Abstract
Many animals make behavioural changes to cope with winter conditions, being gregariousness a common strategy. Several factors have been invoked to explain why gregariousness may evolve during winter, with individuals coming together and separating as they trade off the different costs and benefits of living in groups. These trade-offs may, however, change over space and time as a response to varying environmental conditions. Despite its importance, little is known about the factors triggering gregarious behaviour during winter and its change in response to variation in weather conditions is poorly documented. Here, we aimed at quantifying large-scale patterns in wintering associations over 23 years of the white-winged snowfinch Montifringilla nivalis nivalis. We found that individuals gather in larger groups at sites with harsh wintering conditions. Individuals at colder sites reunite later and separate earlier in the season than at warmer sites. However, the magnitude and phenology of wintering associations are ruled by changes in weather conditions. When the temperature increased or the levels of precipitation decreased, group size substantially decreased, and individuals stayed united in groups for a shorter time. These results shed light on factors driving gregariousness and points to shifting winter climate as an important factor influencing this behaviour.
Keywords: collective movement, climate change, fission–fusion dynamic, gregariousness, Montifringilla nivalis nivalis, snowfinches
1. Introduction
Winter represents a major challenge for a large number of animal species. Even cold-adapted species of temperate regions face challenges like reduction in food availability and have to seek shelter when snowfall arrives. Alternative or complementary strategies for sedentary species include hibernation and topor [1], whereas in other species migration represents a good strategy for overwintering [2]. An additional adaptive response to harsh winter conditions is the adoption of gregarious behaviour [3,4]. Individuals that are extremely territorial throughout the breeding season may in contrast adopt an extended social way of life during the winter.
Gregariousness during winter is such a common strategy in a temperate zone, that it must have marked advantages [3,5]. A variety of ultimate and proximate factors have been proposed to explain why gregariousness may evolve during winter. Among birds, apart from reduced predation [6], wintering association confers considerable advantages for locating suitable feeding grounds (e.g. patches free of snow or with abundant food supply) [2]. Drawbacks of living in groups include increased competition for resources or the spread of diseases [7]. Therefore, group size can be dynamic and fluctuate over time and space [8,9], with individuals separating or gathering together (i.e. fission–fusion dynamics) as they trade off the costs and benefits of living in a group [8,10]. Fission–fusion dynamics [9] certainly influence many ecological and evolutionary aspects, such as habitat selection, space use and migration [11–14], ultimately affecting the dynamic and persistence of animal populations [10]. Despite its importance, little is known about the dynamics of gregariousness during winter nor how these dynamics respond to spatio-temporal variability in climatic conditions.
Animal life-history strategies are adapted to local and global climate conditions [15–18]. In birds, there is good evidence that the changes in climatic conditions (e.g. temperature, precipitation) affect migratory behaviour [19–22] and the timing of reproduction of many species [23–25]. Such behavioural adjustments frequently have severe negative effects on species distribution, abundances and may lead to local extinctions [15,25]. Notably, other behavioural adaptations to new local climatic conditions are key adaptive responses for maintaining populations in a changing world [26,27]. As individual decisions are context dependent [10], we could anticipate that gregariousness may change over space and time as a response to varying environmental conditions. While theoretical studies on animal aggregation have primarily focused on methods to detect the underlying mechanisms leading to its emergence [28], empirical works on gregariousness have often been restricted to small spatial (single location) and short temporal (from few days to few months) scales [29,30]. Discerning patterns and revealing the geographical scope of locally observed dynamics in gregariousness requires spatially and temporally extensive data. Although responses in gregariousness to local weather conditions help understand the short-term impacts of changes in environmental conditions, assessing differences in natural groups along a geographical and climatic gradient can offer deeper insights into how gregariousness may respond to long-term changes in climate.
