Abstract
Purpose
This study investigated whether thymoquinone (TQ) could alleviate central nervous system (CNS) and cardiovascular toxicity of prilocaine, a commonly used local anesthetic.
Methods
Rats were randomized to the following groups: control, prilocaine treated, TQ treated and prilocaine + TQ treated. Electroencephalography and electrocardiography electrodes were placed and trachea was intubated. Mechanical ventilation was initiated, right femoral artery was cannulated for continuous blood pressure measurements and blood-gas sampling while the left femoral vein was cannulated for prilocaine infusion. Markers of myocardial injury, reactive oxygen/nitrogen species (ROS/RNS) generation and total antioxidant capacity (TAC) were assayed by standard kits. Aquaporin-4 (AQP4), nuclear factor(NF)κB-p65 and -p50 subunit in brain tissue were evaluated by histological scoring.
Results
Blood pH and partial oxygen pressure, was significantly decreased after prilocaine infusion. The decrease in blood pH was alleviated in the prilocaine + TQ treated group. Prilocaine produced seizure activity, cardiac arrhythmia and asystole at significantly lower doses compared to prilocaine + TQ treated rats. Thymoquinone administration attenuated levels of myocardial injury induced by prilocaine. Prilocaine treatment caused increased ROS/RNS formation and decreased TAC in heart and brain tissue. Thymoquinone increased heart and brain TAC and decreased ROS/RNS formation in prilocaine treated rats. AQP4, NFκB-p65 and NFκB-p50 expressions were increased in cerebellum, cerebral cortex, choroid plexus and thalamic nucleus in prilocaine treated rats. Thymoquinone, decreased the expression of AQP4, NFκB-p65 and NFκB-p50 in brain tissue in prilocaine + TQ treated rats.
Conclusion
Results indicate that TQ could ameliorate prilocaine-induced CNS and cardiovascular toxicity.
Graphical abstract
Keywords: Prilocaine, Central nervous system, Cardiovascular, Toxicity, Thymoquinone
Introduction
Regional anesthesia is applied frequently and maintains its popularity as it is a more reliable anesthesia method compared to general anesthesia. Therefore, the use and toxicity of local anesthetic agents has become an important factor in the clinic. Central nervous system and cardiovascular toxicity occur as a result of accidental intravascular injections, rapid systemic absorption or high-dose medication [1]. The American Society of Regional Anesthesia and Pain Medicine Practice Advisory identified 93 cases of local anesthetic systemic toxicity (LAST) between the years 1979–2009. Central nervous system symptoms were reported in 83 patients (89%), most of these symptoms were reported as seizure activity (68%). In the same study, 51 patients (55%) had symptoms of cardiovascular system toxicity. Bradycardia-asystole (27%) was the most common symptom of cardiovascular toxicity, followed by hypotension (18%) and bradycardia (16%) [2]. In a study compiling LAST cases between January 2014 and November 2016, a total of 47 LAST cases were evaluated. In this study, the most common side effect was CNS toxicity (77%), while the most common CNS symptom was seizure activity (53%). Cardiovascular system symptoms were observed in 23% of the patients. In 10 patients, cardiac supportive treatment was needed [3].
Synthesized by Löfgren and Tegner in 1953 and used for the first time in the clinic by Eriksson and Gordh in 1960, prilocaine is one of the most frequently used local anesthetics for regional anesthesia today [4]. Prilocaine is commercially available as a stable and water-soluble hydrochloride salt and is chemically designated as N-(2-Methylphenyl)-2-(propylamino) propanamide [5]. The dissociation constant (pKa) of prilocaine is 7.9 and it is approximately 75% ionized at a normal tissue pH of 7.4. Therefore it can dissolve in water and diffuse through tissue with an acceptable rapid onset of anesthesia. Lipid solubility characteristics (partition coefficient) of prilocaine predicts its potency and is reported to be lower compared to other local anesthetics. Protein binding of prilocaine (55%) determines the duration of anesthesia and is also reported to be lower than similar local anesthetics [1]. These pharmacologic characteristics of prilocaine allow higher rate of clearance and a larger volume of distribution. Thus, prilocaine infrequently achieves toxic blood concentrations at doses commonly administered and has a low side-effect profile [5].
Serious and life threatening prilocaine toxicity can occur by excessive dosing, rapid or incorrect administration. In such cases the drug can diffuse away from the site of injection and be absorbed into the systemic circulation where it is metabolized [1]. Toxicity reactions to prilocaine overdose can be associated with severe central nervous system (CNS) and cardiac toxicity leading to morbidity and mortality [6]. Prilocaine, suppresses neural transmission by blocking the voltage-gated fast sodium channels in the neural tissue and inhibiting depolarization [7]. At first, excitatory reactions to prilocaine overdose are observed, such as muscle twitching, tremors, shivering and clonic-tonic convulsions [8]. Central nervous system and respiratory depression follows if blood concentrations of prilocaine continue to increase. Myocardial excitability and conductivity may also be depressed with exceeding high doses. Cardiac toxicity to prilocaine overdose is most often apparent as ectopic cardiac arrhythmia and bradycardia. Significant hypotension is observed following depressed cardiac contractility and peripheral vasodilation [1].
Phytomedicine has garnered notable attention in recent years and is progressively used as alternative treatment. Thymoquinone is the most abundant ingredient isolated from the volatile oil of Nigella sativa seeds. Pharmacological activities of TQ include protection against neurological [9] and cardiovascular disorders [10]. It is predicted to be a promising neuropharmacological agent because it displays potential for attenuating neurological injuries. It has been reported to have beneficial effects in toxin-induced neuroinflammation and neurotoxicity [11]. Likewise, findings of recent studies show the protective effects of TQ against cardiovascular diseases [10].
Since the mechanism of prilocaine induced toxicity is reported to involve neurological and cardiac toxicity [6] and that TQ has previously been shown to have neuroprotective [9] and cardioprotective effects [10], the present study was designed to evaluate the possible protective role of TQ in prilocaine toxicity. The therapeutic effects of TQ on prilocaine-induced CNS and cardiovascular toxicity were investigated in rats by evaluating biomarkers of central CNS and cardiovascular toxicity. In this context, functional alterations toward increased seizure susceptibility were also evaluated in brain tissue. The glial cell water channel AQP4 is implicated in the modulation of neuronal excitability and epilepsy [12]. Thus, the effect of TQ treatment on AQP4 expression in different brain regions were also determined to evaluate potential therapeutic targets of TQ.
Materials and methods
Animals
All animal experiments were performed in accordance with the standards approved by the Institutional Animal Care and Use Committee (ID: 625, Protocol No: 2017.02.02). Forty male wistar rats, aged 3 months weighting 350–450 g were randomized to one of the following groups: sham (n = 10), prilocaine treated (n = 10), TQ treated (n = 10) and prilocaine + TQ treated (n = 10). Rats were anesthetized intraperitoneally with a mixture of ketamine and 2% xylazine hydrochloride (0.02 mg/kg). Electroencephalography (EEG) and electrocardiography (ECG) electrodes were placed and tracheostomy was opened to initiate mechanical ventilation. Mechanical ventilation was initiated with a tidal volume of 10 ml/kg (50% O2-Air) and a rate of 50–55 breaths/min. Right femoral artery was cannulated for continuous blood pressure measurements and blood-gas sampling while the left femoral vein was cannulated for prilocaine infusion. Prilocaine (Priloc 2%, VEM Pharmaceuticals, Istanbul, Turkey) was infused at a rate of 8 mg/kg/min. The infusion dose of prilocaine (8 mg/kg/min) was chosen with reference to a previous study in which acute central nervous system and cardiovascular toxicity of prilocaine was investigated in rat [13]. If cardiac arrest did not occur 20 min after infusion, the rate was increased to 12 mg/kg/min. EEG and ECG recordings were performed simultaneously during drug administration. As soon as a change was seen in the EEG and ECG, the infusion time and the infusion dose of prilocaine was recorded. Prilocaine doses causing the occurrence of the initial seizure, isoelectric EEG, arrhythmia and asystole were calculated separately by multiplying the infusion dose with infusion time.
