Abstract
Hyperadiponectemia is paradoxically associated with renal disease progression and mortality in chronic kidney disease (CKD). Its association with health-related quality of life (HR-QOL) is unknown. This study aimed to verify the association between adiponectin and HR-QOL in Korean pre-dialysis CKD cohort. This cross-sectional study analyzed 1551 pre-dialysis CKD patients from KNOW-CKD (KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease). Participants were categorized into three tertiles (T1–T3) according to adiponectin levels. HR-QOL was assessed using SF-36. High physical component summary (PCS) and mental component summary (MCS) were defined as highest quartile of each score. Multivariate logistic regression was used to analyze odds ratio (OR) and 95% confidence interval (CI) for high PCS and MCS. Prevalence of high PCS were 33.3%, 27.5%, and 17.0% and that of high MCS were 31.7%, 24.8%, and 21.3% for T1, T2, and T3 (both p for trend < 0.001). The adjusted OR [95% CI] of T1 and T2 in reference to T3 were 1.56 [1.09–2.23] and 1.19 [0.85–1.68] for high PCS and 1.19 [0.85–1.68] and 0.94 [0.68–1.29] for high MCS. Serum adiponectin level was inversely associated with physical HR-QOL in Korean pre-dialysis CKD patients. This relationship was independent of various cardiovascular risk factors.
Subject terms: Medical research, Nephrology
Introduction
Adiponectin is a 30-kDa, 244 amino acid polypeptide hormone secreted by adipocytes. It is the most abundant adipokine with insulin-sensitizing, anti-inflammatory, and anti-atherogenic properties1. Several epidemiological studies have shown that hypoadiponectinemia is associated with increased metabolic and cardiovascular disease prevalence2–4. On the other hand, recent studies have shown that hyperadiponectinemia is associated with high mortality5,6. Moreover, there are conflicting results as to whether adiponectin plays a protective role in kidney disease. Some studies have shown that hypoadiponectinemia in CKD is associated with insulin resistance, inflammation, and increased cardiovascular risks7,8. However, majority of the studies have shown that hyperadiponectinemia is paradoxically a risk factor for renal disease progression and high mortality in CKD9,10.
There is a growing interest in evaluating the health-related quality of life (HR-QOL) in CKD patients. HR-QOL is defined as an individual or group’s perceived physical and mental health over the duration of a disease11. Since HR-QOL is relative to the severity of the patient’s perceived symptoms, HR-QOL itself can be a clinical outcome indicator12. CKD is a disease with particularly low HR-QOL. In CKD patients, HR-QOL decreases after initiation of hemodialysis or peritoneal dialysis and increases after kidney transplantation13. Low HR-QOL is a risk factor for CKD progression, cardiovascular disease prevalence, hospitalization, and mortality14–16.
In previous studies, high adiponectin level has been associated with malnutrition or anemia, which can affect HR-QOL17,18. Therefore, we hypothesized that adiponectin would affect HR-QOL in CKD. Despite enormous interest in adiponectin and HR-QOL, the relationship between serum adiponectin level and HR-QOL is unknown in CKD. Therefore, this cross-sectional study was conducted to investigate the association between serum adiponectin level and HR-QOL in the Korean pre-dialysis CKD cohort.
Methods
Study design and population
This cross-sectional study was designed to examine the association between serum adiponectin level and HR-QOL in pre-dialysis CKD patients. ‘KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease’ (KNOW-CKD) is an ongoing multicenter, prospective study which includes pre-dialysis CKD patients from nine nephrology centers of major university hospitals in Korea. A total of 2238 adults between 20 and 75 years of age were enrolled from ‘KNOW-CKD’ study from 2011 to 2016. Among them, 472 and 215 participants were excluded due to missing HR-QOL information and missing data for variables of interest. Finally, 1551 patients were included for analysis in this study (Fig. 1).
Figure 1.
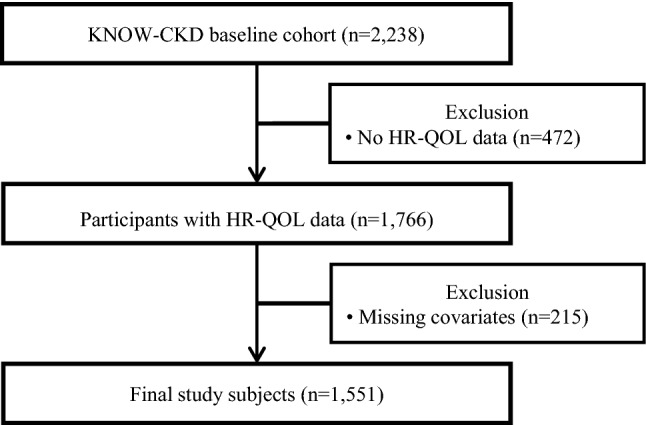
Algorithm for study patient selection from the KNOW-CKD cohort. HR-QOL, health-related quality of life.
