Abstract
Purpose
The present study compared the efficacy of mycophenolate mofetil (MMF) with that of azathioprine (AZA) in Korean patients with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA).
Materials and Methods
The medical records of 69 patients with MPA and GPA who received cyclophosphamide and subsequently received AZA or MMF for remission maintenance therapy were reviewed. All-cause mortality, relapse, end-stage renal disease (ESRD), cerebrovascular accident, and cardiovascular disease were evaluated as poor outcomes. Having a lower Birmingham Vasculitis Activity Score (BVAS) was defined as the lowest tertile of BVAS (BVAS ≤11 in this study).
Results
In comparative analysis of the occurrence of poor outcomes among patients taking AZA only, MMF only, and MMF after AZA, patients taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than patients taking AZA only (p=0.028). In terms of ESRD occurrence between the groups based on BVAS at diagnosis, among patients with MPA and GPA with higher BVAS at diagnosis, patients taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than those taking AZA only (p=0.047). Among patients with MPA and GPA with the lowest tertile of BVAS at diagnosis, cumulative ESRD-free survival rates did not differ.
Conclusion
With regard to ESRD occurrence, the efficacy of MMF in remission maintenance therapy was less effective than AZA in patients with MPA and GPA. However, among patients with lower BVAS, there was no difference in the occurrence of poor outcomes between patients taking MMF and those taking AZA.
Keywords: Mycophenolate mofetil, azathioprine, antineutrophil cytoplasmic antibody-associated vasculitis, poor outcomes, end-stage renal disease
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is characterized by necrotizing vasculitis in capillaries, arterioles, and venules and few apparent immune complex deposits in perivascular tissues. Based on clinical and pathological features, AAV comprises three subtypes: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA).1,2
The European League Against Rheumatism (EULAR)/European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) recommendations for the management of AAV currently recommend a combination of low-dose glucocorticoid and either azathioprine (AZA), rituximab (RTX), methotrexate (MTX) or mycophenolate mofetil (MMF) for remission maintenance therapy.3 Among the four recommended immunosuppressive drugs, AZA is the most widely used for remission maintenance of MPA and GPA because it has the highest level of evidence (1B) and grade of recommendation (A). However, the level of evidence and grade of recommendation for AZA in remission maintenance therapy for EGPA are 3 and C, respectively, which are lower than those for MPA and GPA.3
The recommendation strength for RTX, MTX, and MMF in remission maintenance therapy ranges from 53% to 59%, far less than the value of 94% for AZA.3 Nevertheless, in actual clinical situations, there may be cases in which it is difficult to prescribe AZA, such as in patients with elevated liver enzyme levels or leukocytopenia.4,5 In these cases, one of the remaining three drugs should be selected. However, RTX in remission maintenance therapy for MPA and GPA is not covered by the Korean National Health Insurance despite its proven efficacy.6 In addition, MTX is not sufficient to maintain the remission of MPA and GPA with major organ involvement.7 Therefore, MMF may emerge as an alternative remission maintenance therapy for MPA and GPA in cases in which AZA is contraindicated. However, the efficacy of MMF is not as high as expected: a clinical trial conducted by the European Vasculitis Study Group demonstrated a significantly higher relapse-preventive potential for AZA than MMF in patients with MPA and GPA.8
Therefore, the present study was undertaken to re-evaluate and compare the efficacy of MMF and AZA in remission maintenance therapy among Korean patients with MPA and GPA.
