Abstract
Increased climate variability as a result of anthropogenic climate change can threaten the functioning of ecosystem services. However, diverse responses to climate change among species (response diversity) can provide ecosystems with resilience to this growing threat. Measuring and managing response diversity and resilience to global change are key ecological challenges. Here, we develop a novel index of climate resilience of ecosystem services, exemplified by the thermal resilience of predator communities providing biological pest control. Field assays revealed substantial differences in the temperature-dependent activity of predator species and indices of thermal resilience varied among predator communities occupying different fields. Predator assemblages with higher thermal resilience provided more stable pest control in microcosms where the temperature was experimentally varied, confirming that the index of thermal resilience developed here is linked to predator function. Importantly, complex landscapes containing a high number of non-crop habitat patches were more likely to contain predator communities with high thermal resilience. Thus, the conservation and restoration of non-crop habitats in agricultural landscapes—practices known to strengthen natural pest suppression under current conditions—will also confer resilience in ecosystem service provisioning to climate change.
Keywords: ecosystem service, ecosystem function, pest, predator, land use, climate resilience
1. Introduction
A major determinant of the vulnerability of ecosystem services to environmental change is their resilience to disturbance [1,2]. Resilient ecosystem services can absorb a substantial amount of disturbance before transitioning to an alternate state, whereas services of low resilience are likely to transition when exposed even to a relatively small level of disturbance [2,3]. In addition, resilient ecosystem services can recover faster and/or persist better in response to disturbance than their low resilience counterparts [2,3]. Resilient services are characterized by two complementary properties of their service-providing communities: functional redundancy, defined as the diversity of functionally equivalent species [4–6], and response diversity, defined as the diversity in responses to changes in environmental conditions within a community [4,7]. The resilience of ecosystem services is expected when functionally redundant species differ in their responses to environmental change so that some species can ensure the continuation of the service when other species are lost, or their efficiency is reduced [8–10].
Accelerating rates of environmental change are increasing the need for the development of metrics to quantify the resilience of ecosystem services [11], and efforts to measure resilience have been numerous [1]. Yet, our understanding of its drivers in natural systems remains limited and quantifying resilience has proven notoriously difficult [12]. As a result, estimates of the resilience of ecosystem services of high economic importance [13] are commonly based on the effects of disturbance on taxonomic diversity rather than quantifications of diversity in process-relevant traits and response diversity to a disturbance within communities [2,14]. Because the trait and response diversity of ecosystem service providers are directly related to how they function, and taxonomic diversity is only indirectly related, a focus on the former may better enable ecologists to engineer ecosystems for resilience through carefully targeted conservation strategies [2].
Here, we describe a new approach to estimate climate resilience of ecosystem services that directly addresses this problem by basing resilience estimates on both, redundancy in process-relevant functions and response diversity to variation in ambient temperature within functional groups. We exemplify the approach by calculating the climate resilience of insect pest control by naturally occurring predators in cereal fields. Biological pest control is an ecosystem service that is of critical importance for sustainable agriculture [13,15,16] and both habitat degradation and climate change can affect it negatively [16,17]. Independently, it has been demonstrated that the presence of natural habitats in human-dominated landscapes can increase species diversity, redundancy and response diversity [6,18]. Nevertheless, it remains unclear whether conservation strategies at the landscape level can increase both redundancy and response diversity within the same community and thus improve the chances of continued provisioning of valuable ecosystem services in the face of global change.
Increased variability in temperatures resulting from anthropogenic climate change can dramatically affect ecosystems and threaten important ecosystem services humans are relying on [19]. Changes in ambient temperature can influence prey consumption rates of ectotherm predators [20,21], with their ability to maintain functioning constrained by their thermal tolerance, i.e. the ability to maintain homeostasis under varying temperatures [22]. As a result, exposure to temperature regimes outside of temperature optima can reduce the performance of species [23,24]. Ectotherm predators are therefore likely to increase foraging when ambient temperatures are within their preferred range and decrease foraging when ambient temperatures are outside of their preferred range. Facing increased climate variability under future scenarios [25], realistic predictions of the effects of climate change on biological control services thus need to incorporate effects of climate variability on the performance of individual predator species and the resulting climate resilience of entire predator communities.
Here, we extend our recently introduced metric of functional redundancy in biological control services under current conditions [6] by including a measure of response diversity to ambient temperatures in order to calculate the resilience of biological control services to increased temperature variability under future climate conditions. We measured the field activity of predators of aphids, which are globally important pests, in relation to ambient temperature to assess diversity in temperature niches among species. We then quantified the climate resilience of biological control in different predator communities using a novel index that combines functional redundancy with response diversity to climate variability (figure 1). To test whether temperature-dependent activity in the field has functional relevance for prey consumption and the climate resilience of biological control, we conducted feeding trials in environmentally controlled climate chambers with selected predators. We then tested whether climate resilience of biological control is higher in complex compared with simplified agricultural landscapes.