The study was conducted in accordance with the principles of the Declaration of Helsinki and supervised by the Korea Centers for Disease Control and Prevention. All study participants were provided with written consent forms. ‘KNOW-CKD’ was approved by the Institutional Review Boards of Kangbuk Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary’s Hospital, Severance Hospital, Gil Hospital, Eulji General Hospital, Chonnam National University Hospital and Pusan Paik Hospital in 2011. A detailed protocol of this study has been previously published19.
Clinical and laboratory measurements
All clinical and laboratory measurement were collected from patients on their initial visit to each hospital. Sociodemographic data, including education, marital status, and income, underlying medical history, medication, and lifestyle patterns, including cigarette smoking and alcohol consumption were collected using self-reported questionnaires with the assistance of a trained staff.
Blood samples were collected after at least 8 h of fasting. Serum adiponectin level was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Adipogen Corp., San Diego, CA, USA). This method had intra- and inter-assay coefficients of variations of ≤ 3.84 and ≤ 5.50%, respectively. Random urine samples were collected midstream for the urine protein-to-creatinine ratio (PCR) measurement. Serum creatinine, cystatin C, and 25-(OH)-vitamin D were measured at the Lab Genomics, Seoul, Republic of Korea. Serum creatinine was measured using an isotope dilution mass spectrometry (IDMS)-traceable method. Other biochemical analyses were conducted in each participating hospital’s laboratory. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Blood pressure (BP) was measured by a trained nurse using an electronic sphygmomanometer after 5 min of rest in the sitting position. Hypertension was defined as: (a) systolic blood pressure (SBP) > 140 mmHg or diastolic blood pressure > 90 mmHg or (b) previous diagnosis of hypertension. Diabetes mellitus (DM) was defined as: (a) fasting serum glucose > 126 mg/dL or (b) previous diagnosis of DM.
HR-QOL assessment
Kidney Disease Quality of Life-Short Form (KDQOL-SF) instrument was used to assess comprehensive HR-QOL in CKD patients20. The KDQOL-SF contains a 36-item short form survey (SF-36) that includes a total of 36 items from each of the four domains of physical and mental health measurements. The Korean versions of KDQOL-SF and SF-36, which were verified as valid HR-QOL instruments in previous studies, were used in this study21,22. The questionnaires were completed with the aid of trained personnel if help was needed. The SF-36 is composed of two dimensions: physical component summary (PCS) and mental component summary (MCS). PCS includes the following four domains: (1) physical function, (2) role limitations due to physical problems, (3) body pain, and (4) general physical health. MCS includes the following four domains: (1) vitality, (2) limitation due to emotional problems, (3) social function, and (4) general mental health. Response to each PCS and MCS dimension was converted to the SF-36 equivalent score ranging from 0 to 100. Higher numerical scores indicate better HR-QOL or less impairment. In this study, the PCS and MCS scores were the primary outcomes of interest. High PCS and MCS were defined as the highest quartile score of each dimension.
Statistical analysis
Participants were categorized into three groups (T1, T2, and T3) according to serum adiponectin tertile. Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range. Continuous variables were analyzed using the Kruskal–Wallis test or one-way analysis of variance. Categorical variables were expressed as percentages. Comparison of categorical variables between each adiponectin tertile group was performed using the Chi-square test.
We constructed generalized linear model and plotted adjusted predictions of PCS and MCS according to serum adiponectin tertile using the Stata command, marginsplot. We used multivariate logistic regression analysis to estimate the odds ratio (OR) and 95% confidence interval (CI) for the high PCS and MCS. These regression models were adjusted for age, sex, body mass index (BMI), marriage, economy, education, systolic BP, DM, dyslipidemia, cardiovascular disease, eGFR, urine PCR, C-reactive protein (CRP), hemoglobin, current smoking status, alcohol intake, and physical activity measured as metabolic equivalent (MET).
All statistical analyses were performed using the Stata version 15.0 (StataCorp LP, College Station, TX, USA). Two-sided P-values < 0.05 were considered statistically significant.
