ketamine impairs dendritic arbor development in GABAergic interneurons, including dendritic retraction and branching point elimination [10]. During cortical maturation, ketamine exposure persistently affects the integration of GABAergic neurons by reducing their survival and differentiation [11, 39], further affecting the establishment of neuronal networks. Ketamine also acts as a potent caspase-3 activator in immature GABAergic neurons [40], indicating that ketamine might induce apoptosis of GABAergic neurons. In the current study, we demonstrated that ketamine decreases growth and branching of dendrites in a dose- and time-dependent manner but does not specifically induce death of MSNs.
Early exposure to general anesthetics can alter the number of dendritic spines, and these effects depend on the stage of brain development [41, 42]. Application of 100 mg/kg ketamine to neonatal mice decreases dendritic spine density in the hippocampal CA1 region [43]. Moreover, ketamine reduces the number and length of dendritic spines in cultured rat hippocampal neurons in a dose-dependent manner [44]. Consistently, we found that ketamine reduced the dendritic spine density of immature MSNs in a time- and dose-dependent manner. Moreover, reductions in total dendritic length, the number of dendritic branches, and dendritic spine density indicate the structural plasticity and maturation of neurons [45]. These findings suggest that morphological maturation of MSNs is negatively regulated in response to ketamine.
HDAC6 regulates synaptic activity and is involved in neural development and neurodegenerative diseases. Unlike other HDACs, HDAC6 primarily resides in the cytoplasm and modulates a variety of nonhistone substrates [46]. α-Tubulin, one of the building blocks of microtubules, is a substrate of HDAC6 [47]. Stable microtubules are acetylated in neurons. HDAC6 inhibition reduces the growth rate of microtubules [25, 48] and slows axonal growth [49]. Sheu et al. observed a similar phenomenon in newly generated neurons in mice; dysfunction of HDAC6 led to hyperacetylation of α-tubulin and resulted in reduced dendritic growth after ischemia [50]. Our results showed that HDAC6 was primarily localized in the cytoplasm of MSNs. Translocation of HDAC6 to the nucleus and decreases in HDAC6 levels in response to ketamine exposure may have contributed to the hyperacetylation of α-tubulin. In addition, pharmacological suppression of HDAC6 reduced the growth of MSN dendrites and spines. Thus, altering microtubule stability and dynamics after HDAC6 inhibition is involved in ketamine-induced impairment of dendritic and spine growth in MSNs.
We found that the impact of ketamine was reversible and lasted for a limited period of time. Furthermore, the reversible changes in acetylated tubulin levels were inversely correlated with changes in HDAC6 expressing in MSNs. These findings suggest that HDAC6 activity is necessary for dendritic growth in MSNs. The changes in acetylated tubulin levels after HDAC6 inhibition may explain the lack of dendritic growth and spine density induced by ketamine exposure. However, other cellular mechanisms might synergistically regulate ketamine’s effects on the dendrites and spines of MNSs. For example, previous reports indicate that the downregulation of GSK-3β rescues ketamine-induced neurotoxicity by preventing neurite degeneration [51]. Activation of the RhoA/Rho-associated kinase (ROCK) signaling pathway is involved in dendritic spine loss and shortening in hippocampal neurons after ketamine treatment [44]. Finally, how ketamine exposure decreases HDAC6 expression and the association between ketamine and the HDAC6/α-tubulin pathway and other pathways in MSNs need to be further clarified.
iPSCs are heterogeneous [52, 53]. We used MSNs derived from iPSCs from a healthy male and H9 cells to examine the effects of ketamine on dendrites and spines. However, our findings do not necessarily indicate that ketamine universally affects the growth of dendrites and spines. Therefore, exploring ketamine’s effects on MSNs from other iPSC lines with different origins, including lymphocytes and epithelia, is necessary. The loss of GABAergic projection neurons is a pathological characteristic of Huntington’s disease (HD) [54]. Administration of intermediate or high doses of ketamine to HD patients exacerbate the symptoms of the patients [20]. Elucidating the effect of ketamine on developing or mature MSNs in HD patients might provide more insight into ketamine’s role in MSN pathology and development. GABAergic neurons and glutamatergic neurons act in coordination to regulate excitability in the human brain. In this study, we did not examine the effects of ketamine on glutamatergic neurons. Exposure to sub-anesthetic doses of ketamine increases dendritic spine density and dendritic arborization in mature human dopaminergic neurons [55]. These data imply that ketamine affects the development of both GABAergic neurons and glutamatergic neurons. However, ketamine’s effects on glutamatergic neuronal development remain unclear. In the future, exploring ketamine’s effects on glutamatergic neurons derived from iPSCs might provide more insight into ketamine’s role in developing and mature human neurons.
