Abstract
The mechanisms that contribute to variation in lifetime reproductive success are not well understood. One possibility is that telomeres, conserved DNA sequences at chromosome ends that often shorten with age and stress exposures, may reflect differences in vital processes or influence fitness. Telomere length often predicts longevity, but longevity is only one component of fitness and little is known about how lifetime reproductive success is related to telomere dynamics in wild populations. We examined the relationships between telomere length beginning in early life, telomere loss into adulthood and lifetime reproductive success in free-living house sparrows (Passer domesticus). We found that females, but not males, with longer telomeres during early life had higher lifetime reproductive success, owing to associations with longevity and not reproduction per year or attempt. Telomeres decreased with age in both sexes, but telomere loss was not associated with lifetime reproductive success. In this species, telomeres may reflect differences in quality or condition rather than the pace of life, but only in females. Sexually discordant selection on telomeres is expected to influence the stability and maintenance of within population variation in telomere dynamics and suggests that any role telomeres play in mediating life-history trade-offs may be sex specific.
Keywords: longevity, lifetime reproductive success, pace-of-life hypothesis, quality hypothesis, stressors, house sparrow
1. Introduction
Darwinian fitness is influenced by variance in longevity and in reproductive output. Understanding the mechanisms that contribute to this variation is important to diverse biological disciplines. Accumulating evidence suggests that telomeres are part of a suite of mechanisms that impact vital processes or are reflective of organismal performance [1]. Telomeres are highly conserved, repetitive sequences of non-coding DNA that form protective caps at chromosome ends that enhance genome integrity, but limit cellular lifespan [2]. In the absence of restoration, telomeres shorten during cell division [2]. Once telomeres become critically short, cells stop dividing and can undergo apoptosis [2] and this process is expected to contribute to age-related declines in organismal function [3]. In support of these ideas, telomeres shorten with age in diverse tissues and their length often predicts subsequent longevity, although this pattern is not universal [4]. In some species, this relationship is already present during early life, with juveniles having longer telomeres living longer [5–8].
Importantly, although longevity is often positively related to telomere length, survival is only one component of fitness and little is known about the relationships between telomeres and lifetime reproductive success. However, this information will be essential for predicting how selection is likely to act on telomeres and the potential role that telomeres play in shaping the evolution of life-history strategies. Some of the variation in telomere length is owing to genetic factors [8–12], but environmental circumstances including exposure to stressors and metabolically demanding activities can also impact the rate of telomere loss [13]. During early life, both rapid growth [14] and exposure to stressors [15] including sibling competition [16], reduced access to food [17,18], and parental neglect [19,20] often predict greater telomere loss and shorter telomeres. In adulthood, reproduction and other stressors can increase telomere loss [21]. In humans [22] and in birds [5], females that produced offspring had shorter telomeres than nulliparous females, and the production and/or rearing of a greater number of young was associated with greater telomere loss and/or shorter telomeres [23–26]. Thus, telomeres may be reflective of past life experiences and could play an important role in mediating life-history trade-offs [27–29].
Several processes could lead to individuals with longer telomeres living longer and having more offspring. Individuals with longer telomeres could be of higher quality or have experienced less stressful circumstances during development that then could have positive effects on both longevity and reproductive performance, both of which would increase lifetime reproductive success [7,30]. Alternatively, individuals may exhibit a different pace of life, with telomeres being a correlated characteristic [7,29]. Individuals with longer telomeres may exhibit lower reproductive output but live longer if they allocate less to reproduction and thereby suffer lower reproductive costs [7,28,29]. Thus, how telomeres and lifetime reproductive success are related to one another will depend on the mechanisms linking telomeres to increased lifespan and reproduction. Importantly, many of these relationships could differ between the sexes as sensitivity to stress, reproductive rates, and longevity often differ between males and females [31]. Sex differences in telomere dynamics across taxa are highly variable [32], but few non-human studies have investigated the relationships between early-life telomeres and lifetime fitness components.
Research examining the relationship between telomeres and lifetime reproductive success in natural populations has been limited because it requires long-term studies in which individuals have been repeatedly sampled and tracked across the lifespan. In purple-crowned fairy wrens (Malurus coronatus), a cooperatively breeding bird, individuals of both sexes with longer telomeres early in life lived longer and had higher lifetime reproductive success, the latter occurring primarily through longer lifespan [7]. However, this study only included telomeres collected from early life, thus how variation in telomere loss relates to lifetime fitness remains unknown. Furthermore, given the diversity of associations between telomere dynamics and longevity in the two sexes across taxa [32], a general understanding of the potential links between telomeres and life histories requires study in diverse systems.
Here, we examined the relationship between telomeres and lifetime reproductive success in free-living house sparrows (Passer domesticus), a monogamous, biparental songbird distributed globally [33]. Both sexes exhibit similar natal philopatry before breeding, but males show higher breeding philopatry than females, probably driven by higher mortality in females [33] (D. F. Westneat 1993–2014, unpublished data). We used data from a long-term study [34,35] in which birds were sampled at 10 days of age and then followed through their reproductive lives to test between two major hypotheses about links between telomere dynamics and lifetime reproductive success. The ‘pace-of-life' hypothesis predicts that individuals with long telomeres early in life would reproduce at a slower rate, live longer, and have slower rates of telomere shortening than those with shorter telomeres early in life. The quality hypothesis, in which differences in telomeres reflect either intrinsic quality differences or differences in early-life conditions, predicts positive correlations between telomere length and either longevity or reproductive rate or both. We also tested for sex differences in all relationships but had no a priori expectation about differences.