MATERIALS AND METHODS
Patients
Inclusion and exclusion
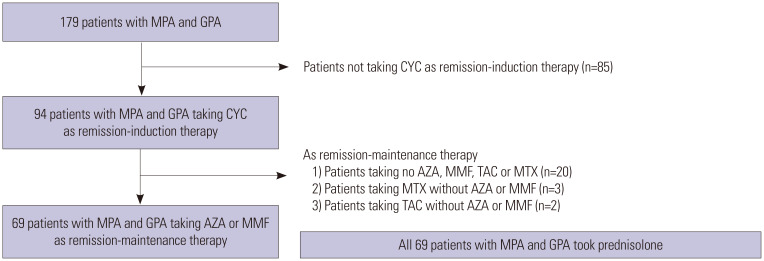
The algorithm of inclusion and exclusion of patients is described in Fig. 1. The medical records of 179 patients with MPA and GPA enrolled in the Severance Hospital ANCA-associated VasculitidEs (SHAVE) cohort were retrospectively reviewed. The SHAVE cohort is a prospective observational cohort of patients with MPA, GPA, and EGPA established in November 2016. All patients were first classified to have MPA or GPA according to the 2007 European Medicines Agency algorithm for AAV and polyarteritis nodosa and the 2012 revised Chapel Hill Consensus Conference Nomenclature of Vasculitides.1,2 The patients had never received remission induction or maintenance therapies for AAV and had no medical conditions, such as malignancies, infectious diseases, or hematological disorders, at diagnosis. They were followed up for ≥3 months from their AAV diagnosis. Of 179 patients, 85 were excluded because they did not receive cyclophosphamide (CYC) in remission induction therapy. Of the 94 patients taking CYC, 20 were excluded owing to the administration of only glucocorticoid without the administration of any immunosuppressive drug. Furthermore, five patients were excluded owing to the use of tacrolimus or MTX instead of AZA or MMF in remission maintenance therapy along with glucocorticoid therapy. Finally, this study included 69 patients who received CYC for remission induction therapy and AZA or MMF for remission maintenance therapy along with glucocorticoid. For remission induction therapy, all patients received glucocorticoid pulse therapy (1 g for 3 days or 500 mg for 5 days), followed by high-dose prednisolone (1 mg/kg/day) for 4 weeks and tapered to 5 to 10 mg per day over 3 months. CYC was administered as intravenous pulse therapy [15 mg/kg - maximum pulse dose 1.2 g, dose adjusted by estimated glomerular filtration rate (eGFR) and age] with six cycles of infusion for 3 to 6 months.9 AZA was orally administered with a target dose of 2 mg/kg/day, and MMF was administered with a target dose of 2–3 g/day depending on the patient's condition during the maintenance therapy. This study was approved by the Institutional Review Board of Severance Hospital (4-2017-0673), which waived the need for written informed consent from the patients owing to the retrospective nature of the study.
Fig. 1. Algorithm for the inclusion and exclusion of patients MPA and GPA. MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; CYC, cyclophosphamide; AZA, azathioprine; MMF, mycophenolate mofetil; TAC, tacrolimus; MTX, methotrexate.
Clinical and laboratory data at diagnosis
Data on age, sex, body mass index (BMI), and smoking history (or current smoker) were collected as demographic data. AAV subtypes and ANCA positivity were reviewed, and the presence of clinical manifestations according to nine items of Birmingham Vasculitis Activity Score (BVAS) was recorded. BVAS and five-factor score (FFS) were also assessed as AAV-specific indices. Chronic kidney disease (CKD) (stage 3–5), diabetes mellitus (DM), hypertension (HTN), and dyslipidemia were identified, and erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were evaluated.
Poor prognosis and follow-up period
All-cause mortality, relapse, end-stage renal disease (ESRD), cerebrovascular accident (CVA), and cardiovascular disease (CVD) during follow-up were defined and evaluated as poor outcomes of MPA and GPA. The follow-up duration was defined as the period between the date of AAV diagnosis and the date of the last visit for surviving patients. For deceased patients, the follow-up duration based on all-cause mortality was defined as the period between the initial AAV diagnosis and the time of death. For patients with poor outcomes, the follow-up duration based on each poor outcome was defined as the period starting from AAV diagnosis until the occurrence of each poor outcome.
Medications administered during follow-up
During the follow-up period, the numbers of patients taking AZA only, those taking MMF only, and those taking MMF after AZA were counted. Since CYC was administered to all patients for remission induction therapy and glucocorticoid was also provided to all patients in combination with AZA or MMF in remission maintenance therapy, the subgroups were simply labelled AZA only, MMF after AZA, and MMF only.
Higher and lower BVAS at diagnosis
When patients were divided into two groups based on a BVAS of 11, which is the upper limit of the lowest tertile of BVAS, 47 and 22 patients, respectively, were assigned to the higher (BVAS >11) and the lower (BVAS ≤11) BVAS groups.
Statistical analyses
All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as medians (interquartile range) and categorical variables as numbers (%). Significant differences in categorical variables between the two groups were analyzed using the chi-square and Fisher's exact tests. Significant differences in continuous variables between the two groups were compared using the Mann Whitney U test. Comparison of the cumulative poor outcome-free survival rates between the two groups was performed by Kaplan-Meier survival analysis with log-rank test. A multivariable Cox hazards model including variables with statistical significance in a univariable Cox hazards model was used to obtain hazard ratios (HRs) during the follow-up period. p values less than 0.05 were considered statistically significant.