In conclusion, exposure to a single dose of ketamine impaired the morphological maturation of MSNs in both a dose- and time-dependent manner, reducing dendritic growth, dendrite number, and dendritic spine density. Inhibition of HDAC6 and its aberrant nuclear translocation induced by ketamine mediated hyperacetylation of α-tubulin, which may have reduced the dynamic flexibility of microtubules and ultimately delayed dendritic growth in MSNs. Moreover, the reduction in HDAC6 expression, nuclear translocation of HDAC6 and hyperacetylation of α-tubulin were reversible, suggesting that the effects of ketamine on dendritic growth in MSNs are transient. Our findings reveal that HDAC6 is a regulator of ketamine-induced impairment of dendritic and spine growth in human MSNs and new intervention pathways and targets underlying the preventive and treatment effects of ketamine are expected to be identified in the clinic.
Supplementary information
Author contributions
XL, WML, and QY, helped conceive and design the experiments. XL, and HS, performed the experiments. XL, and JHZ, analyzed the data. XL, HS, and QY, wrote the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Xuan Li, Hexige Saiyin.
Contributor Information
Qiong Yu, Email: yu_qiong816@sina.com.
Wei-min Liang, Email: chiefliang@sina.cn.
Supplementary information
The online version of this article (10.1038/s41401-020-00521-3) contains supplementary material, which is available to authorized users.
References
- 1.Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;10:612–26. doi: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persson J. Ketamine in pain management. CNS Neurosci Ther. 2013;19:396–402. doi: 10.1111/cns.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 5.Ng LHL, Huang Y, Han L, Chang RC-C, Chan YS, Lai CSW. Ketamine and selective activation of parvalbumin interneurons inhibit stress-induced dendritic spine elimination. Transl Psychiatry. 2018;8:272–87. doi: 10.1038/s41398-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun. 2020;11:72–87. doi: 10.1038/s41467-019-13809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–47. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 8.Forrest MP, Parnell E, Penzes P. Dendritic structural plasticity and neuropsychiatric disease. Nat Rev Neurosci. 2018;19:215–34. doi: 10.1038/nrn.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng M, Hofacer RD, Jiang C, Joseph B, Hughes EA, Jia B, et al. Brain regional vulnerability to anaesthesia-induced neuroapoptosis shifts with age at exposure and extends into adulthood for some regions. Br J Anaesth. 2014;113:443–51. doi: 10.1093/bja/aet469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vutskits L, Gascon E, Tassonyi E, Kiss JZ. Effect of ketamine on dendritic arbor development and survival of immature GABAergic neurons in vitro. Toxicol Sci. 2006;91:540–9. doi: 10.1093/toxsci/kfj180. [DOI] [PubMed] [Google Scholar]
- 11.Aligny C, Roux C, Dourmap N, Ramdani Y, Do-Rego JC, Jegou S, et al. Ketamine alters cortical integration of GABAergic interneurons and induces long-term sex-dependent impairments in transgenic Gad67-GFP mice. Cell Death Dis. 2014;5:1311–25. doi: 10.1038/cddis.2014.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn KC. The cytoskeleton and neurite initiation. Bioarchitecture. 2013;3:86–109. doi: 10.4161/bioa.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–32. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 14.Howes SC, Alushin GM, Shida T, Nachury MV, Nogales E, Zheng Y. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol Biol Cell. 2014;25:257–66. doi: 10.1091/mbc.E13-07-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iaconelli J, Xuan L, Karmacharya R. HDAC6 modulates signaling pathways relevant to synaptic biology and neuronal differentiation in human stem-cell-derived neurons. Int J Mol Sci. 2019;20:1605–23. doi: 10.3390/ijms20071605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batchu SN, Brijmohan AS, Advani A. The therapeutic hope for HDAC6 inhibitors in malignancy and chronic disease. Clin Sci. 2016;130:987–1003. doi: 10.1042/CS20160084. [DOI] [PubMed] [Google Scholar]