Here, we used a long-term dataset to quantify large-scale patterns in wintering associations of one of the most emblematic songbird species of alpine ecosystems in Europe, the white-winged snowfinch (Montifringilla nivalis; hereafter snowfinch). The snowfinch is a Palaearctic alpine species, with a subspecies (M. n. nivalis) distributed in Europe from the Spanish Cantabrian Mountains in the northwest of the Iberian Peninsula, through the Pyrenees, the Alps, Corsica, the Apennines, eastwards to the Dinaric Alps and the south-western Balkans [31,32]. Even though the snowfinch is classified as a Least Concern species by the Global International Union for Conservation of Nature Red List Category Criteria, data for population and trend estimation is currently unknown and remain poorly known in more than 90% of the European countries [31]. Surveys conducted in part of its range however point to recent range contractions [33,34], at the same time that high-elevation ecosystems in Europe are facing dramatic changes induced by global warming [35–38]. Little is known about whether variation in climate affects the social behaviour of cold-adapted species, and the snowfinch, with its marked gregarious behaviour [39] during the non-breeding season (hereafter winter), is an ideal biological model for the purposes of this study. Specifically, the aims of our study were to address: (i) to what extent does the variation in fission–fusion dynamics and in wintering group size follow abiotic gradients such as latitude or elevation (here represented by mean site temperature and mean site precipitation over the extent of the study period)? and (ii) have wintering group size and fission–fusion dynamics changed as a response to varying weather conditions? In mountain areas with harsher and longer wintering conditions, where living in groups might benefit individuals for e.g. locating food resources, large group sizes are predicted to occur. However, because within-group individual competition is expected to increase with group size, we can expect individuals in large groups to stay together for shorter times. Moreover, if flocking behaviour is sensitive to climate variation across winter, we can expect that ongoing changes in climate might be impacting the gregarious behaviour of this alpine bird species. In particular, we might predict that mild winters could lead to a decrease in the size of snowfinch wintering associations and to a reduction of the time individuals stay together.
2. Material and methods
(a) . Data collection
From 1990 to 2013, 10 843 observations on snowfinches were collected in Switzerland, Italy and Spain, in the framework of different studies carried out by the authors [39–42] and by national parks and local institutions. In addition, data collected by the public (citizen science) and gathered through online databases (www.ornitho.at, www.ornitho.ch, www.ornitho.it, www.ornitho.cat) were obtained after official requests for the purpose of the study. All data were collected in the form of spatially georeferenced observations (figure 1a,b; for more information, see the electronic supplementary material, Data collection).
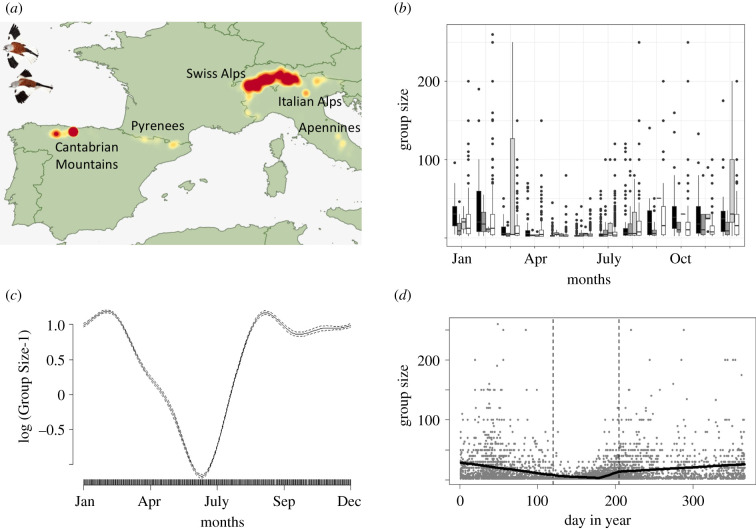
Figure 1.
(a) Map of the study areas. Colour tonalities represent the number of observations recorded in each snowfinch population, from dark to light colours, respectively, representing the areas with fewer and higher number of snowfinch observations collected; (b) boxplots representing the size of snowfinch groups between the areas (black: Cantabrian Mountains; dark grey: Italy; light grey: Pyrenees; white: Swiss Alps) and over the months; (c) snowfinches show marked fission–fusion dynamics across the annual cycle, as evidenced by fitting the general additive model of the group size-1 as a function of the smoothing factor for day in year (i.e. Julian dates). In particular, once the nestlings fledge, family groups gather in large flocks during the non-breeding season (i.e. from July to early April); (d) representation of the two internal knots (vertical dotted lines) estimated by fitting the general additive model of the group size-1 as a function of the smoothing factor for day in year (i.e. Julian dates). The two internal knots represent the inflection points where the linear regression could be separated into different segments with different slopes. (Online version in colour.)