Results
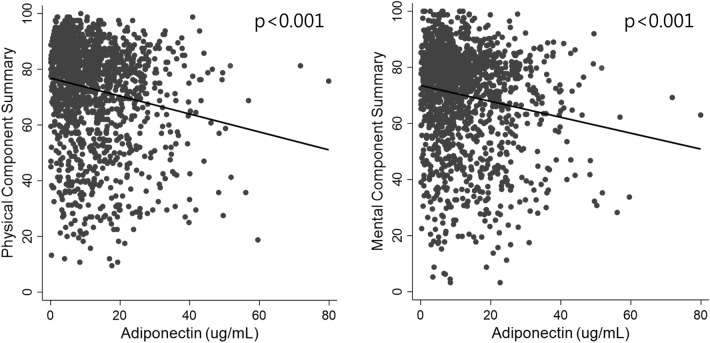
The mean age of the overall study population was 52.5 ± 12.4 years, and 61.25% of them were male. The mean serum adiponectin level was 72.87 ± 18.26 ug/mL, and the range was between 0.12 and 79.89 ug/mL. In comparison of the baseline characteristics of the study population according to adiponectin tertiles, the higher tertile groups had higher percentage of females and urine PCR levels, and lower BMI, eGFR, CRP, hemoglobin, and serum albumin. In the sociodemographic and lifestyle pattern domains, the higher tertile groups had lower percentage of those with high education, high income, current smokers, and those who consumed alcohol more than twice a week. Furthermore, the PCS score was lower and the percentage of those with high PCS was also lower in the higher adiponectin tertile groups. Similar trend was seen with MCS, as the mean MCS score was lower and the percentage of those with high MCS was also lower in the higher adiponectin tertile groups (Table 1). The scatter plot of each PCS and MCS scores versus serum adiponectin level shows their inverse correlations (Fig. 2).
Table 1.
Baseline characteristic of participants according to serum adiponectin tertile.
| Adiponectin tertile | p for trend | ||||
|---|---|---|---|---|---|
| Total (0.12–79.89) | T1 (0.12–6.54) | T2 (6.55–13.71) | T3 (13.74–79.89) | ||
| Total number of patients | 1551 | 517 | 517 | 517 | |
| Adiponectin, ug/mL | 72.87 ± 18.26 | 3.74 ± 1.80 | 9.80 ± 2.17 | 23.28 ± 8.92 | |
| Age, years | 52.5 ± 12.4 | 51.4 ± 12.2 | 53.2 ± 12.1 | 53 ± 12.7 | 0.039 |
| Male sex | 950 (61.3) | 389 (75.2) | 312 (60.4) | 249 (48.2) | < 0.001 |
| BMI, kg/m2 | 24.5 ± 3.4 | 25.4 ± 3.1 | 24.6 ± 3.4 | 23.4 ± 3.4 | < 0.001 |
| Systolic BP, mmHg | 128 ± 16 | 128 ± 16 | 130 ± 18 | 129 ± 16 | 0.06 |
| Diastolic BP, mmHg | 77 ± 11 | 77 ± 11 | 76 ± 11 | 78 ± 11 | 0.404 |
| eGFR, mL/min/1.73 m2 | 54.1 ± 31.5 | 63.5 ± 31.6 | 54.5 ± 29.1 | 44.3 ± 30.8 | < 0.001 |
| Urine PCR, g/g | 0.5 (0.1–1.5) | 0.4 (0.1–1) | 0.5 (0.1–1.4) | 0.7 (0.2–2.3) | < 0.001 |
| CRP, mg/dL | 0.6 (0.2–1.6) | 0.8 (0.3–2) | 0.6 (0.2–1.5) | 0.5 (0.2–1.4) | < 0.001 |
| Hemoglobin, g/dL | 12.9 ± 2 | 13.7 ± 2 | 13 ± 1.8 | 11.9 ± 1.7 | < 0.001 |
| Serum albumin, g/dL | 4.2 ± 0.4 | 4.3 ± 0.3 | 4.2 ± 0.4 | 4.0 ± 0.5 | < 0.001 |
| High educationa | 682 (44.0) | 267 (51.6) | 227 (43.9) | 188 (36.4) | < 0.001 |
| Diabetes | 497 (32.0) | 174 (33.7) | 152 (29.4) | 171 (33.1) | 0.842 |
| Dyslipidemia | 831 (53.6) | 284 (54.9) | 286 (55.3) | 261 (50.5) | 0.152 |
| Cardiovascular disease | 200 (12.9) | 64 (12.4) | 75 (14.5) | 61 (11.8) | 0.781 |
| Married | 1286 (82.9) | 423 (81.8) | 443 (85.7) | 420 (81.2) | 0.804 |
| High incomeb | 367 (23.7) | 132 (25.5) | 133 (25.7) | 102 (19.7) | 0.028 |
| Current smoker | 255 (16.4) | 109 (21.1) | 83 (16.1) | 63 (12.2) | < 0.001 |
| Alcohol intakec | 218 (14.1) | 87 (16.8) | 75 (14.5) | 56 (10.8) | 0.006 |
