Abstract
Background
With the advances of modern medicine and technology there has been an increase of indications of shoulder reconstruction techniques and shoulder arthroplasty. Consequently, the number of complications and failures have increased in parallel. Not negligible number of cases are driven to an end-stage situation where salvage procedures, such as glenohumeral arthrodesis (GHA) and shoulder resection arthroplasty (SRA), are the only remaining solution.
Methods
The current literature on glenohumeral arthrodesis and shoulder resection arthroplasty was reviewed to determine the indications, surgical technique, complications and outcomes. The electronic search was conducted using the MEDLINE and EMBASE databases and the strategies used were “glenohumeral arthrodesis”, “glenohumeral fusion”, “shoulder arthrodesis” and “shoulder resection arthroplasty”.
Results
Indications for glenohumeral arthrodesis (GHA) include brachial plexus injury, tumor resections, chronic infection, failed prosthetic arthroplasty, persistent refractory instability or pseudoparalysis of the shoulder with combined irreparable rotator cuff and deltoid injuries. GHA provides good stability, pain resolution, although function is markedly compromised and relying mostly on scapulothoracic joint. The gold standard surgical technique continues to be open shoulder arthrodesis and still has a high complication rate. Shoulder resection arthroplasty (SRA) indications have evolved through the years, being nowadays a salvage procedure for recalcitrant infection of shoulder arthroplasty the main indication. Shoulder function after SRA is often severely compromised, but has a high infection rate resolution. SRA is not technically demanding and complications are rare, being the persistence of infection the most common one.
Discussion
Despite GHA and SRA having negative connotations, in selected patients, these procedures can diminish pain, resolve persistent infections and provide an acceptable shoulder function. Hence, they should be retained as part of the treatment algorithm for complex shoulder pathology.
Keywords: Salvage procedure, Glenohumeral arthrodesis, Shoulder resection arthroplasty, Indications, Complications
Introduction
With the advances of modern medicine and technology there has been an increase of indications for shoulder reconstruction techniques and shoulder arthroplasty. This tendency has led to a worrying increase in number of complications and failures. Not negligible number of cases are driven to an end-stage situation where salvage procedures are necessary. Glenohumeral arthrodesis (GHA) [1–4] and shoulder resection arthroplasty (SRA) [5–8] are salvage procedures that are indicated in patients with marked shoulder dysfunction as a valuable salvage option when other surgical options are limited or not possible. These patients often have paralytic disorders, soft-tissue deficiency and deformity, bone loss or infection that compromise the options for surgical reconstruction [1–3, 6].
Glenohumeral Arthrodesis
Indications
Despite GHA has often been associated with negative outcomes, mainly due to compromised shoulder function, in selected patients it can improve function, diminish pain, and actually provide acceptable range of motion. The most common indications for GHA include brachial plexus injury [9], tumor resections [4], chronic infection [6, 10], failed arthroplasty [3], persistent refractory instability [11], post traumatic arthritis in the young patient [12], inflammatory arthritis with irreparable rotator cuff tear or pseudoparalysis of the shoulder from combined rotator cuff and deltoid deficiency [4, 13].
To obtain the optimal results, it is necessary to assess preoperatively the scapulothoracic mobility and the periscapular muscle function [1]. Although the glenohumeral joint account for the majority of shoulder motion (2:1 ratio in a normal patient), the scapulothoracic joint has an extraordinary ability to compensate and provide significant mobility and function [14]. It is therefore contraindicated a GHA in patients with paralysis of trapezius, levator scapulae, serratus anterior, latissimus dorsi, or rhomboid muscles. These scapula-stabilizing muscles are required to provide motor function to the extremity. Richards reported that, if these muscles are nonfunctional, the extremity will be severely impaired despite successful joint fusion [15, 16]. Charcot arthropathy has also been reported as a contraindication to shoulder arthrodesis. The rates of nonunion and infection are stated to be higher in patients with Charcot arthropathy, and thus GHA is discouraged [17]. Other contraindications to GHA are ipsilateral elbow arthrodesis or contralateral shoulder arthrodesis [18]. Bilateral GHA severely inhibits the patient’s functional abilities, including the ability to perform activities of daily living [19].
Surgical Technique
There have been described multiple surgical techniques for GHA [3, 20, 21]. The main variations are focused on the fixation method, the use of bone grafts, the fusion position and the postoperative rehabilitation protocol.
Surgical and Fixation Options
In the first reports of GHA, the technique did not include internal fixation with hardware and was postoperatively followed by 3 months of spica casting [2]. The use of glenohumeral compression screws was one of the first internal fixation techniques that allowed to reduce significantly the postoperative immobilization period [22]. Later on Richards et al. [23] were the first to report an arthrodesis technique by means of a dynamic compression plate (DCP) and to include both the glenohumeral and acromiohumeral arthrodesis. A contoured plate was utilized over the spine of the scapula, acromion and lateral humerus obtaining 100% fusion rate after 8 weeks of immobilization. Subsequent studies have used a 4.5 mm pelvic reconstruction plate because of the ease of contouring it to fit the shoulder [19, 24, 25]. They demonstrated that the reconstruction plate provided sufficient stability to achieve a high union rate. Further innovative changes to the technique have been described like subacromial position of the plate [26] or arthroscopic assisted procedures [11, 27].
