Abstract
Background:
To evaluate the efficacy of gabapentin at 20 mg/kg per day in the treatment of vincristine-related neuropathic pain
Procedure:
Children aged 1 to 18 years who developed vincristine-induced neuropathy on a St. Jude frontline acute lymphoblastic leukemia trial were prospectively enrolled on a randomized, double-blind, placebo-controlled, phase II trial with two treatment arms: gabapentin plus opioid versus placebo plus opioid. Daily evaluations of morphine dose (mg/kg per day) and pain scores were conducted for up to 21 days; the values of the two arms were compared to assess analgesic efficacy.
Results:
Of 51 study participants, 49 are eligible for analyses. Twenty-five participants were treated with gabapentin, with a mean (SD) dose of 17.97 (2.76) mg/kg per day (median 18.26, range 6.82–21.37). The mean (SD) opioid doses taken, expressed as morphine equivalent daily (mg/kg per day), were 0.26 (0.43) in the gabapentin group (25 patients, 432 days) and 0.15 (0.22) in the placebo group (24 patients, 411 days; p=0.15). Only the risk classification of acute lymphoblastic leukemia was significantly associated with the daily morphine dosage (p=0.0178): patients in the lower-risk arm received higher daily morphine dosages. Multivariate analyses revealed a significant difference between the groups’ average daily scores for the previous 24 hours and “right now”.
Conclusion:
In this population of children with vincristine-related neuropathic pain, opioid consumption and pain scores were higher in the gabapentin group than in the placebo group. Future randomized, double-blind, placebo-controlled studies should test gabapentin given longer or at a higher dose.
Keywords: support care, pediatric oncology, ALL, neuropathic pain, vincristine, gabapentin
Introduction
Children with cancer experience significant treatment-related complications,1,2 with pain being the most frequent, severe, and distressful symptom. Pain during treatment for cancer has long-term implications after completion of therapy.3,4 Neuropathic pain (NP) and related neuropathy symptoms are directly correlated with chemotherapeutic agents,5 particularly vincristine (VCR),6 being given during therapy for childhood cancers.7,8 Neurotoxicity, the dose-limiting side effect of VCR, most often causes mixed sensorimotor neuropathy (loss of deep tendon reflexes, paresthesia, neuritic pain, and wrist or foot drop) or autonomic neuropathy (constipation, abdominal pain, paralytic ileus, bladder atony with retention of urine, and orthostatic hypotension). Occasionally, VCR-associated neurotoxicity involves the cranial nerves and manifests as transient blindness, oculomotor nerve dysfunction with ptosis and diplopia, jaw pain, facial palsy, hearing loss, and vocal cord paresis or paralysis.5
Studies in adult NP syndromes, such as diabetic neuropathy and postherpetic neuralgia, indicate that dual-therapy using opioid and gabapentin has better analgesic efficacy than either drug used individually;9 nevertheless, no clinical trials have explored therapies for NP in the pediatric oncology population. In our institutional experience, the incidence of vincristine-related peripheral neuropathy during treatment of childhood acute lymphoblastic leukemia (ALL) has been 17.5% among the 240 patients treated in the Total Therapy XIIIB study10 and 34.9% among the 498 children treated in the Total Therapy XV study.11 Our retrospective analysis of the Total XV study did not conclusively demonstrate gabapentin’s analgesic efficacy.11 However, gabapentin continues to be used for VCR-related NP in various treatment regimens despite a lack of data from relevant pediatric clinical trials. We performed the current study to prospectively investigate gabapentin’s value for treatment of VCR-related NP in children with ALL treated on Total XVI protocol.12
Methods
Study Design
This is a prospective, randomized, double-blind, placebo-controlled, phase-II trial with two treatment arms: gabapentin plus opioid versus placebo plus opioid. The study was approved by the institutional review board, and written informed consent was obtained from the parents, guardians, or patients, with assent from the patients, as appropriate. The enrollment goal was 60 participants randomized into the treatment (gabapentin) or the placebo groups (30 per group), with daily evaluations for up to 21 days. The daily data collection was based either on face-to-face interaction with the family and the patient or on telephone follow up. The following data were collected daily: number of study drug doses and opioid doses taken in the previous 24 hours, pain score (PS) “right now”, and average PS over the previous 24 hours.