Rats in prilocaine groups had cardiac arrest at the end of the experiment. Rats in the sham and TQ groups were euthanized by decapitation. Measurement of serum myoglobin and creatine kinase-MB were done on blood samples collected just after cardiac arrest or decapitation. Likewise, total antioxidant and ROS/RNS measurements in heart and brain samples were done in tissues collected after rats were sacrificed.
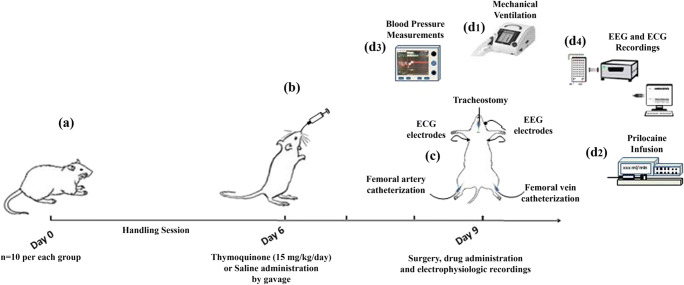
Thymoquinone (Sigma-Aldrich, St. Louis, MO, USA) was given by gavage (15 mg/kg per day) for 3 days prior to drug administration. The given dose of TQ was in reference to what was previously described as protective in rats [14]. Water solubility of TQ is reported to be >0,5 mg/mL which is enough to exert pharmacologic effects [15]. The last TQ dose was administered intraperitoneally on the fourth day of the experimental period, before prilocaine infusion and sacrification. Sham group animals received an equal volume of i.p. saline and distilled water via gavage. Body weight was determined before and after the treatment period. Arterial blood sample for blood gas analysis was drawn before drug infusion, during drug infusion and after cardiac arrest. Schematic presentation of prilocaine and thymoquinone treatment schedule is presented in Fig. 1.
Fig. 1.
Schematic presentation of prilocaine and thymoquinone treatment schedule. Thymoquinone (TQ) was given by gavage for 3 days prior to drug administration. The last TQ dose (forth dose) was administered intraperitoneally, before prilocaine infusion. Sham group animals received an equal volume of i.p. saline and distilled water via gavage. On the day of prilocaine infusion, electroencephalography (EEG) and electrocardiography (ECG) electrodes were placed and tracheostomy was opened to initiate mechanical ventilation (d1). Left femoral vein was cannulated for prilocaine infusion (d2) and right femoral artery was cannulated for continuous blood pressure measurements (d3) and blood-gas sampling. EEG and ECG recordings were performed simultaneously during drug administration (d4)
Electrophysiological recordings and analysis
The details of surgical protocols have been described previously [16, 17]. Rats were anesthetized with a mixture of ketamine-based anesthetics (ketamine [50 mg/kg] and xylazine [10 mg/kg], intraperitoneally (i.p.)) and fixed on a stereotaxic instrument. For the EEG recordings, stainless steel screw electrodes (E363/20 /1.6/ SP, Plastics One INC. USA) were inserted bilaterally into the frontal (AP: 4.5 mm, ML: +2 and −2 mm) and parietal (AP: −4.5 mm, ML: +3.5 and −3.5 mm) cortex regions, reference electrode to cerebellum (AP: −12.72 mm (ML: 2.5 mm) and the ground electrode (AP: −12.72 mm, ML: −2.5 mm) were placed on the coordinates. For the ECG recordings, stainless steel needle electrodes were inserted subcutaneously to the right and left anterior extremities to receive recordings from the II derivation after carefully shaving the ventral thoracic region. All recordings were taken in an electromagnetically shielded room in the morning, and each recording session lasted almost 30 min for each rat. Before recording, rats were allowed to adapt to ambient conditions. Following the adaptation process, EEG and ECG recordings were recorded by using Brainamp EEG/EP amplifier at 500 Hz sampling rate and were filtered with a 48 dB/octave band at 0.01–250 Hz (Brainamp EEG / EP Amplifier, Brain Products, Munich, Germany). All electrode impedances were kept less than 5 kΩ during the recording session. Later, the EEG and ECG data analysis were conducted offline by using the BrainVision Recorder software (Brain Products, Munich, Germany). In addition, alterations in both EEG and ECG activity were monitored simultaneously during experimental procedures.
Blood gas measurements
Blood gas measurements were performed on ABL80 FLEX CO-OX blood gas measuring device (Radiometer Medical ApS, Denmark) using standard kits. Carbon dioxide partial pressure (pCO2), oxygen partial pressure (pO2), fraction of methemoglobin (FMetHb) and blood pH measurements were made from arterial blood samples taken at first catheterization, 15 min and after rats were sacrificed.
Measurement of serum myoglobin and Creatine kinase-MB
Serum myoglobin levels were assayed by rat myoglobin ELISA kit (SUNLONG BIOTECH CO., LTD Catalog #SL0515Ra, Hangzhou, China) according to manufacturer’s instructions. A standard curve of absorbance values of known concentrations of rat myoglobin standards was plotted as a function of standard concentrations using the GraphPad Prism Software program for windows version 5.03. (GraphPad Software Inc.). The amount of myoglobin (ng/ml) in serum samples were calculated from their corresponding absorbance values via the standard curve. The absorbance values were measured on a spectrophotometric plate reader (MicroQuant Plate Reader, Bio-Tek Instruments Inc. Vermont, USA). For serum creatine kinase-MB (CK-MB) analyses, 2.0 mL blood samples were collected into tubes without an anticoagulant and centrifuged at 3000 rpm for 10 min at room temperature to obtain serum. Serum CK-MB levels were assayed using Fujifilm kits and analyzed on Fujifilm DRI-CHEM NX500 (FUJIFILM Co.,Tokyo, Japan).
Total antioxidant capacity measurement
Heart and brain tissue samples were homogenized in cold PBS and centrifuged at 10,000 x g for 10 min at 4 °C. The supernatants were stored at −80 °C for protein determination and TAC assay. Total antioxidant capacity was measured by OxiSelect Total Antioxidant Capacity Assay Kit (Cell Biolabs, Inc. San Diego,CA, USA).
Reactive oxygen and nitrogen species measurement
Heart and brain samples were homogenized in cold PBS (10–50 mg/mL) and centrifuged at 10,000 x g for 5 min. The supernatants were stored at −80 °C for protein determination and ROS/RNS assay. Reactive oxygen species and reactive nitrogen species were measured by OxiSelect in vitro ROS/RNS assay kit (Cell Biolabs, Inc. San Diego,CA, USA).
Protein measurements
Protein concentrations were measured at 595 nm by a modified Bradford assay using Coomassie Plus reagent with bovine serum albumin as a standard (Pierce, Thermo Fisher Scientific, Roskilde, Denmark). The absorbance values were measured on a spectrophotometric plate reader (MicroQuant Plate Reader, Bio-Tek Instruments Inc. Vermont, USA).