Arthroscopic assisted arthrodesis was described aiming for a less invasive approach sparing the deltoid. Through a regular intraarticular arthroscopic approach and with the help of the shaver and the burr, glenoid and humeral head are debrided of cartilage and skeletonized to bleeding surfaces. Thereafter, intraarticular fixation is performed with screws under arthroscopic control. Generally, arthroscopic techniques fuse only glenohumeral joint, sparing the acromiohumeral interface. Morgan and Casscells in 1992 were the first who described an arthroscopic technique for shoulder arthrodesis [28]. Porcellini et al. compared clinical and radiographic outcomes of open and mini-open arthroscopic assisted arthrodesis [29]. The authors showed equal results in satisfaction and complications rate. Recently, Lädermann et al. [30] showed the value of O-arm navigation in arthroscopic arthrodesis for precise screw fixation, which may be particularly useful in cases of limited bone stock and/or bony deformity.
Lastly, there are a few reports of glenohumeral arthrodesis using external fixation [31–33]. This arthrodesis method can be augmented with the use of glenohumeral compression screws.
Bone Grafting
Like arthrodesis of other joints, GHA nonunion rate is high and the use of different types of bone graft have been described to enhance consolidation. For no bone defect or for small bone defect, the local graft obtained from the humeral head or from the iliac crest is sufficient. Rühmann et al. [14] noted that the use of plates and bone-grafting improved the union rate compared to the use of screws and no graft. Atlan et al. demonstrated that the use of an iliac corticocancellous autograft in the subacromial space significantly increased the union rate when compared with the use of cancellous bone alone [12]. Other studies have reinforced the need for plate fixation and a large amount of autograft [13]. For more extensive bone loss, mainly due to infection or osteolysis after failed arthroplasty [3] or tumor resection, a structural allograft like tricortical autologous iliac crest graft, or vascularized fibular grafts may be required to replace bone stock deficiency.
Position of Arthrodesis
The position of arthrodesis is argued to be most important factor in determining postoperative extremity function [34], although this has been debated extensively in the literature [13]. Conversely, Cofield and Briggs found that shoulder position had no impact on function [35]. The main goal is to be functional for daily living activities and hygiene (i.e., eating, showering, dressing, reaching back for bathroom hygiene). Initial recommendations exaggerated the arthrodesis position in 50º of abduction. But soon in 1974, Rowe et al. advocated for 20–25º of abduction, 30º of flexion, and 45–50º of internal rotation [36]. They argued that high degrees of abduction lead to periscapular muscle fatigue, hence triggering pain and winging at rest. In these days most studies have agreed that the optimal arthrodesis position is 15–25° of forward flexion, 15–25º of abduction and 40–45° of internal rotation [37–39]. This position enables the patient to reach from the top of the head to the ipsilateral back pocket, as well as rest the arm at the side comfortably without scapular winging [2].
Author’s Preferred Technique
The authors preferred GHA technique depends on the selected patient and the hypothetical future conversion to a shoulder replacement (Table 1). When the indication is persistent refractory shoulder instability, inflammatory arthritis, or post traumatic early arthritis in the young patient and there is an intact rotator cuff, our preference is an open intraarticular GHA with screws (Fig. 2). Three cannulated 6.5 mm screws are placed under fluoroscopic control. In this procedure, the rotator cuff and acromiohumeral interface are spared. This will allow an easier conversion to shoulder arthroplasty in the future. When the indication is neurologic paralysis, inflammatory arthritis, septic arthritis or pseudoparalysis with combined rotator cuff and deltoid damage in the elderly patient, our preference is an open GHA using a plate fixation. Through an extended deltopectoral approach to the acromion and scapula spine, decortication of acromiohumeral and glenohumeral surfaces to bleeding subchondral bone is performed to increase the surface area available. The arthrodesis is fused by means of a 4.5 mm reconstruction plate over the spine, acromion and lateral humerus (Fig. 1). Regardless of the technique, our fusion position preference is 20–30º flexion, 10–15º abduction and 40–45º internal rotation. Also cortico-cancellous allograft from a fermoral head or tricortical iliac crest autograft is used to promote consolidation.
Table 1.
Tips and tricks
| Shoulder arthrodesis | Shoulder resection arthroplasty |
|---|---|
| Spare the acromio-humeral interface in cases where conversion to shoulder arthroplasty is possible in the future | Use previous incisions and avoid tissue devitalization |
| Decorticate surfaces to bleeding bone and achieve congruency to increase surface contact area | Extensive debridement, irrigation and bony resection to good quality bone are essential |
| For screw fixation, use at least three 6.5 mm cannulated screws and check the position with fluoroscopy or O-arm | Extraction of cemented implants may be assisted by mechanical ultrasound. When the humeral stems are well-fixed a cortical window may be needed |
| Avoid fusion position over 20º of abduction or 25º flexion. Check functional position intraoperatively | Avoid violating humerus tuberosities and rotator cuff insertion to reach better functional outcome |
| Supply cortico-cancellous auto- or allograft | Collecting multiple biopsies for microbiological and anatomopathological study is mandatory |
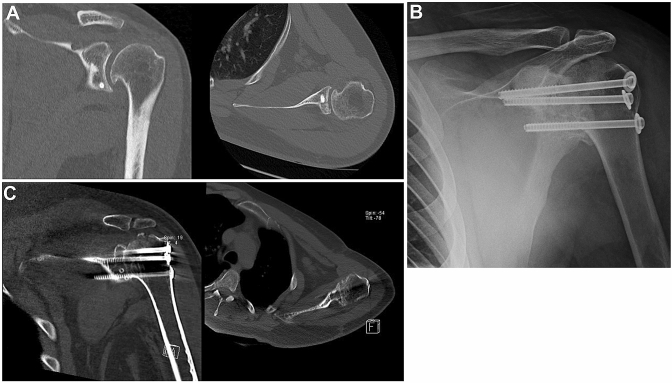
Fig. 2.