RESULTS
Characteristics at diagnosis
The median age of patients was 59.0 years, and 42 were female. The median BMI was 22.3 kg/m2, and only three patients were ex-smokers. Of the 69 patients, 54 were diagnosed with MPA, and 15 were diagnosed with GPA. ANCA was detected in 62 patients. The most frequently observed clinical manifestation was renal manifestation (76.8%), followed by pulmonary (53.6%) and general (50.7%) manifestations. The median BVAS, FFS, ESR, and CRP levels were 14.0, 1.0, 76.0 mm/h, and 19.1 mg/L, respectively. Thirty patients had CKD (stage 3–5), and 24 had HTN (Table 1).
Table 1. Characteristics and Comparison of Variables at Diagnosis and During Follow-Up in Patients with MPA and GPA.
| Variables | All patients (n=69) | AZA (n=45) | MMF (n=24) | p value | ||
|---|---|---|---|---|---|---|
| At the time of diagnosis | ||||||
| Demographic data | ||||||
| Age (yr) | 59.0 (18.5) | 64.0 (16.5) | 54.0 (24.8) | 0.022 | ||
| Female sex | 42 (60.9) | 23 (51.1) | 19 (79.2) | 0.037 | ||
| Body mass index (kg/m2) | 22.3 (3.6) | 22.7 (3.6) | 21.2 (3.8) | 0.036 | ||
| Smoking history (ex-smoker) | 3 (4.3) | 3 (6.7) | 0 (0) | 0.547 | ||
| AAV subtypes | 0.550 | |||||
| MPA | 54 (78.3) | 34 (75.6) | 20 (83.3) | |||
| GPA | 15 (21.7) | 11 (24.4) | 4 (16.7) | |||
| ANCA positivity | ||||||
| MPO-ANCA (or P-ANCA) positive | 53 (76.8) | 33 (73.3) | 20 (83.3) | 0.390 | ||
| PR3-ANCA (or C-ANCA) positive | 10 (14.5) | 7 (15.6) | 3 (12.5) | 1.000 | ||
| Both ANCA positive | 1 (1.4) | 0 (0) | 1 (4.2) | 0.348 | ||
| ANCA negative | 7 (10.1) | 5 (11.1) | 2(8.3) | 1.000 | ||
| Clinical features based on BVAS | ||||||
| General manifestations | 35 (50.7) | 24 (53.3) | 11 (45.8) | 0.553 | ||
| Cutaneous manifestations | 10 (14.5) | 8 (17.8) | 2 (8.3) | 0.475 | ||
| Mucous and ocular manifestations | 3 (4.3) | 1 (2.2) | 2 (8.3) | 0.276 | ||
| Otorhinolaryngologic manifestations | 21 (30.4) | 13 (28.9) | 8 (33.3) | 0.702 | ||
| Pulmonary manifestations | 37 (53.6) | 22 (48.9) | 15 (62.5) | 0.280 | ||
| Cardiovascular manifestations | 17 (24.6) | 9 (20.0) | 8 (33.3) | 0.221 | ||
| Gastrointestinal manifestations | 3 (4.3) | 2 (4.4) | 1 (4.2) | 1.000 | ||
| Renal manifestations | 53 (76.8) | 35 (77.8) | 18 (75.0) | 0.795 | ||
| Nervous systemic manifestations | 15 (21.7) | 14 (31.1) | 1 (4.2) | 0.013 | ||
| AAV-specific indices | ||||||
| BVAS | 14.0 (8.0) | 12.0 (7.5) | 15.5 (9.0) | 0.468 | ||
| FFS | 1.0 (1.0) | 1.0 (1.5) | 1.0 (1.0) | 0.707 | ||
| Acute phase reactants | ||||||
| ESR (mm/hr) | 76.0 (80.5) | 74.0 (84.0) | 77.5 (88.3) | 0.437 | ||
| CRP (mg/L) | 19.1 (69.4) | 21.0 (69.2) | 16.5 (81.6) | 0.786 | ||
| Comorbidities | ||||||
| CKD (stage 3–5) | 30 (43.5) | 21 (46.7) | 9 (37.5) | 0.464 | ||
| DM | 19 (27.5) | 14 (31.1) | 5 (20.8) | 0.363 | ||
| HTN | 24 (34.8) | 15 (33.3) | 9 (37.5) | 0.729 | ||
| Dyslipidemia | 13 (18.8) | 6 (13.3) | 7 (29.2) | 0.109 | ||
| During the follow-up period | ||||||
| Poor outcomes and follow-up periods | ||||||
| All-cause mortality | 11 (15.9) | 8 (17.8) | 3 (12.5) | 0.736 | ||
| Follow-up period based on all-cause mortality (months) | 32.9 (55.3) | 19.8 (39.5) | 60.8 (61.8) | 0.003 | ||
| Relapse | 29 (42.0) | 14 (31.1) | 15 (62.5) | 0.012 | ||
| Follow-up period based on relapse (months) | 16.4 (34.5) | 14.0 (34.1) | 29.3 (38.6) | 0.409 | ||
| ESRD | 18 (26.1) | 7 (15.6) | 11 (45.8) | 0.006 | ||
| Follow-up period based on ESRD (months) | 19.8 (41.7) | 17.0 (32.5) | 33.1 (78.1) | 0.279 | ||
| CVA | 7 (10.1) | 4 (8.9) | 3 (12.5) | 0.687 | ||
| Follow-up period based on CVA (months) | 28.8 (45.6) | 17.1 (38.6) | 37.8 (50.7) | 0.061 | ||
| CVD | 5 (7.2) | 4 (8.9) | 1 (4.2) | 0.652 | ||
| Follow-up period based on CVD (months) | 32.0 (46.4) | 19.8 (40.9) | 43.4 (57.1) | 0.006 | ||
MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; AZA, azathioprine; MMF, mycophenolate mofetil; AAV, ANCA-associated vasculitis; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; P, perinuclear; PR3, proteinase 3; C, cytoplasmic; BVAS, Birmingham vasculitis activity score; FFS, five-factor score; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; ESRD: end-stage renal disease; CVA, cerebrovascular accident; CVD, cardiovascular disease.