Immunohistochemistry
Rats were sacrificed and tissues from a single hemisphere of the brain were fixed with 10% formalin and embedded in paraffin. Five μm thick serial tissue sections were taken from total hemisphere of the brain tissue and placed on positive charged slides (cat no# 1014356190; Thermo Fisher Scientific, Waltham, MA, USA) and incubated overnight at 45 °C. Sections were deparaffinized in xylene, rehydrated in a decreasing gradient of ethanol and finally rinsed in distilled water. An antigen-retrieval procedure was performed by heating the samples with citric acid buffer (Merck, cat# 1–00244-1000; Darmstadt, Germany), pH 7 in a microwave oven at 800 W for 10 min. After cooling for 20 min at room temperature, the sections were washed three times in PBS and endogenous peroxidase activity was blocked (3% H2O2 for 15 min). The sections were washed three times in PBS prior to incubation with universal blocking reagent (Thermo, TA-125-UB) for 7 min to block non-specific binding. Later, tissues were incubated with anti-AQP4 (Millipore; #AB3594, 1/1000 dilution), Anti-NFkB p50 antibody (Abcam; #ab32360, 1/50 dilution) and anti NFkB-p65 (Cell signaling; #8242S, 1/200 dilution). Antibodies were diluted in dilution buffer (Abcam; #ab64211) and applied for overnight at 4 °C in a humidified chamber. After several washes in PBS, sections were incubated with biotinylated goat anti-rabbit secondary antibody (Vector Lab; #BA1000, 1/400 dilution) for 1 h at room temperature. Following washing steps with PBS, sections were incubated with HRP streptavidin-peroxidase complex (Invitrogen; 43-4323) for 20 min at room temperature. The sections were washed several times and diaminobenzidine (DAB) tablets (D-4168;Sigma Aldrich, MO, USA) were used for chromogenic detection. The sections were counterstained with Mayer’s Hematoxylin (1.09249.1000; Merck, Darmstadt, Germany) and mounted on glass slides. For negative controls, instead of primary antibody, slides were incubated with normal rabbit IgG. The sections were visualized with a Zeiss-Axioplan (Oberkochen, Germany) microscope. Analysis of immunostaining intensities were done by capturing immunohistochemistry images using Spot Imaging software version 4.6 (Diagnostic Instruments, Inc., MI, USA) at 20 X magnification. Ten non-overlapping fields in each tissue were randomly selected for each group and analyzed by ImageJ Version 1.46 (NIH, Bethesda, MD). Briefly, Images were captured onto the hard drive by using Spot Imaging software and captured images were opened in ImageJ software for evaluating the staining density. Staining was assessed by setting a “threshold” using the thresholding tool. The “Limit to Threshold” option was also selected, because otherwise the entire image would be measured, rather than the selected area. Thus, increased density was a result of increased staining levels. Measurement of the area that resulted in positive antigen staining in brain tissue of experimental groups were analyzed. Integrated density values of immunohistochemically stained areas were normalized to the integrated density values of the total area. The calculated ratio of the immunostained area was reported as percentage of the total area.
Statistical analysis
Statistical analysis was performed using SigmaStat statistical software version 3.0 (Sigma, St. Louis, MO, USA) and GraphPad Prism Software program for windows version 5.03. (GraphPad Software Inc.). A p value <0,05 was considered to be statistically significant.
Result
Electrophysiological parameters
Electrophysiological recordings were used to evaluate and compare the time to first seizures, isoelectric EEG, first arrhythmia, asystole and the corresponding prilocaine doses between groups (Fig. 2a). In the prilocaine group, the first seizure dose (mean ± SD) was found to be 39.10 ± 6,07 mg/kg. In the TQ + prilocaine group, the first seizure dose (mean ± SD) was 60.79 ± 6.79 mg/kg. The dose (mean ± SD) for isoelectric EEG was 57.71 ± 25.05 mg/kg in the prilocaine group; and the mean dose was 94.59 ± 10.43 mg/kg in the TQ + prilocaine group. In the prilocaine group, the first arrhythmia was observed with 89,95 ± 12.44 mg/kg dose. The first arrhythmia in the TQ + prilocaine group was observed with 177.38 ± 19.98 mg/kg dose. Asystole was seen at a dose (mean ± SD) of 122.83 ± 15.18 mg/kg in the prilocaine group and 198.99 ± 16.91 mg/kg in the TQ + prilocaine group. The electrophysiological recordings of Sham and TQ groups did not show any change (seizure, arrhythmia, asystole). There were significant changes in other groups due to prilocaine toxicity (Fig. 2b). Prilocaine dose (mean ± SD) of time to first seizure was significantly higher in the TQ + prilocaine group compared to prilocaine group (p = 0,0285). Similarly, the rates of isoelectric EEG were also significantly higher in the TQ + prilocaine group compared to prilocaine group (p = 0.0003). The infusion dose (mean ± SD) for the first arrhythmia (p = 0.0014) and asystole (p = 0.0036) were significantly higher in the TQ + prilocaine group than the prilocaine group (Fig. 2b).
Fig. 2.
a The representative 30-s EEG and ECG traces for all groups are presented. Normal EEG and ECG recordings were seen in sham and thymoquinone groups. Arrows indicate 4 different time points (beginning of epileptic activity, isoelectric EEG formation, first arrhythmia, asystole) due to prilocaine toxicity in prilocaine and thymoquinone + prilocaine group. Infussion doses are expressed as mean ± standard deviations. b The comparison of prilocaine doses (mg/kg) for time to first epileptic activity, isoelectric EEG, arrhythmia and asystole in prilocaine and prilocaine + thymoquinone groups. Results are expressed as mean ± standard deviations. Statistical analysis was by student t-test. p < 0.05 was considered as statistically significant. c Comparison of heart beat and blood pressure changes between prilocaine and prilocaine + thymoquinone groups. Results are expressed as mean ± standard deviations. Statistical analysis was by student t-test. p < 0.05 was considered as statistically significant
Hemodynamic parameters
No change was observed in the pulse and blood pressure values in the sham and TQ groups. Whereas in the groups given prilocaine, changes due to prilocaine infusion dose were observed (Fig. 2c). Prilocaine and TQ + prilocaine groups were compared for doses that made a change between 20% and 50% in heart rate and blood pressure. The doses that made 50% change in systolic (p = 0.0052), diastolic (p = 0.0003) and mean (p = 0.0004) arterial pressures in the TQ + prilocaine group, were significantly higher than the prilocaine group. No significant difference was observed in other variables (Fig. 2c).
Blood gas analyzes
Blood gas analysis revealed significant differences observed both within and between experimental groups (Table 1). In the prilocaine group, blood pH value was significantly lower at 15 min (1st analysis) and after arrest (2nd analysis) compared to basal blood pH (p < 0.01). Likewise, the blood pH value at 15 min was significantly lower in the prilocaine group (p < 0.01) compared to sham, TQ and prilocaine +TQ groups. The significant decrease observed in blood pH in prilocaine treated groups after arrest was accompanied by significantly decreased partial oxygen pressure.
Table 1.