Rationale for gabapentin dose selection and duration of therapy
The gabapentin dose selection was based on the limited published studies about the clinical use of gabapentin in children with NP at the time the study was designed13–20 and on our institutional experience with gabapentin in a retrospective study evaluating NP in children with ALL during Total XV therapy.11
Dosing and safety information for the pediatric use of gabapentin has been defined only for anti-seizure therapy.21 Most pediatric pain specialists recommend the anti-seizure dose regimen for children with NP, starting at 10 mg/kg per day and titrating up to 50 to 70 mg/kg per day. Gabapentin is the first-line therapy for NP at our institution. In our retrospective review of NP during the Total XV study for ALL, we found treatment data for 180 of 207 episodes of NP in 153 of 174 patients: gabapentin was used to treat 62.2% of episodes (112 of 180) in 65.4% of patients (100 of 153); the remaining 37.8% of episodes (68 of 180) in 34.6% of patients (53 of 153) were treated with opioids. The selection of gabapentin or opioids did not appear to be influenced by the pain intensity score at the time of diagnosis of NP (P=0.91). The mean starting dose used for the 112 episodes was 15.5 mg/kg (SD 7.9) per day, and the median starting dose was 14.2 mg/kg per day.11 Some evidence indicates that concurrent gabapentin and morphine treatment provide better analgesia at doses lower than those used for single-agent therapy.9
The gabapentin dose regimen applied in this study was 20 mg/kg per day as a stable dose throughout study participation, divided in 3 daily doses and rounded up to the nearest 100 mg for capsules and to the nearest 10 mg for liquid preparation. Participants randomized to the placebo treatment arm received look-alike capsules or liquid in a respective capsule size or liquid measure equivalent to those given to patients on the active treatment arm.
We selected the phases of the Total XVI study during which VCR was administered most intensely, in 3 to 4 consecutive weekly doses, based on evidence from Total XV and preliminary data from Total XVI supporting higher cumulative incidence of NP at the time of induction and at reinduction I and II treatment phases. The duration of gabapentin therapy in this study was up to 21 days based on the pattern of administration of VCR, the trigger agent for NP in ALL.
Breakthrough Pain Control
All study participants, regardless of treatment arm assignment, had access to open-label oral morphine, available as standard doses (0.15 mg/kg per dose every 2 hours, rounded to the nearest tablet size or measurable liquid quantity), as needed for pain. Substitution of other opioid medication and/or routes was generally discouraged. If necessary, equianalgesic doses were prescribed by the pain specialist principal investigator.
Study Objectives
We compared two analgesic efficacy measures of patients enrolled in the two treatment arms: 1) the morphine daily dose (mg/kg per day) used to control breakthrough pain and 2) two pain scores, PS “right now” and the average PS for the previous 24 hours.
Research Participant Recruitment and Screening
Study participants met the following inclusion criteria: enrollment on Total XVI protocol for ALL; aged 1 year or older; presence of symptoms of NP within 7 days after VCR doses during protocol week 1 or week 2 (induction), week 7 (reinduction I), or week 17 (reinduction II); expectation to receive 2 more subsequent weekly doses of VCR per protocol; and ability to take oral medications. Exclusion criteria included previous participation in this study, receiving gabapentin for another indication at the time of diagnosis of NP, previous gabapentin treatment, decreased glomerular filtration rate <60 mL/min as estimated by the revised Schwartz equation),22 and allergy or other contraindication for morphine or gabapentin therapy.
Participants were referred to the study team (Pain Service) from the clinical team (Leukemia Service) upon diagnosis of NP during the 7 days following a VCR dose. Additionally, the study team screened the clinical notes of potentially eligible patients for documentation of new-onset NP to evaluate eligibility. With the agreement of the clinical team, a study team member approached the eligible participant or their guardian to initiate informed consent discussions. If informed consent was obtained, then the patient was enrolled, the randomization procedure was initiated in the pharmacy, and the pharmacy order sets were activated for the study drug and open-label oral morphine.
Randomization Process
Randomization was performed by a research pharmacist using a randomization program developed by our institution’s Department of Biostatistics, with stratification by three age ranges for which distinct pain assessment tools are applied (1–3, 4–7, >7 years) and by two categories of the baseline pain score (<5, ≥5, on pain scales of 1 to 10). For each subject, a pre-treatment, baseline pain score was obtained and used in the stratified randomization.
Statistical Analysis
The efficacy measurements analyzed were the average daily morphine dosage (morphine equivalent, mg/kg per day) and the average daily PS during the maximum 21-day treatment period, both as PS “right now” and average PS during the previous 24 hours. We tested the null hypothesis that the expected average daily morphine dosage and PS are the same in the gabapentin and the placebo group vs. a one-sided hypothesis that the expected average daily morphine dosages and PS are lower in the gabapentin group. The daily morphine dosage was modeled as longitudinal observations with the treatment (gabapentin vs. placebo) group, and if necessary, with other clinical factors such as age range for pain assessment, baseline pain score, and ALL risk classification, as explanatory factors, using repeated measure linear models. Multiple test adjustment was not made.
The joint distribution of the age ranges and baseline PS categories in the enrolled patients may have been skewed with either null or small odd-numbered (e.g., 1, 3, 5) cell sizes, and consequently, stratification may not have guaranteed balance; therefore, the age ranges and baseline PS were included in the longitudinal model as covariates. Participants whose doses of VCR were delayed or those who were taken off study drug were included in the analysis according to “intent to treat”.
