Abstract
Background
If osteomyelitis is confined to the proximal humerus, arthroscopic debridement with multiple punctures at the infected bone might be sufficient to eradicate the septic shoulder with bone involvement.
Materials and Methods
From 2005 to 2017, 15 patients received arthroscopic debridement with multiple punctures. We included patients with septic shoulder arthritis with proximal bone involvement and excluded patients with glenohumeral joint destruction or extension of bone involvement to the diaphysis of the humerus. We performed multiple punctures for drainage of proximal humerus after complete arthroscopic debridement of septic soft tissue. Infection laboratory studies and postoperative magnetic resonance image were evaluated. For clinical outcome measurements, range of motion, pain visual analog scale, functional visual analog scale, American shoulder elbow surgeon scores, constant scores, and simple shoulder test were evaluated.
Results
There were 11 males and 4 females with a mean age of 53 years (range 28–73 years). Mean follow-up was 32 months (range 12–115 months). There was no reinfection case. The postoperative C-reactive protein levels were normalized in all. The postoperative magnetic resonance image showed no bony involvement of the proximal humerus in all patients except one patient. The clinical scores and range of motion were significantly improved postoperatively. Six patients underwent secondary surgery for rotator cuff tear at a mean time period of 25 months (range 4–104 months) from the index period.
Conclusion
Septic shoulder with proximal bone involvement can be successfully treated with arthroscopic debridement with multiple punctures.
Level of Evidence
Level IV, treatment study
Keywords: Shoulder, Infection, Osteomyelitis, Arthroscopy, Debridement
Introduction
Shoulder infection is an orthopedic emergent disease that should be treated promptly to avoid the destruction of the cartilage of the shoulder joint and the life-threatening situation [1]. The incidence of bacterial joint infection is reported to be 4 to 12 out of 100,000 per year [2, 3]. A joint infection may damage cartilage directly by bacterial enterotoxins and indirectly from the host immune response to bacteria [4]. Delayed treatment of septic arthritis can result in joint destruction, osteomyelitis, or joint instability [5, 6].
Surgical treatment of septic arthritis of the shoulder performed arthroscopically with lavage and debridement has been reported in early stage of infection without joint articulation damage [7–10]. An open surgical approach is more commonly performed in cases involving either delayed diagnosis or late stages of infection with joint articulation damage and bone involvement [10, 11]. When bony and cartilage destruction or osteomyelitis is present, joint preservation is typically not possible and treatment options such as resection arthroplasty with antibiotic impregnated cement spacer [12] or two stage arthroplasty operation is necessary [8]. However, it is very conflicting to make decision on those patients who have infected joint with proximal bone signal change in MRI especially to the upper 1/3 of proximal humerus. Although it might be related to some bone edema, we cannot rule out osteomyelitis of proximal humerus. This gives us the dilemma of the treatment to include bone resection or not. Nonetheless, bone resection is very destructive surgery with severe injury to remaining rotator cuff, especially the subscapularis tendon. Moreover, the remaining surgery is usually left with arthroplasty in very young age.
There is no study on arthroscopic treatment for osteomyelitis of the proximal humerus in septic shoulder arthritis without bone destruction. We hypothesized that if osteomyelitis is confined to the proximal humerus without involvement of the entire diaphysis of the humerus, arthroscopic debridement with multiple punctures at the infected bone such as greater tuberosity, lesser tuberosity, and humeral head might be sufficient to eradicate the septic shoulder with bone involvement. The purpose of this study was to report the clinical results after arthroscopic debridement with multiple punctures for septic shoulder arthritis with proximal bone involvement.