Arterial blood gas parameters
| Group | Blood pH | pCO2 (mmHg) | pO2 (mmHg) | FMetHb (%) | |
|---|---|---|---|---|---|
| SHAM (n = 10) | Baseline | 7,37 ± 0,01 | 46,78 ± 3,96 | 82,00 ± 7,12 | 3,88 ± 0,10 |
| 1st analysis | 7,34 ± 0,07 | 48,68 ± 11,42 | 95,50 ± 14,11 | 4,08 ± 0,31 | |
| 2nd analysis | 7,34 ± 0,04 | 47,33 ± 5,53 | 99,75 ± 14,06 | 4,25 ± 0,24 | |
| TQ (n = 10) | Baseline | 7,31 ± 0,12 | 56,18 ± 19,52 | 77,38 ± 19,83 | 2,29 ± 0,25 |
| 1st analysis | 7,31 ± 0,08 | 50,24 ± 9,93 | 85,13 ± 10,38 | 2,41 ± 0,19 | |
| 2nd analysis | 7,35 ± 0,10 | 43,56 ± 12,35 | 96,63 ± 22,10 | 2,49 ± 0,21 | |
| PRL (n = 10) | Baseline | 7,33 ± 0,13 | 48,41 ± 19,51 | 81,33 ± 32,27 | 2,30 ± 0,79 |
| 1st analysis | 7,06 ± 0,27a,* | 65,86 ± 33,24 | 66,27 ± 48,55 | 2,23 ± 0,77 | |
| 2nd analysis | 7,09 ± 0,14a | 63,13 ± 32,15 | 51,00 ± 50,08# | 2,97 ± 1,07 | |
| PRL + TQ (n = 10) | Baseline | 7,34 ± 0,09 | 42,59 ± 11,62 | 101,78 ± 29,48 | 2,67 ± 0,82 |
| 1st analysis | 7,22 ± 0,16 | 51,14 ± 24,52 | 102,11 ± 43,92 | 3,14 ± 1,00 | |
| 2nd analysis | 6,92 ± 0,24b,** | 77,48 ± 33,82 | 29,50 ± 11,50c,# | 3,60 ± 0,94 |
Values are mean ± SD. TQ thymoquinone treated, PRL prilocaine treated. pCO2 carbon dioxide partial pressure, pO2 oxygen partial pressure
FMetHb fraction of methemoglobin. A p value of <0,01 was considered statistically significant
ap < 0,01 vs. PRL baseline blood pH. Statistical analysis was done by Paired t-test
bp < 0,001 vs. PRL + TQ baseline blood pH. Statistical analysis was done by Paired t-test
cp < 0,001 vs. PRL + TQ baseline and PRL + TQ 1st pO2 analysis. Statistical analysis was done by Paired t-test
*, p < 0,01 vs. SHAM, TQ and PRL + TQ 1st blood pH analysis. Statistical analysis was done by Kruskal-Wallis one way analysis of variance on ranks with multiple comparisons by Dunn’s method
**, p < 0,01 vs. SHAM and TQ 2nd blood pH analysis. Statistical analysis was done by one way analysis of variance with multiple comparisons by Dunnett’s method
#, p < 0,01 vs. SHAM and TQ 2nd pO2 analysis. Statistical analysis was done by one way analysis of variance with multiple comparisons by Dunnett’s method
Markers of myocardial injury
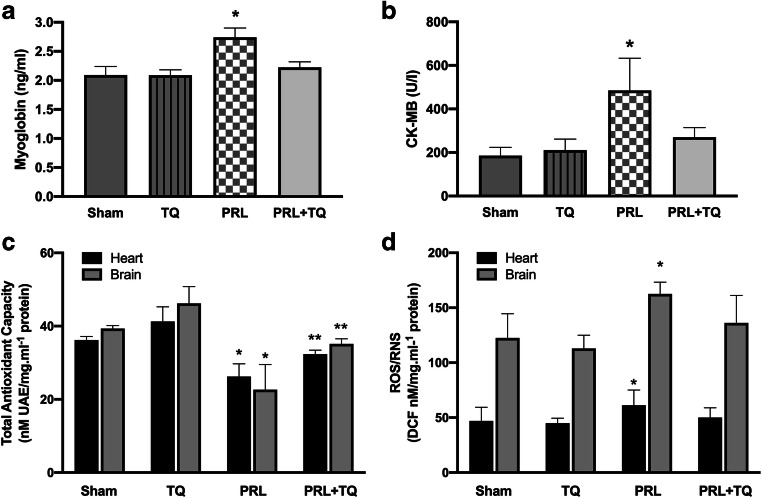
Myoglobin and CK-MB levels were significantly higher in the prilocaine group compared to sham, TQ, prilocaine +TQ groups (p < 0.001) (Fig. 3a and b, respectively). In addition, no significant change was observed in myoglobin and CK-MB levels among sham, TQ and prilocaine +TQ groups.
Fig. 3.
TQ, thymoquinone treated; PRL, prilocaine treated a Serum myoglobin levels. Values are mean ± SD. Statistical analysis was by One Way Analysis of Variance with all pairwise multiple comparison procedures done via Tukey Test. *, p < 0,001 vs. sham, TQ, PRL + TQ groups. b Serum creatine kinase-MB (CK-MB) levels. Values are mean ± SD. Statistical analysis was by Kruskal-Wallis One Way Analysis of Variance on Ranks with all pairwise multiple comparison procedures done by Dunn’s Method. *, p < 0,05 vs. sham, TQ, PRL + TQ groups. c Total antioxidant capacity (TAC). Values are mean ± SD. Statistical analysis was by Kruskal-Wallis One Way Analysis of Variance on Ranks with all pairwise multiple comparison procedures done by Dunn’s Method. *, p < 0,05 vs. sham, TQ, PRL + TQ groups. **, p < 0,05 vs. TQ group. d Total reactive oxygen species (ROS) and reactive nitrogen species (RNS). Values are mean ± SD. Statistical analysis was by Kruskal-Wallis One Way Analysis of Variance on Ranks with all pairwise multiple comparison procedures done by Dunn’s Method. *, p < 0,05 vs. sham, TQ, PRL + TQ groups
Total antioxidant capacity and reactive oxygen-nitrogen species
Total antioxidant capacity and total reactive oxygen and nitrogen species levels in heart and brain tissue are shown in Fig. 3c and d, respectively. Prilocaine group had significantly lower TAC compared to sham, TQ, prilocaine +TQ groups (p < 0.001). Thymoquinone treatment increased TAC in heart and brain tissue and no significant difference was observed between sham and prilocaine +TQ groups. Total antioxidant capacity was significantly different between TQ and prilocaine +TQ groups (p < 0.05). Total reactive oxygen and nitrogen species levels in heart and brain tissue were found to be significantly higher (p < 0.05) in the prilocaine group compared to the other groups (Fig. 3d).
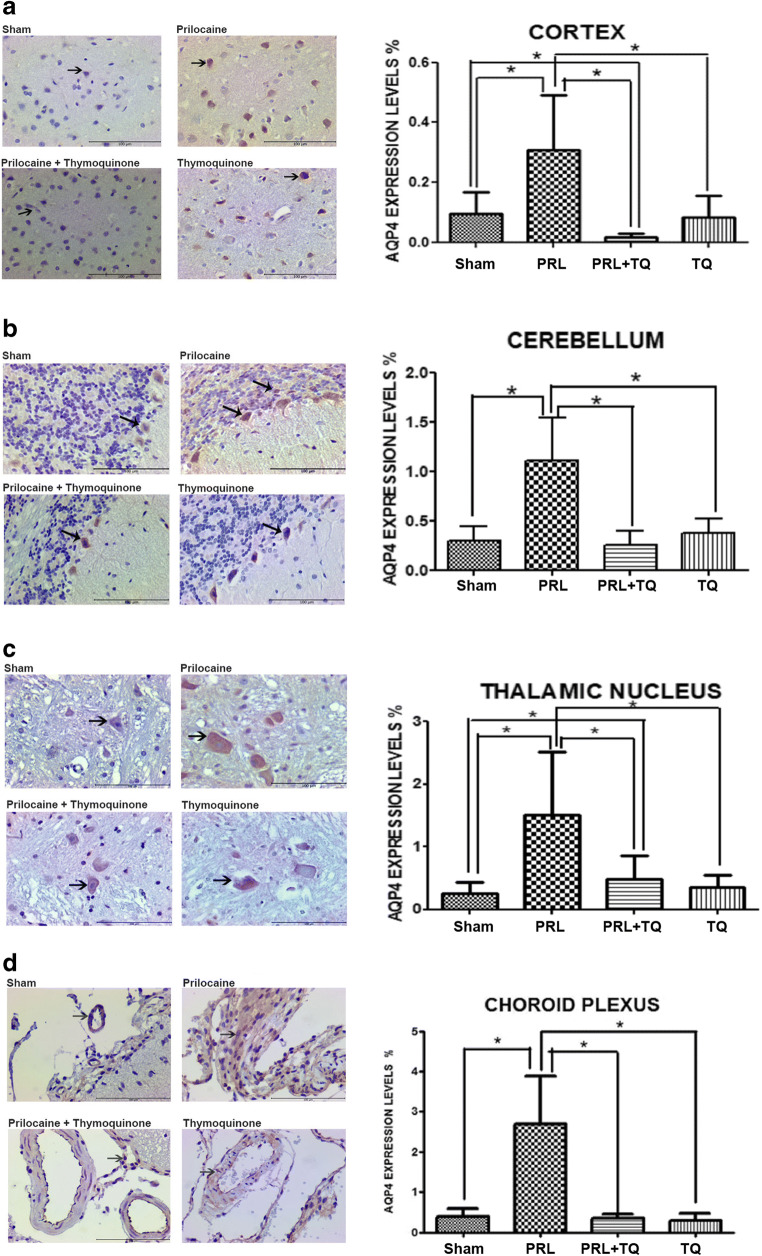
Immunolocalization and expression levels of Aquaporin-4
Studies have identified the presence of AQP4 expression in the cerebral cortex [18]. The cerebral cortex (neocortex) consists of six layers. We focused on lamina granularis externa (2nd layer) and lamina pyramidalis interna (5th layer), instead of the entire cerebral cortex. In these two laminas, the difference in expression between the groups was characterized by an increase in the prilocaine group and a decrease in the prilocaine + TQ group compared to prilocaine treated rats (p < 0.05) (Fig. 4a). Aquaporin-4 expression in the cerebellum was increased in the prilocaine group due to edema, while in the prilocaine + TQ group, it appears to decrease with the therapeutic effect of TQ (p < 0.05) (Fig. 4b). The results obtained suggest that TQ has an edema-reducing effect in rats with experimental epilepsy. The expression of AQP4 in Purkinje cells rather than astrocytic feet may be an indication that the cerebellum is severely affected. Aquaporin-4 expression in the thalamic nucleus was low in sham and TQ groups (Fig. 4c), whereas a significant increase was observed in prilocaine treated rats. Thymoquinone treatment decreased expression of AQP4 in the thalamic nucleus of prilocaine treated rats when compared to the prilocaine group (p < 0.05). To the best of our knowledge, AQP4 expression has not been previously evaluated in the choroid plexus. However, the difference in AQP4 staining between the groups encouraged us to evaluate the expression of AQP4 in the choroid plexus. Similar expression of AQP4 was observed in the sham and TQ groups. In the prilocaine group, AQP4 staining was increased compared to sham, TQ and prilocaine +TQ groups (p < 0.05) (Fig. 4d).