Results
Study Participants
Of the 51 patients enrolled in the study, 2 patients who were randomized to the placebo arm were not evaluable because they withdrew from the study immediately after enrollment and randomization (Fig. 1). Of the 49 evaluable patients, 25 were treated in the gabapentin arm and 24, in the placebo arm. Of them, 45 patients completed all therapy and outcome assessments, and 4 participants (3 in the gabapentin arm and 1 in the placebo arm) were taken off the study early and received partial treatment for 7, 13, 17, or 18 days. Two participants terminated the study early due to patient/parental request based on difficulties taking oral medications, and 2 participants terminated the study early as decided by the principal investigator because critical illness unrelated to the study drug developed, which prompted holding of the subsequent VCR doses. (Fig. 1). The study closed enrollment before reaching the goal of 60 participants because all patients treated for ALL on this protocol had completed their cancer therapy, and no further participants were expected to be exposed to VCR therapy in this clinical trial.
FIGURE 1.
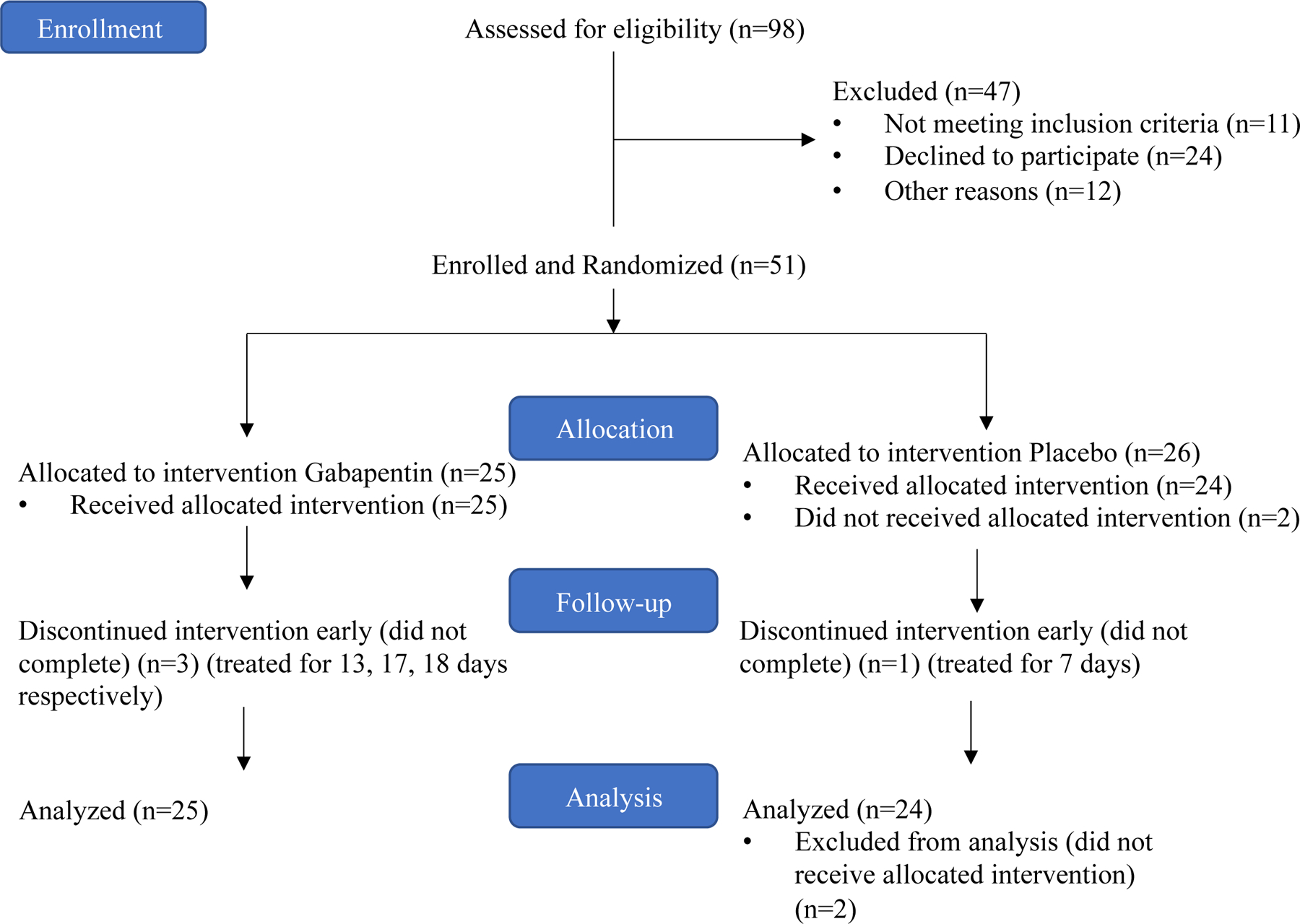
Patient enrollment flow
The percentages of patients in each randomization arm who did not complete all protocol assessments are similar (P=1.000). Distributions of most collected demographic variables as well as VCR dose groups were similar between the two randomization arms, except that more patients with standard/high-risk disease received placebo than received gabapentin (63% vs. 37%, P=0.048) (Table 1).
TABLE 1.