2. Material and methods
(a) . Study system and data collection
The samples we used came from a long-term study of a free-living population of house sparrows (P. domesticus) breeding in artificial nestboxes located at the University of Kentucky's Agricultural Experiment Station North Farm complex in Lexington, Kentucky (see [35]). During 1992–2013, between the months of April–August, nearly all offspring were given a US Fish and Wildlife numbered aluminium band and had a blood sample collected at 10 days after hatching. Periodic trapping using seed-baited traps throughout the year or nest-traps during the breeding season resulted in recaptures of some banded birds that were then marked with colour bands and blood sampled again. In the focal study population, both sexes recruit to breed at low but similar rates, and most individuals do so in their first adult year. Nestboxes were censused at least once per week through the entire breeding season in all years, and adults identified from their colour-bands at least once per month, making it possible to establish which adults were associated with each breeding attempt and to monitor adult survival. Some breeding dispersal occurs; movement between locations is more common for females than males but is still rare (less than 10%). However, only two males and three females in our sample of 104 breeders had a gap year between bouts of breeding in our boxes (and so may have bred elsewhere). Our methods thus allowed us to estimate longevity (time from day 10 sampling until last sighting) and to have a good measure of lifetime reproductive success (total eggs produced or offspring reaching day 10, the last day nests were checked, for the focal bird), reproductive rate (eggs or offspring produced per year) and reproductive performance (date of first egg in the season, and clutch size and number of offspring reaching day 10 per reproductive attempt).
After collection, whole blood samples were suspended in 100–200 ul Tris–NaCl-EDTA buffer, stored on ice for less than or equal to 6 h in the field, and then transferred to −80°C freezers at the University of Kentucky. For the current study, we selected frozen whole blood samples of individuals that had a 10-day sample and at least one subsequent sample and transported them on dry ice to the Heidinger Laboratory at North Dakota State University where they were then stored in −80°C freezers until telomere analyses. Birds that met these criteria were a small fraction of all birds followed in the study (greater than 15 000).
(b) . DNA extraction and telomere measurement
To measure relative telomere length, DNA was extracted from whole blood samples using DNeasy tissue and blood kits and following the manufacturer's instructions (Qiagen). DNA quantity was measured using a Nanodrop 8000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) and DNA quality was verified by electrophoresis on a 2% agarose gel.
Relative telomere length was measured using quantitative polymerase chain reaction (qPCR) on an Mx3000P (Stratagene, San Diego, CA, USA), following the methods of [36] and adapted for house sparrows. The single copy control gene used for this study was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The suitability of GAPDH was verified using a melt curve analysis, which confirmed that the dissociation curve had a single peak at the expected melting temperature (Tm) of 82.0°C. In addition, PCR product was also run on a 2% agarose gel to verify that a single product was amplified at the expected 98 bp.
Reactions for GAPDH and telomere were run in triplicate on separate plates. The total volume for each reaction was 25 µl and contained 20 ng of DNA and either telomere or GAPDH primers at a 200 nM forward/200 nM reverse concentration mixed with 12.5 µl of perfeCTa SYBR green supermix Low ROX (Quantabio). The following primers were used to amplify the telomere and GAPDH reactions respectively: telomeres - forward tel1b (5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′) and reverse tel2b (5′- GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′) and GAPDH - forward (5′-AACCAGCCAAGTACGATGACAT-3′) and reverse GAPDH (5′-CCATCAGCAGCAGCCTTCA-3′). Thermal profiles for the qPCR reactions were as follows: telomeres—10 min at 95°C, followed by 27 cycles of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C, finishing with 1 min at 95°C, 30 s at 58°C, and 30 s at 95°C; and GAPDH—10 min at 95°C, followed by 40 cycles of 30 s at 95°C and 30 s at 60°C, finishing with 1 min at 95°C, 30 s at 55°C, and 30 s at 95°C.
The number of PCR cycles necessary to accumulate sufficient fluorescent signal to cross a threshold (Ct) was measured and average Ct values were used to calculate the relative telomere length (T/S ratio) according to the following formula: 2ΔΔCt, where ΔΔCt = (Ct telomere − Ct GAPDH) reference sample – (Ct telomere − Ct GAPDH) focal sample (Stratagene 2007).
Each plate also included a house sparrow reference sample that was serially diluted to create a 5-point standard curve (40, 20, 10, 5, 2.5 ng), which allowed us to measure reaction efficiencies and ensure that all samples fell within the bounds of the standard curve. The average reaction efficiencies for GAPDH 94.4 ± 0.80 (mean ± s.e.m) and telomere 96.0 ± 0.81 (mean ± s.e.m) plates were similar and in all cases were between the recommended 85–115%. At the time of assay optimization, the repeatability of the T/S ratio was calculated by running samples collected from 28 individuals in this long-term study in random well locations across two plates. The intraclass correlation coefficient (two-way, single measurement, absolute agreement, random effects model) was 0.86, p < 0.001, 95% confidence interval lower bound 0.723 and upper bound 0.932 [37].
(c) . Statistical analyses
We tested predictions from the two main hypotheses and also the potential for these to differ between the sexes using four related datasets. The initial dataset (telomere) consisted of 544 telomere measures for 250 individuals; for three of these individuals their day 10 sample did not provide DNA suitable for telomere measurement, and for two we only had their day 10 measures. This dataset was used to assess technical influences on telomere measures, and to evaluate if there was sufficient individual variation in telomere length or change in telomere length to warrant further analysis. We employed linear mixed models (in SAS 9.4) to explore factors affecting relative telomere length measures. In all models, subject identity was included as a random effect. We ran initial models to assess the impact of plate identity and the number of years the samples sat in the freezer (fixed effects) on relative telomere length. Plate identity was significant (F26,231 = 4.5, p = 0.0001) and so was included in all subsequent models of telomere length. Years stored had no effect (estimate = −0.007 ± 0.006, F1,283 = 1.3, p = 0.26) and so was ignored in all subsequent analyses.
We estimated repeatability of telomere length using a mixed model with bird identity as a random effect and plate identity as a fixed effect; repeatability and bootstrapped confidence limits were estimated with the rptR progam in R [38] as the variance among individuals divided by the sum of residual plus variance among individual. To provide an initial view of the biological variation in telomere length, we then modelled it with plate, sex, and age in years at the time of sampling as fixed effects, and subject identity and the by-subject slope with age as random effects. We also asked if the pattern of variation in telomere length within and among individuals differed by sex. We present results using a frequentist approach in which fixed effects were tested with F-tests using degrees of freedom estimated according to the Kenward–Rogers method, and random effects were tested using the likelihood ratio test (LRT). A Bayesian version of the model was also run and is presented in the electronic supplementary material, table S3.