Fig. 4.
Immunolocalization and expression levels of aquaporin-4 (AQP4) in the a Cerebral cortex b Cerebellum c Thalamic nucleus d Choroid plexus tissue from all groups. TQ, thymoquinone treated; PRL, prilocaine treated. Scale bar 100 μm. Arrows point to the expression regions. Values for the expression levels of AQP4 are mean ± SD (n = 5). Statistical analysis was performed by One Way Analysis of Variance and all pairwise multiple comparison procedures were done by Tukey test. *, p < 0,05
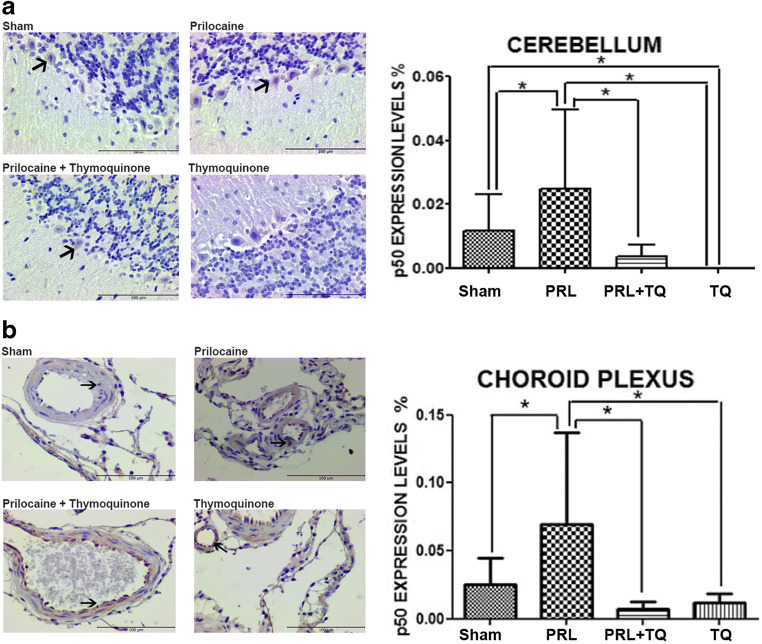
Immunolocalization and expression levels of nuclear factor κB-p65 and -p50 subunit
In light of the expression pattern of AQP4 in the cerebral cortex, cerebellum, thalamic nucleus and choroid plexus we reasoned that neuroinflammation may occur due to edema. Thus we evaluated NFκB-p65 and -p50 subunits in the same brain regions. We observed p50 immunostaining only in the cerebellum and choroid plexus while p65 expression was observed in all the aforementioned brain regions. Cerebral cortex NFκB-p65 expression, even though not much, was seen at a level that was different between the groups (p < 0.05). It was noted that the increase observed in the prilocaine group decreased in the prilocaine +TQ group (Fig. 5a). Cerebellum NFκB-p65 expression was rarely observed in sections of the sham group and an increase was observed in the prilocaine group (p < 0.05). Thymoquinone treatment decreased NFκB-p65 expression in the prilocaine + TQ group (Fig. 5b). It was noteworthy that cerebellum NFκB-p50 expression was low in all groups (Fig. 6a). It is observed that cerebellum NFκB-p50 expression, which was rarely observed in the sham group, increased due to toxicity-induced neuroinflammation in the prilocaine group (p < 0.05). This increase was seen to decrease in the prilocaine + TQ group. This suggests that TQ has an anti-inflammatory effect on neuroinflammation associated edema in epilepsy. The absence of cerebellum NFκB-p50 expression in the TQ group, suggests that it completely suppresses possible neuroinflammation. Thalamic nucleus NFκB-p65 expression that was not observed in the sham group and rarely seen in the TQ group, significantly increased in prilocaine treated rats (Fig. 5c). Increased thalamic nucleus NFκB-p65 expression due to prilocaine toxicity was decreased in the prilocaine + TQ group. Choroid plexus NFκB-p65 and -p50 expression level was low in all groups (Figs. 5d and 6b, respectively). However, there was a significant difference between prilocaine and prilocaine + TQ groups (p < 0.05). It was observed that immunostaining of both p65 and p50 subunits was decreased in prilocaine + TQ groups.
Fig. 5.
Immunolocalization and expression levels nuclear factorκB-p65 (NFκB-p65) in the a Cerebral cortex b Cerebellum c Thalamic nucleus d Choroid plexus tissue from all groups. TQ, thymoquinone treated; PRL, prilocaine treated. Scale bar 100 μm. Arrows point to the expression regions. Values for the expression levels of NFκB-p65 are mean ± SD (n = 5). Statistical analysis was performed by One Way Analysis of Variance and all pairwise multiple comparison procedures were done by Tukey test. *, p < 0,05
Fig. 6.
Immunolocalization and expression levels nuclear factorκB-p50 (NFκB-p50) in the a Cerebellum b Choroid plexus. Tissue from all groups. TQ, thymoquinone treated; PRL, prilocaine treated. Scale bar 100 μm. Arrows point to the expression regions. Values for the expression levels of NFκB-p50 are mean ± SD (n = 5). Statistical analysis was performed by One Way Analysis of Variance and all pairwise multiple comparison procedures were done by Tukey test. *, p < 0,05
Discussion
We investigated whether TQ had protective effects on CNS and cardiovascular toxicity seen in a rat model of prilocaine systemic toxicity. A recent study revealed that thymoquinone had cardioprotective effect against bupivacaine, a local anesthetic commonly used in regional anesthesia [19]. In this study we used prilocaine which is frequently applied in infiltration anesthesia. Systemic toxicity of prilocaine can be seen not only in intravenous applications, but also after regional anesthesia applications such as infiltration anesthesia, peripheral block applications and spinal anesthesia [20].
In a study performed on sheep, 350 mg iv. prilocaine was applied for 3 min. Central nervous system toxicity was observed in all sheep, one of the sheep died due to polymorphic ventricular tachycardia and cardiac collapse [21]. In a study by Rosenberg et al., cardiovascular toxicity of 2-chloroprocaine and prilocaine were compared in rat. The first cardiac changes and asystole doses were evaluated [13]. Thymoquinone has been previously reported to be cardioprotective on cyclosporin-A-induced cardiotoxicity in rats [22] and in rat a model of myocardial ischemic reperfusion injury [23]. Likewise, Nigella sativa has been shown to reduce fibrosis in rats with inflammation-induced myocardial fibrosis, and it has been thought that this effect may show through its antioxidant properties [24]. Studies investigating the effects of the Nigella sativa plant on the cardiovascular system have also shown that it has a vasorelaxant effect, decreases heart rate and restores endothelial function [25, 26].