To study the spatio-temporal variation in fission–fusion dynamics, we modelled the number of snowfinches within groups (Q) as a function of the Julian date for each particular site and year (figure 1c,d). We treated Q as a Poisson distributed response variable and fitted a general additive model (GAM, wrapped seasonally to match 31 December to 1 January) to allow the relationship to be nonlinear, i.e. the smoothing function f(Q) could potentially take any shape [43,44]. A model was fitted for each area and year separately. For each model, we estimated the two internal knots of the linear regressions, representing the inflection points where the linear regression could be separated into different segments with different slopes (figure 1d). We used these knots as a proxy of the fission–fusion dynamic, representing for each site and each year the time when individuals separate (fission), and when individuals come together in large flocks during the non-breeding season (fusion). We could not include the Pyrenees area because for some months, there was only one observation (see the electronic supplementary material, Data collection), and thus we could not build the generalized additive mixed models (GAMMs) to estimate the internal knots. After removing some potential influential observations, and considering those observations for which we had information on climatic variables, our final dataset consisted of a total of 33 fission–fusion dates (Cantabrian Mountains = 9, Italy = 9 and Switzerland = 15). Further, for studying the spatio-temporal variation in group size during winter, we considered only those observations where more than five individuals (this number corresponding to the mean of the first interquartile range; see the electronic supplementary material, table S1) were simultaneously observed (total number of observations = 6164) and selected the 1295 observations collected in winter (i.e. from September to March, both included; see the electronic supplementary material, table S1). Typically, larger group sizes of animals are easier to observe than isolated individuals. However, in our study, we did not consider the potential introduced bias to play a major role (figure 1b). Snowfinches are larger, more conspicuous and inhabit a more open habitat than other alpine bird species and are readily observed in isolation (e.g. over 34% of all observations were of individual animals).
To study the potential influence of weather conditions in wintering group fission–fusion and group size dynamics, we used the potentially relevant weather data for the snowfinch as those related to mean ambient temperature and mean precipitation gathered by the CHELSA database at a 30 arc sec-resolution [45]. In particular, we estimated two different types of weather variables: (i) for each area, we estimated the mean temperature over the observation period (hereafter, mean site temperature) and the mean precipitation over the observation period (mean site precipitation). Thus, we characterized each area by one mean site temperature and one mean site precipitation over the observation period; and (ii) for each observation, we recorded the mean monthly temperature and mean monthly precipitation.
(b) . Statistical analysis
To quantify the dynamics of wintering associations, we fitted three GAMMs, treating the number of snowfinches within a group observed during winter as a Poisson and the two internal knots (representing group fission and fusion) as two normal distributed response variables. Because the number of snowfinches within a group was count data with no zeros, we modelled the response variable group size-1 as a simple way to technically consider it as a zero-truncated Poisson regression. To account for both snowfinch fission–fusion and wintering group size varying with abiotic gradients, we included the linear effect of mean site temperature and mean site precipitation. Both of these variables were standardized to facilitate comparisons of effect sizes. To measure how fission–fusion dynamic and the size of groups are changing as a response to temporal varying weather conditions during the winter, we further included mean monthly temperature, mean monthly precipitation and year, as well as its interactions as smoothing variables using the default thin-plate regression spline in the GAMM4 package in R [46,47]. When adding the nonlinear effects, we checked the effective degrees of freedom (EDF) of the variables. Those variables showing an EDF of less than 2 were otherwise included as a linear effect [46].
It is important to note that the different areas considered here vary in snowfinch population sizes, and therefore differences in population size could potentially influence the upper limits of flock size. However, as the observed maximum flock sizes were always well below the size of populations in each area, we consider that differences in this variable could not directly affect the results of this study. Yet, to account for any potential bias owing to differences in the number of observations collected among years and areas, we included both the area identification (ID) and year ID as random intercept factors. By doing so, we accounted at the same time for any other potential influential factor varying with site or year that could otherwise be overlooked. For fission–fusion group dynamics, area ID and year ID variance were estimated as zero; we therefore proceeded with linear models without random effects for those cases.
Once we generated the sets of competing models, we employed the Akaike information criterion (AIC), using the values of ΔAIC of less than 2 as the criterion for selecting the most parsimonious model [48]. Following standard procedures, we calculated the Akaike weight for each candidate model (wi) as the relative strength of evidence, i.e. the probability of model i being the best-approximating model from the entire set of candidate models, and evidence ratios of the best models as the ratio of model weights. Models were finally evaluated by checking diagnostic plots. All analyses were performed using R v. 3.5.0. [49].
3. Results
Snowfinches show a marked seasonal pattern in group size (figure 1c). While fusion takes place at the beginning of July, i.e. around the mean (±s.d.) Julian day of 220.6 ± 17.9 days (range = 181–268.8 days; figure 1c), fission occurs in April, i.e. around the mean (±s.d.) Julian day of 147.3 ± 44.3 days (range = 46–217.5 days; figure 1c). Overall, group fission–fusion dynamic tends to follow the abiotic gradient among the study sites, with fusion occurring slightly earlier at warmer sites with abundant precipitation (electronic supplementary material, table S2 and figure S1) and fission occurring later at warmer sites with low precipitation (electronic supplementary material, table S2 and figure S1). During the winter, the median size (±s.d.) of snowfinch group is 20 (first interquartile range (IQR) = 10, third IQR = 40). The number of individuals within a group is also related to the mean site temperature and mean site precipitation (electronic supplementary material, table S2). At locations with lower mean site temperatures, especially when associated with abundant mean site precipitation, winter groups tend to be larger (figure 2a).