Because most juvenile house sparrows disperse (approx. 3% of banded nestlings were seen breeding over the course of the study, D. F. Westneat 1993–2014, unpublished data), many of the 250 individuals were not seen to breed on the study site. To analyse relationships between telomere length and longevity or reproductive success metrics, we restricted the data to 104 local recruits (56 males and 48 females) who had at least two measures, one of which was at day 10 (n = 75 sampled twice, n = 23 sampled three times and n = 6 sampled four times). Of these 104 individuals, 77 were sampled at least once in adulthood (greater than or equal to 10 months after the first sample was collected).
To examine the relationships between telomere length and longevity, we ran a Cox regression survival analysis including the telomere length at 10 days post-hatching as a fixed effect. In these models, we also included sex and the interaction between sex and 10-day telomere length. Neither variable violated the proportional hazards assumption (electronic supplementary material). We analysed two metrics of lifetime reproductive success, the total eggs laid (for males, these are eggs laid by their mates) and total offspring produced, both calculated from all attempts associated with the focal bird, for both sexes. We assumed 100% paternity by males in this analysis, and so eggs and offspring are those for social fathers. Extra-pair paternity (EPP) occurs in house sparrows [39–42], but at a relatively low frequency (10–15%) in all examined populations including this one (11%) [43]. Given this low rate, we assume for now that any possible association between EPP and telomere length would not influence relationships between telomere length and apparent reproductive success in males.
Both lifetime number of eggs and offspring are count variables, but neither fits a Poisson distribution. Both had some level of overdispersion, so we used a generalized linear model (GLM) with a negative binomial and a logit link, run in Proc Genmod in SAS which includes a scale parameter to control for over-dispersion.
The quality and pace-of-life hypotheses make contrasting predictions about the relationship between telomeres and reproductive performance, including the date in the season when birds started breeding, clutch size, and the number of offspring reared to day 10. Because we had repeated measures data on all of these metrics, we analysed each (using dataset ‘Breeding’) using generalized linear mixed models with subject identity as a random effect. Covariates included telomere length at 10 days, sex, and their interaction in all models. The year was included as a factor in all models to control for yearly differences in reproductive performance. For the date a subject had their first egg of the season, we included bird age as a covariate. For clutch size and day 10 brood size, we included lay date for each attempt as a covariate, as it is previously known to be important [35]. Offspring produced from each nest was modelled with clutch size as an offset fixed effect. Date of seasonal first egg and clutch size were analysed with a Gaussian distribution, and for brood size we used a negative binomial. All analyses were done in Proc Glimmix in SAS.
We also explored links between measures of telomere change and lifetime reproductive success. The ideal method for analysing emergent variables such as the rate of telomere length change with age is a bivariate mixed model [44], as this incorporates the observed distributions of individual measures and avoids problems with doing statistics on statistics [45]. We performed separate bivariate mixed models with both telomere length and each of the three life-history metrics as dependent variables. In this way, we could potentially estimate the correlation between individual intercept (estimated mean telomere length at 10 days of age) and individual slope with respect to age at sampling to the life-history variables. The model fitted for longevity and telomere length contained common elements to both as follows:
where βl0 is the mean, Il is individual j's deviation in longevity from the population mean and eij is the residual for a given observation. The telomere length equation additionally included the terms: (β1 + St1j) ageij, in which ageij is bird j's age at sample i, β1 is the population slope of telomere length with age at sampling, and St1 is individual j's deviation from the population slope.
Our primary interest was the among-subject variance-covariance matrix, as follows:
The diagonals are the variance in longevity, variance in telomere length intercepts, and variance in slopes of telomere length in response to age, respectively. We were specifically interested in two covariances, that between individual longevity and individual telomere length intercept (), and between individual longevity and individual slope (). We fit similar models with lifetime number of eggs and offspring in place of longevity, both also ln transformed (note: we attempted models with the negative binomial, but these models failed to converge). The analysis was coded into SAS 9.4 as in the electronic supplementary material, text S17.C in [44] and is provided in this case in the electronic supplementary material Part VI using the dataset called ‘Telostack’.
Bivariate models are data hungry, and they may be sensitive to limiting information. As outlined above, the majority of the cases in our study had only two measures of telomere length. This may affect resolution of some covariance parameters (see Results and the electronic supplementary material) limiting use of these methods, so we also analysed telomere change using two commonly used methods (in the dataset ‘TelomereChange'). For the first, we took the difference in telomere length measures between the first and last measure we had for each individual and divided by the subject's age (years) at the last sampling. Second, because measurement error could be an issue in measures of telomere change with age, we calculated a modified measure of telomere change adjusted for potential measurement error [46]. The equation published in [46] centres individual values to the mean values at two time points. Because our second measures varied considerably in the ages they were taken, we instead calculated the centred values relative to the predicted value using the regression of telomere length on age in years for the full dataset. Hence, our modified equation was
where TL1 is the relative telomere measure at time 1 (day 10 measure), is the mean telomere measure at day 10 (defined as the intercept of the regression on age), TLlast is the last observed TL, slope is the population mean change in TL with age in years, agelast is the age in years at last sampling, and ρ is
where S1 is the observed standard deviation in TL at the first observation, S2 is the s.d. of the regression-centred last observation, and r is the correlation between TL1 and TL2.
3. Results
(a) . Age and individual variation in telomere length
House sparrows exhibited significant repeatable individual variation in telomere length over time (r = 0.28 ± 0.08 (range: 0.18, 0.50), LRT = 5.9, df = 1, p = 0.008). We also obtained evidence that on average telomere length declined with age after the first sampling at 10 days of age, and that sparrows exhibit individual variation in telomere loss (table 1 and figure 1). We found no difference between the sexes in either mean telomere length (sex, F-M: −0.03 ± 0.04, F1,225 = 0.6, p = 0.46) or the mean rate of decline with age (sex by age, F-M: 0.03 ± 0.04 units per year, F1,36.8 = 1.5, p = 0.23). We also found no difference in the variance matrix between the two sexes even though variance in telomere change with age was an order of magnitude higher in females (electronic supplementary material, table S2). Finally, estimates of individual slopes were negatively collinear with intercepts in both sexes (F: −0.78, M: −1.0). A Bayesian version of this model produced similar results (electronic supplementary material, table S3).
Table 1.