In the study by Rosenberg et al., acute central nervous system toxicity of 2-chloroprocaine and prilocaine were also compared in rats. [13]. Although there are many theories on how local anesthetics cause central nervous system toxicity, the most accepted is the specific receptor theory [27]. According to the specific receptor theory, local anesthetics cross the membrane in the phospholipid structure covering the outside of the nerve axon and bind to specific receptors in the internal part of the voltage-dependent Na + channels in the membrane, preventing sodium passage through these channels. Due to this inhibition, action potential (depolarization) cannot develop and the conduction is blocked by stabilizing the nerve fiber membrane. Selective depression of central inhibitory tracts allows excitatory tracts to run out-of-control or behave in an unrestrained manner leading to epilepsy. The depressive phase of neurological toxicity is also accompanied by loss of consciousness, coma, and respiratory arrest [27].
Various studies have reported that Nigella sativa has neuroprotective properties through inhibitory neurotransmitters such as gamma aminobutyric acid (GABA), opiate-like substances and nitric oxide [28, 29]. Indeed, Nigella sativa has been reported to show protective activity through GABA and glycine neurotransmitters in an epilepsy model created with ciprofloxacin [30].
Antiepileptic efficacy of Nigella sativa plant has been investigated in several studies using experimental epilepsy models. In two studies, where experimental epilepsy was created with pentylenetetrazole, it was reported that Nigella sativa plant showed antiepileptic properties and prevented neuronal damage by showing antioxidant properties [31, 32]. Nigella sativa seed constituents have also been shown to potentiate valproate-induced anticonvulsant response through GABA receptors in rats subjected to pentylenetetrazole-induced convulsions [33]. In a study by Noor et al., pilocarpine model of epilepsy was accomplished in rats, Nigella sativa and valproic acid were compared in terms of antiepileptic activity. The referenced study reported that Nigella sativa can be used as adjuvant with antiepileptic agents, thereby reducing the doses of antiepileptic drugs [34]. In a double-blinded crossover clinical trial study on children with refractory epilepsy, TQ was administered as an adjunctive therapy and its effects on frequency of seizures were compared with those of a placebo. According to the results of this study, TQ was evaluated as an effective and tolerable agent in children with persistent epilepsy [35].
Inflammation occurs as a result of acute epilepsy, it contributes to epileptogenesis and worsens the consequences of status epilepticus [36]. Studies in animal models have shown that there is an intense cytokine release from neurons, reactive astrocytes, activated microglia, vascular endothelial cells and circulating monocytes during status epilepticus [37]. Reactive astrocytes also increase AQP4 expression, which leads to edema formation and spread [38]. Increased edema associated with AQP4 expression exacerbates epilepsy, astrogliosis and further triggers neuroinflammation [12]. Aquaporin-4 is most commonly found in the brain. It is a small molecule of 30 kDa and it expands cell membranes. The glial membrane water channel protein AQP4 is largely adjacent to the cerebral capillaries and pial membranes covering the subarachnoid space [39]. It is also present in neurons of the supraoptic and paraventricular nuclei of the hypothalamus [40]. This localization and high water permeability of the channel make AQP4 an important molecule in water transport. In our study we performed immunohistochemical assessment of AQP4 in brain tissue. We found that TQ reduced the expression of AQP4 which increased due to prilocaine toxicity. Reduced AQP4 expression following TQ treatment may provide partial protection against prilocaine induced epileptiform activity as observed herein.
The main components of the NF-kB pathway activated in neuroinflammation are p50 and p65 proteins [41]. Thus, we also performed immunohistochemical assessment of NF-kB-p65 and -p50 in brain tissue. We found that TQ decreased prilocaine-induced p50 and p65 expression in brain tissue sections. It has been reported that TQ modulates gene expressions in the NFκB pathway and as a result, prevents neuroinflammation by inhibiting proinflammatory cytokine production [42]. In studies investigating the effects of TQ on neuroinflammation, TQ has been shown to inhibit cytokine formation via acting on various signaling pathways including 5’ AMP-activated protein kinase (AMPK), sirtuin 1 (SIRT1), nuclear erythroid 2 related factor 2 (Nrf2) and antioxidant response element (ARE) which regulate the activation of microglia cells [43, 44]. Protective effects of TQ have been reported against convulsant activity induced by lithium-pilocarpine in a model of status epilepticus. The results of this study showed that TQ pretreatment significantly down regulated the protein levels of cyclooxygenase (COX)-2 and tumor necrosis factor (TNF)-α in the brain, by NF-κB signaling pathway [45]. Prilocaine-induced p50 and p65 expression in brain tissue sections decreased following TQ administration which may also contribute to alleviated epileptiform activity as observed in our experimental model.
The choroid plexus is located within the cerebral ventricles, it is established by epithelial cells, loose connective tissue and permeable capillaries. The choroid plexus produces cerebrospinal fluid and has seldom been investigated in disease conditions influenced by inflammatory stimulus. We reasoned that epilepsy could lead to inflammation of the choroid plexus and of the epithelial lining of the ventricle, and that these changes would be associated with altered expression of AQP4 and NFκB-p65 and -p50. To the best of our knowledge, AQP4 expression in the choroid plexus has not been previously evaluated. We found that TQ reduced the expression of AQP4 which increased due to prilocaine toxicity. Treatment with TQ also decreased prilocaine-induced p50 and p65 expression in the choroid plexus.
In addition to the above mentioned properties of TQ, it also has a wide range of antioxidant effects reported in the literature. In most studies, it has been reported that TQ exhibits some of its beneficial effects due to its antioxidant properties. Shao et al., showed that TQ attenuated brain injury in an epilepsy model in rats via an anti-oxidative pathway [46]. In studies conducted on isoproterenol induced myocardial injury, it was shown that TQ was protective via its antioxidant actions [47, 48]. Ojha et al., reported that TQ protects against myocardial ischemic injury by mitigating oxidative stress and inflammation [49]. The findings of a study by Lu et al., demonstrated that TQ was efficient in attenuating myocardial ischemia/reperfusion injury through activation of the SIRT1 signaling pathway, which can thus reduce mitochondrial oxidative stress damage and cardiomyocyte apoptosis [50]. Studies investigating the antioxidant effect of TQ reported that it improves cardiovascular function, and attenuates oxidative stress, inflammation and apoptosis by mediating the phosphatidylinositol 3-kinase (PI3K)/ phosphorylated-protein kinase B (Akt) pathway in diabetic rats [51]. Likewise, it was shown that TQ reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via peroxisome proliferator-activated receptor γ (PPAR-γ) and PI3K/Akt pathways [52].
One of the common clinical findings associated with prilocaine toxicity in humans is methemoglobinemia caused by human carboxylesterase and drug metabolising enzymes cytochrome P450 (CYP)-2E1 and -3A4 [53]. However, we found that prilocaine did not generate methemoglobinemia in rats. Our findings confirm a previous study which has reported that prilocaine did not elevate methemoglobin levels in rat blood [54]. Thus, our study did not show any protective effect of TQ in this regard.
In our experimental model of prilocaine toxicity, we first observed epileptic activity with subsequent cardiac side effects including hypotension and bradycardia. With the onset of cardiac side effects, circulatory disorders occurred in animals, which resulted in altered blood gas measurements. Circulatory disturbance resulted from hemodynamic instability and caused increased pCO2 and decreased blood pH values. The resulting decrease in blood pH began to occur after epileptic seizures. Emerging acidosis can normally exacerbate epileptic seizures and it is reported that respiratory and metabolic acidosis lowers the seizure threshold of local anesthetics [55]. However, epileptic seizures in our experimental model did not occur due to the resulting acidosis, but rather developed from local anesthetic toxicity. Isoelectric EEG occured in rats along with increasing prilocaine infusion in tandem with strengthened acidosis and more severe cardiac side effects. Mechanical ventilation was applied to rats in order to minimize respiratory acidosis. Therefore, we aimed to minimize the effect of acidosis on epileptic seizures to better observe prilocaine toxicity.