Values are expressed as medians (interquartile range) or n (%).
Characteristics during follow-up
Of the 69 patients, 11 (15.9%) died during the median follow-up period of 32.9 months. Relapse, ESRD, CVA, and CVD were observed in 42.0%, 26.1%, 10.1%, and 7.2% of the study population, respectively. In remission maintenance therapy, AZA was administered to 58 patients, whereas MMF was administered to 24 patients, of which 11 were maintained on MMF only and 13 received MMF after AZA (Table 1).
Comparison variables at diagnosis
Patients taking AZA were older and had higher BMI than those taking MMF (64 years vs. 54 years and 22.7 kg/m2 vs. 21.2 kg/m2, respectively). Among the clinical manifestations, patients taking AZA exhibited a significantly higher frequency of nervous systemic manifestations than those taking MMF (31.1% vs. 4.2%). No other significant differences in clinical features at diagnosis were observed between the two groups (Table 1).
Comparison variables during follow-up
In terms of poor outcomes of MPA and GPA, patients taking MMF exhibited significantly higher frequencies of relapse (62.5% vs. 31.1%) and ESRD occurrence (45.8% vs. 15.6%) than those taking AZA during follow-up. In addition, patients taking MMF had longer follow-up periods based on all-cause mortality and CVD, but no significant differences in the frequencies of all-cause mortality, CVA, and CVD were observed between the two groups (Table 1).
Comparison of cumulative poor outcome-free survival rates
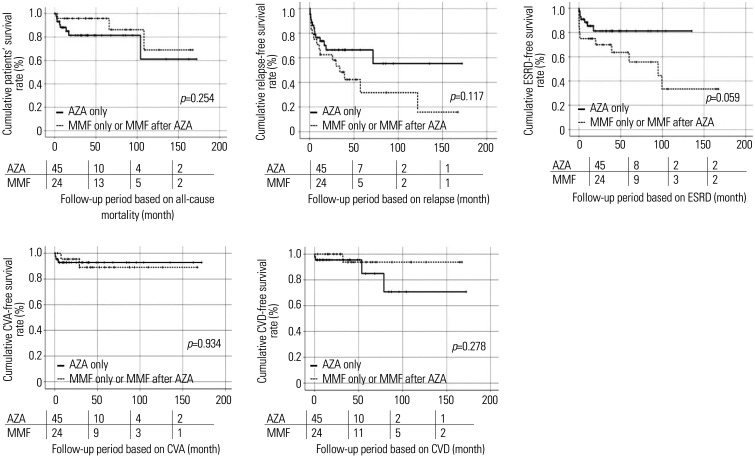
Among the five poor outcomes of MPA and GPA, patients taking MMF only or MMF after AZA tended to show lower cumulative relapse-free (p=0.117) and ESRD-free (p=0.059) rates during follow-up, compared to those in patients taking AZA only. However, the difference was not statistically significant (Fig. 2).
Fig. 2. Comparison of cumulative poor outcome-free survival rates. Patients taking MMF only or MMF after AZA tended to show lower cumulative relapse-free (p=0.117) and ESRD-free (p=0.059) rates during follow-up, compared to those in patients taking AZA only. However, they did not reach statistical significance. The numbers below are the number of patients followed at each period (0, 50, 100, 150 months). MMF, mycophenolate mofetil; AZA, azathioprine; ESRD, end-stage renal disease; CVA, cerebrovascular accident; CVD, cardiovascular disease.