Variance components and fixed effects from a linear mixed model of relative telomere length obtained from 250 individual house sparrows as nestlings and again at least once post-independence.
| component | estimate ± s.e. | statistic (d.f.)a | p-value |
|---|---|---|---|
| random effects | |||
| individual intercept | 0.046 ± 0.011 | 15.8 (1) | 0.0005 |
| individual slope: age | 0.003 ± 0.002 | 9.0 (2) | 0.03 |
| cov (intercept, slope) | −0.012 ± 0.005 | ||
| residual | 0.094 ± 0.008 | ||
| fixed effectsb | |||
| age (years) | −0.029 ± 0.014 | 4.0 (1,43.5) | 0.053 |
aStatistic is χ2 (likelihood ratio test) for random effects and F for fixed effects.
bThe model also included plate identity as a fixed effect factor.
Figure 1.
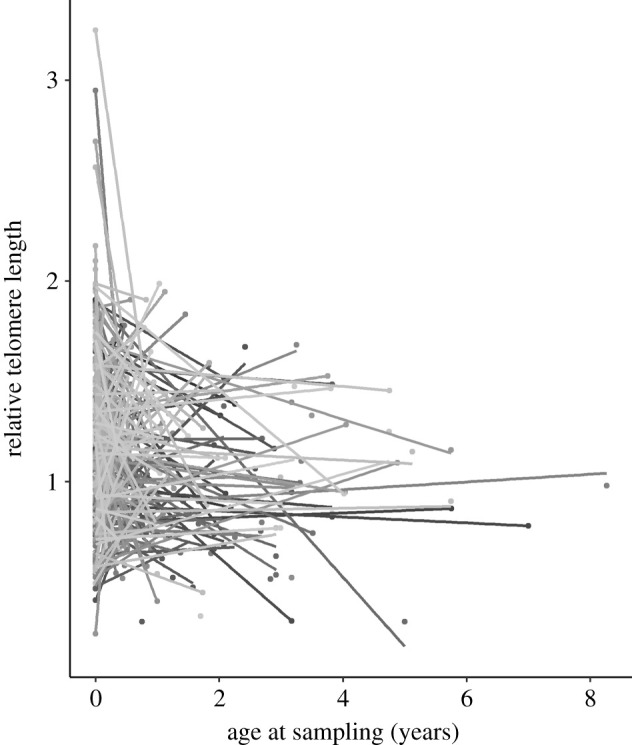
Plot of 250 individual linear trajectories (shaded differently) of relative telomere length sampled from nestling house sparrows at 10 days post-hatch (age = 0 years) and again one to three times later in life, with age at sampling indicated in years. Mixed model analysis (see text) revealed significant intercept and slope variation.
We compared early-life telomere measures between individuals who later recruited into the breeding population and those who did not. We found no significant differences in either the mean telomere length (bred: 1.16 ± 0.03, did not breed: 1.21 ± 0.03; F1,230 = 1.33, p = 0.25) or the change in telomere length with age (bred: −0.02 ± 0.02; did not breed: −0.06 ± 0.04, F1,56.5 = 1.1, p = 0.29), suggesting that the subset which stayed on the study site and bred was no different than those that did not recruit to the local population.
(b) . Telomere length, longevity and measures of lifetime reproductive success
We examined the relationship between telomeres and life-history characteristics in the subset of birds sampled at 10 days of age that recruited into the breeding population (n = 104), controlling for cohort effects. Once recruited, males (n = 56) had a lower probability of disappearing per unit time than females (n = 48) difference in hazard ratio = −3.1 ± 0.9; χ2 = 14.8, p < 0.0001; electronic supplementary material, figure S1). Importantly, telomere length at 10 days predicted survival differently between the sexes (visualized as longevity in figure 2a; difference in hazard ratio = 2.4 ± 0.7, χ2 = 12.6, p = 0.0004), with longer early-life telomere length in females predicting a significantly higher chance of surviving (effect = −1.6 ± 0.5, χ2 = 11.6, p = 0.0006), whereas in males there was a non-significant pattern for survival to decline with longer early-life telomere length.
Figure 2.
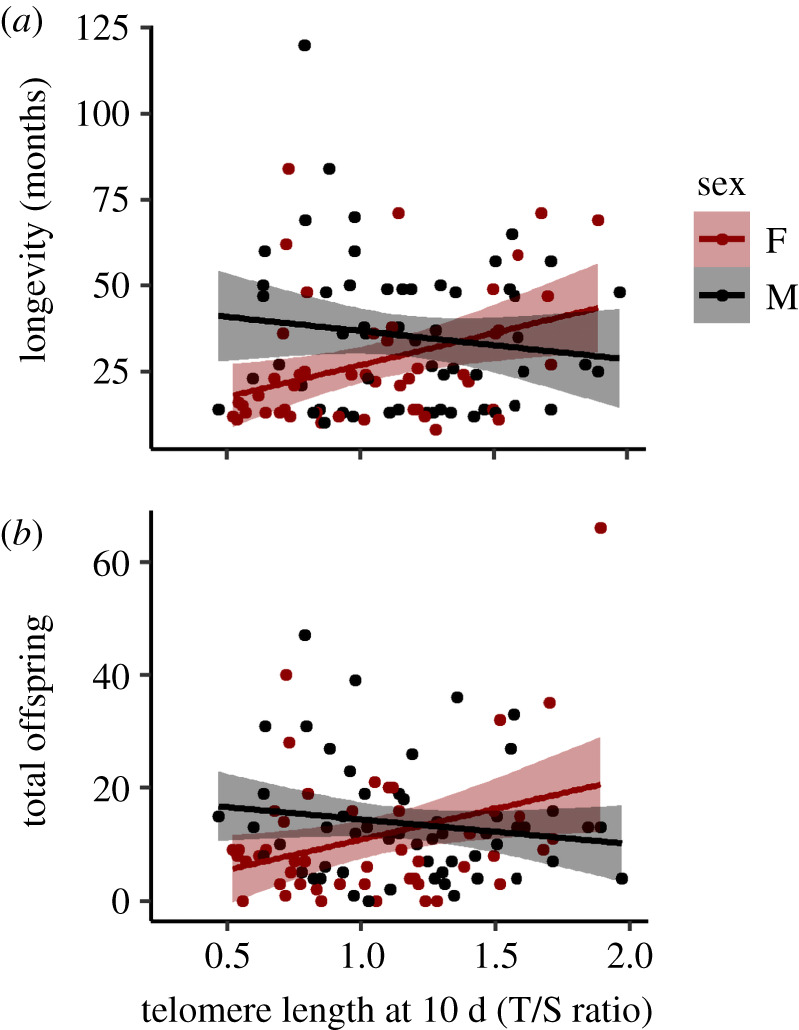
The relationship between early-life telomere length (at 10 days post-hatching) and (a) longevity (months seen alive), and (b) lifetime reproductive success (total offspring produced across the lifetimes) of 104 house sparrows (Passer domesticus) who bred at least once. The sexes are separated by colour (48 females are red and 56 males are black) and their separate linear regressions indicated with solid lines and 95% confidence limits by the shaded areas. Significance (see text) in (a) was assessed using a Cox regression and in (b) using a GLM with a negative binomial distribution. (Online version in colour.)