In conclusion, in line with the findings of our study, we showed cardioprotective, antiarrhythmic, neuroprotective and antiepileptic efficacy of TQ in local anesthetic systemic toxicity. Our experimental model created with prilocaine was a hyperacute toxicity model that caused hemodynamic instability (arrhythmia, bradycardia, hypotension) and cardiac arrest within minutes. The intravenous administered form of TQ is not yet available. At present, TQ can be applied to rats by gastric gavage or intraperitoneal route. It is not possible for TQ to be absorbed into the systemic circulation and to create an effective blood concentration within minutes when given by gavage or intraperitoneal route. For this reason, TQ was applied 4 days before poisoning so that it could reach an effective blood concentration before hyperacute prilocaine toxicity. Future studies may support the use of TQ as an alternative to intravenous lipid emulsions traditionally used in the treatment of local anesthetic toxicity [56]. However, further studies are needed to apply TQ in clinical use.
Acknowledgements
The authors would like to thank Erol Nizamoğlu for his assistance in surgical procedures conducted on rats. This study was supported by a grant (Grant/Contract Number: TTU-2017-2551) from Akdeniz Uni. Research Foundation.
Abbreviations
- Akt
Phosphorylated-protein kinase B
- AMPK
5’ AMP-activated protein kinase
- AQP4
Aquaporin-4
- ARE
Antioxidant response element
- CK-MB
Creatine kinase-MB
- CNS
Central nervous system
- COX
Cyclooxygenase
- DCF
Dichlorodihydrofluorescein
- ECG
Electrocardiographic
- EEG
Electroencephalography
- FMetHb
Fraction of methemoglobin
- GABA
Gamma aminobutyric acid
- LAST
Local anesthetic systemic toxicity
- NO
Nitric oxide
- Nrf2
Nuclear erythroid 2 related factor 2
- ONOO
Peroxynitrite anion
- pCO2
Partial pressure
- PI3K
Phosphatidylinositol 3-kinase
- pO2
Oxygen partial pressure
- ROO
Peroxyl radical
- ROS/RNS
Oxygen/nitrogen species
- SIRT1
Sirtuin 1
- TQ
Thymoquinone
- TAC
Total antioxidant capacity
- TNF
Tumor necrosis factor
- UAE
Uric acid equivalents
Compliance with ethical standards
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Barış Akgül and İlker Öngüç Aycan contributed equally to this work
Contributor Information
Barış Akgül, Email: barisakgul1503@hotmail.com.
İlker Öngüç Aycan, Email: ilkeraycan@gmail.com.
Enis Hidişoğlu, Email: enishidisoglu@akdeniz.edu.tr.
Ebru Afşar, Email: ebrukiracc@gmail.com.
Sendegül Yıldırım, Email: sendegul.yildirim@gmail.com.
Gamze Tanrıöver, Email: gamzetanriover@akdeniz.edu.tr.
Nesil Coşkunfırat, Email: nesildgr@yahoo.com.
Suat Sanlı, Email: suatsanli@akdeniz.edu.tr.
Mutay Aslan, Email: mutayaslan@akdeniz.edu.tr.
References
- 1.Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin N Am. 2010;54:587–599. doi: 10.1016/j.cden.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Neal JM, Bernards CM, Butterworth JF, 4th, Di Gregorio G, Drasner K, Hejtmanek MR, Mulroy MF, Rosenquist RW, Weinberg GL. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152–161. doi: 10.1097/AAP.0b013e3181d22fcd. [DOI] [PubMed] [Google Scholar]
- 3.Gitman M, Barrington MJ. Local anesthetic systemic toxicity: a review of recent case reports and registries. Reg Anesth Pain Med. 2018;43:124–130. doi: 10.1097/AAP.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 4.Singh P. An emphasis on the wide usage and important role of local anesthesia in dentistry: a strategic review. Dent Res J (Isfahan) 2012;9:127–132. doi: 10.4103/1735-3327.95224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manassero A, Fanelli A. Prilocaine hydrochloride 2% hyperbaric solution for intrathecal injection: a clinical review. Local Reg Anesth. 2017;10:15–24. doi: 10.2147/LRA.S112756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier FJ, Bouaziz H, Samii K. Major complications of regional anesthesia in France: the SOS regional anesthesia hotline service. Anesthesiology. 2002;97:1274–1280. doi: 10.1097/00000542-200211000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Boublik J, Gupta R, Bhar S, Atchabahian A. Prilocaine spinal anesthesia for ambulatory surgery: a review of the available studies. Anaesth Crit Care Pain Med. 2016;35:417–421. doi: 10.1016/j.accpm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Cherobin ACFP, Tavares GT. Safety of local anesthetics. An Bras Dermatol. 2020;95:82–90. doi: 10.1016/j.abd.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaev NK, Chetverikov NS, Stelmashook EV, Genrikhs EE, Khaspekov LG, Illarioshkin SN. Thymoquinone as a potential Neuroprotector in acute and chronic forms of cerebral pathology. Biochemistry (Mosc) 2020;85:167–176. doi: 10.1134/S0006297920020042. [DOI] [PubMed] [Google Scholar]
- 10.Farkhondeh T, Samarghandian S, Borji A. An overview on cardioprotective and anti-diabetic effects of thymoquinone. Asian Pac J Trop Med. 2017;10:849–854. doi: 10.1016/j.apjtm.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Jakaria M, Cho DY, Ezazul Haque M, Karthivashan G, Kim IS, Ganesan P, Choi DK. Neuropharmacological potential and delivery prospects of thymoquinone for neurological disorders. Oxidative Med Cell Longev. 2018;2018:1209801. doi: 10.1155/2018/1209801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder DK, Nagelhus EA, Ottersen OP. Aquaporin-4 and epilepsy. Glia. 2012;60:1203–1214. doi: 10.1002/glia.22317. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg PH, Zou J, Heavner JE. Comparison of acute central nervous system and cardiovascular toxicity of 2-chloroprocaine and prilocaine in the rat. Acta Anaesthesiol Scand. 1993;37:751–755. doi: 10.1111/j.1399-6576.1993.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 14.Aycan İÖ, Elpek Ö, Akkaya B, Kıraç E, Tuzcu H, Kaya S, Coşkunfırat N, Aslan M. Diclofenac induced gastrointestinal and renal toxicity is alleviated by thymoquinone treatment. Food Chem Toxicol. 2018;118:795–804. doi: 10.1016/j.fct.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Salmani JM, Asghar S, Lv H, Zhou J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules. 2014;19:5925–5939. doi: 10.3390/molecules19055925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hidisoglu E, Kantar-Gok D, Er H, Acun AD, Yargicoglu P. Alterations in spontaneous delta and gamma activity might provide clues to detect changes induced by amyloid-β administration. Eur J Neurosci. 2018;47:1013–1023. doi: 10.1111/ejn.13832. [DOI] [PubMed] [Google Scholar]
- 17.Hidisoglu E, Kantar-Gok D, Ozen S, Yargicoglu P. Short-term 2.1 GHz radiofrequency radiation treatment induces significant changes on the auditory evoked potentials in adult rats. Int J Radiat Biol. 2018;94:858–871. doi: 10.1080/09553002.2018.1492166. [DOI] [PubMed] [Google Scholar]
- 18.Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002;22(4):367–378. doi: 10.1097/00004647-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gonca E, Çatli D, Erdem S. The effects of thymoquinone on bupivacaine-induced cardiotoxicity. Karaelmas Fen ve Müh Derg. 2019; 10.7212/zkufbd.v9i1.1246.