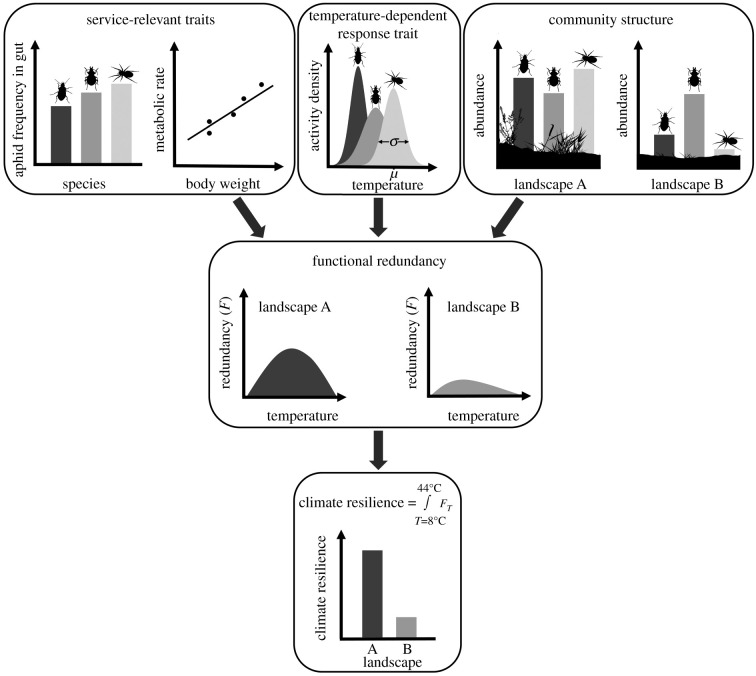
Figure 1.
Flowchart illustrating the links between species-specific traits, redundancy and climate resilience of biological control services. Service-relevant traits (i.e. aphid preference based on aphid frequency in gut contents and body weight-dependent metabolic rate), response traits (i.e. temperature-dependent activity density determined by temperature optimum µ and temperature-niche breadth σ) and taxonomic diversity are combined to calculate functional redundancy of biological control along a gradient of ambient temperature. Climate resilience of biological control is then expressed as the integral of functional redundancy across temperatures and allows for a direct comparison of climate resilience between communities.
2. Methods
(a) . Study location and period
The study was conducted in spring-sown barley fields surrounding the city of Uppsala (59.86° N, 17.64° E) in south-central Sweden. We selected 10 fields located along a gradient of landscape complexity ranging from complex (i.e. landscapes with a high proportion of semi-natural habitat) to simple landscapes (i.e. landscapes dominated by arable land) with five fields under conventional management and the other five managed organically for a minimum consecutive period of 10 years. Selected fields were arranged in pairs with one conventionally and one organically managed field, with a mean distance of 1.6 km (ranging from 1.1 to 2.2 km) within each pair and a maximum distance of 52.7 km between pairs. Differences between conventional and organic barley fields in our study region are mainly due to the use of herbicides and inorganic fertilizers under conventional management with only a limited application of insecticides [26].
Field sampling was carried out over periods of five weeks from the end of May until the beginning of July in 2011 and 2017, respectively. This period covers the two phases most critical for biological control of the bird cherry-oat aphid Rhopalosiphum padi by economically important generalist arthropod predators [27], initial colonization during the crop tillering stage and population build-up during the stem extension and heading stages [28]. We sampled the activity density of two key taxonomic groups of actively hunting ground-dwelling arthropod predators, wolf spiders (Araneae: Lycosidae) and carabid beetles (Coleoptera: Carabidae) in each field along two 75 m long transects located approximately 5 m from, and in parallel with, randomly selected field margins. Along each transect, we placed 30 pitfall traps (11.5 cm diameter × 11 cm depth; Noax Lab, Farsta, Sweden) spaced 5 m apart. Pitfall traps were filled with water and a small quantity of detergent (Yes, Procter & Gamble, Stockholm, Sweden) to reduce surface tension.
(b) . Sampling of predator abundance and molecular gut content analysis
In 2011, we monitored the activity density as a proxy for the abundance of wolf spiders and carabid beetles. Pitfall traps were kept open for the entirety of the sampling period of five weeks and emptied once per week with the exception of one field (JC) which was only sampled during weeks 2–5 of the study. We collected a total of 2043 wolf spiders belonging to 15 species and a total of 5122 carabid beetles belonging to 45 species, respectively. Furthermore, we collected a total of 3755 specimens of the same species for molecular gut content analysis (MGCA) (for bioassay specificity, material and methods description, see [29]).
(c) . Sampling of temperature-dependent activity density
In 2017, we monitored temperature-dependent activity densities for the calculation of species-specific temperature niches in five spring barley fields located in the same region as the previous sampling. Each field was sampled 1 day per week over a total period of five weeks. During each day of sampling, traps were open for one 2 h period in the morning (10.00–12.00) and one 2 h period in the afternoon (13.00–15.00), emptied immediately after each 2 h period and kept closed before and after sampling sessions.
We used miniature temperature loggers (SL54TH, Signatrol, Tewkesbury, UK) to monitor ambient temperature during the surveys. Six loggers were placed at equal distance along each transect and programmed to take temperature readings in 5 min intervals. Each logger was located approx. 2 cm above the soil surface and protected from direct sunlight by a plastic tray located an additional 5 cm above the logger. For each transect and sampling session, the average of temperature readings of the six loggers was calculated and used as ambient temperature for the subsequent analysis of species-specific temperature niches. Over the course of sampling, predators were collected at ambient temperatures near the soil surface ranging from 8.0 to 44.0°C.
(d) . Species-specific temperature niches
We calculated species-specific temperature niches by translating temperature-dependent activity patterns recorded in the field into a weighted thermal optimum (mean, µ) and weighted temperature niche breadth (standard deviation, σ):
where n is the number of captures of predator i at temperature T and N the total number of captures of predator i across all temperatures.