Distribution of demographic variables by randomization arms
| Clinical Feature (n) | Gabapentin Arm, n (%) | Placebo Arm, n (%) | P |
|---|---|---|---|
| Protocol Completion? | 1.0 | ||
| Yes (45) | 22 (49) | 23 (51) | |
| No (6) | 3 (50) | 3 (50) | |
| Sex | |||
| Male (30) | 14 (47) | 16 (53) | 0.779 |
| Female (21) | 11 (52) | 10 (48) | |
| Primary ALL Diagnosis | |||
| Early Pre B (2) | 1 (50) | 1 (50) | 0.765 |
| Early T Cell (1) | 0 (0) | 1 (100) | |
| Pre-B (42) | 20 (48) | 22 (52) | |
| Pre-T Cell (6) | 4 (67) | 2 (33) | |
| Race | |||
| Asian and White (1) | 0 (0) | 1 (100) | 0.771 |
| Black (3) | 1 (33) | 2 (67) | |
| Black and White (3) | 1 (33) | 2 (67) | |
| White (44) | 23 (52) | 21 (48) | |
| Vincristine dose group | |||
| Week 1-Induction (31) | 13 (42) | 18 (58) | 0.318 |
| Week 2-Induction (13) | 9 (69) | 4 (30) | |
| Week 7-Reinduction I (6) | 3 (50) | 3 (50) | |
| Week 17-Reinduction II (1) | 0 (0) | 1 (100) | |
| Total risk group | |||
| Low (21) | 14 (67) | 7 (33) | 0.048* |
| Standard/High (30) | 11 (37) | 19 (63) | |
| Age range | |||
| 1–3 y (16) | 8 (50) | 8 (50) | 1.0 |
| 4–7 y (15) | 7 (47) | 8 (53) | |
| >7 y (20) | 10 (50) | 10 (50) | |
| Baseline Pain Score in the previous 24 hours | |||
| <5 (33) | 17 (52) | 16 (48) | |
| ≥5 (18) | 8 (44) | 10 (56) | |
| Baseline Pain Score now | |||
| <5 (40) | 20 (50) | 20 (50) | 1.0 |
| ≥5 (11) | 5 (45) | 6 (54) | |
Abbreviations: ALL, acute lymphoblastic leukemia
Two-sided P<0.05 by Chi-squared test
Gabapentin Doses
Twenty-five participants were treated with gabapentin, with a mean (SD) dose of 17.97 (2.76) mg/kg per day (median 18.26, range 6.82–21.37).
Analgesic Efficacy of Gabapentin vs. Placebo as Reflected by Morphine Consumption
Opioids were taken by all 25 patients in the gabapentin group for a median of 17.28 days (range, 13 to 21 days, IQR days) and by all 24 patients in the placebo group for 17.12 days (range, 7 to 23 days, IQR days) (P=0.9433). The mean (SD) opioid doses taken, expressed as morphine equivalent daily (mg/kg/day), were 0.26 (0.43) in the gabapentin group (25 patients, 432 days) and 0.15 (0.22) in the placebo group (24 patients, 411 days) (P=0.15, Table 2 and Fig. 2).
TABLE 2.
Analgesic outcome measures
| Measurements | Treatment | Mean (95% CI) | P |
