Abstract
Background
Drug benefit policies are an important determinant of a population's use of prescription drugs. This study was undertaken to determine whether a change in a provincial drug benefit policy, from a fixed deductible and copayment system to an income-based deductible system, resulted in changes in receipt of prescriptions for inhaled corticosteroids by Manitoba children with asthma.
Methods
Using Manitoba's health care administrative databases, we identified a population-based cohort of 10 703 school-aged children who met our case definition for asthma treatment before and after the province's drug benefit policy was changed in April 1996. The effects of the program change on the probability of receiving a prescription for an inhaled corticosteroid and on the mean number of inhaled corticosteroid doses dispensed were compared between a group of children insured under other drug programs (the comparison group) and 2 groups of children insured under the deductible program: those living in low-income neighbourhoods and those living in higher-income neighbourhoods. All analyses were adjusted for a measure of asthma severity.
Results
For higher-income children with severe asthma who were covered by the deductible program, the probability of receiving an inhaled corticosteroid prescription and the mean annual number of inhaled corticosteroid doses declined after the change to the drug policy. A trend toward a decrease in receipt of prescriptions was also observed for low-income children, but receipt of prescriptions was unaltered in the comparison group. Before the policy change, among children with severe asthma, the mean annual number of inhaled corticosteroid doses was lowest for low-income children, and this pattern persisted after the change. Among children with mild to moderate asthma, those covered by the deductible program (both low income and higher income) were less likely to receive prescriptions for inhaled corticosteroids than those in the comparison group, and this difference was statistically significant for the higher-income children.
Interpretation
The change to an income-based drug benefit policy was associated with a decrease in the use of inhaled corticosteroids by higher-income children with severe asthma and did not improve use of these drugs by low- income children.
Drug benefit policies play an important role in determining a population's use of pharmaceuticals. Policies that impose limits on reimbursement for prescriptions or that increase the cost that must be borne by the patient can promote optimal use of pharmaceuticals,1,2 but they have also led to unintended effects, such as reductions in the use of essential drugs3,4,5,6 and substitution with less effective drugs.1 Negative health outcomes of these unintended effects have been documented.4,5,6 Low-income Canadians who must pay for their prescriptions are less likely to use prescription drugs than recipients of income assistance, who receive prescription drugs at no charge.7 A recent change to the drug benefit policy in Quebec, which required a deductible payment from social assistance recipients (who previously received prescription drugs at no cost), resulted in a decrease in the consumption of inhaled corticosteroids for asthma.6,8 The impact of this type of policy change is clinically relevant, since use of inhaled corticosteroids significantly reduces illness and death resulting from asthma.9,10,11
Until March 1996, the prescription reimbursement policy for Manitoba's drug benefit program, Pharmacare, required a fixed annual deductible payment of $237 per family plus 40% copayment on prescription costs over $237. In April 1996, the policy was changed, and the deductible is now based on income.12 Under the income-based policy, families with an annual income of $15 000 or less, adjusted for dependents, are required to pay a deductible equivalent to 2% of income, whereas those with higher incomes pay a deductible of 3% of income. Once the deductible is reached, the provincial government pays 100% of all prescription costs. There has been no change in the 100% reimbursement benefit available to households receiving income assistance and to federal-treaty First Nations households.
This study was undertaken to determine if the change in Manitoba's drug benefit policy, from a fixed deductible and copayment system to an income-based deductible system, affected the receipt of prescriptions for inhaled corticosteroids among children with asthma living in different socioeconomic environments.
Methods
We conducted a population-based cohort study of the receipt of prescriptions for inhaled corticosteroids, before and after introduction of the income-based drug policy, among school-aged children (5 to 15 years old) treated for asthma. The data were obtained from 4 computerized databases maintained by the Manitoba Health Service Insurance Plan (MHSIP): registration files, physician reimbursement claims, hospital discharge abstracts and prescriptions dispensed in retail pharmacies. The MHSIP registration file contains a unique numeric identifier for every registrant, which allows linkage of the health care records. MHSIP's prescription and health care administrative databases have been shown to have good reliability and validity.13,14 The study protocol was approved by the Human Research Ethics Committee, University of Manitoba, and permission to access the data was obtained from the Manitoba Health Access and Confidentiality Committee.