(e) . Climate resilience of biological control
Analyses of the climate resilience of biological control were conducted on a subset of the arthropod predator communities and comprised seven wolf spider species (1775 individuals, 86.9% of all captures) and nine carabid beetle species (4722 individuals, 92.2% of all captures) for which we were able to gather information on all three field-monitored components of the resilience metric: abundance, gut content and temperature niche.
We defined the climate resilience of biological control as the sum of redundancy within aphid predator communities along a gradient of ambient temperature. In a first step, we calculated the risk of predation PT,i for aphids to predator species i for a given ambient temperature T, weighted by an approximation of metabolic rates and the temperature-dependent likelihood of predator activity (figure 1):
where p is the probability of predator i feeding on aphids (expressed as the fraction of specimens within a species tested positive for aphid DNA in their gut content), N is the total number of captures of predators belonging to species i over the duration of one sampling period, b0 is a taxon-specific normalization constant [30], M is the average body weight of species i, a is the taxon-specific allometric exponent [30], μ is the temperature optimum and σ the temperature-niche breadth of species i.
Adapting the redundancy metric described by Feit et al. [6], we then calculated functional redundancy FT,i within predator community i for a given ambient temperature T, expressed as the exponential of the Shannon entropy (figure 1):
Finally, we calculated the climate resilience of biological control of aphids Ri within a predator community i as the integral of functional redundancy along the temperature gradient (figure 1):
Similar to the method to quantify redundancy presented in Feit et al. [6], this approach of summing re-transformed entropy has a doubling property that allows for a direct comparison of resilience between communities. A community with an Ri of 2 is considered to have double redundancy of a community with an Ri of 1, etc.
(f) . Functional relevance of temperature niches
We conducted feeding trials in environmentally controlled climate chambers to test whether temperature-dependent activity patterns determine prey consumption and whether response diversity provides service stability under varying temperature scenarios. We used two common carabid species in our study system, Poecilus cupreus, a predator preferably active under warm conditions (µ = 30.2°C, σ = 8.9°C), and Pterostichus melanarius, a predator preferably active under cold conditions (µ = 16.5°C, σ = 8.1°C). Like all other predators in our study, both carabids employ active hunting strategies and exhibit distinct temperature-dependent activity niches in the field. Therefore, it is reasonable to expect that the results of the feeding trials can be generalized to other predators in our study system. The likelihood of predation on aphids, based on MGCA, was similar between the two species with 42.1% of P. cupreus specimens and 51.9% of P. melanarius specimens tested positive for aphid predation. To assess prey consumption, we manufactured sentinel aphid cards from conventional 360-grain sandpaper. We glued clutches of 10 live adult aphids (R. padi) central onto 1 × 1 cm pieces of sandpaper using egg white as glue [31]. After gluing, the cards were immediately frozen at −15.0°C and used within 24 h.
Over a period of three weeks in June 2019, we collected live predators in the field using pitfall traps in a similar set-up as for the activity density monitoring in 2017, with the exception of replacing water and detergent with wood chips to provide shelter and reduce the likelihood of predation. Traps were emptied twice a week. During this collection phase, captured carabids were separated by species and stored in large plastic containers at 4.0°C with ad libitum access to water and dry dog food (Royal Canin, Aimargues, France). Two days prior to experiments, carabids were separated into small plastic containers and starved at room temperature with ad libitum access to water.
During the experiments, carabids were kept in plastic containers (11.5 cm diameter × 11 cm depth; Noax Lab, Farsta, Sweden) filled 4 cm with conventional potting soil and provided with shelter in the form of two 2 × 4 cm strips of bent sandpaper. In each container, two carabids were placed in either a single-species (two P. cupreus (n = 30) or two P. melanarius (n = 30)) or multi-species design (one P. cupreus and one P. melanarius (n = 30)). The containers were then placed in environmentally controlled climate chambers (KB8000FL, Termaks AS, Bergen, Norway) with half of the containers of each design kept at the temperature optimum of P. cupreus (30.2°C) and the other half at the temperature optimum of P. melanarius (16.5°C). In addition, light conditions in the climate chambers were set to mimic natural conditions at the ground level in barley fields under both temperature scenarios with 3750–7500 lux on a hot, sunny day and 375–750 lux on a cold, cloudy day [32].
After an acclimatization phase of 1 h, we placed a sentinel aphid card in each of the containers. Subsequently, aphid cards were checked for signs of predation in 30 min intervals over a total duration of 5 h (n = 10 aphid cards). After each interval, the occurrence of either partial or complete predation was recorded as a positive predation event and attacked aphid cards were replaced.
To investigate differences in aphid card attack rates between temperature treatments in feeding trials, we used generalized linear mixed models (GLMM) with a binomial distribution and container identity as a random factor. To test the robustness of our climate resilience modelling approach, we employed one-sample t-tests to compare measured variation in aphid card attack rates at the respective optimum temperature of each multi- and single-species design against predicted mean aphid card attacks. To do this, we first calculated average aphid card attack rates for each design during each temperature treatment. Predicted mean attack rates (PM) were then calculated for each design by multiplying measured mean attack rates at the optimal experimental temperature with expected differences in predation rates between temperatures as predicted by the approximated risk of predation (P) at both temperatures:
where M is the measured attack rate of species, i at its temperature optimum Topt and P the approximated risk of predation by species i at their respective temperature optima Topt and sub-optima Tsubopt.