| Physical activity, MET min/wk | 1188 (264–2754) | 1332 (297–2853) | 1188 (297–2613) | 990 (198–2754) | 0.855 |
| PCS | 72.9 ± 18.3 | 76.1 ± 16.9 | 73.9 ± 17.8 | 68.7 ± 19.3 | < 0.001 |
| MCS | 70 ± 18.1 | 72.5 ± 17.4 | 71.2 ± 17 | 66.4 ± 19.2 | < 0.001 |
| High PCS | 402 (25.9) | 172 (33.3) | 142 (27.5) | 88 (17.0) | < 0.001 |
| High MCS | 402 (25.9) | 164 (31.7) | 128 (24.8) | 110 (21.3) | < 0.001 |
Values for categorical variables are reported as number (percentage) and for continuous variables as mean ± standard deviation or median (interquartile range).
The p values are for the Kruskal–Wallis tests or one-way analysis of variance for continuous variables and Chi-square tests for categorical variables.
PCS physical component summary; MCS mental component summary; BMI body mass index; SBP systolic blood pressure; DBP diastolic blood pressure; LDL low-density lipoprotein; CRP C-reactive protein; eGFR estimated glomerular filtration rate; PCR protein to creatinine ratio; MET metabolic equivalent.
aCollege diploma or more.
bMore than 4.5 million won per month.
cAlcohol intake frequency ≥ 2/week.
Figure 2.
Scatter plot of the HR-QOL scores versus serum adiponectin (A; physical component summary, B; mental component summary).
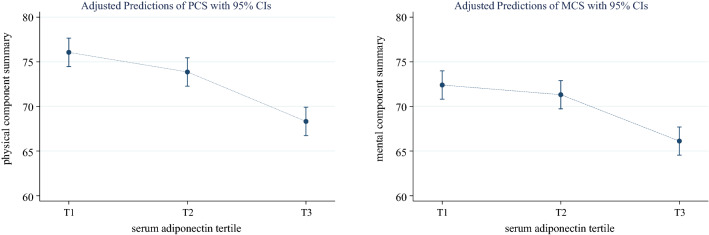
Figure 3 shows the adjusted predictions of PCS and MCS scores according to serum adiponectin tertiles, and both scores were lower in T3. In the multivariate logistic regression analysis, lower adiponectin levels were associated with high PCS scores. As shown in Table 2, the OR (95% CI) for high PCS was 1.44 (1.03–2.01) and 1.56 (1.09–2.23) in T2 and T1, respectively, compared with T3 (Reference). However, serum adiponectin level was not associated with high MCS. (Table 3).
Figure 3.
Adjusted predictions of physical component summary and mental component summary according to serum adiponectin tertile. Adjusted for age, sex, body mass index, marriage, economy, education, systolic blood pressure, diabetes, dyslipidemia, cardiovascular disease, estimated glomerular filtration rate, urine protein to creatinine ratio, C-reactive protein, hemoglobin, albumin current smoking, alcohol, and physical activity.
Table 2.
Multivariate analysis of the association between serum adiponectin tertile and high physical component summary (PCS).
| Adiponectin tertile | Unadjusted model | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR [95% CI] | p value | OR [95% CI] | P value | OR [95% CI] | p value | |
| T1 | 2.43 [1.81–3.26] | < 0.001 | 1.98 [1.44–2.73] | < 0.001 | 1.56 [1.09–2.23] | 0.015 |
| T2 | 1.85 [1.37–2.49] | < 0.001 | 1.65 [1.20–2.25] | 0.002 | 1.44 [1.03–2.01] | 0.033 |
| T3 | Reference | Reference | Reference | |||
Model 1: Adjusted for age, sex, body mass index, marriage, economy, education, systolic blood pressure, diabetes, dyslipidemia, cardiovascular disease, estimated glomerular filtration rate, urine protein to creatinine ratio, C-reactive protein, hemoglobin, and albumin.