- 17.Landwehrmeyer GB, Standaert DG, Testa CM, Penney JB, Jr., Young AB. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci. 1995;15:5297–307. doi: 10.1523/JNEUROSCI.15-07-05297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W, Buchele F, Papazoglou A, Dobrossy M, Nikkhah G. Ketamine anaesthesia interferes with the quinolinic acid-induced lesion in a rat model of Huntington’s disease. J Neurosci Methods. 2009;179:219–23. doi: 10.1016/j.jneumeth.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Luk KC, Kennedy TE, Sadikot AF. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23:2239–54. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murman DL, Giordani B, Mellow AM, Johanns JR, Little RJ, Hariharan M, et al. Cognitive, behavioral, and motor effects of the NMDA antagonist ketamine in Huntington’s disease. Neurology. 1997;49:153–61. doi: 10.1212/wnl.49.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 22.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–82. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8:1670–9. doi: 10.1038/nprot.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;10:455–64. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci. 2009;122:3531–41. doi: 10.1242/jcs.046813. [DOI] [PubMed] [Google Scholar]
- 26.Van Den Bosch L. HDAC6 and Miro1: another interaction causing trouble in neurons. J Cell Biol. 2019;218:1769–70. doi: 10.1083/jcb.201904016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70:621–60. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Dominof SE, et al. Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesth Analg. 1982;61:87–92. [PubMed] [Google Scholar]
- 29.Grant IS, Nimmo WS, McNicol LR, Clements JA. Ketamine disposition in children and adults. Br J Anaesth. 1983;55:1107–11. doi: 10.1093/bja/55.11.1107. [DOI] [PubMed] [Google Scholar]
- 30.McLean RF, Baker AJ, Walker SE, Mazer CD, Wong BI, Harrington EM. Ketamine concentrations during cardiopulmonary bypass. Can J Anaesth. 1996;43:580–4. doi: 10.1007/BF03011770. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Uchida T, Makita K. Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons. PLoS One. 2015;10:3651–71. doi: 10.1371/journal.pone.0128445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng X, Lin C, Li Y, Ye J, Zhou J, Guo P. Long noncoding RNA BDNF-AS regulates ketamine-induced neurotoxicity in neural stem cell derived neurons. Biomed Pharmacother. 2016;82:722–8. doi: 10.1016/j.biopha.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 33.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, et al. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg. 2013;116:869–80. doi: 10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz A, Weiss M, Kellenberger C, O’Gorman Tuura R, Klaghofer R, Scheer I, et al. Sedation for magnetic resonance imaging using propofol with or without ketamine at induction in pediatrics—a prospective randomized double-blinded study. Pediatr Anaesth. 2018;28:264–74. doi: 10.1111/pan.13315. [DOI] [PubMed] [Google Scholar]
- 35.Hayes J, Matava C, Pehora C, El-Beheiry H, Jarvis S, Finkelstein Y. Determination of the median effective dose of propofol in combination with different doses of ketamine during gastro-duodenoscopy in children: a randomised controlled trial. Br J Anaesth. 2018;121:453–61. doi: 10.1016/j.bja.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 36.Frey TM, Florin TA, Caruso M, Zhang N, Zhang Y, Mittiga MR. Effect of intranasal ketamine vs fentanyl on pain reduction for extremity injuries in children: the PRIME randomized clinical trial. JAMA Pediatr. 2019;173:140–6. doi: 10.1001/jamapediatrics.2018.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt M, Johnson DW, Chan J, Taljaard M, Barrowman N, Farion KJ, et al. Risk factors for adverse events in emergency department procedural sedation for children. JAMA Pediatr. 