Materials and Methods
Patients
We retrospectively reviewed data from consecutive patients who were treated at our department because of a septic arthritis of the shoulder between December 2005 and February 2017. During the study period, 91 septic shoulder arthritis patients were treated by arthroscopic debridement, open debridement, or open resection arthroplasty. Among a total of 91 cases, we included our study patients based on the following inclusion criteria: (1) those who had infection in glenohumeral joint; (2) those who had proximal humerus involvement (until metaphysis of humerus) without glenohumeral joint destruction in magnetic resonance imaging (MRI) (Fig. 1); (3) those who treated by arthroscopic debridement and multiple punctures for involvement of proximal humerus. The exclusion criteria of this study were: (1) diaphysis involvement, (2) abnormal immune system, (3) atypical infection (such as tuberculosis, fungus, etc.), (4) follow-up < 2 years. The diagnosis of a septic arthritis had to be confirmed by a positive culture of the joint fluid. In negative culture of the joint fluid, the diagnosis of a septic arthritis was made by clinical symptoms (such as pain at rest, severe limited range of motion, redness, and heating sense), pus like joint fluid, increased inflammatory parameters (such as C-reactive protein), and MRI findings suggesting septic arthritis. Of 91 patients, 44 patients underwent arthroscopic debridement only and 28 patients underwent open resection arthroplasty with antibiotic impregnated cement spacer. A total of 19 patients underwent arthroscopic debridement and multiple punctures and were included this study. One patient was excluded due to additional open debridement for diaphysis involvement, two patients were excluded due to immunosuppressive treatment, and one patient was excluded due to tuberculosis infection. After exclusion, 15 patients met the criteria for this study.
Fig. 1.
T1-weighted fat suppression contrast-enhanced coronal view and axial view showing high signal change around the greater tuberosity and humeral head (a). There is no high signal change at the proximal diaphysis of the humerus (b). Yellow line indicates axial plane
Surgical Technique
All surgical procedures were performed by a single experienced surgeon. Under general anesthesia, the patient was placed in semi-lateral decubitus position with 30° posterior tilt. Evaluation of motion range was done and gentle manipulation was used in where motion was limited. Posterior viewing portal was made, and routine glenohumeral joint examination was done. Before starting intra-articular saline inflow, joint fluid for microbiological culture was collected in all patients.
A thorough synovectomy was performed at infected capsules of the joint. Anterior gutter, humeral insertion of the anterior capsule, and posterior gutter were thoroughly debrided. After synovectomy was finished at intra-articular space, the arthroscopy was inserted into the subacromial space. Thorough subacromial bursectomy was also done. The bursa of the subcoracoid space at the front, the bursa of the teres minor at the posterior, and the bursa of the deltoid were thoroughly removed. After synovectomy, all areas showing signal change in MRI were punctured. If there was rotator cuff tear, debridement and multiple punctures were done mainly through the greater tuberosity of tear site. If there were anchors, all foreign materials were thoroughly removed. Multiple punctures were performed using 4.0 mm punch and turbid fat globulin material and fluid were gush out from the holes (Fig. 2). An adequate number of holes were made to provide sufficient drainage in the signal change area of the MRI. In most patients, cartilage destruction of greater tuberosity was observed and we performed punctures 5–6 times through this. After making holes using punch, a careful debridement was performed by inserting a shaver though the hole. If there is no tear, multiple punctures were done at the lateral anchor site which is distal to the greater tuberosity at the subacromial space and Hill-Sachs area at the intra-articular side.