Model 2: Adjusted for Model 1 + current smoking, alcohol, and physical activity.
Table 3.
Multivariate analysis of the association between serum adiponectin tertile and high mental component summary (MCS).
| Adiponectin tertile | Unadjusted model | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR [95% CI] | p value | OR [95% CI] | P value | OR [95% CI] | p value | |
| T1 | 1.72 [1.30–2.28] | < 0.001 | 1.41 [1.04–1.92] | 0.028 | 1.19 [0.85–1.68] | 0.312 |
| T2 | 1.22 [0.91–1.63] | 0.184 | 1.04 [0.77–1.41] | 0.795 | 0.94 [0.68–1.29] | 0.694 |
| T3 | Reference | Reference | Reference | |||
Model 1: Adjusted for age, sex, body mass index, marriage, economy, education, systolic blood pressure, diabetes, dyslipidemia, cardiovascular disease, estimated glomerular filtration rate, urine protein to creatinine ratio, C-reactive protein, hemoglobin, and albumin.
Model 2: Adjusted for Model 1 + current smoking, alcohol, and physical activity.
In the subgroup analyses of clinically relevant groups, the association between serum adiponectin level and high PCS was similar across all subgroups with no significant interactions between sex (female vs. male), age (< 55 vs. ≥ 55 years), BMI (< 23.5 vs. ≥ 23.5 kg/m2), diabetes (no vs. yes), proteinuria (< 1.0 vs. ≥ 1.0 g/day), and CRP (< 0.5 vs. ≥ 0.5 mg/dL). Although the association was modified by the eGFR level (< 45 vs. ≥ 45 mL/min/1.73 m2), the trend was similar (Table 4).
Table 4.
Association between serum adiponectin tertile and high PCS in clinically relevant subgroups.
| Adiponectin tertile | ||||
|---|---|---|---|---|
| T1 | T2 | T3 | p for interaction | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Sex | 0.591 | |||
| Female (n = 601) | 1.25 [0.67–2.34] | 1.40 [0.83–2.35] | Reference | |
| Male (n = 950) | 1.69 [1.07–2.68] | 1.45 [0.93–2.28] | Reference | |
| Age, years | 0.678 | |||
| < 55 (n = 820) | 1.68 [1.03–2.73] | 1.34 [0.85–2.11] | Reference | |
| ≥ 55 (n = 731) | 1.38 [0.79–2.40] | 1.59 [0.96–2.65] | Reference | |
| BMI, kg/m2 | 0.564 | |||
| < 23.5 (n = 605) | 1.15 [0.65–2.04] | 1.08 [0.67–1.76] | Reference | |
| ≥ 23.5 (n = 946) | 1.96 [1.17–3.27] | 1.81 [1.10–2.99] | Reference | |
| Diabetes | 0.532 | |||
| Diabetes (−) (n = 1054) | 1.80 [1.18–2.74] | 1.66 [1.13–2.45] | Reference | |
| Diabetes ( +) (n = 497) | 1.11 [0.55–2.26] | 0.87 [0.43–1.76] | Reference | |
| eGFR, mL/min/1.73 m2 | 0.029 | |||
| < 45 (n = 734) | 2.73 [1.54–4.85] | 2.22 [1.30–3.79] | Reference | |
| ≥ 45 (n = 817) | 1.02 [0.64–1.64] | 1.04 [0.67–1.63] | Reference | |
| Urine protein/creatinine, g/g | 0.925 | |||
| < 1.0 (n = 1039) | 1.74 [1.12–2.70] | 1.50 [0.99–2.26] | Reference | |
| ≥ 1.0 (n = 512) | 1.28 [0.66–2.46] | 1.23 [0.67–2.26] | Reference | |
| CRP, mg/dL | 0.452 | |||
| < 0.5 (n = 632) | 1.50 [0.87–2.60] | 1.22 [0.75–1.98] | Reference | |
| ≥ 0.5 (n = 919) | 1.56 [0.95–2.56] | 1.60 [0.99–2.58] | Reference | |
BMI body mass index; eGFR estimated glomerular filtration rate; CRP C-reactive protein.