2017;171:160–8. doi: 10.1001/jamapediatrics.2017.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci Off J Soc Neurosci. 2015;35:1089–105. doi: 10.1523/JNEUROSCI.2279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Shen> FY, Zou R, Zheng JJ, Yu X, Wang YW. Ketamine-induced apoptosis in the mouse cerebral cortex follows similar characteristic of physiological apoptosis and can be regulated by neuronal activity. Mol Brain. 2017;10:24–39. doi: 10.1186/s13041-017-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desfeux A, El Ghazi F, Jegou S, Legros H, Marret S, Laudenbach V, et al. Dual effect of glutamate on GABAergic interneuron survival during cerebral cortex development in mice neonates. Cereb Cortex. 2010;20:1092–108. doi: 10.1093/cercor/bhp181. [DOI] [PubMed] [Google Scholar]
- 41.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2009;17:179–88. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puskarjov M, Fiumelli H, Briner A, Bodogan T, Demeter K, Lacoh CM, et al. KCl cotransporter 2-mediated cl- extrusion determines developmental stage-dependent impact of propofol anesthesia on dendritic spines. Anesthesiology. 2017;126:855–67. doi: 10.1097/ALN.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 43.Tan H, Ren R-r, Xiong Z-q, Wang Y-w. Effects of ketamine and midazolam on morphology of dendritic spines in hippocampal CA1 region of neonatal mice. Chin Med J (Engl) 2009;122:455–9. [PubMed] [Google Scholar]
- 44.Jiang S, Hao Z, Li X, Bo L, Zhang R, Wang Y, et al. Ketamine destabilizes growth of dendritic spines in developing hippocampal neurons in vitro via a Rho-dependent mechanism. Mol Med Rep. 2018;18:5037–43. doi: 10.3892/mmr.2018.9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt U, Beyer C, Oestreicher AB, Reisert I, Schilling K, Pilgrim C. Activation of dopaminergic D1 receptors promotes morphogenesis of developing striatal neurons. Neuroscience. 1996;74:453–60. doi: 10.1016/0306-4522(96)00201-1. [DOI] [PubMed] [Google Scholar]
- 46.Ellmeier W, Seiser C. Histone deacetylase function in CD4+ T cells. Nat Rev Immunol. 2018;18:617–34. doi: 10.1038/s41577-018-0037-z. [DOI] [PubMed] [Google Scholar]
- 47.Southwood CM, Peppi M, Dryden S, Tainsky MA, Gow A. Microtubule deacetylases, SirT2 and HDAC6, in the nervous system. Neurochem Res. 2007;32:187–95. doi: 10.1007/s11064-006-9127-6. [DOI] [PubMed] [Google Scholar]
- 48.Hanson K, Tian N, Vickers JC, King AE. The HDAC6 inhibitor trichostatin A acetylates microtubules and protects axons from excitotoxin-induced degeneration in a compartmented culture model. Front Neurosci. 2018;12:872–83. doi: 10.3389/fnins.2018.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS One. 2010;5:129–42. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheu J-R, Hsieh CY, Jayakumar T, Lin GY, Lee HN, Huang SW, et al. HDAC6 dysfunction contributes to impaired maturation of adult neurogenesis in vivo: vital role on functional recovery after ischemic stroke. J Biomed Sci. 2019;26:27–44. doi: 10.1186/s12929-019-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Cui C, Li Y, Xu H. Inhibition of GSK-3beta signaling pathway rescues ketamine-induced neurotoxicity in neural stem cell-derived neurons. NeuroMolecular Med. 2017;20:54–62. doi: 10.1007/s12017-017-8472-8. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi Y, Ohnuma K, Furue MK. Pluripotent stem cell heterogeneity. Adv Exp Med Biol. 2019;1123:71–94. doi: 10.1007/978-3-030-11096-3_6. [DOI] [PubMed] [Google Scholar]
- 53.Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14:357–68. doi: 10.1038/nrm3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Philpott AL, Fitzgerald PB, Cummins TD, Georgiou-Karistianis N. Transcranial magnetic stimulation as a tool for understanding neurophysiology in Huntington’s disease: a review. Neurosci Biobehav Rev. 2013;37:1420–33. doi: 10.1016/j.neubiorev.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018;23:812–23. doi: 10.1038/mp.2017.241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.