|---|---|---|---|
| Morphine Equivalent Daily | Gabapentin | 0.27 (0.23–0.31) | 0. 15 |
| (mg/kg per day) | Placebo | 0.15 (0.13–0.17) | |
| Pain Score Right Now | Gabapentin | 1.41 (1.22 – 1.60) | 0.04 |
| (scale, 0–10) | Placebo | 0.76 (0.63 – 0.90) | |
| Pain Score Average for Previous | Gabapentin | 2.58 (2.37 – 2.81) | 0.06 |
| 24 Hours (scale, 0–10) | Placebo | 1.73 (1.54 – 1.93) |
Notes: P values from longitudinal models
FIGURE 2.
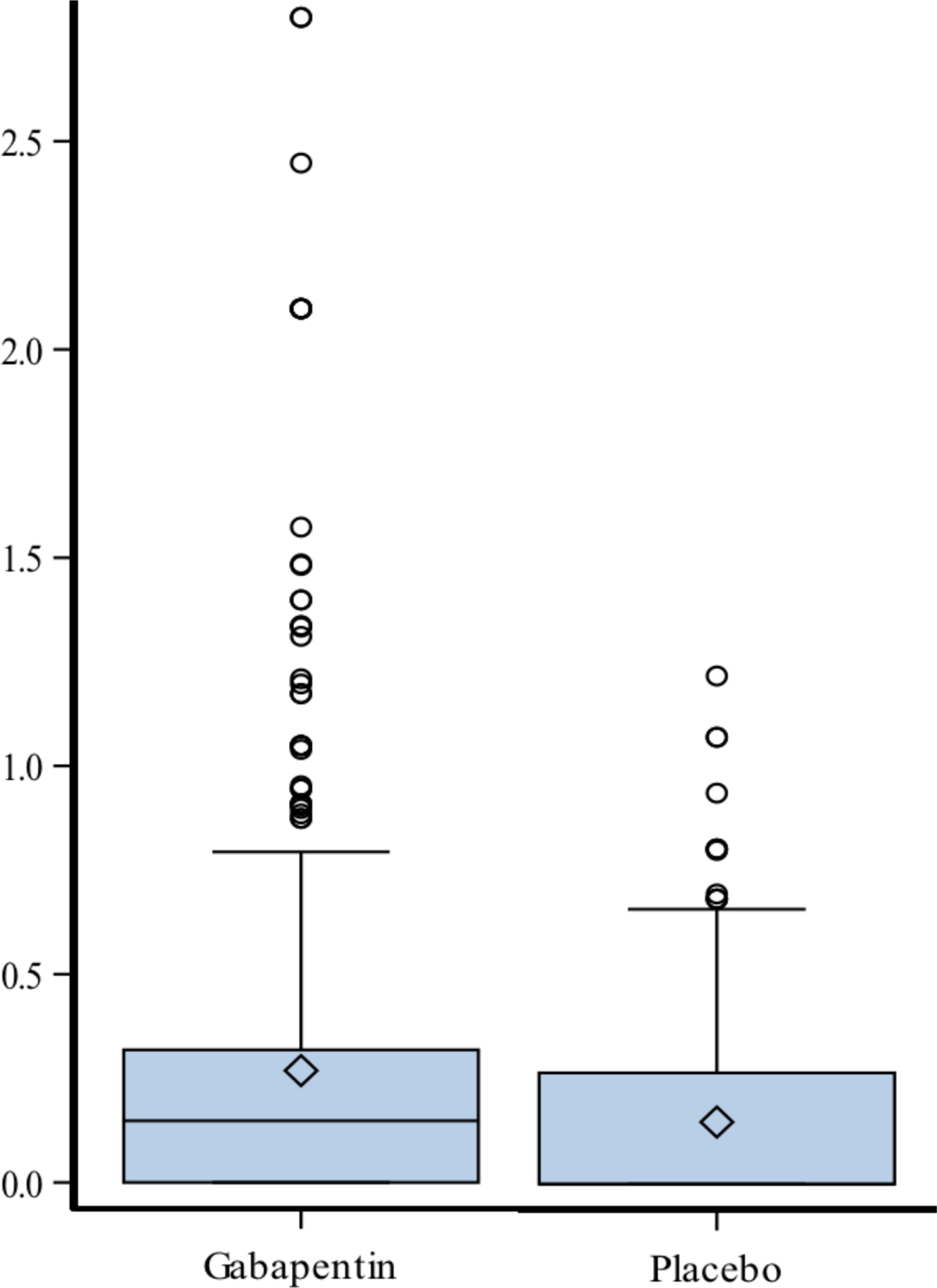
Morphine equivalent daily dose (mg/kg per day) by randomization arms
When the daily morphine dosage was modeled as longitudinal observations with treatment (gabapentin vs. placebo) group and other clinical factors such as age range for pain assessment, baseline pain score, and ALL risk classification, as explanatory factors, by using repeated measure linear models without multiple test adjustment, we found that ALL risk classification was the only factor significantly associated with the daily morphine dosage (P=0.0178). Patients in the lower-risk arm tended to receive a higher daily morphine dosage.
Analgesic Efficacy of Gabapentin vs. Placebo as Reflected by Pain Scores
We compared the PS in the gabapentin and placebo groups collected as PS “right now” and average PS for the previous 24 hours, using longitudinal models, with pain treatment group, and if necessary, other clinical factors as explanatory variables. Multivariate analyses of the daily PS of the gabapentin and placebo groups performed using longitudinal models with Poisson link function indicated no significant differences in the average PS for the previous 24 hours and PS “right now” (Table 2 and Fig. 3).
FIGURE 3.
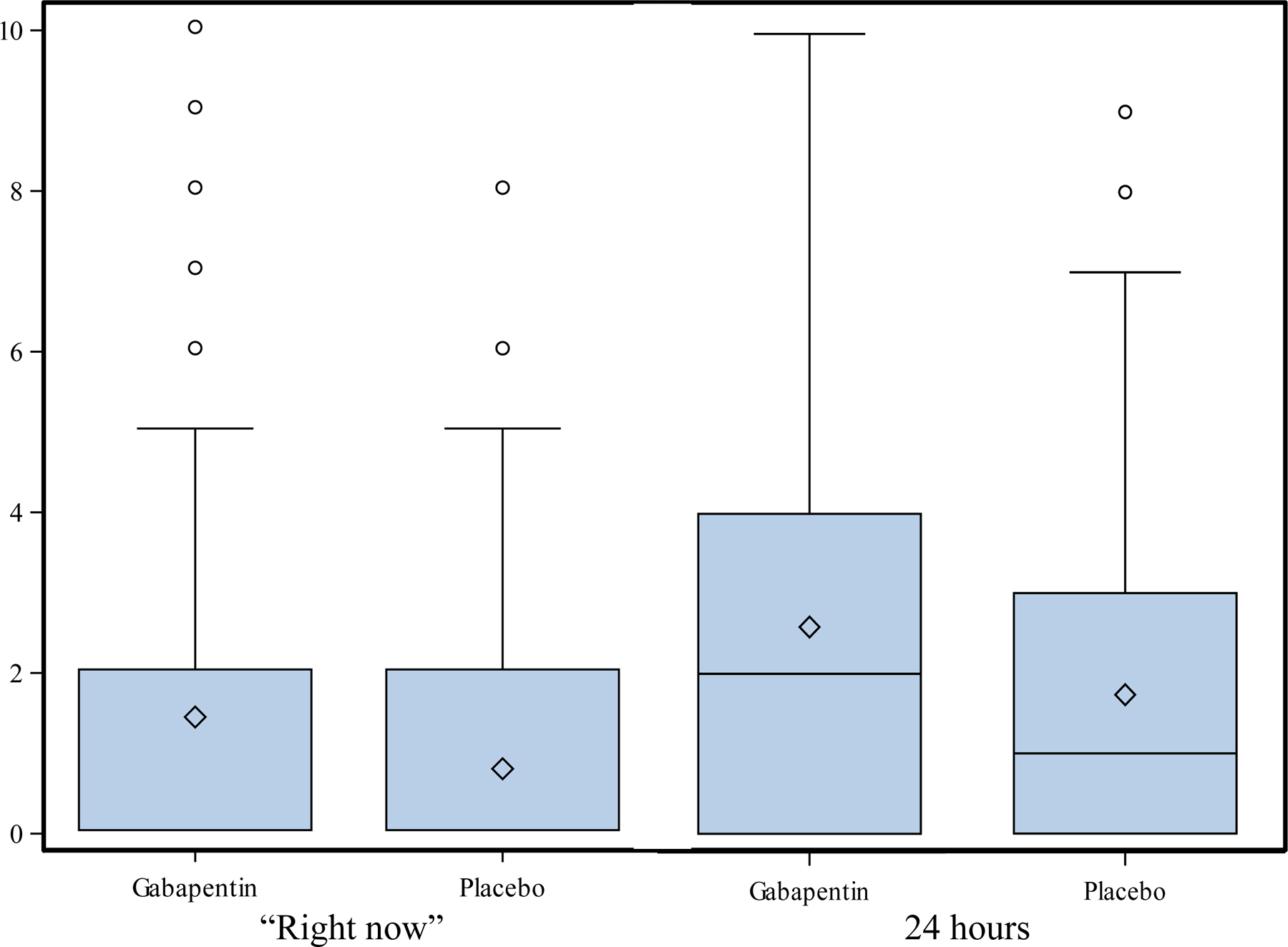
Pain score “right now” and “previous 24 hours” by randomization arms
Discussion
This randomized, double-blind, placebo-controlled, single-center study was designed to compare the analgesic efficacy of gabapentin in the treatment of NP related to administration of VCR during therapy for ALL in children aged 1 to 18 years while allowing use of opioid doses as needed for breakthrough pain. Evaluating study efficacy measures of both opioid use (morphine equivalent, mg/kg per day) and pain scores (pain “right now” and average pain score over the previous 24 hours) shows that therapy with gabapentin plus opioid did not demonstrate better analgesic efficacy than therapy with placebo plus opioid. In this pediatric population of children with ALL and VCR-related NP, opioid consumption and pain scores were both higher in the gabapentin group than in the placebo group.