Comparison of cumulative relapse-free and ESRD-free survival rates among patients taking AZA only, MMF only, and MMF after AZA
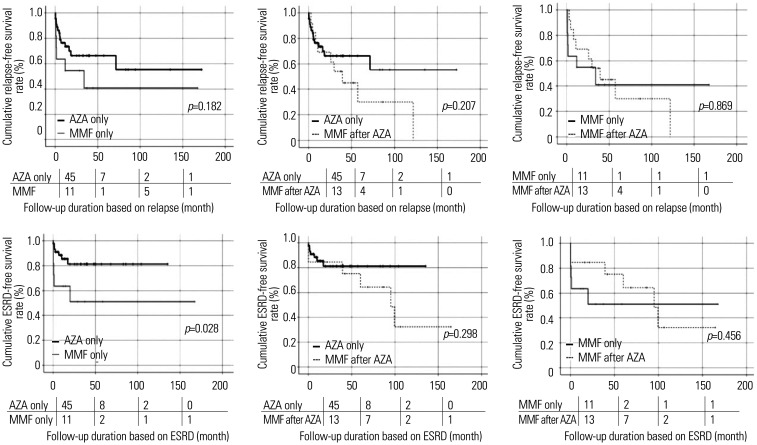
No significant differences in cumulative relapse-free survival rates were observed not only between patients taking AZA only and those taking MMF only, but also between those taking AZA only and those taking MMF after AZA. Furthermore, cumulative relapse-free survival rates between patients taking MMF only and those taking MMF after AZA also did not differ. However, regarding ESRD, patients taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than patients taking AZA only (p=0.028). No significant differences in the cumulative ESRD-free survival rates were observed among the other groups (Fig. 3).
Fig. 3. Comparison of cumulative relapse-free and ESRD-free survival rates. Among patients taking AZA only, MMF only, and MMF after AZA, only patients taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than patients taking AZA only. The numbers below are the number of patients followed at each period (0, 50, 100, 150 months). ESRD, end-stage renal disease; AZA, azathioprine; MMF, mycophenolate mofetil.
Cox hazard model analyses
To evaluate the independent predictive value of each variable at diagnosis for the occurrence of ESRD during follow-up, Cox hazards model analysis was conducted. In univariate analysis, smoking history (HR 0.611) decreased the occurrence of ESRD, whereas BVAS (HR 1.079) and HTN (HR 2.728) increased the occurrence of ESRD. However, renal manifestation and CKD (stage 3–5) at diagnosis were not significantly associated with the occurrence of ESRD during follow-up. In multivariate analysis, both BVAS [HR 1.087, 95% confidence interval (CI) 1.018, 1.161] and HTN (HR 3.201, 95% CI 1.186, 8.638) independently increased the occurrence of ESRD during follow-up (Table 2). In contrast, the addition of the variable of MMF only to the Cox hazards model analysis showed that MMF only increased the occurrence of ESRD over AZA only (HR 1.835) in univariable analysis. In multivariable analysis, including smoking history, BVAS, HTN, and MMF only over AZA only, both BVAS (HR 1.172, 95% CI 1.068, 1.285) and MMF only over AZA only (HR 4.823, 95% CI 1.338, 17.384) could independently predict the occurrence of ESRD (Table 2).
Table 2. Cox Hazards Model Analysis of Variables at Diagnosis for the Occurrence of ESRD during Follow-Up in Patients with MPA and GPA.