Fig. 2.
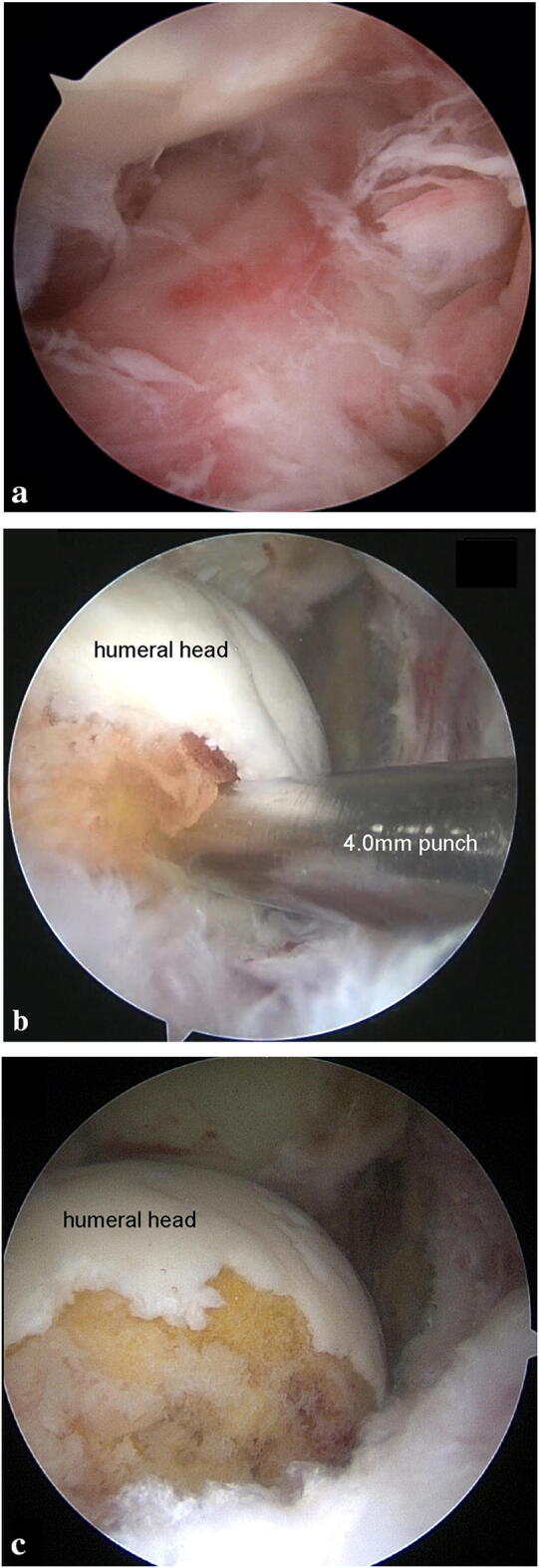
Arthroscopic images with 30° arthroscope in the subacromial space at anterolateral portal (a). After decortication and multiple punctures of greater tuberosity, turbid fat globulin materials and fluid were gush out (b) and cancellous bone was exposed (c)
After surgery, arm sling was applied and the patients were allowed to do daily activity without restrictions. Based on the results of intraoperative culture, we consulted with the department of infectious diseases for selection of appropriated antibiotics choice. Antibiotics were routinely administered for 4–6 weeks after surgery. During the hospitalization period, intravenous antibiotics were administered and they were replaced with oral antibiotics after discharge.
Data Collection and Clinical Assessment
Data from each patient’s demographic data (sex, age at surgery, dominant arm involvement), medical history, and comorbidities were collected. Preoperative injection history and operative history were reviewed. Post-operative antibiotics type, dosage, and period were reviewed.
Clinical evaluation was performed preoperatively and most recently at outpatient clinic. Range of motion (ROM) was measured in forward elevation (FE), external rotation at side (ERS), and internal rotation in vertebral level (IRV). For statistical analysis, we converted vertebral level to a number: T4 ~ T7 to 10, T8 ~ T12 to 8, L1 ~ L3 to 6, L4 ~ L5 to 4, sacrum and buttock to 2. Pain visual analog scale (PVAS), functional visual analog scale (FVAS), American shoulder elbow surgeons scores (ASES) [13], Constant scores [14], and Simple shoulder test (SST) [15] were collected pre- and post-operatively. Clinical evaluations were all performed by a single physiotherapist who was unaware of this study.
Six of fifteen patients underwent additional secondary surgery for cuff pathology after all infections had been treated. We classified patients who underwent additional cuff surgery as a secondary surgery group and those without additional cuff surgery as non-secondary surgery group. For secondary surgery group, clinical scores after cuff surgery were also analyzed.
Laboratory and Radiologic Evaluation
Before starting saline inflow to arthroscopy, we collected intraoperative joint fluid for microbiological culture in all patients using a culture bottle. Inflammatory parameters, such as fever, white blood cell (WBC, in 103/μl) count, C-reactive protein (CRP, in mg/L), and Erythrocyte sedimentation rate (ESR, in mm/hr) were analyzed pre- and post-operatively.
Radiographs of the affected shoulder were performed in all patients. All patients did not show joint destruction of shoulder in plain radiographs. All patients underwent preoperative MRI before surgery. Thirteen patients underwent MRI at our institution and two patients underwent MRI at other institution and brought the images to the hospital.
All preoperative MRIs showed that intra-articular fluid collection with rim enhancement and high signal change around the greater tuberosity and humeral head (Fig. 1). But they showed no high signal in the humerus diaphysis, confirming that bone involvement was limited to proximal humerus only. To evaluate the signal change after arthroscopic treatment, the postoperative MRI was performed in all patients except one. All MRI was performed with a 3.0T MR imager (Gyroscan Interna Achieva, Best, Netherlands).
Our Institutional Review Board approved this retrospective study and waived the requirement for informed patient consent.