The sexes also differed in how early-life telomere length predicted metrics of lifetime reproductive success. In females, longer telomere length at 10 days positively predicted lifetime production of eggs (effect on natural scale: 28.4 ± 10.0 eggs per unit of telomere length, Wald χ2 = 13.7, p = 0.002) and offspring that survived to leave the nest (figure 2b; effect on natural scale: 13.7 ± 4.7 offspring per unit of telomere length, Wald χ2, using negative binomial = 9.8, p = 0.002). In our sample, males contributed to more total eggs (sex, M-F, natural scale: 40.6 ± 16.0 eggs, GLM with negative binomial, Wald χ2 = 10.4, p = 0.001) and offspring that survived to leave the nest (sex, M-F on natural scale: 20.2 ± 6.6 offspring per unit, Wald χ2 = 9.6, p = 0.002). However, the association between telomere length at 10 days and total eggs assigned to males was negative and significantly different from females (sex by telomere length, M-F on natural scale: −25.5 ± 14.9 eggs per unit, Wald χ2 = 10.1, p = 0.002). In males, the relationship between telomere length at 10 days and lifetime production of offspring was significantly less than in females (sex by telomere length, M-F on natural scale: −17.9 ± 6.0 offspring per unit telomere length, Wald χ2 = 9.8, p = 0.002) and trended negatively (−6.1 ± 3.9 offspring per unit telomere length, Wald χ2 = 1.8, p = 0.18).
Both the quality and pace-of-life hypothesis predict a possible link between telomere length and the rate of reproduction, albeit in opposite directions. To assess this, we added longevity as a covariate to the above models, thereby assessing if telomere lengths predicted residual reproduction controlling for longevity. We found that this removed any relationships between early-life telomere length and the difference between the sexes for both total eggs and total offspring that survived to leave the nest (electronic supplementary material, tables S7 and S8), indicating no evidence of reproductive rate differing with telomere length.
(c) . Early-life telomere and reproductive performance
To further test the possibility that telomere length may reflect differences in reproductive rate, we assess correlations between day 10 telomere length and the timing of first breeding in the season or reproduction per breeding attempt. We found telomere length at 10 days did not predict any individual reproductive performance measure in either sex (table 2).
Table 2.
Analysis of relationships between early-life telomere length and three metrics of reproductive performance in 104 male and female house sparrows. (All models included individual as a random effect and year as a fixed effect factor.)
| metrics of reproductive performance | effect ± s.e. | statistic (d.f.) | p-value |
|---|---|---|---|
| date of first egg in season | |||
| sex | 0.03 (1,81.1) | 0.87 | |
| female (n = 48) | 155.2 ± 26.8 | ||
| male (n = 56) | 155.9 ± 27.3 | ||
| sex by telomere length at day 10 | 1.4 (1,67.2) | 0.23 | |
| female | −4.9 ± 8.0 | ||
| male | 8.2 ± 7.5 | ||
| subject age | −3.5 ± 1.3 | 7.3 (1,181) | 0.008 |
| clutch size per attempt | |||
| sex | 1.5 (1,62.8) | 0.22 | |
| female | 4.5 ± 0.6 | ||
| male | 4.4 ± 0.7 | ||
| sex by telomere length at day 1 | 0.03 (1,47.9) | 0.87 | |
| female | 0.17 ± 0.18 | ||
| male | −0.14 ± 0.17 | ||
| first egg date of each breeding attempt | −0.01 ± 0.0009 | 111.0 (1,583) | <0.0001 |
| offspring per attempta | |||
| sex | 0.7 (1,515) | 0.41 | |
| female | −0.3 ± 1.4 | ||
| male | −0.4 ± 1.4 | ||
| clutch size (offset) | 0.4 ± 0.08 | 19.2 (1,515) | <0.0001 |
| date of first egg for attempt | −0.004 ± 0.002 | 2.5 (1,515) | 0.12 |
| sex by telomere length at 10 days | 0.7 (1,515) | 0.41 | |
| female | 0.18 ± 0.33 | ||
| male | −0.12 ± 0.31 | ||
aEffect sizes are in natural scale but statistical tests used logits. Individual explained no variance, so d.f. assume observations are independent.
(d) . Telomere change and measures of lifetime reproductive success
Bivariate analysis using all measures of telomere length would in theory provide the most effective test [44] of the independent influence of early-life telomere (individual intercept) or telomere loss (individual slope with respect to age) on life-history traits. Our attempt with this approach yielded the same patterns as described above for intercept values (electronic supplementary material, table S21). However, we could not separate the influence of slope and intercept owing to their collinearity (strongly negative, see the electronic supplementary material, VI).
We thus analysed longevity and lifetime reproductive success as a function of two other metrics of telomere change, raw change per year and change per year adjusted for the regression to the mean (D) [46]. None of the six tests revealed any indication that the three metrics of lifetime reproductive success were correlated with measures of telomere change (table 3). We also found no difference between the sexes in these relationships (table 3).
Table 3.