- 20.Naguib M, Magboul MM, Samarkandi AH, Attia M. Adverse effects and drug interactions associated with local and regional anaesthesia. Drug Saf. 1998;18:221–250. doi: 10.2165/00002018-199818040-00001. [DOI] [PubMed] [Google Scholar]
- 21.Copeland SE, Ladd LA, Gu XQ, Mather LE. The effects of general anesthesia on the central nervous and cardiovascular system toxicity of local anesthetics. Anesth Analg. 2008;106:1429–1439. doi: 10.1213/ane.0b013e31816d12af. [DOI] [PubMed] [Google Scholar]
- 22.Ebru U, Burak U, Yusuf S, Reyhan B, Arif K, Faruk TH, Emin M, Aydin K, Atilla II, Semsettin S, Kemal E. Cardioprotective effects of Nigella sativa oil on cyclosporine A-induced cardiotoxicity in rats. Basic Clin Pharmacol Toxicol. 2008;103:574–580. doi: 10.1111/j.1742-7843.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- 23.Seif AA. Nigella sativa attenuates myocardial ischemic reperfusion injury in rats. J Physiol Biochem. 2013;69:937–944. doi: 10.1007/s13105-013-0272-5. [DOI] [PubMed] [Google Scholar]
- 24.Norouzi F, Abareshi A, Asgharzadeh F, Beheshti F, Hosseini M, Farzadnia M, Khazaei M. The effect of Nigella sativa on inflammation-induced myocardial fibrosis in male rats. Res Pharm Sci. 2017;12:74–81. doi: 10.4103/1735-5362.199050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebi M, Zeggwagh N, Hajj L, Bouhali BE, Eddouks M. Cardiovascular effect of Nigella sativa L. aqueous extract in normal rats. Cardiovasc Hematol Disord Drug Targets. 2016;16:47–55. doi: 10.2174/1871529x16666160729115249. [DOI] [PubMed] [Google Scholar]
- 26.Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al TH. Cardiovascular benefits of black cumin (Nigella sativa) Cardiovasc Toxicol. 2013;13:9–21. doi: 10.1007/s12012-012-9181-z. [DOI] [PubMed] [Google Scholar]
- 27.El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018;11:35–44. doi: 10.2147/LRA.S154512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beheshti F, Khazaei M, Hosseini M. Neuropharmacological effects of Nigella sativa. Avicenna J Phytomed. 2016;6:104–116. [PMC free article] [PubMed] [Google Scholar]
- 29.Khazdair MR. The protective effects of Nigella sativa and its constituents on induced neurotoxicity. J Toxicol. 2015;2015:841823. doi: 10.1155/2015/841823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arafa NM, Abdel-Rahman M, El-khadragy MF, Kassab RB. Evaluation of the possible epileptogenic activity of ciprofloxacin: the role of Nigella sativa on amino acids neurotransmitters. Neurochem Res. 2013;38:174–185. doi: 10.1007/s11064-012-0905-z. [DOI] [PubMed] [Google Scholar]
- 31.Seghatoleslam M, Alipour F, Shafieian R, Hassanzadeh Z, Edalatmanesh MA, Sadeghnia HR, Hosseini M. The effects of Nigella sativa on neural damage after pentylenetetrazole induced seizures in rats. J Tradit Complement Med. 2015;6:262–268. doi: 10.1016/j.jtcme.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M. Antiepileptogenic and antioxidant effects of Nigella sativa oil against pentylenetetrazol-induced kindling in mice. Neuropharmacology. 2005;49:456–464. doi: 10.1016/j.neuropharm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Raza M, Alghasham AA, Alorainy MS, El-Hadiyah TM. Potentiation of valproate-induced anticonvulsant response by Nigella sativa seed constituents: the role of GABA receptors. Int J Health Sci (Qassim). 2008;2:15–25. [PMC free article] [PubMed] [Google Scholar]
- 34.Noor NA, Aboul Ezz HS, Faraag AR, Khadrawy YA. Evaluation of the antiepileptic effect of curcumin and Nigella sativa oil in the pilocarpine model of epilepsy in comparison with valproate. Epilepsy Behav. 2012;24:199–206. doi: 10.1016/j.yebeh.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Akhondian J, Kianifar H, Raoofziaee M, Moayedpour A, Toosi MB, Khajedaluee M. The effect of thymoquinone on intractable pediatric seizures (pilot study) Epilepsy Res. 2011;93:39–43. doi: 10.1016/j.eplepsyres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Zador Z, Bloch O, Yao X, Manley GT. Aquaporins: role in cerebral edema and brain water balance. Prog Brain Res. 2007;161:185–194. doi: 10.1016/S0079-6123(06)61012-1. [DOI] [PubMed] [Google Scholar]
- 40.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 41.Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr. 2014;14:3–7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cobourne-Duval MK, Taka E, Mendonca P, Soliman KFA. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J Neuroimmunol. 2018;320:87–97. doi: 10.1016/j.jneuroim.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velagapudi R, El-Bakoush A, Lepiarz I, Ogunrinade F, Olajide OA. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol Cell Biochem. 2017;435:149–162. doi: 10.1007/s11010-017-3064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velagapudi R, Kumar A, Bhatia HS, El-Bakoush A, Lepiarz I, Fiebich BL, Olajide OA. Inhibition of neuroinflammation by thymoquinone requires activation of Nrf2/ARE signalling. Int Immunopharmacol. 2017;48:17–29. doi: 10.1016/j.intimp.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Shao Y, Feng Y, Xie Y, Luo Q, Chen L, Li B, Chen Y. Protective effects of thymoquinone against convulsant activity induced by lithium-pilocarpine in a model of status epilepticus. Neurochem Res. 2016;41:3399–3406. doi: 10.1007/s11064-016-2074-y. [DOI] [PubMed] [Google Scholar]
- 46.Shao YY, Li B, Huang YM, Luo Q, Xie YM, Chen YH. Thymoquinone attenuates brain injury via an anti-oxidative pathway in a status epilepticus rat model. Transl Neurosci. 2017;8:9–14. doi: 10.1515/tnsci-2017-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randhawa MA, Alghamdi MS, Maulik SK. The effect of thymoquinone, an active component of Nigella sativa, on isoproterenol induced myocardial injury. Pak J Pharm Sci. 2013;26:1215–1219. [PubMed] [Google Scholar]
- 48.Al-Nimer MS, Rajab BR, Al-Aani HA. Thymoquinone protects the heart against isoproterenol-induced myocardial ischemia in mice: a histopathological study. Indian J Pharmacol. 2016;48:97–98. doi: 10.4103/0253-7613.174585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ojha S, Azimullah S, Mohanraj R, Sharma C, Yasin J, Arya DS, Adem A. Thymoquinone protects against myocardial ischemic injury by mitigating oxidative stress and inflammation. Evid Based Complement Alternat Med. 2015;2015:143629. doi: 10.1155/2015/143629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Y, Feng Y, Liu D, Zhang Z, Gao K, Zhang W, Tang H. Thymoquinone attenuates myocardial ischemia/reperfusion injury through activation of SIRT1 signaling. Cell Physiol Biochem. 2018;47:1193–1206. doi: 10.1159/000490216. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Liu HY, Jiang YN, Li N. Protective effect of thymoquinone improves cardiovascular function, and attenuates oxidative stress, inflammation and apoptosis by mediating the PI3K/Akt pathway in diabetic rats. Mol Med Rep. 2016;13:2836–2842. doi: 10.3892/mmr.2016.4823. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Wang B, Zhao H. Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways. Exp Ther Med. 2018;15:4987–4994. doi: 10.3892/etm.2018.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higuchi R, Fukami T, Nakajima M, Yokoi T. Prilocaine- and lidocaine-induced methemoglobinemia is caused by human carboxylesterase-, CYP2E1-, and CYP3A4-mediated metabolic activation. Drug Metab Dispos. 2013;41:1220–1230. doi: 10.1124/dmd.113.051714. [DOI] [PubMed] [Google Scholar]
- 54.Heinonen J, Ahtee L. Influence of enzyme inducer on methaemoglobinaemia caused by prilocaine. Acta Anaesthesiol Scand. 1968;12:23–29. doi: 10.1111/j.1399-6576.1968.tb05453.x. [DOI] [PubMed] [Google Scholar]
- 55.Perks A, Cheema S, Mohanraj R. Anaesthesia and epilepsy. Br J Anaesth. 2012;108:562–571. doi: 10.1093/bja/aes027. [DOI] [PubMed] [Google Scholar]
- 56.Motayagheni N, Phan S, Nozari A, Atala A. Lipid emulsion, more than reversing bupivacaine Cardiotoxicity: potential organ protection. J Pharm Pharm Sci. 2017;20:329–331. doi: 10.18433/J30D2V. [DOI] [PubMed] [Google Scholar]