Discussion
In this cross-sectional study, we analyzed the association between serum adiponectin level and HR-QOL in the Korean pre-dialysis CKD patients. Patients with lower serum adiponectin level had higher PCS and MCS scores than those with high serum adiponectin level. According to multivariate logistic regression analyses, low serum adiponectin level was significantly and independently associated with high PCS. This association was preserved in clinically relevant subgroups. However, there was no statistically significant association between serum adiponectin level and high MCS.
Adiponectin is a 30-kDa, 244 amino acid polypeptide hormone released by adipocytes, which exists as low, medium, and high molecular weight proteins23. It has insulin-sensitizing, anti-inflammatory, and anti-atherogenic functions towards different cell types. Several previous studies have reported that higher serum adiponectin level has a protective function against metabolic and cardiovascular diseases, as it is associated with higher HDL-cholesterol levels and lower DM and cardiovascular disease risks1,2,9,24. However, in CKD patients, higher serum adiponectin level is paradoxically associated with proteinuria, CKD progression, and all-cause mortality9,10,23,25,26.
Previous studies have investigated the clinical implication of HR-QOL in CKD13–15,27. In patients with end-stage renal disease, low HR-QOL was generally associated with higher risks of mortality and hospitalization16,28,29. According to a study that assessed the HR-QOL in 3837 CKD patients, low HR-QOL was associated with higher risk of cardiovascular event and mortality, but not with CKD progression15. On the other hand, according to a Korean study that assessed the impact of HR-QOL in CKD progression, lower PCS score was associated with increased CKD progression risk14.
To our knowledge, there is not yet a study that has assessed the association between serum adiponectin level and HR-QOL in pre-dialysis CKD patients. According to our study, low serum adiponectin level was associated with better physical HR-QOL. The mechanism underlying this association is unknown; however, we can suggest several explanations for our finding.
First, previous studies have reported association between high adiponectin levels and malnutrition in pre-dialysis CKD patients17,30,31. A large percentage of CKD patients are in a ‘malnutrition-inflammation’ status caused by reduced caloric intake due to symptoms of uremia, reduced absorption of nutrients, cachexia, and persistent inflammatory status. In CKD, protein-energy wasting is caused by a complex interaction of persistent inflammation, malnutrition, acidosis status, and increased catabolism, resulting in sarcopenia32. It is assumed that serum adiponectin levels are increased to counteract this ‘malnutrition-inflammation’ status33. Furthermore, according to a Korean study, mildly obese CKD patients were more likely to be in a state of adequate nutrition without inflammation than those with low BMI and total body fat34. Consistent with the above finding, results from our study showed that the highest adiponectin tertile group (T3) had significantly lower BMI and serum albumin level than the lower adiponectin tertile groups (T1,T2). The combined results of reduced muscle mass, body fat mass, and symptoms of fatigue caused by malnutrition would have contributed to limitations in physical activity, as reflected by lower PCS scores in the high adiponectin tertile group.
Second, serum adiponectin exists mainly as two types of hexamers: as a low molecular weight complex (LMW) and as a high molecular weight (HMW) complex consisting of 12–18 subunits35. The ratio between the two complexes (HMW to LMW complex) determines its insulin-sensitizing activity36. In CKD patients, the altered ratios of the adiponectin complexes result in a decreased insulin-sensitizing activity. Subsequently, this leads to an increased insulin resistance that ultimately increases muscle proteolysis resulting in an increased skeletal muscle wasting and weakness associated with poor physical QOL36.
Third, previous studies have shown that high serum adiponectin levels were associated with anemia in CKD patients18,37. Anemia is associated with low HR-QOL, especially physical activity impairment, due to its symptoms, including fatigue, dizziness, dyspnea as well as being in an inflammatory state38–40. Consistent results were found in our study as hemoglobin levels were significantly lower in the high adiponectin tertile group than in the lower tertile groups.
It is unclear why low adiponectin level was only associated with high PCS and not high MCS. We hypothesize the mechanism behind this finding as the following. Previous studies have reported that low serum adiponectin level was associated with various mental disorders, including insomnia and depression, and neurodegenerative disorders, including Alzheimer’s disease41–43. Insomnia, depression, and Alzheimer’s disease are comorbidities that can affect the MCS score. As CKD patients are in a relatively hyperadiponectemia state, the increased adiponectin level may have had neuroprotective effects and influenced the association between adiponectin and mental HR-QOL.
There are a few limitations of this study that include the following. First, as this is a cross-sectional study, we cannot determine the causal relationship between serum adiponectin level and HR-QOL. Further longitudinal studies would be helpful to verify the causality. Second, adiponectin level and HR-QOL are both affected by body compositions, such as adiposity and muscle mass. We included BMI in the analytical model, but unfortunately, we have no data on body composition analysis.