Test of the relationship between two measures of telomere change and each of three measured components of lifetime reproductive success in both sexes using 104 breeding house sparrows with multiple telomere measurements.
| models | metric of lifetime successa |
||
|---|---|---|---|
| longevity (months)b | lifetime eggsc | lifetime offfspringc | |
| raw change per year | |||
| female (n = 48) | 22.7 ± 38.8 | 32.1 ± 51.3 | 28.9 ± 20.8 |
| male (n = 56) | −12.3 ± 21.4 | −25.6 ± 28.3 | −6.9 ± 11.5 |
| test of interaction | χ2 = 0.9, p = 0.35 | χ2 = 1.4, p = 0.24 | χ2 = 2.2, p = 0.14 |
| adjusted D per year | |||
| female | 3.7 ± 10.0 | −12.0 ± 13.2 | −6.2 ± 5.4 |
| male | 3.6 ± 8.0 | 2.4 ± 10.5 | 4.5 ± 4.3 |
| test of interaction | χ2 = 0.001, p = 0.97 | χ2 = 0.6, p = 0.46 | χ2 = 2.1, p = 0.15 |
aAll effects sizes are in the natural scale.
bSignificance from Cox regression (χ2).
cSignificance from a negative binomial with a log link (χ2).
4. Discussion
We tested predictions of two hypotheses about the potential links between telomere dynamics and lifetime reproductive success. In house sparrows, the relationship between telomeres during early life and lifetime reproductive success is sex specific. Females with longer telomeres as nestlings had greater longevity and higher lifetime reproductive success than females with shorter telomeres. In males the opposite was true; birds with longer telomeres during early life tended to have shorter lifespans and lower reproductive output. However, early-life telomere length did not significantly predict annual or per episode measures of reproductive performance including the timing of first breeding, clutch size, or the number of young that survived to leave the nest in either sex, providing no support for the ‘pace-of-life' hypothesis. Thus, the positive relationship between early-life telomere length and lifetime reproductive success in females appears to occur primarily through the relationship between early-life telomere length and longevity, suggesting that early-life quality or condition differences linked to telomeres only impact survival. Telomere length declined with age in both sexes but was not associated with longevity or lifetime reproductive success in either males or females. Taken together, these results are more consistent with the idea that telomeres are reflective of variation in individual quality than differences in the pace of life and that those quality differences apply to females but not males. Furthermore, these results provide novel evidence that selection on telomeres is likely to be sexually discordant, which may help to explain the stability of both mean and within-population variation in telomere dynamics over time, and suggests that any role which telomeres play in mediating or reflecting life-history trade-offs may be complex and sex-specific.
Intriguingly, although longevity often differs between the sexes, this variation is not consistently associated with sex differences in telomere length and loss [32,47]. In house sparrows, although females had shorter lifespans than males, a pattern commonly reported in birds [48–50], they did not have shorter telomeres or experience more telomere shortening than males. Several factors could have contributed to these sexually divergent relationships between telomere dynamics and lifetime reproductive success. In birds, females are the heterogametic sex and consequently are expected to be more vulnerable to the unguarded expression of deleterious alleles on the sex chromosome than males, which could influence the relationship between telomeres and longevity [32]. There may also be important sex differences in growth profiles, stress exposure, or sensitivity to stress during development that could influence both telomeres and future fitness measures [31]. For example, if telomeres in females are more sensitive to the effects of developmental stress this may result in negative effects on longevity and lifetime reproductive success in females, but not in males [31]. Few studies have examined whether the relationships between telomeres and reproductive success differ between the sexes. Consistent with our results, a recent study in Cory's shearwaters (Calonectris borealis), reported that females with longer telomeres in adulthood produced more offspring, while the opposite was true in males [51], but it is unclear how telomeres and longevity or lifetime fitness are related in this species or whether this results from early-life events as suggested here, or from processes that act only in adulthood.
Although telomere loss is also often correlated with longevity and reproductive strategies [25,52], we found no association between the change in telomere length and either longevity or lifetime reproductive success in either sex. Thus, in female house sparrows, telomere length during early life seems to be a better predictor of lifetime fitness than the loss that occurs after post-natal development. Generally, early-life telomere length will be influenced by variation in starting length and any loss that occurs during pre- and post-natal growth [1]. Longitudinal studies [5,53,54] suggest that the pace of telomere loss tends to be greater during early life than it is at later life stages. Thus, although loss after post-natal development is not predictive of lifetime reproductive success in female house sparrows, loss that occurs prior to this time, during pre- or post-natal development, may contribute to variation in early-life telomere length and impact lifetime reproductive success. It is also possible that reproductive investment increases the rate of telomere loss, but this would be best evaluated using experimental studies as individuals often plastically modify reproductive effort to match their own state [21].
Importantly, our results do not provide support for the idea that early-life telomere length is reflective of intrinsic pace-of-life differences whereby individuals with longer telomeres have greater longevity but lower levels of reproductive output [29]. Instead, our findings are more consistent with the idea that the positive relationship between early-life telomere length and lifetime fitness in females is driven by differences in individual quality. Telomeres may directly affect longevity, which could occur if telomeres decline with age and once they reach a critically short length contribute to declines in organismal function [1]. In this case, early-life telomere length may function as a countdown timer where individuals with longer telomeres live longer and have higher lifetime reproductive success simply because they have more time on their biological clocks than individuals with shorter telomeres. Alternatively, variation in the quality of the developmental environment could affect both early-life telomeres and other aspects of condition that impact longevity. This could occur if individuals that experience better developmental circumstances have longer telomeres and are in better condition and able to allocate more to mechanisms that extend longevity. Yet why this would impact longevity but not reproductive output during each breeding event, and why these effects differed between the sexes is unclear.
In conclusion, our results demonstrate that in females, telomeres are reflective of differences in quality or condition rather than the pace of life. Further, they suggest that selection on telomeres will be sexually discordant, with no apparent effect on sex differences in telomere dynamics. Our results raise challenging questions about the evolutionary stability of mean telomere length in the two sexes and the maintenance of within population variation in telomere dynamics. They also suggest that how telomeres influence or reflect life-history trade-offs may be sex specific. Investigating what factors contribute to this sex-specific relationship between early-life telomeres and lifetime reproductive success and whether this pattern is common across organisms with disparate life histories are important areas of future study.
Supplementary Material
Acknowledgements
We thank the many students who helped with the field study of sparrows, especially M. I. Hatch, T. Kinnard, J. P. Poston, S. Sloane, I. R. K. Stewart, and D. P. Wetzel. We also thank the Westneat group, D. R. Rubenstein, T. E. Martin, and two anonymous reviewers for helpful comments on the manuscript.