Statistical Analysis
Preoperative and postoperative values were analyzed using the Wilcoxon signed rank test. Comparisons between secondary surgery group and non-secondary surgery group were analyzed using the Mann–Whitney test and Fisher’s exact test. All statistical analysis was performed with the significance level set at P = 0.05 and using the software SPSS (Windows Release 20.0, SPSS Inc, Armonk, NY, USA).
Results
Preoperative patient data were shown in Table 1. There were 11 males and 4 females. Mean age of patients was 53 years (range, 28–73 years) at the time of surgery. Patients underwent arthroscopic treatment on average 74 days (17–167) after symptom onset. Eleven patients were dominant arm involved. Four patients had a diabetes. Mean follow-up was 32 months (range 12–115 months). All patients were followed up for 1 year, and 12 patients were followed up for more than 2 years.
Table 1.
Preoperative patient data
| Case | Sex | Age, years | Symptom duration, day | Infection cause | Other hospital treatment before our treatment | Preoperative CRPa (mg/L) | Joint fluid culture |
|---|---|---|---|---|---|---|---|
| 1 | Male | 53 | 37 | A/S RC repair | A/S debridement | 145 | Enterobacter cloacae |
| 2 | Male | 56 | 35 | A/S RC repair | IV antibiotics | 12.1 | Serratia marcescens |
| 3 | Male | 59 | 17 | Injection | IV antibiotics | 139 | Negative |
| 4 | Male | 32 | 48 | A/S RC repair | IV antibiotics | 1.5 | MSSA |
| 5 | Male | 28 | 161 | Injection | A/S debridement | 0.3 | Negative |
| 6 | Male | 44 | 73 | A/S RC repair | A/S debridement | 5.8 | Negative |
| 7 | Female | 60 | 30 | Injectionb | none | 9.3 | Negative |
| 8 | Female | 66 | 52 | Injection | A/S debridement | 5.8 | MRSA |
| 9 | Male | 69 | 167 | Injection | none | 23.1 | Negative |
| 10 | Male | 53 | 106 | Injection | IV antibiotics | 8.5 | MRSA |
| 11 | Female | 53 | 28 | Injection | IV antibiotics | 18.6 | Negative |
| 12 | Male | 50 | 57 | A/S SLAP repair | A/S debridement | 14.7 | Bacillus species |
| 13 | Female | 73 | 123 | Injection | IV antibiotics | 9.9 | Negativec |
| 14 | Male | 55 | 90 | Injection | A/S debridement | 44.1 | MSSA |
| 15 | Male | 50 | 90 | A/S unknown operation | none | 134 | MSSA |
| Mean | 53 | 74 | 38.1 |
CRP C-reactive protein, RC rotator cuff, A/S arthroscopic, IV intravenous, MSSA methicillin-sensitive S. aureus, MRSA methicillin-resistant S. aureus
aNormal range 0–3
b6 years ago, rotator cuff repair was done
cMRSA was cultured in other hospital
Nine patients received intra-articular injections prior to septic shoulder arthritis. Six patients had a history of shoulder arthroscopic surgery at other hospital and had infection symptom immediately after surgery. One patient underwent rotator cuff repair at our hospital 6 years ago and had infection symptom one month ago after shoulder injection. Eight patients did not have a history of shoulder surgery, but all had a history of shoulder injection. After the diagnosis of infection, six patients underwent arthroscopic debridement at other hospital, but failed to control infection and were transferred to our hospital (Table 1).
The preoperative ESR and CRP increased by an average of 75.5 (mm/hr) (range 5–120 mm/hr), 38.1 (mg/l) (range 0.3–145 mg/l), respectively. Thirteen patients had elevated ESR and CRP, but two patients showed normal range. The mean preoperative WBC was 7.66 (×103/μl) (range 2.5–12.4×103/μl) and only two patients showed elevation. There were one patient who had fever (> 37.4°) before surgery. The other 14 patients had no fever. One of the two patients with normal ESR and CRP was confirmed as a septic shoulder by positive joint fluid culture. The other one was diagnosed by clinical symptoms, turbid joint fluid and MRI findings.