Case 2: a 25 year-old patient with recalcitrant post instability that after several failed stabilization procedures underwent GHA. a CT scan of the left shoulder showing coronal (left) and axial (right) projections of posterior screwed bone block procedure. Chronic instability led to early osteoarthritis with osteophytes in the infero-medial humeral head. b Radiograph of a left shoulder showing GHA using three cannulated screws with washers transfixing the glenohumeral joint. Fusion to the acromion was avoided. Post-operative CT scan of the left shoulder showing successful gleno-humeral fusion in coronal (left) and axial views (right)
Fig. 1.
Case 1: anteroposterior (a) and lateral (b) view radiographs of glenohumeral arthrodesis using a plate over the acromion and subacromial bone-grafting
Outcomes
While there have been numerous reports concerning the poor results of glenohumeral arthrodesis, it can yield satisfactory outcomes in selected patients with severe shoulder disfunction by diminishing pain, improving motion and overall function (Tables 2, 3). Interestingly, there is no consensus in the literature about the indications associated with improved outcomes. Richards et al. [38] noted that GHA using a ten-hole plate led to the highest patient satisfaction in those with brachial plexus injuries, osteoarthritis and failed total shoulder arthroplasties. The worst results were found in those with a history of multidirectional shoulder instability. Rühmann et al. [14] examined 43 GHAs at a mean of seven years follow-up with patients achieving 60º of active flexion. The Constant score improved postoperatively by 30 points, reaching a final score of 57 and 91% of patient satisfaction. Atlan et al. [12] reported on 54 GHAs in patients with brachial plexus palsy at a mean time of 37 months finding a 94% fusion rate at the last follow-up. Although patient pain and satisfaction were not reported, mean abduction was 59º and mean external rotation was > 45°. In a more recent study, Wagner et al. [13] reported outcomes of 29 GHA and at a mean follow-up of 12 years the postoperative scores were 35 points for the SSV, 58 points for DASH questionnaire, and 54 points for the Short Form-36. They noted that patients with neurologic injuries had worse functional outcomes, and a GHA position in abduction of ≥ 25° yielded better functional outcomes. Although patients obtained reasonable pain relief and shoulder stability, they experienced marked limitations in their upper extremity function.
Table 2.
Studies including patients in whom glenohumeral arthrodesis was performed
| Author/year | Number of patients | Average follow-up | Position of arthrodesis | Postoperative Shoulder score | ROM | Complication rate |
|---|---|---|---|---|---|---|
| Cofield and Briggs (1979) [35] | 71 | 9 years | 45º Abd, 25º Flex | – | – | 35% |
| Hawkins and Neer (1987) [34] | 17 | 3.3 years | 30º Flex, 25 Abd, 25º IR | – | – | |
| Richards et al. (1993) [38] | 57 | 3.7 years |
30º Abd, 30º Flex, 30º IR |
– | – | 14% |
| Rühmann et al. (2005) [14] | 43 | 6.7 years |
20-60º Abd, 20-40º Flex, 0-50º IR |
57 CMS | 60º Flex, 56º Abd | 28% |
| Scalise and Iannotti (2008) [3] | 7 | 4 years | 10-20º Abd, 10-20º Flex, 35-45º IR | 58 Penn score | – | 29% |
| Atlan et al. (2012) [12] | 45 | 3 years | 26º Abd, 36º IR | – | 59 ABD, > 45 ER | 17% |
| Porcellini et al. (2014) [29] | 12 | 2 years | - | 64 SPADI | – | 17% |
| Wagner et al. (2018) [66] | 29 | 12 years | 24º Abd, 25 Flex, 34º IR | 58 DASH, 35 SSV, 54 Short form 36 | 60º Flex, 13º ER | 41% |
Abd abduction, Flex flexion, IR internal rotation, ER external rotation, CMS Constant-Murley Score, DASH disabilities of the arm, shoulder and hand, SSV subjective shoulder value, SPADI shoulder pain and disability index
Table 3.
Advantages and Disadvantages
| Shoulder arthrodesis | Shoulder resection arthroplasty | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Fair functional outcomes | High complication rate | High resolution rate of infection and pain | Poor functional outcomes |
| Effective resolution of pain | May require revision surgeries | Surgical technique not demanding | Challenging revision surgery in case of failure |
| Convertible to shoulder replacement | Surgical technique demanding | Low cost/benefit ratio | |
| Low complication rate | |||
The conversion of shoulder arthroplasty to GHA is an extremely challenging situation due to the extensive bone loss and soft tissue deficiency. Scalise and Iannotti [3] reviewed seven patients who underwent GHA after prosthetic arthroplasty failures. Two of the patients experienced persistent nonunions, and another two patients underwent revision bone-grafting for delayed union. However, at the time of the latest follow-up, the patients’ subjective shoulder function and pain were significantly improved (p < 0.03). Another review by Miller et al. examined 11 children who experienced a flail shoulder secondary to polio and underwent GHA [40]. Although six patients underwent hardware removal secondary to pain, two required osteotomies to correct rotation, and one underwent revision for nonunion. All patients reported satisfaction and minimal pain at a mean follow-up of 41 months.
Complications
Although the outcomes vary among studies, GHA is associated with a high rate of complications [1–3, 19, 41] (Table 2). Rühmann et al. [14] reported an overall complication rate of 28% in a series of 43 cases using two different techniques: screws and plate fixation. Nonunions appeared to be most frequent complication (12%), and more related to screws fixation. Infection (11%) and fracture of the humerus (9%) were more related to plate fixation. In 2018 Wagner et al. [13] evaluated the long-term results of 29 shoulders who underwent primary GHA for varied indications, reporting an overall complication rate of 41% had postoperative complications, including six periprosthetic fractures, seven nonunions, and three infections. Cofield and Briggs [35] reported on 71 patients who underwent GHA for multiple etiologies and had a 35% complication rate. In another review of 17 patients who underwent GHA, Hawkins and Neer found that only four patients (24%) were without pain at the time of the latest follow-up, and that seven of the 17 patients were dissatisfied with their functional disability [34].