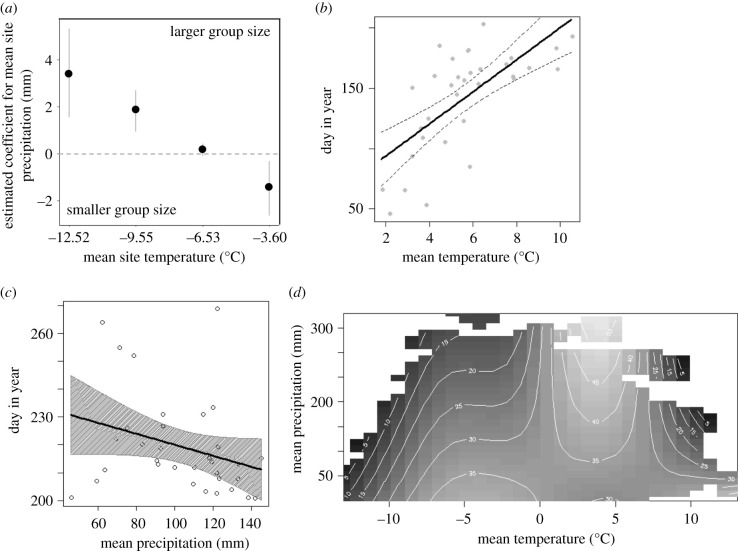
Figure 2.
(a) Plot of marginal effects of the interaction between mean site temperature and mean site precipitation on wintering group size showing that at colder sites, especially when associated with high levels of precipitation, winter groups tend to be larger; linear effect of temperature (b) and precipitation (c) on group fission and fusion dates, respectively. Each point corresponds, respectively, to each fission and fusion date for each year in each site considered (i.e. Cantabrian Mountains (n = 9), Italy (n = 9) and Switzerland (n = 15)). Overall, when ambient temperature increased and the level of precipitation decreased, individuals stayed united in groups for a shorter time; (d) counterplot representing the effect of the interaction between mean monthly temperature and mean monthly precipitation on group size-1 from a generalized additive mixed model. The axes represent the values of the predictor variables, and the interior is a topographic map of the predicted values. The light colours represent larger predictions, and the dark colours the smaller prediction. We set-up the ‘too.far’ argument in the vis.gam function to 0.07 to remove those predicted values that were not well represented by our data. Drawings: Giulia Bombieri.
The different mountain regions in our study have experienced an uneven increase in temperature, especially outside the breeding season, while precipitation has remained stable or declined (electronic supplementary material, figure S2). We observed that fission occurs later when mean monthly temperature increased (figure 2b; electronic supplementary material, table S2), whereas fusion shifts earlier when the level of mean monthly precipitation increased (figure 2c; electronic supplementary material, table S2). The resulting models yielded moderate mean explanatory power (fission: adjusted R-squared = 49%; fusion: marginal R-squared = 22%). When weather conditions result in warmer mean monthly temperature, independently of the amount of mean monthly precipitation, snowfinches form smaller groups (figure 2d; electronic supplementary material, table S2). Notably, beyond the observed influence of weather, the low mean explanatory power (adjusted R-squared = 4%) of the resulting model suggests that other factors not accounted for here probably are determining group size variation.
4. Discussion
Gregariousness is essential in allowing individuals to interact, transfer information and cope with changing environmental conditions [14]. Here, we found that individuals belonging to an alpine species gather in larger groups especially at sites where wintering conditions are harsher, i.e. under cold ambient temperature and high levels of precipitation. At these sites, individuals reunite later and separate earlier in the season than at warmer sites. However, our results revealed that temporary changes in wintering associations (i.e. group size and fission–fusion dynamic) are affected by weather conditions. Specifically, we found that when ambient temperatures are warm and precipitation is low, the size of wintering groups substantially decreased, with the group fission occurring later when temperature increased and the group fusion shifting to earlier dates when the level of precipitation increased. Together, this sensitivity of flocking behaviour to climate variation across winter indicate that ongoing changes in climate, which are particularly affecting high-elevation ecosystems, will probably impact on the gregarious behaviour of alpine species.