Children were selected for inclusion if they had received prescriptions for asthma drugs in the 1-year period before and the 2-year period after introduction of the income-based policy on Apr. 1, 1996, as follows: at least 1 prescription for a bronchodilator, inhaled corticosteroid or cromone, ketotifen, or oral corticosteroid, in conjunction with at least one physician visit or admission to hospital related to a diagnosis of asthma or bronchitis; or, in the absence of such a diagnosis, at least 1 prescription for an inhaled corticosteroid or cromone or for ketotifen in conjunction with a bronchodilator, or at least 2 prescriptions for a bronchodilator. Because our definition excluded children with one-time bronchodilator prescriptions and no diagnosis of asthma,15 it was more stringent than definitions used by others.16
In the absence of direct measures of household income, we used 1996 census information on average household income reported for enumeration areas to rank households into quintiles, from the 20% of the population in the lowest-income neighbourhoods to the 20% of the population in the highest-income neighbourhoods.17 Three groups were identified: children in households receiving prescriptions reimbursed in full by the income assistance and treaty First Nations prescription programs, as defined in the prescription database (the non-Pharmacare group), children in households receiving Pharmacare benefits that were located in neighbourhoods in the lowest-income quintile (the low-income Pharmacare group) and children in households receiving Pharmacare benefits that were located in neighbourhoods in the 4 higher-income quintiles (the higher-income Pharmacare group). The non-Pharmacare group was the comparison group, because there was no change in the drug reimbursement policy for this group.
To diminish confounding by disease severity,18 the children were stratified by asthma severity, as derived from the asthma prescription drug profile19 and the history of hospital admissions, as follows: mild to moderate asthma was defined as use of bronchodilators with or without inhaled corticosteroids or cromones, and severe asthma was defined as high use of bronchodilators (greater than the 90th percentile of doses) with use of inhaled corticosteroids or cromones or admission to hospital for asthma, or use of oral corticosteroids. Children who did not receive any inhaled corticosteroids were classified as having severe asthma if they used oral corticosteroids or high doses of bronchodilators. The severity measure was found to have good reliability (kappa = 0.82) and validity through its association with other markers of severity, such as admission to an intensive care unit.20 By applying the severity criteria to health care data from before and after introduction of the income-based drug policy, we obtained 2 subgroups: 6612 children with mild to moderate asthma before and after introduction of the income-based policy (referred to here as stable, mild to moderate asthma) and 1420 children with severe asthma before and after introduction of the new policy (referred to here as stable, severe asthma). Children with decreasing (n = 1223) or increasing (n = 1448) severity of asthma over time were excluded because of the difficulty in distinguishing changes in prescriptions for inhaled corticosteroids that were secondary to changes in asthma severity from changes associated with the drug benefit policy.
Two measures of inhaled corticosteroid use, computed for the 1-year period before and the 2 consecutive 2-year periods after the policy change, were determined: the proportion of children who received a prescription and the mean number of doses per child-year among children whose prescriptions were filled, derived from the prescription quantity and the standard unit sizes of inhalers. Inhaled corticosteroid drugs included beclomethasone, budesonide, fluticasone, flunisolide and triamcinolone. Comparisons between time periods of the likelihood of receiving an inhaled corticosteroid prescription, adjusted for monthly variation and stratified by income status and asthma severity, were assessed with Mantel–Haenszel odds ratios (ORs) and 95% confidence intervals (CIs). The Breslow–Day test of heterogeneity was applied to assess whether the likelihoods for the non-Pharmacare and the low- income and higher-income Pharmacare groups were statistically different. A split-unit analysis, reported as least-square means and 95% CI, was conducted to determine the mean number of inhaled corticosteroid doses before and after the policy change in relation to income status. Assuming an α of 0.05, a β of 0.2, and one-sided and paired (before and after) comparisons, 496 children were required per stratum to find an OR of 0.80, and 371 children were required per stratum to detect a 10% decrease in dose.