Is There Anything After Failed Glenohumeral Arthrodesis?
Yet performing GHA is not an "endroad" procedure for the shoulder. Despite a primary successful result a number of patients may complain of increasing symptoms on the scapulo-thoracic joint. In cases with chronic pain and presence of a functional deltoid, a conversion of a GHA to an arthroplasty may be indicated. Since the first report by Neer [42], several authors have reported successful outcome on such procedure. Hertel et al. [43] reported the case of GHA for a rotator cuff arthropathy using a plate over the acromion with a solid fusion. Three years later it was converted to an anatomic hemiarthroplasty due to persistent pain around the scapula. Although function was poor after the arthroplasty, complete and permanent pain relief was achieved. More recently, Alta et al. [44] presented the conversion of four cases of GHA to a reverse shoulder arthroplasty after a longstanding fusion (5–11 years). Satisfactory results were achieved by decreasing pain and improving mobility, especially rotations. In our opinion, whenever there is a potential conversion to an arthroplasty, the GHA should avoid fusion of the acromiohumeral interface and spare the rotator cuff (Fig. 2). Providing enough bone graft to the glenohumeral joint and stabilization by means of several transfixing screws through the joint usually leads to successful fusion.
Shoulder Resection Arthroplasty
Indications
Historically, shoulder resection arthroplasty (SRA) was a widely used procedure for glenohumeral pathology in the late nineteenth and early twentieth centuries [5]. The main indications of SRA were complex fractures-dislocations, septic arthritis with osteomyelitis, glenohumeral osteoarthritis and other processes such as war wounds or tuberculosis [20, 45]. However, the emergence of alternative treatments for these pathologies such as shoulder arthroplasties (SA) [46] and subsequently reverse shoulder prostheses (RSA) [47] drove the SRA to be abandoned or at least relegated to exceptional salvage situations.
In recent years, the rapid development on shoulder arthroplasty components led to an exponential increase in the use of total prostheses and especially of reverse shoulder arthroplasties (RSA) [48–50]. Although the overall success rate is high [51, 52], the number of failures has proportionally increased. The main cause for revision is instability, followed by peri-prosthetic infection, aseptic loosening of components and fractures [53]. Currently, the incidence of infection after SA reach up to 2.9% for primary SA and up to 15.4% for revision arthroplasty [54] with devastating consequences. There are several options for the treatment of failed shoulder arthroplasty, including antibiotics, arthroscopic debridement, open debridement with and without preservation of components, one stage revision and two staged revision with or without a spacer. However, revision surgery for recalcitrant infected arthroplasty or persistent component loosening is challenging and the options are limited due to the loss of bone stock and soft tissue tension [7]. In many of these cases where preservation of prosthetic components carries a high failure risk or even jeopardize patient’s life due to disseminated sepsis, SRA stands as an option for infection eradication [6, 55] (Fig. 3).
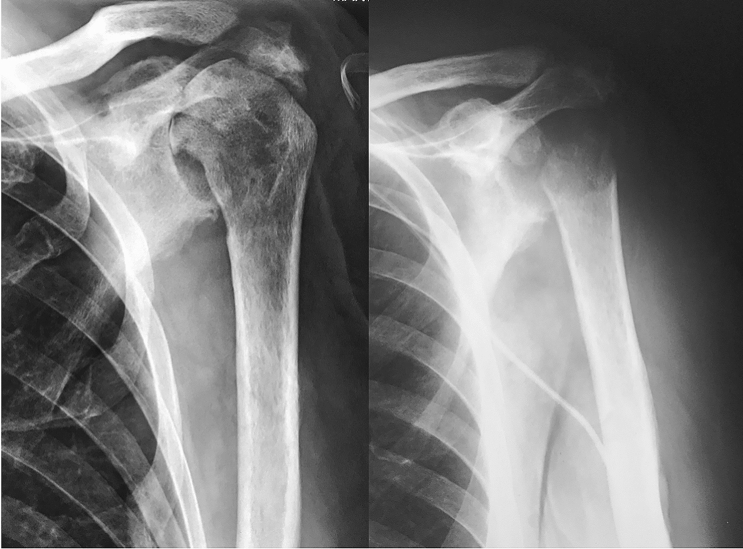
Fig. 3.

Case 3: 52-year-old male diagnosed with schizotypal disorder presented after a seizure with a glenohumeral fracture-dislocation. a Left: anteroposterior radiograph of left shoulder showing fracture-dislocation of proximal humerus. a Right: He was initially treated by plate osteosynthesis. b Left: some weeks later underwent revision surgery, with hardware removal and hemiarthroplasty. In the immediate postoperative period, he began with general sepsis signs and purulent wound effusion. b Right: SRA was performed due to failure to solve periprosthetic infection after two surgical revisions, worsening of the patient’s general condition, low functional demand of the patient and being the non-dominant arm. c At 24 months mark, the patient was free of infection, had complete pain relief and was satisfied with the functional outcome to develop activities of daily life
Surgical Technique
The surgical technique for SRA is usually a standardized and not technically demanding procedure, including variants proposed by some authors. The most commonly used approach is the deltopectoral or through previous surgical incisions. Extensive debridement and removal of the prosthesis and cement are performed, followed by thorough irrigation of the surrounding tissue. For cement extraction, the use of multiple osteotomes and even an ultrasound device is recommended to ensure elimination of cement particles and bone that could persist the infection [56]. When managing infection, bone resection should be aggressive to the level of non-suspicious bone [45] (Fig. 4). Seemingly, it is important to take biopsies for microbiological cultures and anatomical pathology study. Verhelst et al. [45] recommended preservation of the tuberosities and rotator cuff remnant, since it enhances functional results by avoiding antero-superior escape of the humerus.