(g) . Effects of landscape heterogeneity
We used raster-based land-use data from the national land cover data project (NMD) of the Swedish Environmental Protection Agency for the reference year of 2012 (no data were available for the sampling year of 2011) to identify the structure and composition of the landscape surrounding each field. NMD accounts for 25 classes of vegetation and land use with a resolution of 10 m. We merged classes into five larger habitat categories: arable land, grassland, forest, water bodies and settlement. Using the ‘buffer’ tool in ArcGIS (v. 10.5.1), we created circular buffers of 1 km radius around the centre of each field, a relevant scale to understand population dynamics of arthropod predators in crop fields [33,34]. Within each polygon, we quantified landscape heterogeneity by calculating the exponential of the Shannon diversity (eH’) to quantify the variance in the proportion of area covered by each habitat category and by counting the number of distinct habitat patches irrespective of habitat type as an expression of landscape patchiness.
We used GLMM with a normal distribution to investigate the respective effects of landscape complexity (i.e. number of distinct habitat patches and exponential Shannon diversity (eH’)), farming system (i.e. conventional or organic), and their interactions, on climate resilience of biological control. Field identity was included as a random factor in each model. We selected models based on Akaike's information criteria corrected for small sample sizes (AICc) [35]. We determined empirical support for each model by calculating the relative likelihood of a model (Akaike weight, AICw) [35] with the weight of any particular model depending on the entire set of candidate models, varying from 0 (no support) to 1 (complete support). All multivariable generalized linear modelling was carried out using IBM SPSS Statistics 26.0.
3. Results
(a) . Species-specific temperature niches
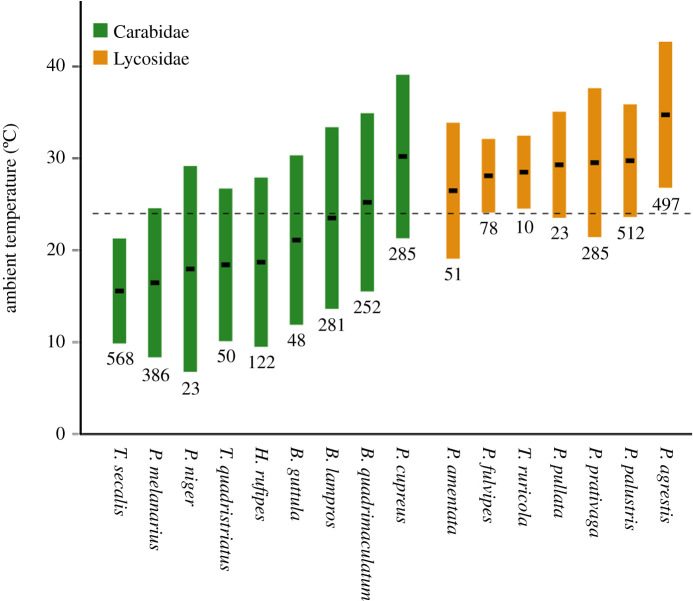
We calculated temperature niches for 16 actively hunting arthropod predator species, nine carabids and seven wolf spiders (figure 2). On average, the weighted thermal optimum (mean, µ) was higher for ground-dwelling spiders (29.5°C, ranging from 26.5 to 34.8°C) than carabid beetles (20.8°C, ranging from 15.6 to 30.2°C; Mann–Whitney U = 16.0, p < 0.01; figure 2). By contrast, weighted temperature-niche breadth (standard deviation, σ) was, on average, greater for carabid beetles (8.9°C, ranging from 5.7 to 11.2°C) than spiders (6.2°C, ranging from 4.0 to 8.1°C; Mann–Whitney U = 76.0, p < 0.05; figure 2). As neither µ (ANOVA, F = 0.10 p = 0.76) nor σ were affected by sample size (ANOVA, F = 0.01, p = 0.92), we are confident that our method of calculating temperature niches was robust and is adequately reflecting actual behavioural patterns of predators in the field.
Figure 2.
Temperature niches of ground-dwelling generalist arthropod predators in south-central Sweden. Weighted thermal optima (mean, µ; black bars) and weighted temperature-niche breadths (standard deviation, σ; coloured bars) of nine carabid beetles (Coleoptera: Carabidae) and seven wolf spider species (Araneae: Lycosidae) are shown. The dashed line indicates the average ambient temperature of 24.1°C across all field surveys. Numbers below the bars indicate the total number of specimens collected for temperature-niche calculations. (Online version in colour.)
(b) . Functional relevance of temperature niches
Attack rates on aphid cards in single-species P. cupreus trials were, on average, 2.9 times higher under warm (aphid card attack rate = 0.35 ± 0.08) than cold conditions (0.12 ± 0.06; GLMM, F1,298 = 6.66, p < 0.05; figure 3). By contrast, attack rates on aphid cards in single-species P. melanarius trials, were, on average, 3.3 times higher under cold (0.20 ± 0.04) than warm conditions (0.06 ± 0.02; GLMM, F1,298 = 23.18, p < 0.001; figure 3). Attack rates on aphid cards in trials with a multi-species P. cupreus and P. melanarius design did not differ between warm (0.24 ± 0.08) and cold conditions (0.23 ± 0.04; GLMM, F1,298 = 0.001, p = 0.97; figure 3). Importantly, variations in measured aphid card attack rates at their respective optimum temperature did not differ from predicted mean attack rates generated from our climate niche models for the P. cupreus (mean predicted rate at 30.2°C = 0.44, mean measured rate = 0.35; t = −1.09, p = 0.29), P. melanarius (mean predicted rate at 16.5°C = 0.22, mean measured rate = 0.20; t = −0.45, p = 0.66) or diverse designs (mean predicted rate at 30.2°C = 0.29, mean measured rate = 0.24; t = −1.47, p = 0.16).