Our results indicate that the variation in snowfinch group size and its fission–fusion dynamics substantially follow an environmental gradient, importantly confirming the basic expectation of a general pattern of variation in wintering associations along latitudinal or elevational gradients. The pattern that wintering associations tend to be larger at colder sites with high levels of precipitation (typically higher latitude or elevation) is in accordance with the hypothesis that living in the group might help individuals to locate food resources [9], which in alpine environments gradually changes as the season progresses. During the breeding season, snowfinches move upslope following the phenology of their most important items, i.e. larvae of Diptera and Lepidoptera, as well as adults of Arachnida, Diptera and Lepidoptera [50]. However, in autumn and winter, they exclusively feed on seeds of alpine plants [50]. Depending on the amount of snow cover, feeding grounds in winter are fairly unpredictable and are patchily and heterogeneously distributed [39]. Foraging in large groups may benefit snowfinches when moving in a quite nomadic manner to locate prime feeding grounds of alpine forbs or shrubs that need to be exposed to be accessible. However, because within-group individual competition is expected to increase with group size, maintaining long-term groups during the non-breeding period may reduce individual fitness and increase the levels of stress [9]. This might be particularly important in areas close to human surroundings (e.g. refuges and ski-areas) that are visited by snowfinches in very harsh weather conditions [51]. The occurrence and intensity of artificial feeding vary among the snowfinch populations and is much more common in the Alps than in the other snowfinch populations [50]. Significant detrimental ecological effects of providing feed to birds have been documented [52], including disease transmission and individual physical condition (e.g. high levels of cholesterol and triglycerides). Understanding whether and how artificial feeding might cause a disruption of snowfinches movement patterns, ultimately affecting their gregariousness during winter, is still a pertinent open question that needs further research.
While part of the variation in wintering associations can be attributed to a simple environmental gradient, we observed that winter association responses to weather conditions are specific to local regions. Certainly, variations in climatic events may depend on average temperature and mean levels of precipitation and change as well differently over time. Therefore, we could expect site-specific variation in wintering associations attributable to changes in average temperature and/or levels of precipitation. In areas where climate has experienced warming, resources probably occur broadly over larger areas, such that the costs of living in a group are not compensated by the benefits of cooperation [11,12]. Moreover, when snowfinches aggregate in large flocks during the winter, they adopt a partial-migration strategy [53]. Stable isotope data suggest that some individuals may move from Switzerland to the Spanish Pyrenees and to the Cantabrian mountains. The probability and magnitude of those migratory movements are related to the local winter conditions in Switzerland [53]. In particular, the migratory propensity of snowfinches is higher when winter conditions in the Alps are harsh. As the migratory propensity of snowfinches depends on climate [53], we can expect the migratory behaviour, and consequently, the size of the groups of snowfinches, to decrease under the ongoing global warming. Taken as whole, however, our results suggest that, as climate warming continues, large wintering associations could revert to smaller groups.
As the costs of living in a group may not be compensated by the benefits of cooperation under warming weather conditions, individuals might coordinate decisions to fuse into a short-term group. Although increasing temperatures might positively influence food availability, temporary changes in group fission–fusion dynamics, such as when and why groups separate and reunite, could result in individuals having to re-establish their social relationships, thus taking time away from other tasks like foraging or breeding [54]. These relationships are worthy of exploring by future studies, to assess whether fission–fusion dynamics generated by e.g. varying climatic conditions might lead to group instability, ultimately disrupting the social organization of populations [55].
Even though our data cannot directly measure breeding activity, we could expect that the latter may be linked with the observed delay in the timing of group fission. In a warming climate, mild winters and early springs are associated with unpredictable extreme weather events, resulting in unexpected cold temperature episodes later in spring [56]. This is particularly common in alpine environments, which are among the most affected by climate change [57]. Staying together longer during winter might indeed represent an adaptive response of alpine bird species to cope with these extreme climatic events. However, snowfinches might need to adjust their breeding period to match the peak of particular food resources [41]. If the spring arrives early but wintering groups separate later, birds might be delayed in relation to the phenology of food resources [58], consequently shortening their reproductive activity and/or lowering their breeding performance.
Variation in the duration of the reproductive season in birds as a response to climate change has been previously reported [23,24]. Notably, elevational clines have generally received far less attention than latitude [59], though alpine birds are declining more severely than other passerine birds, with the exception of farmland birds. Given the ongoing rapid environmental change, more studies disentangling the relative role of climatic factors in driving wintering associations and its effects on the breeding activity of alpine birds could help understand how these species might maintain viable populations in changing environments.
Supplementary Material
Acknowledgements
We are very grateful to National/Natural Parks in Switzerland, Italy and Spain, and to the many volunteers who uploaded their snowfinch observations on ornitho websites, park databases, for help with data collection. We thank H. Schmid, R. Lardelli, D. Rubolini and the management group of ornitho.it, ornitho.at and ornitho.ch for helpful collaboration. We are also indebted to the Institut Català d'Ornitologia (ICO) for providing the species observations from ornitho.cat. The authors wish to extend their particular thanks to Vincenzo Penteriani for providing useful comments on early drafts, and to Enrique González, Antonio L. Orta (project no. IDI/2018/000151) and Rubén Tarifa (these latest ones being part of the Crustacean Team) for their wonderful help and good mood when working in the mountains with snowfinches.