Fig. 4.
Case 4: a 75 year-old female patient presented with hematogenous osteomyelitis of the left shoulder. Left: anteroposterior radiograph shows osteolysis and cystic lesions of the humeral head. Right: after 6 months of non-responsiveness to antibiotics and four surgical procedures for debridement and thorough irrigation, a SRA was performed to resolve the infection
Author’s Preferred Surgical Technique
The surgical technique preferred by the authors is an approach through previous surgical incisions (usually deltopectoral) to minimize soft tissue devitalization and skin necrosis (Table 1). It is crucial in recalcitrant infections to take at least six biopsies (including joint fluid, soft tissue and bone), extensive soft tissue debridement, bone resection to clear margins and thorough wash with povidone-iodine for internal use. After taking the biopsies, combined wide spectrum antibiotic therapy is started, which will be modified according to the antibiogram result and maintained for at least 6 weeks or until the infection parameters normalize. We always intend to preserve the tuberosities to improve postoperative function if the bone and rotator cuff are free of infection. A drain is placed for 24–48 h after surgery and the arm is immobilized in a sling. The patients is allowed to start passive-assisted physiotherapy exercises from the day after the intervention.
Outcomes
Previous reports on outcomes are based on short series (Table 4). Despite having poor functional outcome with limited mobility and strength infection control and pain improvement are remarkable. The infection remission rates are between 70 and 100% [8, 57–61] and improvement in the visual analog scale (VAS) pain score between 4 and 5 points [7, 8]. Range of motion is consistently compromised after SRA, but it varied among series. The best results were reported by Rispoli et al. [8], reaching a mean active forward flexion of 70° and external rotation of 31° in a series of 18 cases after total shoulder arthropasty removal, improving preoperative motion. Verhelst et al. [45] reported an average of 85° for active forward flexion and 21° of external rotation. Most series presenting RSA removal, nevertheless, showed decreased postoperative motion, ranging from 30° to 45° of flexion and a maximum of 10° of external rotation [6, 7, 59, 62, 63]. It is important to highlight that SRA after anatomic SA present better functional results than after RSA, which could be attributed to a higher humeral head resection, therefore preserving the remaining rotator cuff insertions. All in all, taking the poor postoperative function, the SRA is reserved for elderly, low demand patients with poor physical condition unresponsive to other types of surgery.
Table 4.
Studies including patients in whom resection arthroplasty was performed as a treatment after a prosthetic shoulder infection
| Author/year | Number of patients | Average follow-up (months) | Patients free of infection | Percentage of patients free of infection | Constant-Murley score |
|---|---|---|---|---|---|
| Coste et al. (2004) [64] | 10 | 32.0 | 7 | 70.0 | 30 |
| Sperling et al. (2001) [57] | 21 | 20.4 | 15 | 71.4 | – |
| Braman et al. (2006) [67] | 7 | 48.0 | 7 | 100 | – |
| Ghijselings et al. (2013) [58] | 8 | 43.8 | 7 | 87.5 | 28 |
| Jacquot et al. (2015) [68] | 3 | 36.0 | 2 | 66.6 | 27 |
| Ortmaier et al. (2014) [69] | 4 | 73.7 | 4 | 100 | 17 |
| Rispoli et al. (2007) [8] | 13 | 99.6 | 13 | 100 | – |
| Romano et al. (2012) [60] | 6 | 42.0 | 6 | 100 | 32 |
| Verhelst et al. (2011) [45] | 3 | 46.8 | 2 | 66.6 | 38 |
| Weber et al. (2011) [61] | 5 | 48.0 | 5 | 100 | 33 |
| Debeer et al. (2006) [6] | 7 | 9 | 6 | 85.7 | 25.7 |
Complications
Intraoperative complications are rare. There are two documented cases of intraoperative fractures during implant removal though, which consolidated satisfactorily during the immobilization period [8]. The main postoperative complication is the persistence of infection, which can reach up to 33% in some series [59]. It is very rare that these patients require revision surgeries [64].
Is There Anything After Failed Shoulder Resection Arthroplasty?
The SRA is usually the end-stage procedure for a shoulder [64] performed in extreme situations aiming to resolve recalcitrant infection and improve pain [6, 55], knowing beforehand that the expected functional result will be poor [57, 63, 64] (Table 3). However, there are options for SRA reversal after resolution of the infection or in situations of tumor resections, aiming to improve shoulder function and pain. The conversion is usually performed using reverse shoulder-allograft prosthesis composite [59, 60]. Recently. Boileau et al. [65] have reported a series of 25 cases of SRA or with extensive bone defects converted to a reverse shoulder prosthesis using structural allograft. They reported good results in around 75% of patients and requiring revision surgery in 32% of the cases. Sanchez-Sotelo et al. [66] using a similar technique on 26 cases showed improved function for elevation, external rotation and improvement in pain levels. It must be noted that although the shoulder function can be improved, these are challenging procedures with high rates of complications even in expert hands.
Conclusions
Glenohumeral arthrodesis is a salvage procedure that is indicated in patients with marked shoulder dysfunction when other surgical options are limited. To date, there is no consensus in the literature about the indications associated with improved outcomes or the surgical technique. Although GHA is able to improve instability, diminish pain and preserve shoulder function, it is associated with a high rate of complications. Both the position for fusion and the surgical technique have been shown to influence the surgical outcomes after GHA.