Figure 3.
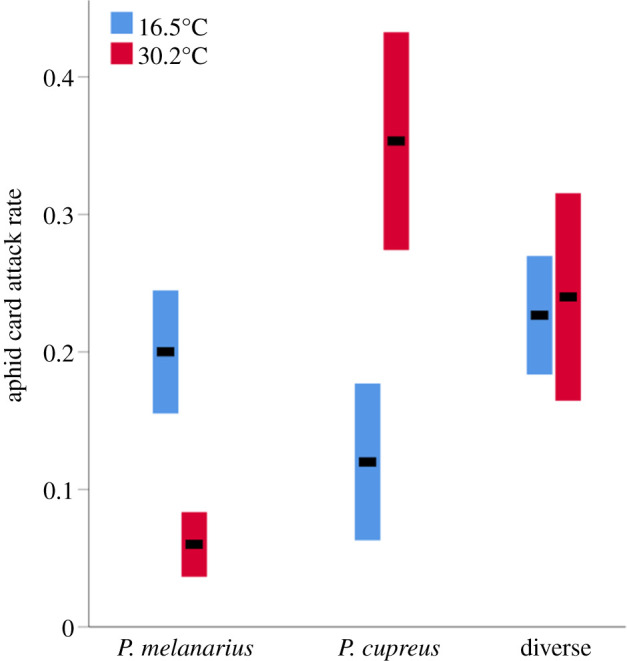
Attack rates on aphid cards in temperature-controlled mesocosm experiments. Mean (black bars) and standard deviations (coloured bars) of aphid card attack rates in single species (Pterostichus melanarius or Poecilus cupreus) and diverse (P. melanarius and P. cupreus) designs under cold and warm conditions in climate chambers are shown. (Online version in colour.)
(c) . Landscape effects on climate resilience of biological control
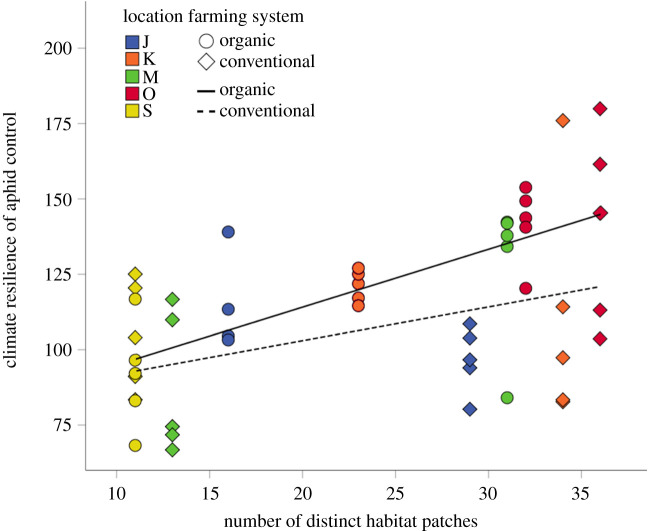
Landscape complexity had a positive effect on the climate resilience of biological control of aphids. Predator communities in landscapes with low numbers of distinct habitat patches and low habitat diversity exhibited, on average, lower climate resilience compared to predator communities in landscapes characterized by high numbers of distinct habitat patches and high habitat diversity (figures 4 and 5 and table 1). We found no difference in climate resilience of biological control between organic and conventional fields when farming system was the sole predictor (table 1). However, the model that best-predicted climate resilience of biological control at the landscape level included an interaction term between the number of distinct habitat patches in the landscape surrounding the spring barley fields and farming system and indicated that organic farming had a positive effect on the climate resilience of biological control only in more complex landscapes (AICc = 444.8, AICcw = 0.56; table 1 and figure 5).
Figure 4.
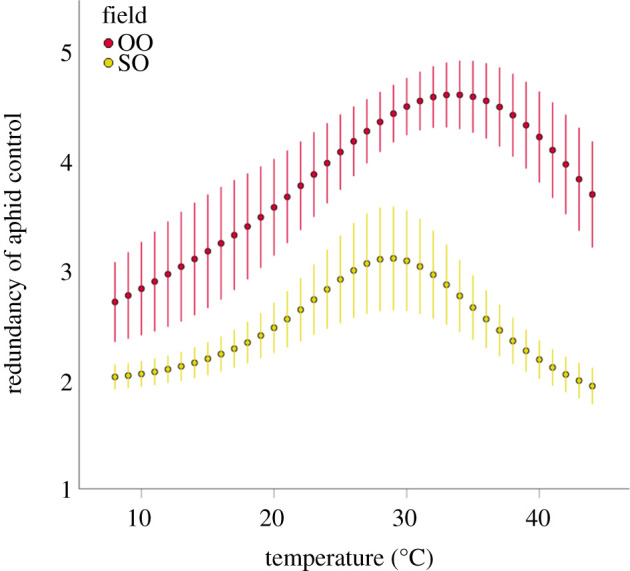
Relationship between redundancy of biological control and ambient temperature at opposing ends of the landscape spectrum. Field OO is located in a landscape of high complexity (32 distinct habitat patches); field SO is located in a landscape of low complexity (11 distinct habitat patches). (Online version in colour.)
Figure 5.