The average operation time was 74 min (range 50–120 min). For irrigation, and mean used saline for surgery were 31 l (range 12–60 l). The causative organism was cultured in 8 patients. Three of them were methicillin-sensitive S. aureus (MSSA), two was methicillin-resistant S. aureus (MRSA), one was Enterobacter cloacae, one was Bacillus species and the other was Serratia marcescens (Table 1). One patient was identified as MRSA at another hospital but negative at our hospital. Although seven patients had negative culture, all patients showed a purulent joint fluid on aspiration and synovial hypertrophy with fibrinous debris on arthroscopic findings (Fig. 2). All patients were seen in consultation by the infectious diseases department and received culture-specific intravenous antibiotic therapy after arthroscopic debridement. The mean duration of antibiotic use was 6 weeks (range 3–8 weeks) after arthroscopic debridement.
Infection Control
The control of infection was considered as follows: (1) relief of preoperative symptoms (pain at rest, severe limited range of motion, redness, and heating sense; (2) normalization of inflammation parameters such as CRP; (3) normalization of signal change in postoperative MRI. No patient underwent a second operation to treat the infection control.
All patients showed relief of preoperative symptoms and there was no recurrence until last follow-up. All patients had normalized CRP after 3 weeks (range 1–7 weeks). The postoperative MRI taken in 13 patients at average of 10 months (range 2–14 months) showed no bony involvement of the proximal humerus in all patients except one patient (Fig. 3). The one patient was 44-year-old man who underwent arthroscopic debridement for septic shoulder arthritis following previous arthroscopic rotator cuff repair at other hospital. The MRI taken at 7 months post-operatively at our institution showed that bony involvement was not totally disappeared and high signal intensity was observed in greater tuberosity area. However, CRP was normalized and no signs of infection were observed. Therefore, we performed a second-stage arthroscopic rotator cuff repair and we could not find evidence of recurrence of infection.
Fig. 3.

The patient presented in Fig. 1 underwent magnetic resonance image at 5 months post-operatively. The proton density fat suppression coronal view (a) and axial view (b) showing no high signal around greater tuberosity and proximal humerus after arthroscopic multiple punctures
Clinical Outcomes
Both clinical scores and range of motion (ROM) were improved significantly after surgery (Table 2). Six patients underwent additional surgery for cuff pathology and we defined these patients as secondary surgery group. All patients showed significantly better results than those before surgery, regardless of whether or not they had a secondary surgery.
Table 2.
Clinical outcomes of the arthroscopic debridement with multiple puncture
| Variable | Preoperative | Final follow-up | P values |
|---|---|---|---|
| Range of motion | |||
| Front elevation (°) | 62 ± 31 | 152 ± 25 | < 0.001 |
| External rotation (°) | 32 ± 19 | 52 ± 16 | 0.008 |
| Internal rotationa (°) | 4 ± 2 | 8 ± 2 | < 0.001 |
| Clinical score | |||
| PVAS | 6.3 ± 1.6 | 1.6 ± 1.6 | < 0.001 |
| FVAS | 2.7 ± 2.0 | 7.3 ± 2.3 | < 0.001 |
| ASES | 29 ± 15 | 75 ± 19 | < 0.001 |
| Constant score | 26 ± 14 | 65 ± 17 | < 0.001 |
| SST | 1.1 ± 1.6 | 7.8 ± 3.0 | < 0.001 |
Values are presented as mean ± standard deviation
PVAS Pain Visual Analog Scale, FVAS Functional Visual Analog Scale, ASES American Shoulder Elbow surgeons Scores, SST Simple Shoulder Test
aConvert vertebral level to a number: T4 ~ T7 to 10, T8 ~ T12 to 8, L1 ~ L3 to 6, L4 ~ L5 to 4, sacrum and buttock to 2
Difference between secondary surgery group and non-secondary surgery group were shown in Table 3. Non-secondary surgery group had more female ratio and older than secondary surgery group, but not statistically significant. Rotator cuff tears were observed during arthroscopic debridement in 11 cases. Secondary surgery group underwent secondary surgery after mean 25 months (range 4–104 months). But secondary surgery was performed after mean 9 months after excluding the one patient who underwent secondary surgery 104 months after the operation. Pain and shoulder function were significantly improved after arthroscopic debridement with multiple punctures but they underwent secondary surgery because of some discomfort after using their shoulder. Except for front elevation, there were no significant differences in ROM and clinical scores between the patients who underwent the second surgery and those who did not (Table 4).
Table 3.