Results
A total of 10 703 children were identified on the basis of the asthma treatment criteria. Seventy-five percent of the children lived in higher-income neighbourhoods and 8% in the lowest-income neighbourhoods; the comparison group, accounting for 17%, consisted of those who lived in households receiving prescriptions at no charge. During the 1-year period before the policy change, 45% of the children had received a prescription for an inhaled corticosteroid, mainly beclomethasone. There was a decreasing trend over the study period in the proportion of children who received prescriptions for inhaled corticosteroids (Fig. 1), with peaks in use observed in May, September and December.
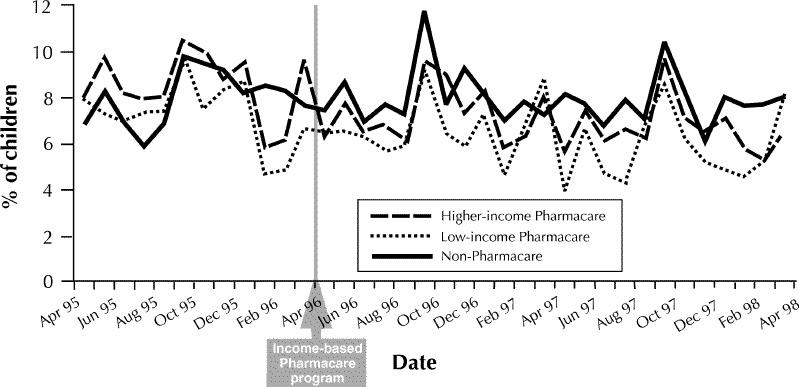
Fig. 1: Monthly percentage of children receiving a prescription for an inhaled corticosteroid, for 1 year before and 2 years after introduction of the income-based drug benefit policy (Pharmacare) in Manitoba.
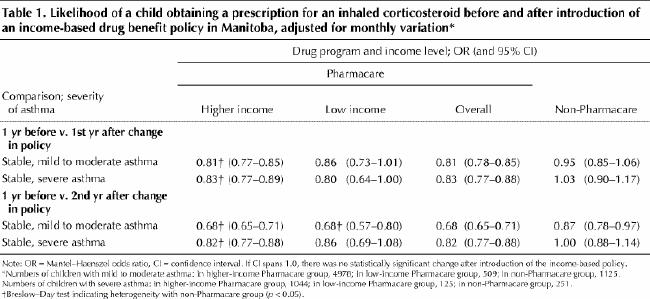
When the second year after the introduction of the income-based policy is compared with the year before this policy was introduced, the likelihood of receiving a prescription for an inhaled corticosteroid decreased for all groups of children with stable, mild to moderate asthma. The same result was observed for both groups of Pharmacare children with stable, severe asthma, although the decreased likelihood was not statistically significant for the low-income group (Table 1). Across groups of children, the likelihood of receiving a prescription was significantly lower for higher-income Pharmacare children than for non-Pharmacare children at both levels of asthma severity (Breslow–Day test for heterogeneity).
Table 1
A total of 7221 children received at least one prescription for an inhaled corticosteroid. In the second year after the change in policy, the mean number of inhaled corticosteroid doses that were dispensed decreased among Pharmacare children with stable, mild to moderate asthma (184 doses/year [95% CI 174–194] during the year before the change in policy v. 131 doses/year [95% CI 122–139] during the second year after the change in policy) and among non-Pharmacare children with this level of asthma severity (151 doses/year [95% CI 129–173] v. 134 doses/year [95% CI 115–153]), but the decrease was statistically significant for the Pharmacare group only. Within the Pharmacare group, the decrease in mean number of corticosteroid doses was statistically significant for the higher-income but not the low-income children (Fig. 2).
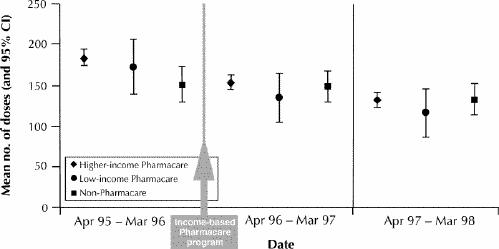
Fig. 2: Mean number of inhaled corticosteroid doses (and 95% confidence interval [CI]) for children with stable, mild to moderate asthma for 1 year before and 2 years after introduction of the income-based policy.