Relationship between landscape simplification and climate resilience of biological control in spring barley fields in south-central Sweden. Regressions (solid line organic, dashed line conventional farming) are fitted by a GLMM. (Online version in colour.)
Table 1.
Model selection results for candidate sets of GLMM for landscape effects within 1 km radius on the level of climate resilience of biological control of aphids by generalist arthropod predators in spring barley fields in south-central Sweden. Parameter estimates (PE) and 95% confidence intervals (CI) are presented for each factor when it was the sole predictor. Significant interactions (i.e. 95% confidence intervals not crossing zero) are highlighted in italics. Farming system includes conventional and organic management. AICc is the Akaike information criterion corrected for small sample sizes. ΔQICc is the difference in AICc in relation to the best model. AICcw is the relative likelihood of the respective model.
| model | AICc | ΔAICc | AICcw | PE (95% CI) |
|---|---|---|---|---|
| landscape complexity | ||||
| farming system × patches | 444.8 | — | 0.56 | |
| farming system (conventional) | 446.4 | 1.6 | 0.25 | −11.3 (−36.1–13.4) |
| patches | 447.8 | 3.0 | 0.12 | 1.3 (0.4–2.3) |
| landscape eH’ | 449.6 | 4.8 | 0.05 | 38.4 (15.2–61.5) |
| farming system × landscape eH’ | 453.7 | 8.9 | 0.01 | |
| null | 454.1 | 9.3 | 0.01 | |
4. Discussion
We have developed a novel approach to quantify the resilience of ecosystem services to climate change that combines measures of functional redundancy and response diversity in a single metric. To exemplify the approach, we quantified the climate resilience of biological control provided by generalist predators as the sum of redundancy along a gradient of ambient temperature. The 10 communities we sampled showed considerable variation in their resilience indices. We found the climate resilience of biological control to increase with landscape complexity. While biological pest control in landscapes of high agricultural intensification, characterized by low numbers of distinct habitat patches and low habitat diversity, was less resilient to climate variability, biological pest control in complex landscapes, characterized by high numbers of distinct habitat patches and high habitat diversity, was, on average, more resilient. A likely explanation could lie within the previously documented negative effects of habitat loss on the diversity of natural predators and the biological control services they provide [15,36]. In contrast with arable land, typically characterized by frequent disturbances, semi-natural habitat is comparatively stable, thus allowing higher diversity of natural predators in complex landscapes with a particular benefit for species that require stable environments for overwintering and reproduction as well as habitat generalists [37–39].
In addition, we found the type of farming system (i.e. conventional and organic farming) to affect the climate resilience of biological control in interaction with landscape complexity. However, while such interactive effects are well established [40], we found positive effects of organic farming on climate resilience of biological control to be strongest in complex landscapes which is in contrast with frequently reported [40,41], but not uncontested [42], stronger increases in taxonomic diversity in organic farms located in simplified landscapes. This discrepancy provides further evidence to the notion that measures of trait and response diversity may better inform conservation strategies targeting the preservation of ecological resilience than taxonomic diversity [2,43–46].
The results of the feeding trials in climate-controlled environments provide further support for the hypothesis that communities exhibiting higher levels of response diversity along a temperature gradient are likely to be more resilient to climate change [10]. First, they show that temperature-dependent activity patterns of aphid predators in the field can translate into temperature-dependent predation pressure on aphids and thus have functional relevance for biological control services under natural conditions. While the species preferably active at warmer field temperatures increased their feeding on aphids when we simulated warm sunny conditions in the laboratory, the species preferably active under colder field temperatures increased feeding on aphids when we simulated cool, cloudy conditions in the laboratory. As variations in aphid card attacks between treatments were in line with predictions generated from our climate niche models, these results provide support for the robustness of our modelling approach. A limitation of this study is that we validated the climate niche models with only two species. We therefore caution that additional experiments are warranted to further corroborate the postulated link between response diversity and resilience of biological control services to future climate conditions. However, all the other predators in our study use an active hunting strategy similar to P. cupreus and P. melanarius and show similarly distinct climate niches, so it is reasonable to expect that their temperature-dependent consumption patterns can be generalized to other predators in our study system. Thus, we can expect predators to increase their contribution to biological control of aphids when ambient temperatures are within their preferred range and to decrease their contribution under climatic conditions outside their preferred range. Second, the similarity between aphid card attacks under warm and cold conditions found in the multi-species design shows that response diversity within the community directly results in more stable biological control of aphids under varying climatic conditions. These findings are consistent with the insurance hypothesis which states that systems characterized by greater response diversity are more resilient to disturbance [47].
5. Conclusion
The ability to predict how drivers of global environmental change will shape the structure and composition of ecosystems in the future is central to the development of mitigation strategies aimed at the protection of ecosystem functionality. Linking process-relevant determinants of functional redundancy with a metric of response diversity to environmental variation in a single model enabled us to compare the thermal resilience of biological control of aphids along a gradient of landscape simplification. Complex landscapes comprising a high number of non-crop habitat patches contained predator communities with higher thermal resilience. Thus, the conservation and restoration of non-crop habitats in agricultural landscapes—practices that are known to increase predator diversity and strengthen natural pest suppression under current conditions [6,18,26,48]—will also confer resilience in ecosystem service provisioning within a changing climate. However, focusing solely on taxonomic diversity as a proxy for the resilience of ecosystem services could lead to inappropriate management advice for conservation and bolstering of ecosystem services under global change. Efforts to enhance the climate resilience of ecosystem services should thus particularly aim to increase functional evenness and response diversity within predator communities. This might be achieved by increasing the availability and diversity of permanent habitat and temporary refugia to bolster diversity of seemingly redundant species already present in the system but could also require assisted migration and/or targeted reintroduction of species [3,49,50].