Shoulder resection arthroplasty indications shifted from complicated fractures of the proximal humerus and war injuries to a salvage procedure for recalcitrant infection of shoulder arthroplasty. With the worrying increase of reverse shoulder arthroplasty and its consequent rise of infections, SRA is a procedure to bear in mind in the present time and in the near future for end-stage situations. It is important to be aware about its indications, expected benefit and its potential functional repercussions. Further studies are needed to establish adequate guidelines to help in the management of the upcoming situation.
Author contributions
All authors contributed to the study. The idea for the paper came from Dr. AA-M. The literature search was done by Dr. FF and Dr. LA-G. The draft was written by Dr. AA-M and Dr. FF. Finally Dr. JDP critically revised the work.
Funding
None of the authors, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
The patients were informed and gave verbal consent to use the anonymized images for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
References
- 1.González-Díaz R, Rodríguez-Merchán EC, Gilbert MS. The role of shoulder fusion in the era of arthroplasty. International Orthopaedics. 1997;21(3):204–209. doi: 10.1007/s002640050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safran O, Iannotti JP. Arthrodesis of the shoulder. Journal of American Academy of Orthopaedic Surgeons. 2006;14(3):145–153. doi: 10.5435/00124635-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Scalise JJ, Iannotti JP. Glenohumeral arthrodesis after failed prosthetic shoulder arthroplasty. Journal of Bone and Joint Surgery. American. 2008;90(1):70–77. doi: 10.2106/JBJS.G.00203. [DOI] [PubMed] [Google Scholar]
- 4.Kamineni S, Unger RZ, Desai R. Shoulder arthrodesis in the management of glenohumeral pathologies. J Shoulder and Elbow Arthroplasty. 2019;3:247154921985065. [Google Scholar]
- 5.Milbrink J, Wigren A. Resection arthroplasty of the shoulder. Scandinavian Journal of Rheumatology. 1990;19(6):432–436. doi: 10.3109/03009749009097632. [DOI] [PubMed] [Google Scholar]
- 6.Debeer P, Plasschaert H, Stuyck J. Resection arthroplasty of the infected shoulder: A salvage procedure for the elderly patient. Acta Orthopaedica Belgica. 2006;72(2):5. [PubMed] [Google Scholar]
- 7.Muh SJ, Streit JJ, Lenarz CJ, McCrum C, Wanner JP, Shishani Y, et al. Resection arthroplasty for failed shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2013;22(2):247–252. doi: 10.1016/j.jse.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Pain relief and functional results after resection arthroplasty of the shoulder. Journal of Bone and Joint Surgery. British. 2007;89-b(9):1184–1187. doi: 10.1302/0301-620X.89B9.19464. [DOI] [PubMed] [Google Scholar]
- 9.Rühmann O, Gossé F, Wirth CJ, Schmolke S. Reconstructive operations for the paralyzed shoulder in brachial plexus palsy: Concept of treatment. Injury. 1999;30(9):609–618. doi: 10.1016/s0020-1383(99)00165-5. [DOI] [PubMed] [Google Scholar]
- 10.Wick M, Müller EJ, Ambacher T, Hebler U, Muhr G, Kutscha-Lissberg F. Arthrodesis of the shoulder after septic arthritis. Long-term results. Journal of Bone and Joint Surgery. British. 2003;85(5):666–670. [PubMed] [Google Scholar]
- 11.Hiersemann K, Patsalis T, Saxler G. Arthroscopy-assisted glenohumeral arthrodesis: A case of uncontrollable shoulder instability. Unfallchirurg. 2007;110(5):456–459. doi: 10.1007/s00113-006-1214-2. [DOI] [PubMed] [Google Scholar]
- 12.Atlan F, Durand S, Fox M, Levy P, Belkheyar Z, Oberlin C. Functional outcome of glenohumeral fusion in brachial plexus palsy: A report of 54 cases. The Journal of Hand Surgery. 2012;37(4):683–688. doi: 10.1016/j.jhsa.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Wagner ER, McLaughlin R, Sarfani S, Cofield RH, Sperling JW, Sanchez-Sotelo J, et al. Long-term outcomes of glenohumeral arthrodesis. Journal of Bone and Joint Surgery. American. 2018;100(7):598–604. doi: 10.2106/JBJS.17.00428. [DOI] [PubMed] [Google Scholar]
- 14.Rühmann O, Schmolke S, Bohnsack M, Flamme C, Wirth CJ. Shoulder arthrodesis: Indications, technique, results, and complications. Journal of Shoulder and Elbow Surgery. 2005;14(1):38–50. doi: 10.1016/j.jse.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Richards R. Redefining indications and problems of shoulder arthrodesis. Philadelphia: Lippincott-Raven; 1997. pp. 319–337. [Google Scholar]
- 16.Richards R. Shoulder arthrodesis. New York: Raven; 1995. pp. 385–396. [Google Scholar]
- 17.Wilde AH, Brems JJ, Boumphrey FR. Arthrodesis of the shoulder. Current indications and operative technique. The Orthopedic Clinics of North America. 1987;18(3):463–472. [PubMed] [Google Scholar]
- 18.Neer C. Glenohumeral arthrodesis. Philadelphia: WB Saunders; 1990. pp. 438–442. [Google Scholar]
- 19.Clare DJ, Wirth MA, Groh GI, Rockwood CA. Shoulder arthrodesis. Journal of Bone and Joint Surgery. American. 2001;83(4):593–600. doi: 10.2106/00004623-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Cofield RH. Shoulder arthrodesis and resection arthroplasty. Instructional Course Lectures. 1985;34:268–277. [PubMed] [Google Scholar]