There was no difference in the mean number of corticosteroid doses dispensed to non-Pharmacare children with stable, severe asthma between the year before and the second year after introduction of the policy (373 doses/year [95% CI 336–411] v. 415 doses/year [95% CI 382–448]), but a decrease was observed among Pharmacare children with stable, severe asthma (344 doses/year [95% CI 326–362] v. 288 doses/year [95% CI 272–304]). This decrease was statistically significant for the higher-income but not the low-income children (Fig. 3). Both before and after introduction of the income-based policy, the mean number of corticosteroid doses used by low-income Pharmacare children with severe asthma was significantly lower than the number of doses used by non-Pharmacare children, whose families received prescriptions at no charge.
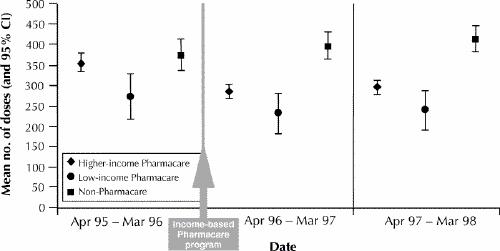
Fig. 3: Mean number of inhaled corticosteroid doses (and 95% CI) for children with stable, severe asthma for 1 year before and 2 years after introduction of the income-based policy.
Interpretation
After a change in drug benefit policy from a fixed deductible and copayment system to an income-based deductible system, children with severe asthma who were covered by the policy were less likely to receive prescriptions for inhaled corticosteroids, and among children with prescriptions for these drugs, there was a reduction of more than 15% in the mean number of annual doses. No change in prescriptions for inhaled corticosteroids was observed in a comparison group of children. These findings were statistically significant for higher-income but not low-income children insured under the income-based deductible program. All children with mild to moderate asthma were less likely to receive prescriptions for inhaled corticosteroids as time went on; this finding is consistent with the natural history of wheezing in childhood, whereby wheezing ceases in some children as they grow older.21 However, among children with this level of asthma severity, the likelihood of using inhaled corticosteroids was significantly lower in the higher-income group than in the comparison group, which suggests that the income-based policy also affected children with less severe forms of the disease.
An analysis of the financial impact of the income-based policy provides possible reasons for our findings. Under the fixed deductible and copayment policy, a family with typical prescription costs of $980 paid $534 out of pocket (i.e., deductible of $237 plus 40% copayment on the remaining $743).22 Under the income-based policy, even among families in the lower income range in our study, higher-income households might have experienced significantly higher out-of-pocket prescription costs; at a 3% deductible for family incomes above $15 000, the cost would be $720 for a family with an adjusted income of $24 000. Thus, the change in the drug benefit policy increased the costs to beneficiaries and had the unintended effect of reducing use of prescription drugs; this type of unintended effect has already been well documented.3,6,8,23 Furthermore, the receipt of prescriptions for inhaled corticosteroids by low-income children did not improve (increase) after introduction of the income-based policy and may in fact have decreased. Under the income-based policy, an adjusted household income of $18 000 would result in a deductible payment of $540, not much different from the payment by a low- income family before the policy was introduced. Prescription costs impose barriers on the ability of low-income families to purchase asthma drugs,24 which may explain why low-income children with severe asthma insured under the deductible policy received fewer corticosteroid doses than the comparison children, who were insured under the 100% reimbursement programs.
Threats to internal validity were minimized in this study through the use of a repeated-measures design in a well- defined population, which included an internal comparison group not exposed to the change in drug benefit policy. Adjustments were made for seasonal variation in inhaled corticosteroid use secondary to exposure to allergens and respiratory viruses.25,26 Intermittent use of high-dose inhaled corticosteroids is recommended in the treatment of acute asthma,19 and parents anticipating seasonal exacerbations may increase their children's supply of corticosteroid inhalers. Findings were also stratified by asthma severity because severity is greater in lower-income children,18 and children with severe asthma are less likely “to grow out of their asthma.”27 There were insufficient numbers of low- income children with severe asthma to detect a 20% decrease in the likelihood of receiving inhaled corticosteroid prescriptions and a 10% decrease in the number of doses. However, because this was a population-based study of a cohort of children with asthma, increases in sample size could only have been achieved by including children from other jurisdictions.