The failure to demonstrate analgesic benefit of gabapentin therapy in this trial may be multifactorial. First, the dose regimen proposed in this study was based on evidence from the literature available at the time of study design and was somewhat conservative.13–20 Since then, more substantial evidence in support of higher-dose regimens has become available. In comparison with gabapentin dose regimens used in adults with NP, those in pediatrics are generally modest. In a review article about NP in pediatric oncology,23 the authors evaluated 3 studies with a cumulative n of 210 children,11,13,20 noting that the gabapentin dose regimen applied for neuropathic pain started at 10 mg/kg per day and was escalated to 30 mg/kg per day over a few days to a week. Although this somewhat-conservative strategy reflected the most common clinical practice, there are reports of substantially higher doses of gabapentin, at 45 mg/kg per day in a case report (n=2) and 43.8 mg/kg per day in a prospective study of post-surgical neuropathic pain after surgery for osteosarcoma treatment (n=30) (Table 3).24,25 Although our study design indicated a gabapentin regimen of 20 mg/kg per day, the actual doses taken were lower (mean, 18 mg/kg per day), most likely due to missed doses secondary to some combination of lack of adherence, concurrent nausea/vomiting related to other chemotherapy, or nil per os status related to anticipated anesthetics for oncological or surgical procedures. Comparisons of regimens for NP treatment with gabapentin in adults and children23 support the concept that under-dosing strategies are common in pediatric populations, whereas the standard recommendation for adults is to escalate doses up to 300 mg/day or 50–70 mg/kg per day. Gabapentin doses between 900 and 3600 mg/day have been reported to reach analgesic efficacy in adults, and pharmacokinetic analyses have demonstrated drug concentrations correlated with efficacy.26
TABLE 3.