- 21.Charnley J. Compression arthrodesis of the ankle and shoulder. Journal of Bone and Joint Surgery. British. 1951;33B(2):180–191. [PubMed] [Google Scholar]
- 22.Beltran JE, Trilla JC, Barjau R. A simplified compression arthrodesis of the shoulder. Journal of Bone and Joint Surgery. American. 1975;57(4):538–541. [PubMed] [Google Scholar]
- 23.Richards RR, Waddell JP, Hudson AR. Shoulder arthrodesis for the treatment of brachial plexus palsy. Clinical Orthopaedics. 1985;198:250–258. [PubMed] [Google Scholar]
- 24.Groh GI, Williams GR, Jarman RN, Rockwood CA. Treatment of complications of shoulder arthrodesis. Journal of Bone and Joint Surgery. American. 1997;79(6):881–887. doi: 10.2106/00004623-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Richards RR, Sherman RM, Hudson AR, Waddell JP. Shoulder arthrodesis using a pelvic-reconstruction plate. A report of eleven cases. The Journal of Bone and Joint Surgery. American. 1988;70(3):416–421. [PubMed] [Google Scholar]
- 26.Klonz A, Habermeyer P. Arthrodesis of the shoulder. A new and soft-tissue-sparing technique with a deep locking plate in the supraspinatus fossa. Unfallchirurg. 2007;110(10):891–895. doi: 10.1007/s00113-007-1337-0. [DOI] [PubMed] [Google Scholar]
- 27.Syal A, MacDonald P. Arthroscopic arthrodesis of the shoulder: a report of two cases. Journal of Shoulder and Elbow Surgery. 2008;17(2):e23–25. doi: 10.1016/j.jse.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Morgan CD, Casscells CD. Arthroscopic-assisted glenohumeral arthrodesis. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 1992;8(2):262–266. doi: 10.1016/0749-8063(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 29.Porcellini G, Savoie FH, Campi F, Merolla G, Paladini P. Arthroscopically assisted shoulder arthrodesis: is it an effective technique? Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2014;30(12):1550–1556. doi: 10.1016/j.arthro.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Lädermann A, Denard PJ. Arthroscopic glenohumeral arthrodesis with o-arm navigation. Arthroscopy Techniques. 2014;3(2):e205–209. doi: 10.1016/j.eats.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kocialkowski A, Wallace WA. Shoulder arthrodesis using an external fixator. Journal of Bone and Joint Surgery. British. 1991;73(1):180–181. doi: 10.1302/0301-620X.73B1.1991766. [DOI] [PubMed] [Google Scholar]
- 32.Nagano A, Okinaga S, Ochiai N, Kurokawa T. Shoulder arthrodesis by external fixation. Clinical Orthopaedics. 1989;247:97–100. [PubMed] [Google Scholar]
- 33.Charnley J, Houston JK. Compression arthrodesis of the shoulder. Journal of Bone and Joint Surgery. British. 1964;46:614–620. [PubMed] [Google Scholar]
- 34.Hawkins RJ, Neer CS. A functional analysis of shoulder fusions. Clinical Orthopaedics. 1987;223:65–76. [PubMed] [Google Scholar]
- 35.Cofield RH, Briggs BT. Glenohumeral arthrodesis. Operative and long-term functional results. Journal of Bone and Joint Surgery American. 1979;61(5):668–677. [PubMed] [Google Scholar]
- 36.Rowe CR. Re-evaluation of the position of the arm in arthrodesis of the shoulder in the adult. Journal of Bone and Joint Surgery. American. 1974;56(5):913–922. [PubMed] [Google Scholar]
- 37.Esenyel CZ, Oztürk K, Imren Y, Ayanoğlu S. Shoulder arthrodesis with plate fixation. Acta Orthopaedica et Traumatologica Turcica. 2011;45(6):412–420. doi: 10.3944/AOTT.2011.2487. [DOI] [PubMed] [Google Scholar]
- 38.Richards RR, Beaton D, Hudson AR. Shoulder arthrodesis with plate fixation: Functional outcome analysis. Journal of Shoulder and Elbow Surgery. 1993;2(5):225–239. doi: 10.1016/S1058-2746(09)80081-5. [DOI] [PubMed] [Google Scholar]
- 39.Stark DM, Bennett JB, Tullos HS. Rigid internal fixation for shoulder arthrodesis. Orthopedics. 1991;14(8):849–855. doi: 10.3928/0147-7447-19910801-08. [DOI] [PubMed] [Google Scholar]
- 40.Miller JD, Pinero JR, Goldstein R, Yen Y-M, Eves W, Otsuka NY. Shoulder arthrodesis for treatment of flail shoulder in children with polio. Journal of Pediatric Orthopedics. 2011;31(6):679–682. doi: 10.1097/BPO.0b013e318229d462. [DOI] [PubMed] [Google Scholar]
- 41.Arntz CT, Matsen FA, Jackins S. Surgical management of complex irreparable rotator cuff deficiency. Journal of Arthroplasty. 1991;6(4):363–370. doi: 10.1016/s0883-5403(06)80189-0. [DOI] [PubMed] [Google Scholar]
- 42.Neer C. Shoulder reconstruction. Philadelphia: WB Saunders; 1990. pp. 253–254. [Google Scholar]
- 43.Hertel R, Ballmer FT. Shoulder arthroplasty after glenohumeral fusion. Journal of Shoulder and Elbow Surgery. 1994;3(6):407–410. doi: 10.1016/S1058-2746(09)80030-X. [DOI] [PubMed] [Google Scholar]
- 44.Alta TDW, Willems WJ. Once an arthrodesis, always an arthrodesis? Journal of Shoulder and Elbow Surgery. 2016;25(2):232–237. doi: 10.1016/j.jse.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Verhelst L, Stuyck J, Bellemans J, Debeer P. Resection arthroplasty of the shoulder as a salvage procedure for deep shoulder infection: does the use of a cement spacer improve outcome? Journal of Shoulder and Elbow Surgery. 2011;20(8):1224–1233. doi: 10.1016/j.jse.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA. Total shoulder arthroplasty. Journal of Bone and Joint Surgery. American. 1987;69(6):865–872. [PubMed] [Google Scholar]