Despite these limitations, our study also has strengths. The KNOW-CKD is a well-organized, multi-center, prospective cohort study that employed a very strict and uniform protocol to collect and record data. In addition, we included various factors in the analysis, including sociodemographic, lifestyle information, and comorbid status, which could affect the HR-QOL.
In conclusion, low adiponectin levels were associated with better physical HR-QOL in pre-dialysis CKD patients, and this association was independent of various metabolic and cardiovascular factors. This study suggests the possible role of measuring serum adiponectin level in evaluating CKD patients. More attention should be given to the HR-QOL and prognosis of patients with low serum adiponectin level. Further studies, including longitudinal studies, are needed to confirm the relationship between serum adiponectin level and HR-QOL and its clinical significance.
Author contributions
J.H.K and J.M.H: Drafting the article, analysis and interpretation of data, revising the article and final approval of the article. H.K, K.B,L, W.C, Y.S.K, S.K.P, D.W.C, C.A and K.H.O: Analysis and interpretation of data, revising the article and final approval of the article. Y.Y.H: Conception or design, analysis and interpretation of data and final approval of the article.
Funding
The KNOW-CKD was supported by the research program of the Korea Centers for Disease Control and Prevention (grants 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, and 2016E3300201).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ji Hye Kim and Ji Min Han.
References
- 1.Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 2.Van de Voorde J, Pauwels B, Boydens C, Decaluwe K. Adipocytokines in relation to cardiovascular disease. Metabolism. 2013;62:1513–1521. doi: 10.1016/j.metabol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Van Berendoncks AM, Garnier A, Ventura-Clapier R, Conraads VM. Adiponectin: Key role and potential target to reverse energy wasting in chronic heart failure. Heart Fail. Rev. 2013;18:557–566. doi: 10.1007/s10741-012-9349-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, et al. Plasma adiponectin levels and type 2 diabetes risk: A nested case-control study in a Chinese population and an updated meta-analysis. Sci. Rep. 2018;8:406–406. doi: 10.1038/s41598-017-18709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzaghi C, Trischitta V. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes. 2018;67:12–22. doi: 10.2337/dbi17-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarale MG, Fontana A, Trischitta V, Copetti M, Menzaghi C. Circulating adiponectin levels are paradoxically associated with mortality rate: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2018;104:1357–1368. doi: 10.1210/jc.2018-01501. [DOI] [PubMed] [Google Scholar]
- 7.Becker B, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The mild and moderate kidney disease study. J. Am. Soc. Nephrol. 2005;16:1091–1098. doi: 10.1681/asn.2004090742. [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J. Am. Soc. Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 9.Lo MM, Mitsnefes M. Adiponectin, cardiovascular disease, chronic kidney disease: Emerging data on complex interactions. Pediatr. Nephrol. 2012;27:521–527. doi: 10.1007/s00467-011-1804-2. [DOI] [PubMed] [Google Scholar]
- 10.Heidari M, Nasri P, Nasri H. Adiponectin and chronic kidney disease; a review on recent findings. J. Nephropharmacol. 2015;4:63–68. [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. HRQOL Concepts, https://www.cdc.gov/hrqol/concept.htm (2018).