Supplementary Material
Acknowledgements
C. Högfeldt and G. Malsher helped with arthropod identification. L. Wu, A. Bailey, G. Altmire and six high-school students from Katedralskolan, Uppsala, assisted with field surveys. E. Klein and D. Bliss conducted mesocosm experiments. A. Feit, Z. Rosin and R. Bommarco provided valuable comments on earlier drafts of the manuscript.
Data accessibility
Data used for calculation of predator-specific climate niches and climate resilience are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.np5hqbzrq [51].
Authors' contributions
B.F.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft, writing-review and editing; N.B.: conceptualization, funding acquisition, methodology, writing-review and editing; E.D.: methodology, writing-review and editing; C.S.S.: conceptualization, funding acquisition, investigation, methodology, writing-review and editing; M.T.: methodology, writing-review and editing; M.J.: conceptualization, funding acquisition, investigation, methodology, project administration, writing-review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests
Funding
This work was supported by the Swedish Research Council FORMAS (2016-01511) and the Centre for Biological Control at the Swedish University of Agricultural Sciences.
References
- 1.Angeler DG, Allen CR. 2016. Quantifying resilience. J. Appl. Ecol. 53, 617–624. [Google Scholar]
- 2.Martin EA, Feit B, Requier F, Friberg H, Jonsson M. 2019. Assessing the resilience of biodiversity-driven functions in agroecosystems under environmental change. Adv. Ecol. Res. 60, pp. 59–123. ( 10.1016/bs.aecr.2019.02.003) [DOI] [Google Scholar]
- 3.Elmqvist T, Folke C, Nyström M, Peterson G, Bengtsson J, Walker B, Norberg J. 2003. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 1, 488–494. ( 10.1890/1540-9295) [DOI] [Google Scholar]
- 4.Rosenfeld JS. 2002. Functional redundancy in ecology and conservation. Oikos 98, 156–162. ( 10.1034/j.1600-0706.2002.980116.x) [DOI] [Google Scholar]
- 5.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 6.Feit B, Blüthgen N, Traugott M, Jonsson M. 2019. Resilience of ecosystem processes: a new approach shows that functional redundancy of biological control services is reduced by landscape simplification. Ecol. Lett. 22, 1568–1577. ( 10.1111/ele.13347) [DOI] [PubMed] [Google Scholar]
- 7.McNaughton SJ. 1977. Diversity and stability of ecological communities: a comment on the role of empiricism in ecology. Am. Nat. 111, 515–525. ( 10.1086/283181) [DOI] [Google Scholar]
- 8.Tilman D. 1999. The ecological consequences of changes in biodiversity: a search for general principles. Ecology 80, 1455–1474. ( 10.2307/176540) [DOI] [Google Scholar]
- 9.Mori AS, Furukawa T, Sasaki T. 2013. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 88, 349–364. ( 10.1111/brv.12004) [DOI] [PubMed] [Google Scholar]
- 10.Kühsel S, Blüthgen N. 2015. High diversity stabilizes the thermal resilience of pollinator communities in intensively managed grasslands. Nat. Commun. 6, 7989. ( 10.1038/ncomms8989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver TH, et al. 2015. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 30, 673–684. ( 10.1016/j.tree.2015.08.009) [DOI] [PubMed] [Google Scholar]
- 12.Pimm SL, Donohue I, Montoya JM, Loreau M. 2019. Measuring resilience is essential to understand it. Nat. Sustain. 2, 895–897. ( 10.1038/s41893-019-0399-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naranjo SE, Ellsworth PC, Frisvold GB. 2015. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 60, 621–645. ( 10.1146/annurev-ento-010814-021005) [DOI] [PubMed] [Google Scholar]
- 14.Gagic V, et al. 2015. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 282, 20142620. ( 10.1098/rspb.2014.2620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson M, Bommarco R, Ekbom B, Smith HG, Bengtsson J, Caballero-Lopez B, Winqvist C, Olsson O. 2014. Ecological production functions for biological control services in agricultural landscapes. Methods Ecol. Evol. 5, 243–252. ( 10.1111/2041-210X.12149) [DOI] [Google Scholar]
- 16.Dainese M, et al. 2019. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 5, eaax0121. ( 10.1126/sciadv.aax0121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson LJ, Macfadyen S, Hoffmann AA. 2010. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Control 52, 296–306. ( 10.1016/j.biocontrol.2009.01.022) [DOI] [Google Scholar]
- 18.Grass I, Jauker B, Steffan-Dewenter I, Tscharntke T, Jauker F. 2018. Past and potential future effects of habitat fragmentation on structure and stability of plant–pollinator and host–parasitoid networks. Nat. Ecol. Evol. 2, 1408–1417. ( 10.1038/s41559-018-0631-2) [DOI] [PubMed] [Google Scholar]
- 19.Nolan C, et al. 2018. Past and future global transformation of terrestrial ecosystems under climate change. Science 361, 920–923. ( 10.1126/science.aan5360) [DOI] [PubMed] [Google Scholar]
- 20.Englund G, Öhlund G, Hein CL, Diehl S. 2011. Temperature dependence of the functional response. Ecol. Lett. 14, 914–921. ( 10.1111/j.1461-0248.2011.01661.x) [DOI] [PubMed] [Google Scholar]
- 21.Lang B, Rall BC, Brose U. 2012. Warming effects on consumption and intraspecific interference competition depend on predator metabolism. J. Anim. Ecol. 81, 516–523. ( 10.1111/j.1365-2656.2011.01931.x) [DOI] [PubMed] [Google Scholar]
- 22.Pörtner HO, Farrell AP. 2008. Ecology: physiology and climate change. Science 322, 690–692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 23.Huey RB, Stevenson RD. 1979. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Integr. Comp. Biol. 19, 357–366. ( 10.1093/icb/19.1.357) [DOI] [Google Scholar]
- 24.Frazier MR, Huey RB, Berrigan D. 2006. Thermodynamics constrains the evolution of insect population growth rates: ‘warmer is better’. Am. Nat. 168, 512–520. ( 10.1086/506977) [DOI] [PubMed] [Google Scholar]
- 25.IPCC. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. See https://www.ipcc.ch/site/assets/uploads/2018/05/SYR_AR5_FINAL_full_wcover.pdf.