Differences between non-secondary surgery group and secondary surgery group
| Variable | Non-secondary surgery group | Secondary surgery group | P values |
|---|---|---|---|
| Patient number | 9 | 6 | |
| Sex, male/female | 5/4 | 6/0 | 0.103 |
| Age (year), mean ± standard deviation | 59 ± 9 | 45 ± 13 | 0.113 |
| Rotator cuff tear | |||
| No tear | 1 | 0 | |
| PTRCT | 3 | 0 | |
| Small | 0 | 2 | |
| Medium | 3 | 2 | |
| Large | 0 | 2 | |
| Massive | 2 | 0 | |
Table 4.
Clinical outcomes of non-secondary surgery group and secondary surgery group
| Variable | Non-secondary surgery group (n = 9) | Secondary surgery group (n = 6) | P values |
|---|---|---|---|
| Follow up period (month) | 21 ± 8 | 22 ± 7a | 0.776 |
| 2ndary surgery | |||
| Arthroscopic capsule release 1 | |||
| Arthroscopic rotator cuff repair | 5 | ||
| Range of motion | |||
| Front elevation (°) | 161 ± 11 | 138 ± 34 | 0.05 |
| External rotation (°) | 52 ± 14 | 54 ± 20 | 0.388 |
| Internal rotationb (°) | 9 ± 2 | 8 ± 3 | 0.607 |
| Clinical score | |||
| PVAS | 1.8 ± 1.9 | 1.3 ± 1.2 | 0.864 |
| FVAS | 7.0 ± 2.3 | 7.7 ± 2.3 | 0.456 |
| ASES | 73 ± 21 | 79 ± 17 | 0.529 |
| Constant score | 64 ± 17 | 66 ± 17 | 0.607 |
| SST | 7.7 ± 3.0 | 8.0 ± 3.1 | 0.776 |
Values are presented as mean ± standard deviation
PVAS Pain Visual Analog Scale, FVAS Functional Visual Analog Scale, ASES American Shoulder Elbow surgeons Scores, SST Simple Shoulder Test
aTime from secondary operation to last follow-up
bConvert vertebral level to a number: T4 ~ T7 to 10, T8 ~ T12 to 8, L1 ~ L3 to 6, L4 ~ L5 to 4, sacrum and buttock to 2
In secondary surgery group, there were two patients in which the tear size at secondary arthroscope surgery was smaller than the previous arthroscope findings. One patient was a 44-year-old man who underwent arthroscopic repair. A medium size tear was observed on previous arthroscopic debridement, but a partial thickness rotator cuff tear was observed on secondary arthroscope surgery. The other patient was 28-year-old man who had secondary stiffness after arthroscopic debridement and multiple punctures. At secondary arthroscopic surgery, the previous small size tear was covered by scar tissue and it was not easy to penetrate with probe. So only arthroscopic capsular release was performed.
Discussion
Ours study showed that the infection did not recur in all 15 patients who underwent arthroscopic debridement with multiple punctures in septic shoulder arthritis with proximal bone signal change. Inflammatory parameters such as CRP were normalized after surgery, and all range of motion and clinical scores were significantly increased compared to before surgery. Our study might give us guideline to treat safely septic joint without bone destruction but proximal humerus signal change in MRI and possible osteomyelitis.
Our results had better results than the previous reports. All our patients had a mean duration of 74 days from symptom onset to treatment and MRI showed proximal bone involvement. It has been treated with open resection arthroplasty instead of arthroscopically, but arthroscopic debridement with multiple punctures eradicated infections in all patients. Factors significantly associated with arthroscopic treatment failure were Gächter stage III or IV [11]. In other words, if the treatment is delayed, it is difficult to obtain eradication of infection through arthroscopic treatment. Other literature showed a recurrence rate of 25–52.4. Abdel et al. [9] reported that repeat irrigation and debridement was required within the first month in 16 of 50 shoulders (32%). Aïm et al. [16] reported 25% required more than one arthroscopic treatment. Jeon et al. [17] reported that 14 out of 19 (reinfection rate, 26.3%) patients showed good results with one arthroscope operation. Böhler et al. [10] reported that reinfection after arthroscopic treatment was 52.4%.