Data accessibility
The data that support the findings of this study are openly available in ‘figshare’ at doi:10.6084/m9.figshare.13084595.
Authors' contributions
M.d.M.D.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing—original draft, writing—review and editing; R.A.: data curation and writing—original draft; C.B.: data curation and writing—original draft; M.B.: data curation and writing—original draft; M.d.G.H.: data curation; Á.E.: data curation; Á.F.-G.: data curation and writing—original draft; A.F.-M.: data curation; J.A.G.: data curation; S.H.-G.: data curation; P.L.: data curation, writing—original draft; J.R.-M.: data curation; J.R.O.: data curation; P.P.: data curation; I.R.-A.: data curation; C.S.: data curation; D.S.: data curation; E.S.: data curation; I.T.: data curation; F.K.-N: data curation and writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
M.d.M.D. was financially supported by the (i) Spanish Ministry of Sciences, Innovation and Universities (no. CGL2016-79764-P) and (ii) a Spanish Ramon y Cajal grant no. RYC-2014-16263. M.B. was partly supported by the project Mediterranean Mosaics II funded by MAVA to Lipu.
References
- 1.Delgado MM, et al. 2018. The seasonal sensitivity of brown bear denning phenology in response to climatic variability. Front. Zool. 15, 41. ( 10.1186/s12983-018-0286-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton I. 2008. The migration ecology of birds. New York, NY: Academic Press. [Google Scholar]
- 3.Evans JC, Morand-Ferron J. 2019. The importance of preferential associations and group cohesion: constraint or optimality. Behav. Ecol. Sociobiol. 73, 1–10. ( 10.1007/s00265-019-2723-7) [DOI] [Google Scholar]
- 4.Spencer R. 1982. Bird study: birds in winter: an outline. Bird Study 29, 169–182. ( 10.1080/00063658209476754) [DOI] [Google Scholar]
- 5.Mcfarland R, Fuller A, Hetem RS, Mitchell D, Maloney SK, Henzi SP, Barrett L. 2015. Social integration confers thermal benefits in a gregarious primate. J. Anim. Ecol. 84, 871–878. ( 10.1111/1365-2656.12329) [DOI] [PubMed] [Google Scholar]
- 6.Riipi M, Alatalo RV, Lindström L, Mappes J. 2001. Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413, 512–514. ( 10.1038/35097061) [DOI] [PubMed] [Google Scholar]
- 7.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302. ( 10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 8.Sueur C, et al. 2011. Collective decision-making and fission–fusion dynamics: a conceptual framework. Oikos 120, 1608–1617. ( 10.1111/j.1600-0706.2011.19685.x) [DOI] [Google Scholar]
- 9.Dhanjal-Adams KL, Bauer S, Emmenegger T, Hahn S, Lisovski S, Liechti F. 2018. Spatiotemporal group dynamics in a long-distance migratory bird. Curr. Biol. 28, 2824–2830.e3. ( 10.1016/j.cub.2018.06.054) [DOI] [PubMed] [Google Scholar]
- 10.del Delgado MM, Miranda M, Alvarez SJ, Gurarie E, Fagan WF, Penteriani V, di Virgilio A, Morales JM. 2018. The importance of individual variation in the dynamics of animal collective movements. Phil. Trans. R. Soc. B 373, 20170008. ( 10.1098/rstb.2017.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loretto MC, Schuster R, Itty C, Marchand P, Genero F, Bugnyar T. 2017. Fission-fusion dynamics over large distances in raven non-breeders. Sci. Rep. 7, 1–9. ( 10.1038/s41598-017-00404-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delellis P, Polverino G, Ustuner G, Abaid N, Macrì S, Bollt EM, Porfiri M. 2014. Collective behaviour across animal species. Sci. Rep. 4, 1–6. ( 10.1038/srep03723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covas R, Griesser M. 2007. Life history and the evolution of family living in birds. Proc. R. Soc. B 274, 1349–1357. ( 10.1098/rspb.2007.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teitelbaum CS, Converse SJ, Fagan WF, Böhning-Gaese K, O'Hara RB, Lacy AE, Mueller T. 2016. Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat. Commun. 7, 1–7. ( 10.1038/ncomms12793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 16.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60. ( 10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 17.Thackeray SJ, et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Chang. Biol. 16, 3304–3313. ( 10.1111/j.1365-2486.2010.02165.x) [DOI] [Google Scholar]