Gabapentin dose regimens in reported pediatric oncology studies
| Reference | Study design (No. of patients) | Patient population | Gabapentin regimen (daily)* | Concurrent pain medications |
|---|---|---|---|---|
| Anghelescu 201725 | Prospective (n=30) | Children with neuropathic pain after limb sparing or amputation for osteosarcoma | Initial dose (mean): 20.2 mg/kg Maximum dose: 43.8 mg/kg |
Amitriptyline (n=5), methadone (n=4) |
| Anghelescu 201111 | Retrospective (n=153) | Patients with childhood ALL and VCR-related neuropathic pain | Initial dose (mean): 14.2 mg/kg | Concurrent medications not evaluated |
| Butkovic 200613 | Case series (n=1) | 18-year-old with neuropathic pain related to metastatic osteosarcoma | Initial dose: 10, 20, or 30 mg/kg Maintenance dose:10 mg/kg for 2–4 weeks at maximum dose |
Morphine, methadone, fentanyl patch, amitriptyline, anti-inflammatory |
| Butkovic 200613 | Case series (n=1) | 17-year-old with pelvic Ewing sarcoma–related neuropathic pain | Initial dose: 30 mg/kg for 3 weeks Maintenance dose: 10 mg/kg |
Tramadol, fentanyl PCA, fentanyl patch, amitriptyline, anti-inflammatory |
| Butkovic 200613 | Case series (n=1) | 14-year-old with metastatic ovarian carcinoma–related neuropathic pain | Dose titrated up to 30 mg/kg for 2 weeks Maintenance dose: 15 mg/kg |
Fentanyl patch, amitriptyline, anti-inflammatory |
| Butkovic 200613 | Case series (n=1) | 15-year-old with osteosarcoma-related neuropathic pain | Dose titrated up to 25 mg/kg for 2 weeks Maintenance dose: 20 mg/kg |
Tramadol, acetaminophen |
| Keskinbora 200420 | Case report (n=1) | 12-year-old with Ewing sarcoma–related neuropathic pain | Initial dose increased daily: 100 mg (~2.9 mg/kg)**, 100 mg BID (~5.7 mg/kg)**, 100 mg TID (~8.6 mg/kg)** Maintenance dose: 300 mg TID (~25.7 mg/kg)** |
|
| Madden 201723 | Case series (n=1) | 19-year-old with ALL and VCR-related neuropathic pain | 45 mg/kg per day | Methadone, amitriptyline |
| Madden 201723 | Case report (n=1) | 6-year-old with ALL and VCR-related neuropathic pain | 45 mg/kg per day | Methadone, morphine |
Abbreviations: BID, twice per day; PCA, patient-controlled analgesia; TID three times per day
Doses in mg/kg per day unless otherwise specified;
Values estimated based on weight
Second, our study was based on a constant gabapentin dose and did not have a dose escalation regimen, which is unlike the usual clinical practice of gradual, slow up-titration of gabapentin on the basis of clinical response of the NP symptoms. This reflects the difficulty posed by dose escalation studies. An ongoing clinical trial with a dose escalation design is evaluating the safety, pharmacokinetics, and efficacy of a new gabapentin liquid formulation as an add-on to morphine in children with severe, chronic mixed or neuropathic pain.27 In this trial, the proposed dose is 45–63 mg/kg per day, dependent on age, for 12 weeks. Dose selection based on pharmacokinetic studies is applied in ongoing multi-institutional clinical trials in pediatric NP.27,28
Third, the study duration was limited to 21 days. However, a therapeutic effect in treating NP may require longer duration of therapy. Furthermore, it is apparent in clinical practice that success in treating NP of various etiologies, in adults and children, usually involves multi-drug therapy and that mono-therapy can rarely resolve this challenging pain entity completely.23
Although the study design was robust, as a randomized, double-blind, placebo-controlled trial, there are limitations to this study, including the discussed considerations about the gabapentin dose and the study duration, as well as the lack of pharmacokinetics data, which could have facilitated dose optimization. The single-institution design of our study created a limitation because enrollment was sub-optimal.
Given the inconclusive results of our clinical trial, future randomized, double-blind, placebo-controlled studies would benefit from higher gabapentin dose regimens, including up-titration regimens, longer duration of therapy, and multi-institutional collaboration, to facilitate larger patient samples.
Acknowledgements
The authors thank Cherise Guess, PhD, ELS, for help with scientific editing and Lane G. Faughnan for support with data collection.
Funding Source:
The preparation of this manuscript has been supported, in part, by the National Cancer Institute Cancer Center Support Core Grant, grant number 2P30CA021765, and by ALSAC.
Abbreviations:
- ALL
acute lymphoblastic leukemia
- NP
neuropathic pain
- VCR
vincristine
- PS
pain score
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Clinical Trial Registration: Therapeutic Interventions for Pain Induced by Vincristine Treatment for Childhood Acute Lymphoblastic Leukemia (TINALL)
ClinicalTrials.gov Identifier: NCT01506453
Data Sharing Statement: Deidentified individual participant data will not be made available.