Our treatments for septic shoulder arthritis had differentiations from other literature’s treatments. First, we performed arthroscopic debridement as well as arthroscopic multiple punctures. All patients included in our study were presumed osteomyelitis of proximal humerus. Therefore, arthroscopic debridement alone was insufficient to eradicate it, so that osteomyelitis of proximal humerus could be treated through arthroscopic multiple punctures. After multiple punctures were performed, we could see the turbid material and fluid were gush out from the holes made by punctures. Second, we performed sufficient irrigation. The Other literature [9, 10, 17, 18] reports 5–20 l is usually used for arthroscopic debridement for septic shoulder arthritis, but we used an average of 31 l during arthroscopic debridement with multiple punctures. Enough amount of normal saline was considered to enable sufficient irrigation of infectious materials. Third, experienced shoulder surgeon performed sufficient debridement of all the infected tissue with synovectomy and bursectomy during surgery. This was reflected in the surgical time of arthroscopic debridement taking mean 74 min for arthroscopic debridement with multiple punctures surgery. We feel that some if not all the referred cases of uncontrolled infection after rotator cuff repair and arthroscopic debridement of second surgery was due to insufficient debridement of the joint. Böhler et al. [10] reported that operative time for arthroscope is 45 min (range 30–55 min). We think that it is important to remove all infected tissues thoroughly and carefully during arthroscopic synovectomy.
Resection arthroplasty with antibiotic impregnated cement spacer can be very effective in successful eradication of the destructive joint shoulder infection but does not significantly improve shoulder ROM compared to the results of the arthroscopic multiple punctures [12, 19]. Garofalo et al. [12] reported that there was no recurrence of infection, mean active FE was 53° and mean active ERs was 35° 1-year post-operatively. Coffey et al. [19] reported that that there was no recurrence of infection, mean active FE was 110° and mean active ERs was 20° 20.5 months post-operatively. Reconstruction surgery such as total shoulder arthroplasty or reverse shoulder arthroplasty remains a good option for patients in whom the infection has been successfully treated with limited potential for improvement. Pain relief was achieved in many patients, but there was an overall rate of unsatisfactory results approaching 40% [20]. Final followed up mean PVAS was 2, mean FE was 118° and mean ER was 41°. If the joint surface is not severely destroyed, we should try to preserve the joint surface as much as possible. In the case of arthroscopic debridement with multiple punctures, good postoperative results were obtained even without secondary repair of the rotator cuff tear.
Our study has several limitations. First, this study is a single center design. Second, this is retrospective study. Third, Patients had different treatment before surgery in other hospital and postoperative managements including antibiotics were different. And fourth, the number of cases is small and heterogeneous. However, even if the number is small, it is meaningful because there is no literature about arthroscopic debridement with multiple punctures in osteomyelitis of the proximal humerus. It is also a strength that all the procedures are carried out by one skilled shoulder surgeon.
Conclusion
Septic shoulder with proximal bone involvement can be successfully treated with arthroscopic debridement with multiple punctures. With our indication selection we can salvage the proximal humerus without bone resection.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors. All authors have seen and approved this manuscript. All authors have participated in the research of this paper. Each author believes that the manuscript represents honest work. This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jae Woo Shim, Email: osdrshim@gmail.com.
Sung Woo Hong, Email: swhongdr@gmail.com.
Jeung Yeol Jeong, Email: inzaghy@naver.com.
Sang Min Lee, Email: sangmin1126.lee@samsung.com.
Jae Chul Yoo, Email: shoulderyoo@gmail.com.