- 47.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16(1):65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 48.Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases. Acta Orthopaedica Belgica. 1995;61(Suppl 1):112–119. [PubMed] [Google Scholar]
- 49.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Journal of Shoulder and Elbow Surgery. 2006;15(5):527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Rockwood CA. The reverse total shoulder prosthesis. The new kid on the block. The Journal of Bone and Joint Surgery American. 2007;89(2):233–235. doi: 10.2106/JBJS.F.01394. [DOI] [PubMed] [Google Scholar]
- 51.Barrett WP, Thornhill TS, Thomas WH, Gebhart EM, Sledge CB. Nonconstrained total shoulder arthroplasty in patients with polyarticular rheumatoid arthritis. Journal of Arthroplasty. 1989;4(1):91–96. doi: 10.1016/s0883-5403(89)80058-0. [DOI] [PubMed] [Google Scholar]
- 52.Bedeir YH, Gawish HM, Grawe BM. Outcomes of reverse total shoulder arthroplasty in patients 60 years of age or younger: a systematic review. The Journal of Hand Surgery. 2019;45:254.e1–254.e8. doi: 10.1016/j.jhsa.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. Journal of Shoulder and Elbow Surgery. 2011;20(1):146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Levy JC, Triplet J, Everding N. Use of a functional antibiotic spacer in treating infected shoulder arthroplasty. Orthopedics. 2015;38(6):e512–e519. doi: 10.3928/01477447-20150603-60. [DOI] [PubMed] [Google Scholar]
- 55.Bonnevialle N, Dauzères F, Toulemonde J, Elia F, Laffosse J-M, Mansat P. Periprosthetic shoulder infection: an overview. EFORT Open Reviews. 2017;2(4):104–109. doi: 10.1302/2058-5241.2.160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Assenmacher AT, Alentorn-Geli E, Dennison T, Baghdadi YMK, Cofield RH, Sánchez-Sotelo J, et al. Two-stage reimplantation for the treatment of deep infection after shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2017;26(11):1978–1983. doi: 10.1016/j.jse.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clinical Orthopaedics and Related Research. 2001;382:206–216. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 58.Ghijselings S, Stuyck J, Debeer P. Surgical treatment algorithm for infected shoulder arthroplasty: A retrospective analysis of 17 cases. Acta Orthopaedica Belgica. 2013;79(6):626–635. [PubMed] [Google Scholar]
- 59.Jacquot A, Sirveaux F, Roche O, Favard L, Clavert P, Molé D. Surgical management of the infected reversed shoulder arthroplasty: a French multicenter study of reoperation in 32 patients. Journal of Shoulder and Elbow Surgery. 2015;24(11):1713–1722. doi: 10.1016/j.jse.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Romanò CL, Borens O, Monti L, Meani E, Stuyck J. What treatment for periprosthetic shoulder infection? Results from a multicentre retrospective series. International Orthopaedics. 2012;36(5):1011–1017. doi: 10.1007/s00264-011-1467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber P, Utzschneider S, Sadoghi P, Andress H-J, Jansson V, Müller PE. Management of the infected shoulder prosthesis: a retrospective analysis and review of the literature. International Orthopaedics. 2011;35(3):365–373. doi: 10.1007/s00264-010-1019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta iii reverse-ball-and-socket total shoulder prosthesis. Journal of Bone and Joint Surgery: American. 2005;87(7):12. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 63.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. Journal of Shoulder and Elbow Surgery. 2001;10(1):17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 64.Coste JS, Reig S, Trojani C, Berg M, Walch G, Boileau P. The management of infection in arthroplasty of the shoulder. The Journal of Bone and Joint Surgery British. 2004;86-B(1):65–69. [PubMed] [Google Scholar]
- 65.Boileau, P., Raynier, J.-L., Chelli, M., Gonzalez, J.-F., Galvin, J. W. (2020). Reverse shoulder–allograft prosthesis composite, with or without tendon transfer, for the treatment of severe proximal humeral bone loss. Journal of Shoulder and Elbow Surgery. [DOI] [PubMed]
- 66.Sanchez-Sotelo J, Wagner ER, Houdek MT. Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. JBJS Essential Surgical Techniques. 2018;8(1):e3. doi: 10.2106/JBJS.ST.17.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braman JP, Sprague Mark, Bishop Julie, Lo Ian K., Lee Edward W., Flatow Evan L. The outcome of resection shoulder arthroplasty for recalcitrant shoulder infections. Journal of Shoulder and Elbow Surgery. 2006;15:549–553. doi: 10.1016/j.jse.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Jacquot A, Sirveaux F, Roche O, Favard L, Clavert P, Molé D. Surgical management of the infected reversed shoulder arthroplasty: a French multicenter study of reoperation in 32 patients. Journal of Shoulder and Elbow Surgery. 2015;24:1713–1722. doi: 10.1016/j.jse.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Ortmaier R, Resch H, Hitzl W, Mayer M, Stundner O, Tauber M. Treatment strategies for infection after reverse shoulder arthroplasty. European Journal of Orthopaedic Surgery & Traumatology. 2014;24:723–731. doi: 10.1007/s00590-013-1251-9. [DOI] [PubMed] [Google Scholar]