This study has demonstrated that altering drug benefits can have unintended consequences on the receipt of prescription drugs for the management of asthma. Lower use of inhaled corticosteroids has been associated with increased admissions to hospital for asthma.10 Hospital admission contributes substantially to the costs of managing the disease and affects patients' quality of life.28,29 Despite more widespread use of inhaled corticosteroids during the past decade,30,31 these drugs remain underused.32,33 It is therefore imperative that drug benefit policies not deter patients and their families from using these drugs.34 In this era of cost containment, income-based drug benefit policies appear to be good choices for equitably distributing the burden of prescription costs. However, their impact on the use of prescription drugs necessitates evaluation of potential unintended effects. Furthermore, reducing prescription cost-sharing for low-income children with asthma, among whom hospital admission rates are highest, has the potential to improve their health.35 These are important considerations in the design of drug benefit policies by provincial governments, third-party payers and the federal government, should it decide to pursue a national pharmacare program.
Footnotes
This article has been peer reviewed.
Acknowledgements: This research was supported by a PhD Fellowship Award from the National Health Research and Development Program, Health Canada.
Competing interests: None declared.
Correspondence to: Dr. Anita Kozyrskyj, Manitoba Centre for Health Policy and Evaluation, S101-750 Bannatyne Ave., Winnipeg MB R3E 0W2; fax 204 789-3910; kozyrsk@cc.Umanitoba.CA
References
- 1.Soumerai SB, Ross-Degnan D, Gortmaker S, Avorn J. Withdrawing payment for nonscientific drug therapy. Intended and unexpected effects of a large-scale natural experiment. JAMA 1990;263:831-9. [PubMed]
- 2.Smalley WE, Griffin MR, Fought RL, Sullivan L, Ray WA. Effect of a prior-authorization requirement on the use of nonsteroidal antiinflammatory drugs by Medicaid patients. N Engl J Med 1995;332:1612-7. [DOI] [PubMed]
- 3.Harris BL, Stergachis A, Ried LD. The effect of drug co-payments on utilization and cost of pharmaceuticals in a health maintenance organization. Med Care 1990;28:907-17. [DOI] [PubMed]
- 4.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of a limit on Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med 1994;331:650-5. [DOI] [PubMed]
- 5.Soumerai SB, Ross-Degnan D, Avorn J, McLaughlin T, Choodnovskiy I. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med 1991;325:1072-7. [DOI] [PubMed]
- 6.Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 2001;285:421-9. [DOI] [PubMed]
- 7.Williamson DL, Fast JE. Poverty and medical treatment: when public policy compromises accessibility. Can J Public Health 1998;89:120-4. [DOI] [PMC free article] [PubMed]
- 8.Blais L, Couture J, Rahme E, Le Lorier J. Impact of the Quebec general drug insurance plan on drug consumption among individuals receiving social assistance [abstract]. Can J Clin Pharmacol 1998;5:55.
- 9.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000;343:332-6. [DOI] [PubMed]
- 10.Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997; 277:887-91. [PubMed]
- 11.Simons FER, for the Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study Group. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. N Engl J Med 1997;337:1659-65. [DOI] [PubMed]
- 12.Income-based Pharmacare. For those who need it most, new guidelines for Pharmacare. Winnipeg: Manitoba Health; 1996.