- 18.Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhov A, Kutenkov A, Kutenkova N, Shcherbakov A, Meyke E, Del Mar Delgado M. 2013. Community-level phenological response to climate change. Proc. Natl Acad. Sci. USA 110, 13 434–13 439. ( 10.1073/pnas.1305533110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotton PA. 2003. Avian migration phenology and global climate change. Proc. Natl Acad. Sci. USA 100, 12 219–12 222. ( 10.1073/pnas.1930548100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordo O. 2007. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 35, 37–58. ( 10.3354/cr00713) [DOI] [Google Scholar]
- 21.Both C, Visser ME. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298. ( 10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- 22.Knudsen E, et al. 2011. Challenging claims in the study of migratory birds and climate change. Biol. Rev. 86, 928–946. ( 10.1111/j.1469-185X.2011.00179.x) [DOI] [PubMed] [Google Scholar]
- 23.Halupka L, Halupka K. 2017. The effect of climate change on the duration of avian breeding seasons: a meta-analysis. Proc. R. Soc. B 284, 20171710. ( 10.1098/rspb.2017.1710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Møller AP, Flensted-Jensen E, Klarborg K, Mardal W, Nielsen JT. 2010. Climate change affects the duration of the reproductive season in birds. J. Anim. Ecol. 79, 777–884. ( 10.1111/j.1365-2656.2010.01677.x) [DOI] [PubMed] [Google Scholar]
- 25.Forchhammer MC, Post E, Stenseth NC. 1998. Breeding phenology and climate. Nature 391, 29–30. ( 10.1038/34070)9422504 [DOI] [Google Scholar]
- 26.Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS. 2004. Species coextinctions and the biodiversity crisis. Science 305, 1632–1634. ( 10.1126/science.1101101) [DOI] [PubMed] [Google Scholar]
- 27.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. ( 10.1111/j.1461-0248.2011.01736.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumpter DJT. 2006. The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22. ( 10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavagna A, Cimarelli A, Giardina I, Parisi G, Santagati R, Stefanini F, Viale M. 2010. Scale-free correlations in starling flocks. Proc. Natl Acad. Sci. USA 107, 11 865–11 870. ( 10.1073/pnas.1005766107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy M, Vásárhelyi G, Pettit B, Roberts-Mariani I, Vicsek T, Biro D. 2013. Context-dependent hierarchies in pigeons. Proc. Natl Acad. Sci. USA 110, 13 049–13 054. ( 10.1073/pnas.1305552110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brambilla M, et al. 2020. Potential distribution of a climate sensitive species, the white-winged snowfinch Montifringilla nivalis in Europe. Bird Conserv. Int. 30, 522–532. ( 10.1017/S0959270920000027) [DOI] [Google Scholar]
- 32.Keller V, et al. 2020. European breeding bird atlas 2 - distribution, abundance and change. Barcelona, Spain: Lynx Edicions, [Google Scholar]
- 33.Scridel D, Bogliani G, Pedrini P, Iemma A, von Hardenberg A, Brambilla M.. 2017. Thermal niche predicts recent changes in range size for bird species. Clim. Res. 73, 207–216. ( 10.3354/cr01477) [DOI] [Google Scholar]
- 34.Knaus P, Antoniazza S, Wechsler S, Guélat J, Kéry M, Strebel N, Sattler T. 2018. Schweizer brutvogelatlas 2013–2016. Verbreitung und bestandsentwicklung der vögel in der schweiz und im fürstentum lichtenstein. 1 band. Sempach, Switzerland: Schweizerische Vogelwarte. [Google Scholar]
- 35.Haeberli W, Hoelzle M, Paul F, Zemp M. 2007. Integrated monitoring of mountain glaciers as key indicators of global climate change: the European Alps. Ann. Glaciol. 46, 150–160. ( 10.3189/172756407782871512) [DOI] [Google Scholar]
- 36.Grunewald K, Scheithauer J. 2010. Europe's southernmost glaciers: response and adaptation to climate change. J. Glaciol. 56, 129–142. ( 10.3189/002214310791190947) [DOI] [Google Scholar]
- 37.López-Moreno JI. 2005. Recent variations of snowpack depth in the central Spanish Pyrenees. Arctic, Antarct. Alp. Res. 37, 253–260. ( 10.1657/1523-0430(2005)037[0253:RVOSDI]2.0.CO;2) [DOI] [Google Scholar]
- 38.Rixen C, Wipf S. 2017. Non-equilibrium in alpine plant assemblages: shifts in Europe's summit floras. In Advances in global change research (eds J Catalan, JM Ninot, M Mercè Aniz), pp. 285–304. Cham, Switzerland: Springer. ( 10.1007/978-3-319-55982-7_12) [DOI]