| Variables | Univariable | Multivariable (without MMF only) | Multivariable (with MMF only) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (yr) | 0.994 | 0.964, 1.026 | 0.726 | ||||||
| Male sex | 0.727 | 0.258, 2.051 | 0.546 | ||||||
| Body mass index | 0.928 | 0.782, 1.100 | 0.388 | ||||||
| Smoking history | 0.611 | 0.000, 6500.122 | 0.046 | 0.000 | 0.000, 0.000 | 0.984 | 0.000 | 0.000, 0.000 | 0.989 |
| MPO-ANCA (or P-ANCA) positivity | 1.719 | 0.496, 5.956 | 0.393 | ||||||
| PR3-ANCA (or C-ANCA) positivity | 0.819 | 0.187, 3.583 | 0.791 | ||||||
| Renal manifestation | 2.354 | 0.654, 8.471 | 0.190 | ||||||
| BVAS | 1.079 | 1.014, 1.147 | 0.016 | 1.087 | 1.018, 1.161 | 0.012 | 1.172 | 1.068, 1.285 | 0.001 |
| FFS | 1.588 | 0.971, 2.598 | 0.066 | ||||||
| ESR | 1.005 | 0.994, 1.017 | 0.387 | ||||||
| CRP | 1.002 | 0.995, 1.009 | 0.584 | ||||||
| CKD (stage 3–5) | 0.880 | 0.344, 2.254 | 0.790 | ||||||
| DM | 1.569 | 0.606, 4.063 | 0.354 | ||||||
| HTN | 2.728 | 1.060, 7.020 | 0.037 | 3.201 | 1.186, 8.638 | 0.022 | 1.778 | 0.547, 5.778 | 0.338 |
| Dyslipidemia | 1.492 | 0.528, 4.220 | 0.450 | ||||||
| MMF only over AZA only | 1.835 | 1.032, 3.264 | 0.039 | 4.823 | 1.338, 17.384 | 0.016 | |||
ESRD, end-stage renal disease; MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; MMF, mycophenolate mofetil; HR, hazard ratio; CI, confidence interval; MPO, myeloperoxidase; ANCA: antineutrophil cytoplasmic antibody; P, perinuclear; PR3, proteinase 3; C, cytoplasmic; BVAS, Birmingham vasculitis activity score; FFS, five-factor score; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; AZA, azathioprine.
Comparison of cumulative ESRD-free survival rates between the higher and lower BVAS groups
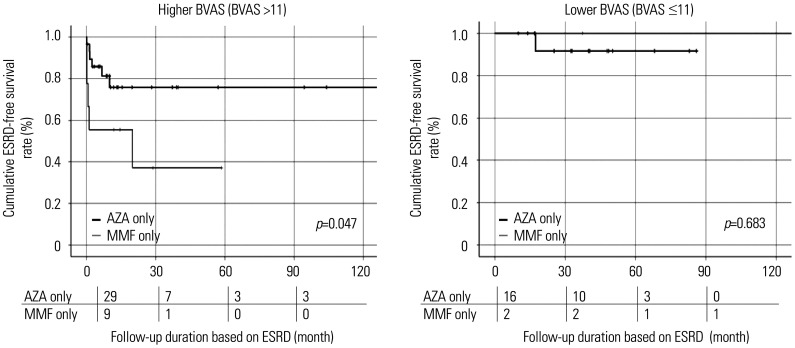
Among patients with MPA and GPA with higher BVAS at diagnosis, those taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than patients taking AZA only (p=0.047). However, among patients with MPA and GPA with a lower BVAS at diagnosis, the cumulative ESRD-free survival rates did not differ between the two groups (p=0.683) (Fig. 4). Combined, these results indicated that in remission maintenance therapy for MPA and GPA, MMF only could be as effective as AZA for preventing the occurrence of ESRD in patients with the lowest tertile of BVAS at diagnosis.
Fig. 4. Comparison of cumulative ESRD-free survival rates between the higher and lower BVAS groups. Among patients with higher BVAS at diagnosis, those taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than patients taking AZA only, whereas among patients with the lowest tertile of BVAS at diagnosis, the cumulative ESRD-free survival rates did not differ between the two groups. The numbers below are the number of patients followed at each period (0, 30, 60, 90 months). ESRD, end-stage renal disease; BVAS, irmingham vasculitis activity score; MMF, mycophenolate mofetil; AZA, azathioprine.
DISCUSSION
This study investigated the preventive potential of MMF against the occurrence of poor outcomes during follow-up in Korean patients with MPA and GPA and compared it with that of AZA. There were three important findings. First, cross-sectional comparative analysis of the frequency of poor outcomes showed that patients taking MMF exhibited significantly higher frequencies of relapse and ESRD occurrence than those taking AZA during follow-up. Second, comparative analysis considering the follow-up period revealed that patients taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than patients taking AZA only. Third, in terms of ESRD occurrence, among patients with MPA and GPA with higher BVAS at diagnosis, patients taking MMF only exhibited a significantly lower cumulative ESRD-free survival rate than those taking AZA only, whereas among patients with MPA and GPA with the lowest tertile of BVAS at diagnosis, the cumulative ESRD-free survival rates did not differ between the two groups.
In Cox hazards model analysis, renal involvement of MPA and GPA at diagnosis was not significantly associated with the occurrence of ESRD. Therefore, the effect of the use of MMF and AZA on the occurrence of ESRD was investigated after excluding the effect of kidney involvement by selecting patients with renal manifestation at diagnosis. In 53 patients with renal manifestation at diagnosis, those taking MMF only showed a lower cumulative ESRD-free survival rate than those taking AZA only (p=0.025), which was similar to the results in all study population. However, the survival rates did not differ significantly between patients taking MMF after AZA and those taking AZA only or between patients taking MMF only and those with MMF after AZA (Supplementary Fig. 1, only online). This suggests that drug selection for remission maintenance therapy was more important with respect to the occurrence of ESRD than renal manifestation at diagnosis.