- 13.Robinson JR, Young TK, Roos LL, Gelskey DE. Estimating the burden of disease: comparing administrative data and self-reports. Med Care 1997; 35: 932-47. [DOI] [PubMed]
- 14.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother 1998;32:1152-7. [DOI] [PubMed]
- 15.Roberts SJ, Bateman DN. Which patients are prescribed inhaled anti-asthma drugs? Thorax 1994;49:1090-5. [DOI] [PMC free article] [PubMed]
- 16.Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockcroft D, et al. The use of β-agonists and the risk of death and near death from asthma. N Engl J Med 1992;326:501-6. [DOI] [PubMed]
- 17.Mustard CA, Derksen S, Berthelot JM, Wolfson M. Assessing ecologic proxies for household income: a comparison of household and neighbourhood level income measures in the study of population health status. Health Place 1999;5:157-71. [DOI] [PubMed]
- 18.Mielck A, Reitmeir P, Wjst M. Severity of childhood asthma by socioeconomic status. Int J Epidemiol 1996;25:388-93. [DOI] [PubMed]
- 19.Boulet LP, Becker A, Bérubé D, Beveridge R, Ernst P, for the Canadian Asthma Consensus Group. Summary of recommendations from the Canadian asthma consensus report, 1999. CMAJ 1999;161(11 Suppl):S1-S12. Available: www.cma.ca/cmaj/vol-161/issue-11/asthma/index.htm [PMC free article] [PubMed]
- 20.Kozyrskyj AL, Mustard CA, Simons FER. Development of a drug treatment-based severity measure in childhood asthma. J Asthma. In press. [DOI] [PubMed]
- 21.Barbee RA, Murphy S. The natural history of asthma. J Allergy Clin Immunol 1998;102:S65-72. [DOI] [PubMed]
- 22.Health Services Insurance Fund, Pharmacare Program. In: Manitoba Health annual report 1996-97. Winnipeg: Manitoba Health. Available: www .gov .mb .ca /health/ann/199697 (accessed 2001 Aug 29).
- 23.Soumerai SB, Avorn J, Ross-Degnan D, Gortmaker S. Payment restrictions for prescription drugs under Medicaid. Effects on therapy, cost, and equity. N Engl J Med 1987;317:550-6. [DOI] [PubMed]
- 24.Watts RW, McLennan G, Bassham I, el-Saadi O. Do patients with asthma fill their prescriptions? A primary compliance study. Aust Fam Physician 1997;26(Suppl 1):S4-6. [PubMed]
- 25.Dales RE, Schweitzer I, Toogood JH, Drouin M, Yang W, Dolovich J, et al. Respiratory infections and the autumn increase in asthma morbidity. Eur Respir J 1996;9:72-7. [DOI] [PubMed]
- 26.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310:1225-9. [DOI] [PMC free article] [PubMed]
- 27.Ulrik CS. Outcome of asthma: longitudinal changes in lung function. Eur Respir J 1999;13:904-18. [DOI] [PubMed]
- 28.Krahn MD, Berka C, Langlois P, Detsky AS. Direct and indirect costs of asthma in Canada, 1990. CMAJ 1996;154:821-31. Available: www.cma.ca/cmaj/vol-154/0821e.htm [PMC free article] [PubMed]
- 29.Taylor WR, Newacheck PW. Impact of childhood asthma on health. Pediatrics 1992;90:657-62. [PubMed]
- 30.Goodman DC, Lozano P, Stukel TA, Chang C, Hecht J. Has asthma medication use in children become more frequent, more appropriate, or both? Pediatrics 1999;104:187-94. [DOI] [PubMed]
- 31.Habbick B, Baker MJ, McNutt M, Cockcroft DW. Recent trends in the use of inhaled β2-adrenergic agonists and inhaled corticosteroids in Saskatchewan. CMAJ 1995;153:1437-43. Abstract available: www.cma.ca/cmaj/vol-153/issue-10/1437.htm [PMC free article] [PubMed]
- 32.Gaist D, Hallas J, Hansen NC, Gram LF. Are young adults with asthma treated sufficiently with inhaled steroids? A population-based study of prescription data from 1991 and 1994. Br J Clin Pharmacol 1996;41:285-9. [DOI] [PMC free article] [PubMed]
- 33.Lang DM, Sherman MS, Polansky M. Guidelines and realities of asthma management. The Philadelphia story. Arch Intern Med 1997;157:1193-200. [PubMed]
- 34.Watt J, Dixon F, Thompson R, Burgess C, Crane J, Beasley R. The effect of the increased prescription charges on the collection of asthma drugs. N Z Med J 1992;105:153-4. [PubMed]
- 35.Erzen D, Carrière KC, Dik N, Mustard C, Roos LL, Manfreda J, et al. Income level and asthma prevalence and care patterns. Am J Respir Crit Care Med 1997;155:1060-5. [DOI] [PubMed]