- 12.Yapa HE, Purtell L, Chambers S, Bonner A. The relationship between chronic kidney disease, symptoms and health-related quality of life: A systematic review. J. Ren Care. 2019 doi: 10.1111/jorc.12303. [DOI] [PubMed] [Google Scholar]
- 13.Avramovic M, Stefanovic V. Health-related quality of life in different stages of renal failure. Artif. Organs. 2012;36:581–589. doi: 10.1111/j.1525-1594.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 14.Oh TR, et al. Association between health related quality of life and progression of chronic kidney disease. Sci. Rep. 2019;9:19595. doi: 10.1038/s41598-019-56102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter AC, et al. Predictors and outcomes of health-related quality of life in adults with CKD. Clin. J. Am. Soc. Nephrol. 2016;11:1154–1162. doi: 10.2215/cjn.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mapes DL, et al. Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Hyun YY, et al. Serum adiponectin and protein-energy wasting in predialysis chronic kidney disease. Nutrition. 2017;33:254–260. doi: 10.1016/j.nut.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, et al. High serum adiponectin is associated with anemia development in chronic kidney disease: The results from the KNOW-CKD study. Cytokine. 2018;103:1–9. doi: 10.1016/j.cyto.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Oh KH, et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): Design and methods. BMC Nephrol. 2014;15:80. doi: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual. Life Res. 1994;3:329–338. doi: 10.1007/bf00451725. [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, et al. Reliability and validity of the Korean version of Kidney Disease Quality of Life instrument (KDQOL-SF) Tohoku J. Exp. Med. 2007;211:321–329. doi: 10.1620/tjem.211.321. [DOI] [PubMed] [Google Scholar]
- 22.Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M. Development of the Korean version of Short-Form 36-Item Health Survey: Health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J. Exp. Med. 2004;203:189–194. doi: 10.1620/tjem.203.189. [DOI] [PubMed] [Google Scholar]
- 23.Sweiss N, Sharma K. Adiponectin effects on the kidney. Best Pract. Res. Clin. Endocrinol. Metab. 2014;28:71–79. doi: 10.1016/j.beem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata R, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon V, et al. Adiponectin and mortality in patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:2599–2606. doi: 10.1681/asn.2006040331. [DOI] [PubMed] [Google Scholar]
- 26.Kollerits B, Fliser D, Heid IM, Ritz E, Kronenberg F. Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: The Mild to Moderate Kidney Disease Study. Kidney Int. 2007;71:1279–1286. doi: 10.1038/sj.ki.5002191. [DOI] [PubMed] [Google Scholar]
- 27.Perlman RL, et al. Quality of life in chronic kidney disease (CKD): A cross-sectional analysis in the Renal Research Institute-CKD study. Am. J. Kidney Dis. 2005;45:658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J. Am. Soc. Nephrol. 2001;12:2797–2806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 29.Lopes AA, et al. Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual. Life Res. 2007;16:545–557. doi: 10.1007/s11136-006-9143-7. [DOI] [PubMed] [Google Scholar]
- 30.Bobin-Dubigeon C, et al. Leptin and adiponectin as new markers of undernutrition in cancer. Clin. Biochem. 2017;50:525–528. doi: 10.1016/j.clinbiochem.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Dervisoglu E, Eraldemir C, Kalender B, Kir HM, Caglayan C. Adipocytokines leptin and adiponectin, and measures of malnutrition-inflammation in chronic renal failure: Is there a relationship? J. Ren. Nutr. 2008;18:332–337. doi: 10.1053/j.jrn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Hara H, et al. Protein energy wasting and sarcopenia in dialysis patients. Contrib. Nephrol. 2018;196:243–249. doi: 10.1159/000485729. [DOI] [PubMed] [Google Scholar]
- 33.Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int. 2009;76:567–575. doi: 10.1038/ki.2009.200. [DOI] [PubMed] [Google Scholar]
- 34.Suh SH, et al. Chronic kidney disease attenuates the impact of obesity on quality of life. Sci. Rep. 2020;10:2375. doi: 10.1038/s41598-020-59382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J. Biol. Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 36.Pajvani UB, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 37.Aso Y, et al. Anemia is associated with an elevated serum level of high-molecular-weight adiponectin in patients with type 2 diabetes independently of renal dysfunction. Transl. Res. 2009;154:175–182. doi: 10.1016/j.trsl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Gerson A, et al. Anemia and health-related quality of life in adolescents with chronic kidney disease. Am. J. Kidney Dis. 2004;44:1017–1023. doi: 10.1053/j.ajkd.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 39.van Haalen H, Jackson J, Spinowitz B, Milligan G, Moon R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: Analysis of multinational real-world data. BMC Nephrol. 2020;21:88. doi: 10.1186/s12882-020-01746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksson D, Goldsmith D, Teitsson S, Jackson J, van Nooten F. Cross-sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol. 2016;17:97. doi: 10.1186/s12882-016-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloemer J, et al. Role of adiponectin in central nervous system disorders. Neural Plast. 2018;2018:4593530. doi: 10.1155/2018/4593530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diniz BS, et al. Reduced serum levels of adiponectin in elderly patients with major depression. J. Psychiatr. Res. 2012;46:1081–1085. doi: 10.1016/j.jpsychires.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Wędrychowicz A, Zając A, Pilecki M, Kościelniak B, Tomasik PJ. Peptides from adipose tissue in mental disorders. World J. Psychiatry. 2014;4:103–111. doi: 10.5498/wjp.v4.i4.103. [DOI] [PMC free article] [PubMed] [Google Scholar]