Ethics
Fieldwork on house sparrows was approved through a succession of protocols overseen by the University of Kentucky's Institutional Animal Care and Use Committee and adhered to all laws and regulations regarding the handling of wild birds.
Data accessibility
All of the data included in these analyses are available on Dryad Digital Repository: https://doi.org/10.5061/dryad.fbg79cntn [55].
Authors' contributions
B.J.H.: conceptualization, formal analysis, funding acquisition, investigation, project administration, resources, writing—original draft, writing—review and editing; A.C.K.: formal analysis, methodology, writing—review and editing; J.D.K.: methodology; D.F.W.: conceptualization, formal analysis, funding acquisition, investigation, project administration, resources, writing—original draft, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
The long-term study of the house sparrows and the telomere analysis were supported by National Science Foundation awards to D.F.W. (IBN-9816989, IBN-0542097 and IOS1257718) and to B.J.H. and D.F.W. (IOS1656194). Indirect support was also provided by the University of Kentucky and North Dakota State University.
References
- 1.Monaghan P. 2010. Telomeres and life histories. the long and the short of it. In Year in evolutionary biology (eds Schlichting CD, Mousseau TA), pp. 130-142. Malden, MA: Wiley-Blackwell. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn EH. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579, 859-862. ( 10.1016/j.febslet.2004.11.036) [DOI] [PubMed] [Google Scholar]
- 3.Hornsby PJ. 2002. Cellular senescence and tissue aging in vivo. J. Gerontol. Ser A-Biol. Sci. Med. Sci. 57, B251-B256. ( 10.1093/gerona/57.7.B251) [DOI] [PubMed] [Google Scholar]
- 4.Wilbourn RV, Moatt JP, Froy H, Walling CA, Nussey DH, Boonekamp JJ. 2018. The relationship between telomere length and mortality risk in non-model vertebrate systems: a meta-analysis. Phil. Trans. R Soc. B 373, 9. ( 10.1098/rstb.2016.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743-1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436-438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 7.Eastwood JR, Hall ML, Teunissen N, Kingma SA, Aranzamendi NH, Fan M, Roast M, Verhulst S, Peters A. 2019. Early-life telomere length predicts lifespan and lifetime reproductive success in a wild bird. Mol. Ecol. 28, 1127-1137. ( 10.1111/mec.15002) [DOI] [PubMed] [Google Scholar]
- 8.Ilska-Warner JJ, et al. 2019. The genetic architecture of bovine telomere length in early life and association with animal fitness. Front. Genet. 10, 13. ( 10.3389/fgene.2019.01048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Njajou OT, et al. 2007. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl Acad. Sci. USA 104, 12 135-12 139. ( 10.1073/pnas.0702703104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atzmon G, et al. 2010. Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl Acad. Sci. USA 107, 1710-1717. ( 10.1073/pnas.0906191106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hjelmborg JB, Dalgard C, Moller S, Steenstrup T, Kimura M, Christensen K, Kyvik KO, Aviv A. 2015. The heritability of leucocyte telomere length dynamics. J. Med. Genet. 52, 297-302. ( 10.1136/jmedgenet-2014-102736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D. 2015. Maternal and genetic factors determine early life telomere length. Proc. R. Soc. B 282, 9. ( 10.1098/rspb.2014.2263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatelain M, Drobniak SM, Szulkin M. 2020. The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecol. Lett. 23, 381-398. ( 10.1111/ele.13426) [DOI] [PubMed] [Google Scholar]
- 14.Geiger S, Le Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500-1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 15.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 7. ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nettle D, Monaghan P, Boner W, Gillespie R, Bateson M. 2013. Bottom of the heap: having heavier competitors accelerates early-life telomere loss in the European starling, Sturnus vulgaris. PLoS ONE 8, 8. ( 10.1371/journal.pone.0083617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 7. ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichert S, Stier A, Zahn S, Arrive M, Bize P, Massemin S, Criscuolo F. 2014. Increased brood size leads to persistent eroded telomeres. Front. Ecol. Evol. 2, 11. ( 10.3389/fevo.2014.00009) [DOI] [Google Scholar]
- 19.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. 2013. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatr. 18, 576-581. ( 10.1038/mp.2012.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalev I. 2012. Early life stress and telomere length: investigating the connection and possible mechanisms. Bioessays 34, 943-952. ( 10.1002/bies.201200084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudyka J. 2019. Does reproduction shorten telomeres? Towards integrating individual quality with life-history strategies in telomere biology. Bioessays 41, 12. ( 10.1002/bies.201900095) [DOI] [PubMed] [Google Scholar]
- 22.Pollack AZ, Rivers K, Ahrens KA. 2018. Parity associated with telomere length among US reproductive age women. Hum. Reprod. 33, 736-744. ( 10.1093/humrep/dey024) [DOI] [PubMed] [Google Scholar]
- 23.Ryan CP, Hayes MG, Lee NR, McDade TW, Jones MJ, Kobor MS, Kuzawa CW, Eisenberg DTA. 2018. Reproduction predicts shorter telomeres and epigenetic age acceleration among young adult women. Sci. Rep. 8, 9. ( 10.1038/s41598-018-29486-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudyka J, Arct A, Drobniak S, Dubiec A, Gustafsson L, Cichon M. 2014. Experimentally increased reproductive effort alters telomere length in the blue tit (Cyanistes caeruleus). J. Evol. Biol. 27, 2258-2264. ( 10.1111/jeb.12479) [DOI] [PubMed] [Google Scholar]