- 39.Bettega C, Fernández-González Á, Ramón Obeso J, Delgado MDM. 2020. Circannual variation in habitat use of the white-winged snowfinch Montifringilla nivalis nivalis. Ibis (Lond. 1859) 162, 1251–1261. ( 10.1111/ibi.12829) [DOI] [Google Scholar]
- 40.Brambilla M, et al. 2018. Past and future impact of climate change on foraging habitat suitability in a high-alpine bird species: management options to buffer against global warming effects. Biol. Conserv. 221, 209–218. ( 10.1016/j.biocon.2018.03.008) [DOI] [Google Scholar]
- 41.Resano-Mayor J, Korner-Nievergelt F, Vignali S, Horrenberger N, Barras AG, Braunisch V, Pernollet CA, Arlettaz R. 2019. Snow cover phenology is the main driver of foraging habitat selection for a high-alpine passerine during breeding: implications for species persistence in the face of climate change. Biodivers. Conserv. 28, 2669–2685. ( 10.1007/s10531-019-01786-9) [DOI] [Google Scholar]
- 42.Strinella E, Scridel D, Brambilla M, Schano C, Korner-Nievergelt F. 2020. Potential sex-dependent effects of weather on apparent survival of a high-elevation specialist. Sci. Rep. 10, 8386. ( 10.1038/s41598-020-65017-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood SN. 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 99, 673–686. ( 10.1198/016214504000000980) [DOI] [Google Scholar]
- 44.Wood SN, Pya N, Säfken B. 2016. Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 111, 1548–1563. ( 10.1080/01621459.2016.1180986) [DOI] [Google Scholar]
- 45.Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M. 2017. Climatologies at high resolution for the earth's land surface areas. Sci. Data 4, 170122. ( 10.1038/sdata.2017.122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuur AF, Saveliev AA, Ieno EN. 2014. A beginner's guide to generalised additive mixed models with R. Newburgh, UK: Highland Statistics Ltd. [Google Scholar]
- 47.Wood S, Scheipl F. 2014. gamm4: generalized additive mixed models using mgcv and lme4. R package version 0.2-3. See https://CRAN.R-project.org/package=gamm4.
- 48.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. A practical information-theoretic approach, 2nd edn. New York: NY: Springer. [Google Scholar]
- 49.R Core Team. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 50.Heiniger PH. 1991. Anpassungsstrategien des Schneefinken Montifringilla nivalis an die extremen Umweltbedingungen des Hochgebirges. Ornithol. Beobachter 88, 193–207. [Google Scholar]
- 51.del Hoyo J, Elliott A, Christie D. 2009. Handbook of the birds of the world. Bush-shrikes to Old World sparrows. Barcelona, Spain: Lynx Edicions. [Google Scholar]
- 52.Van Doren BM, Conway GJ, Phillips RJ, Evans GC, Roberts GCM, Liedvogel M, Sheldon BC. 2021. Human activity shapes the wintering ecology of a migratory bird. Glob. Chang. Biol. 27, 2715–2727. ( 10.1111/gcb.15597) [DOI] [PubMed] [Google Scholar]
- 53.Resano-Mayor J, et al. 2020. Partial migration of White-winged snowfinches is correlated with winter weather conditions. Glob. Ecol. Conserv. 24, e01346. ( 10.1016/j.gecco.2020.e01346) [DOI] [Google Scholar]
- 54.Maldonado-Chaparro AA, Alarcón-Nieto G, Klarevas-Irby JA, Farine DR. 2018. Experimental disturbances reveal group-level costs of social instability. Proc. R. Soc. B 285, 20181577. ( 10.1098/rspb.2018.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 56.Penteriani V, Delgado M del M, Lokki H. 2014. Global warming may depress avian population fecundity by selecting against early-breeding, high-quality individuals in nirthern populations of single-brooded, long-lived species. Ann. Zool. Fennici 51, 390–398. ( 10.5735/086.051.0404) [DOI] [Google Scholar]
- 57.IPCC. 2007. Climate change 2007: synthesis report. Contribution of working groups I, II and III to the fourth assessment. Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- 58.Both C, Visser ME. 2005. The effect of climate change on the correlation between avian life-history traits. Glob. Chang. Biol. 11, 1606–1613. ( 10.1111/j.1365-2486.2005.01038.x) [DOI] [Google Scholar]
- 59.Hille SM, Cooper CB. 2015. Elevational trends in life histories: revising the pace-of-life framework. Biol. Rev. Camb. Phil. Soc. 90, 204–213. ( 10.1111/brv.12106) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in ‘figshare’ at doi:10.6084/m9.figshare.13084595.