References
- 1.Jiang JJ, Piponov HI, Mass DP, Angeles JG, Shi LL. Septic Arthritis of the shoulder: a comparison of treatment methods. Journal of American Academy of Orthopaedic Surgeons. 2017;25:e175–e184. doi: 10.5435/JAAOS-D-16-00103. [DOI] [PubMed] [Google Scholar]
- 2.Geirsson AJ, Statkevicius S, Vikingsson A. Septic arthritis in Iceland 1990–2002: Increasing incidence due to iatrogenic infections. Annals of the Rheumatic Diseases. 2008;67:638–643. doi: 10.1136/ard.2007.077131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy N, Chambers ST, Nolan I, Gallagher K, Werno A, Browne M, et al. Native joint septic arthritis: Epidemiology, clinical features, and microbiological causes in a New Zealand population. Journal of Rheumatology. 2015;42:2392–2397. doi: 10.3899/jrheum.150434. [DOI] [PubMed] [Google Scholar]
- 4.Tahami SM, Aminian A, Azarpira N. Experimental study on protective role of NSAID on articular cartilage destruction in septic arthritis. The Archives of Bone and Joint Surgery. 2020;8:89–93. doi: 10.22038/abjs.2019.37045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wada A, Fujii T, Takamura K, Yanagida H, Urano N, Surijamorn P. Operative reconstruction of the severe sequelae of infantile septic arthritis of the hip. Journal of Pediatric Orthopedics. 2007;27:910–914. doi: 10.1097/bpo.0b013e31815a606f. [DOI] [PubMed] [Google Scholar]
- 6.Sahu KK, Tsitsilianos N, Moselle L, Mishra AK. Septic arthritis of hip joint and its devastating complications. BMJ Case Reports. 2020;13:e233909. doi: 10.1136/bcr-2019-233909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan SF, Sperling JW. Treatment of primary isolated shoulder sepsis in the adult patient. Clinical Orthopaedics and Related Research. 2008;466:1392–1396. doi: 10.1007/s11999-008-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchhoff C, Braunstein V, Buhmann Kirchhoff S, Oedekoven T, Mutschler W, Biberthaler P. Stage-dependant management of septic arthritis of the shoulder in adults. International Orthopaedics. 2009;33:1015–1024. doi: 10.1007/s00264-008-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel MP, Perry KI, Morrey ME, Steinmann SP, Sperling JW, Cass JR. Arthroscopic management of native shoulder septic arthritis. Journal of Shoulder and Elbow Surgery. 2013;22:418–421. doi: 10.1016/j.jse.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Bohler C, Pock A, Waldstein W, Staats K, Puchner SE, Holinka J, et al. Surgical treatment of shoulder infections: A comparison between arthroscopy and arthrotomy. Journal of Shoulder and Elbow Surgery. 2017;26:1915–1921. doi: 10.1016/j.jse.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Stutz G, Kuster MS, Kleinstuck F, Gachter A. Arthroscopic management of septic arthritis: stages of infection and results. Knee Surgery, Sports Traumatology, Arthroscopy. 2000;8:270–274. doi: 10.1007/s001670000129. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskeletal Surgery. 2014;98(Suppl 1):35–39. doi: 10.1007/s12306-014-0317-0. [DOI] [PubMed] [Google Scholar]
- 13.Richards RR, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, et al. A standardized method for the assessment of shoulder function. Journal of Shoulder and Elbow Surgery. 1994;3:347–352. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 14.L'Insalata JC, Warren RF, Cohen SB, Altchek DW, Peterson MG. A self-administered questionnaire for assessment of symptoms and function of the shoulder. Journal of Bone and Joint Surgery. American Volume. 1997;79:738–748. doi: 10.2106/00004623-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Hsu JE, Russ SM, Somerson JS, Tang A, Warme WJ, Matsen FA., 3rd Is the simple shoulder test a valid outcome instrument for shoulder arthroplasty? Journal of Shoulder and Elbow Surgery. 2017;26:1693–1700. doi: 10.1016/j.jse.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Aim F, Delambre J, Bauer T, Hardy P. Efficacy of arthroscopic treatment for resolving infection in septic arthritis of native joints. Orthopaedics and Traumatology: Surgery and Research. 2015;101:61–64. doi: 10.1016/j.otsr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Jeon IH, Choi CH, Seo JS, Seo KJ, Ko SH, Park JY. Arthroscopic management of septic arthritis of the shoulder joint. Journal of Bone and Joint Surgery. American Volume. 2006;88:1802–1806. doi: 10.2106/JBJS.E.00917. [DOI] [PubMed] [Google Scholar]
- 18.Atesok K, MacDonald P, Leiter J, McRae S, Stranges G, Old J. Postoperative deep shoulder infections following rotator cuff repair. World Journal of Orthopedics. 2017;8:612–618. doi: 10.5312/wjo.v8.i8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffey MJ, Ely EE, Crosby LA. Treatment of glenohumeral sepsis with a commercially produced antibiotic-impregnated cement spacer. Journal of Shoulder and Elbow Surgery. 2010;19:868–873. doi: 10.1016/j.jse.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Assenmacher AT, Alentorn-Geli E, Dennison T, Baghdadi YMK, Cofield RH, Sanchez-Sotelo J, et al. Two-stage reimplantation for the treatment of deep infection after shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2017;26:1978–1983. doi: 10.1016/j.jse.2017.05.005. [DOI] [PubMed] [Google Scholar]