- 25.Sudyka J, Arct A, Drobniak SM, Gustafsson L, Cichon M. 2019. Birds with high lifetime reproductive success experience increased telomere loss. Biol. Lett. 15, 4. ( 10.1098/rsbl.2018.0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauch C, Becker PH, Verhulst S. 2013. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B 280, 8. ( 10.1098/rspb.2012.2540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47-53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 28.Young AJ. 2018. The role of telomeres in the mechanisms and evolution of life-history trade-offs and ageing. Phil. Trans. R. Soc. B 373, 12. ( 10.1098/rstb.2016.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giraudeau M, Angelier F, Sepp T. 2019. Do telomeres influence pace-of-life-strategies in response to environmental conditions over a lifetime and between generations? Bioessays 41, 6. ( 10.1002/bies.201800162) [DOI] [PubMed] [Google Scholar]
- 30.Angelier F, Weimerskirch H, Barbraud C, Chastel O. 2019. Is telomere length a molecular marker of individual quality? Insights from a long-lived bird. Funct. Ecol. 33, 1076-1087. ( 10.1111/1365-2435.13307) [DOI] [Google Scholar]
- 31.Geffroy B, Douhard M. 2019. The adaptive sex in stressful environments. Trends Ecol. Evol. 34, 628-640. ( 10.1016/j.tree.2019.02.012) [DOI] [PubMed] [Google Scholar]
- 32.Barrett ELB, Richardson DS. 2011. Sex differences in telomeres and lifespan. Aging Cell 10, 913-921. ( 10.1111/j.1474-9726.2011.00741.x) [DOI] [PubMed] [Google Scholar]
- 33.Anderson TR. 2006. Biology of the ubiquitous house sparrow: from genes to populations, 6th edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 34.Westneat DF, Stewart IRK, Woeste EH, Gipson J, Abdulkadir L, Poston JP. 2002. Patterns of sex ratio variation in house sparrows. Condor 104, 598-609. ( 10.1650/0010-5422(2002)104[0598:Posrvi]2.0.Co;2) [DOI] [Google Scholar]
- 35.Westneat DF, Stewart IRK, Hatch MI. 2009. Complex interactions among temporal variables affect the plasticity of clutch size in a multi-brooded bird. Ecology 90, 1162-1174. ( 10.1890/08-0698.1) [DOI] [PubMed] [Google Scholar]
- 36.Cawthon RM. 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, 6. ( 10.1093/nar/30.10.e47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo TK, Li MY. 2016. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155-163. ( 10.1016/j.jcm.2016.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoffel MA, Nakagawa S, Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639-1644. ( 10.1111/2041-210x.12797) [DOI] [Google Scholar]
- 39.Cordero PJ, Wetton JH, Parkin DT. 1999. Extra-pair paternity and male badge size in the house sparrow. J. Avian Biol. 30, 97-102. ( 10.2307/3677248) [DOI] [Google Scholar]
- 40.Griffith SC, Stewart IRK, Dawson DA, Owens IPF, Burke T. 1999. Contrasting levels of extra-pair paternity in mainland and island populations of the house sparrow (Passer domesticus): is there an 'island effect'? Biol. J. Linnean Soc. 68, 303-316. ( 10.1111/j.1095-8312.1999.tb01171.x) [DOI] [Google Scholar]
- 41.Whitekiller RR, Westneat DF, Schwagmeyer PL, Mock DW. 2000. Badge size and extra-pair fertilizations in the house sparrow. Condor 102, 342-348. ( 10.1650/0010-5422(2000)102[0342:bsaepf]2.0.co;2) [DOI] [Google Scholar]
- 42.Vaclav R, Hoi H, Blomqvist D. 2002. Badge size, paternity assurance behaviours and paternity losses in male house sparrows. J. Avian Biol. 33, 315-318. ( 10.1034/j.1600-048X.2002.330315.x) [DOI] [Google Scholar]
- 43.Stewart IRK, Hanschu RD, Burke T, Westneat DF. 2006. Tests of ecological, phenotypic, and genetic correlates of extra-pair paternity in the house sparrow. Condor 108, 399-413. ( 10.1650/0010-5422(2006)108[399:toepag]2.0.co;2) [DOI] [Google Scholar]
- 44.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39-54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 45.Hadfield JD, Wilson AJ, Garant D, Sheldon BC, Kruuk LE. 2010. The misuse of BLUP in ecology and evolution. Am. Nat. 175, 116-125. ( 10.1086/648604) [DOI] [PubMed] [Google Scholar]
- 46.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for 'regression to the mean'. Eur. J. Epidemiol. 28, 859-866. ( 10.1007/s10654-013-9845-4) [DOI] [PubMed] [Google Scholar]
- 47.Remot F, Ronget V, Froy H, Rey B, Gaillard J-M, Nussey DH, Lamitre J-F. 2020. No sex differences in adult telomere length across vertebrates: a meta-analysis. R. Soc. Open Sci. 7, 200548. ( 10.1098/rsos.200548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sillett TS, Holmes RT. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71, 296-308. ( 10.1046/j.1365-2656.2002.00599.x) [DOI] [Google Scholar]
- 49.Donald PF. 2007. Adult sex ratios in wild bird populations. Ibis 149, 671-692. ( 10.1111/j.1474-919X.2007.00724.x) [DOI] [Google Scholar]
- 50.Promislow DEL, Montgomerie R, Martin TE. 1992. Mortality costs of sexual dimorphism in birds. Proc. R. Soc. Lond. B 250, 143-150. ( 10.1098/rspb.1992.0142) [DOI] [Google Scholar]
- 51.Bauch C, Gatt MC, Granadeiro JP, Verhulst S, Catry P. 2020. Sex-specific telomere length and dynamics in relation to age and reproductive success in Cory's shearwaters. Mol. Ecol. 29, 1344-1357. ( 10.1111/mec.15399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bize P, Criscuolo F, Metcalfe NB, Nasir L, Monaghan P. 2009. Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679-1683. ( 10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bichet C, Bouwhuis S, Bauch C, Verhulst S, Becker PH, Vedder O. 2020. Telomere length is repeatable, shortens with age and reproductive success, and predicts remaining lifespan in a long-lived seabird. Mol. Ecol. 29, 429-441. ( 10.1111/mec.15331) [DOI] [PubMed] [Google Scholar]
- 54.Hall ME, Nasir L, Daunt F, Gault EA, Croxall JP, Wanless S, Monaghan P. 2004. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. B 271, 1571-1576. ( 10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heidinger BJ, Kucera AC, Kittilson JD, Westneat DF. 2021. Data from: Longer telomeres during early life predict higher lifetime reproductive success in females but not males. Dryad Digital Repository. ( 10.5061/dryad.fbg79cntn) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Heidinger BJ, Kucera AC, Kittilson JD, Westneat DF. 2021. Data from: Longer telomeres during early life predict higher lifetime reproductive success in females but not males. Dryad Digital Repository. ( 10.5061/dryad.fbg79cntn) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All of the data included in these analyses are available on Dryad Digital Repository: https://doi.org/10.5061/dryad.fbg79cntn [55].