Unexpectedly, in our data, underlying CKD did not increase the risk of ESRD occurrence. Since we defined underlying CKD as the patient being diagnosed with CKD prior to AAV diagnosis, the initial eGFR of patients with CKD was 41.2, which was not different from eGFR 62.0 for patients without CKD (p=0.289). It is thought that the reason the eGFR showed no difference was that patients without underlying CKD had decreased initial kidney function due to renal involvement of AAV.
Comparative analysis between patients taking AZA and those taking MMF (MMF only plus MMF after AZA) showed statistically significant differences in several variables at diagnosis (Table 1). First, patients taking AZA were older than those taking MMF. The Cox hazards model revealed age as a significant risk factor for all-cause mortality (HR 1.096, 95% CI 1.026, 1.171), similar to that of a previous study in the general population.10 Assuming that the age of patients taking MMF is comparable to that of those taking AZA, there might have been a significant difference in all-cause mortality between the two groups. Since age was not a risk factor for poor outcomes, other than all-cause mortality, particularly ESRD (Table 2), there was no need to adjust the results of this study for age. Second, patients taking AZA had higher BMI than those taking MMF (22.7 kg/m2 vs. 21.2 kg/m2). Unlike age, BMI was not an initial risk factor for all-cause mortality (HR 1.108, 95% CI 0.890, 1.379). This result might be attributable to a U-shape hypothesis of all-cause mortality along with BMI.11,12 BMI was not associated with other poor outcomes in this study.
Third, among clinical manifestations based on BVAS items, nervous systemic manifestations were observed more frequently in patients taking AZA than in those taking MMF. These results might have clinical significance for two reasons. First, the severity of peripheral neuropathy and/or central nervous symptoms or the frequency of motor nerve involvement might have been higher in patients taking AZA than in patients taking MMF.13 Second, life-threatening major organ involvement might have occurred more often in patients taking AZA than in patients taking MMF.3,14 However, comparisons of the numbers of patients exhibiting other serious clinical manifestations between patients with and without nervous systemic manifestation showed no significant differences between the two groups. However, given that plasma exchange is recommended in cases of life-threatening major organ involvement, we also compared the numbers of patients who underwent plasma exchange between patients with and without nervous systemic manifestations3 and observed no differences.
The variable of “MMF only over AZA only” was included and analyzed in multivariable Cox hazards model analysis in Table 2. However, strictly speaking, this variable should not be included in multivariable Cox hazards model analysis, since the durations of MMF and AZA administration did not match the follow-up period based on the poor outcomes of MPA and GPA. Nevertheless, this analysis was performed because all patients received the same CYC infusion protocol and the timing of initiating AZA or MMF as a maintenance therapy after CYC administration was approximately the same.9 We also performed this analysis to identify risk factors at the time of diagnosis that affected the choice between AZA and MMF and to compare the efficacies of AZA and MMF in remission maintenance therapy according to the presence or absence of each risk factor. We found that BVAS at diagnosis, together with the variable of “MMF only over AZA only” predicted the occurrence of ESRD during follow-up in patients with MPA and GPA (Table 2).
Although several studies have favored and advocated the use of MMF in remission maintenance therapy,15,16 research results thus far have consistently reported that the effect of MMF in remission maintenance therapy is inferior to that of AZA.8 Compared to previous studies, our study investigated the progression to ESRD in patients with systemic clinical manifestations that were not confined to renal manifestation. In addition, our study also compared the efficacy of AZA and MMF in remission maintenance therapy for preventing the occurrence of ESRD regardless of renal manifestations at diagnosis. Finally, our results demonstrated that MMF is as effective as AZA in remission maintenance therapy for patients with the lowest tertile of BVAS at diagnosis among patients who need CYC for induction therapy. We also provided a method to obtain the optimal cut-off of BVAS at diagnosis for selecting MMF as a remission maintenance therapeutic regimen.
Our study has several limitations. Since this was a single-center cohort study, the number of patients included in the study was not sufficient to maximize its reliability. However, as patients registered in a single institution, the consistency of the diagnostic criteria and treatment schedule was ensured, and the omission of data was minimized.