Conflict of Interest
The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. The New England journal of medicine. 2000;342(5):326–333. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(10):1717–1723. [DOI] [PubMed] [Google Scholar]
- 3.LeBaron S, Zeltzer L. Assessment of acute pain and anxiety in children and adolescents by self-reports, observer reports, and a behavior checklist. Journal of consulting and clinical psychology. 1984;52(5):729–738. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen PB, Manne SL, Gorfinkle K, Schorr O, Rapkin B, Redd WH. Analysis of child and parent behavior during painful medical procedures. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1990;9(5):559–576. [DOI] [PubMed] [Google Scholar]
- 5.Tuxen MK, Hansen SW. Neurotoxicity secondary to antineoplastic drugs. Cancer treatment reviews. 1994;20(2):191–214. [DOI] [PubMed] [Google Scholar]
- 6.Bradley WG, Lassman LP, Pearce GW, Walton JN. The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. Journal of the neurological sciences. 1970;10(2):107–131. [DOI] [PubMed] [Google Scholar]
- 7.Anghelescu DL, De Armendi AJ, Thompson JW, Sillos EM, Pui CH, Sandlund JT. Vincristine-induced vocal cord paralysis in an infant. Paediatric anaesthesia. 2002;12(2):168–170. [DOI] [PubMed] [Google Scholar]
- 8.Woods WG, O’Leary M, Nesbit ME. Life-threatening neuropathy and hepatotoxicity in infants during induction therapy for acute lymphoblastic leukemia. The Journal of pediatrics. 1981;98(4):642–645. [DOI] [PubMed] [Google Scholar]
- 9.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. The New England journal of medicine. 2005;352(13):1324–1334. [DOI] [PubMed] [Google Scholar]
- 10.Kishi S, Cheng C, French D, et al. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood. 2007;109(10):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anghelescu DL, Faughnan LG, Jeha S, et al. Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatric blood & cancer. 2011;57(7):1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeha S, Pei D, Choi J, et al. Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(35):3377–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butkovic D, Toljan S, Mihovilovic-Novak B. Experience with gabapentin for neuropathic pain in adolescents: report of five cases. Paediatric anaesthesia. 2006;16(3):325–329. [DOI] [PubMed] [Google Scholar]
- 14.Harel L, Mukamel M, Brik R, Blau H, Straussberg R. Peripheral neuropathy in pediatric systemic lupus erythematosus. Pediatric neurology. 2002;27(1):53–56. [DOI] [PubMed] [Google Scholar]
- 15.Lauder GR, White MC. Neuropathic pain following multilevel surgery in children with cerebral palsy: a case series and review. Paediatric anaesthesia. 2005;15(5):412–420. [DOI] [PubMed] [Google Scholar]
- 16.Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. Journal of pediatric orthopedics. 2007;27(5):567–572. [DOI] [PubMed] [Google Scholar]
- 17.Rusy LM, Troshynski TJ, Weisman SJ. Gabapentin in phantom limb pain management in children and young adults: report of seven cases. J Pain Symptom Manage. 2001;21(1):78–82. [DOI] [PubMed] [Google Scholar]
- 18.McGraw T, Stacey BR. Gabapentin for treatment of neuropathic pain in a 12-year-old girl. The Clinical journal of pain. 1998;14(4):354–356. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler DS, Vaux KK, Tam DA. Use of gabapentin in the treatment of childhood reflex sympathetic dystrophy. Pediatric neurology. 2000;22(3):220–221. [DOI] [PubMed] [Google Scholar]
- 20.Keskinbora K, Pekel AF, Aydinli I. The use of gabapentin in a 12-year-old boy with cancer pain. Acta anaesthesiologica Scandinavica. 2004;48(5):663–664. [DOI] [PubMed] [Google Scholar]
- 21.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. Jama. 2003;290(7):905–911. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. The Journal of pediatrics. 1985;106(3):522–526. [DOI] [PubMed] [Google Scholar]
- 23.Anghelescu DL, Tesney JM. Neuropathic Pain in Pediatric Oncology: A Clinical Decision Algorithm. Paediatr Drugs. 2019;21(2):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden K, Bruera E. Very-Low-Dose Methadone To Treat Refractory Neuropathic Pain in Children with Cancer. J Palliat Med. 2017;20(11):1280–1283. [DOI] [PubMed] [Google Scholar]
- 25.Anghelescu DL, Steen BD, Wu H, et al. Prospective study of neuropathic pain after definitive surgery for extremity osteosarcoma in a pediatric population. Pediatr Blood Cancer. 2017;64(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetics of gabapentin after administration of gabapentin enacarbil extended-release tablets in patients with varying degrees of renal function using data from an open-label, single-dose pharmacokinetic study. Clin Ther. 2012;34(1):201–213. [DOI] [PubMed] [Google Scholar]
- 27.de Leeuw TG, Mangiarini L, Lundin R, et al. Gabapentin as add-on to morphine for severe neuropathic or mixed pain in children from age 3 months to 18 years - evaluation of the safety, pharmacokinetics, and efficacy of a new gabapentin liquid formulation: study protocol for a randomized controlled trial. Trials. 2019;20(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaguelidou F, Le Roux E, Mangiarini L, et al. Non-inferiority double-blind randomised controlled trial comparing gabapentin versus tramadol for the treatment of chronic neuropathic or mixed pain in children and adolescents: the GABA-1 trial-a study protocol. BMJ Open. 2019;9(2):e023296. [DOI] [PMC free article] [PubMed] [Google Scholar]