Another limitation of our study is the likelihood of differences in underling vasculitis burden between patients receiving AZA and MMF and the possibility of selection bias owing to retrospective study design. Furthermore, our results should be interpreted with caution because the disease duration and status of diseases, such as relapse, were different for each patient and because there is the possibility of a difference in the cumulative doses of glucocorticoids between patients receiving AZA and those receiving MMF, which might have affected the poor outcomes. Nevertheless, the strength of this study is that, to the best of our knowledge, this is the first pilot study investigating the efficacies of MMF and AZA in remission maintenance therapy among patients with MPA and GPA with lower activity at diagnosis. Moreover, we believe that our study has broadened the choice of remission maintenance therapeutic regimens.
In conclusion, as remission maintenance therapy after induction therapy using CYC, the preventive potential of MMF against ESRD during follow-up, but not death, relapse, CVA or CVD, was lower than that of AZA or MMF after AZA in patients with MPA and GPA. However, among patients with the lowest tertile of BVAS at diagnosis, there was no difference in occurrence of ESRD between AZA and MMF, regardless of the initial renal manifestation. We believe that this study will serve as the basis for conducting further research on the efficacy of MMF as a maintenance therapy in patients with AAV with lower activity.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2019-0184) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung Yoon Pyo, Jason Jungsik Song, Yong-Beom Park, and Sang-Won Lee.
- Data curation: Lucy Eunju Lee and Sung Soo Ahn.
- Formal analysis: Jung Yoon Pyo and Sang-Won Lee.
- Funding acquisition: Sang-Won Lee.
- Investigation: Jung Yoon Pyo and Sang-Won Lee.
- Methodology: Jason Jungsik Song and Yong-Beom Park.
- Project administration: Sang-Won Lee.
- Resources: all authors.
- Software: Jung Yoon Pyo.
- Supervision: Jason Jungsik Song and Yong-Beom Park.
- Validation: Jason Jungsik Song and Yong-Beom Park.
- Visualization: Jung Yoon Pyo and Sang-Won Lee.
- Writing—original draft: Jung Yoon Pyo.
- Writing—review & editing: Sang-Won Lee.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
Comparison of cumulative ESRD-free survival rates in 53 patients with renal manifestation at diagnosis. Patients taking MMF only showed a lower cumulative ESRD-free survival rate than those taking AZA only (p=0.025); however, ESRD-survival rates did not differ between patients taking MMF after AZA and those taking AZA only or between patients taking MMF only and those with MMF after AZA. ESRD, end-stage renal disease; MMF, mycophenolate mofetil; AZA, azathioprine.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 4.Horning K, Schmidt C. Azathioprine-induced rapid hepatotoxicity. J Pharm Technol. 2014;30:18–20. doi: 10.1177/8755122513504078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varma PP, Prasher PK, Madan H, Yashpal B. Azathioprine induced bone marrow suppression in live related renal allograft recipients. Med J Armed Forces India. 1996;52:45–47. doi: 10.1016/S0377-1237(17)30834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 7.Reinhold-Keller E, de Groot K. Use of methotrexate in ANCA-associated vasculitides. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S178–S182. [PubMed] [Google Scholar]
- 8.Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–2388. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 9.de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 10.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wludarczyk A, Szczeklik W. Neurological manifestations in ANCA-associated vasculitis-assessment and treatment. Expert Rev Neurother. 2016;16:861–863. doi: 10.1586/14737175.2016.1165095. [DOI] [PubMed] [Google Scholar]
- 14.Suppiah R, Hadden RD, Batra R, Arden NK, Collins MP, Guillevin L, et al. Peripheral neuropathy in ANCA-associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology (Oxford) 2011;50:2214–2222. doi: 10.1093/rheumatology/ker266. [DOI] [PubMed] [Google Scholar]
- 15.Silva F, Specks U, Kalra S, Hogan MC, Leung N, Sethi S, et al. Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement--a prospective, open-label pilot trial. Clin J Am Soc Nephrol. 2010;5:445–453. doi: 10.2215/CJN.06010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Gao E, Yang L, Liu X, Li K, Liu Z, et al. Long-term outcome of mycophenolate mofetil treatment for patients with microscopic polyangiitis: an observational study in Chinese patients. Rheumatol Int. 2016;36:967–974. doi: 10.1007/s00296-016-3492-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of cumulative ESRD-free survival rates in 53 patients with renal manifestation at diagnosis. Patients taking MMF only showed a lower cumulative ESRD-free survival rate than those taking AZA only (p=0.025); however, ESRD-survival rates did not differ between patients taking MMF after AZA and those taking AZA only or between patients taking MMF only and those with MMF after AZA. ESRD, end-stage renal disease; MMF, mycophenolate mofetil; AZA, azathioprine.