- 26.Weibull AC, Östman Ö, Granqvist Å. 2003. Species richness in agroecosystems: the effect of landscape, habitat and farm management. Biodivers. Conserv. 12, 1335–1355. ( 10.1023/A:1023617117780) [DOI] [Google Scholar]
- 27.Östman Ö, Ekbom B, Bengtsson J. 2003. Yield increase attributable to aphid predation by ground-living polyphagous natural enemies in spring barley in Sweden. Ecol. Econ. 45, 149–158. ( 10.1016/S0921-8009(03)00007-7) [DOI] [Google Scholar]
- 28.Chiverton PA. 1987. Predation of Rhopalosiphum padi (Homoptera: Aphididae) by polyphagous predatory arthropods during the aphids' pre-peak period in spring barley. Ann. Appl. Biol. 111, 257–269. ( 10.1111/j.1744-7348.1987.tb01452.x) [DOI] [Google Scholar]
- 29.Roubinet E, Birkhofer K, Malsher G, Staudacher K, Ekbom B, Traugott M, Jonsson M. 2017. Diet of generalist predators reflects effects of cropping period and farming system on extra- and intraguild prey. Ecol. Appl. 27, 1167–1177. ( 10.1002/eap.1510) [DOI] [PubMed] [Google Scholar]
- 30.Ehnes RB, Rall BC, Brose U. 2011. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000. ( 10.1111/j.1461-0248.2011.01660.x) [DOI] [PubMed] [Google Scholar]
- 31.Boetzl FA, Konle A, Krauss J. 2020. Aphid cards — useful model for assessing predation rates or bias prone nonsense? J. Appl. Entomol. 144, 74–80. ( 10.1111/jen.12692) [DOI] [Google Scholar]
- 32.Borger CPD, Hashem A, Pathan S. 2010. Manipulating crop row orientation to suppress weeds and increase crop yield. Weed Sci. 58, 174–178. ( 10.1614/ws-09-094.1) [DOI] [Google Scholar]
- 33.Thies C, Tscharntke T. 1999. Landscape structure and biological control in agroecosystems. Science 285, 893–895. ( 10.1126/science.285.5429.893) [DOI] [PubMed] [Google Scholar]
- 34.Rusch A, et al. 2016. Agricultural landscape simplification reduces natural pest control: a quantitative synthesis. Agric. Ecosyst. Environ. 221, 198–204. ( 10.1016/j.agee.2016.01.039) [DOI] [Google Scholar]
- 35.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 36.Geiger F, et al. 2010. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105. ( 10.1016/j.baae.2009.12.001) [DOI] [Google Scholar]
- 37.Niemela J, Halme E. 1992. Habitat associations of carabid beetles in fields and forests on the Åland Islands, SW Finland. Ecography (Cop.). 15, 3–11. ( 10.1111/j.1600-0587.1992.tb00001.x) [DOI] [Google Scholar]
- 38.Veres A, Petit S, Conord C, Lavigne C. 2013. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 166, 110–117. ( 10.1016/j.agee.2011.05.027) [DOI] [Google Scholar]
- 39.Sarthou JP, Badoz A, Vaissière B, Chevallier A, Rusch A. 2014. Local more than landscape parameters structure natural enemy communities during their overwintering in semi-natural habitats. Agric. Ecosyst. Environ. 194, 17–28. ( 10.1016/j.agee.2014.04.018) [DOI] [Google Scholar]
- 40.Tuck SL, Winqvist C, Mota F, Ahnström J, Turnbull LA, Bengtsson J. 2014. Land-use intensity and the effects of organic farming on biodiversity: a hierarchical meta-analysis. J. Appl. Ecol. 51, 746–755. ( 10.1111/1365-2664.12219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batary P, Baldi A, Kleijn D, Tscharntke T. 2011. Landscape-moderated biodiversity effects of agri-environmental management: a meta-analysis. Proc. R. Soc. B 278, 1894–1902. ( 10.1098/rspb.2010.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichtenberg E, et al. 2017. A global synthesis of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob. Change Biol. 23, 4946–4957. ( 10.1111/gcb.13714) [DOI] [PubMed] [Google Scholar]
- 43.McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. ( 10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 44.Cadotte MW, Carscadden K, Mirotchnick N. 2011. Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087. ( 10.1111/j.1365-2664.2011.02048.x) [DOI] [Google Scholar]
- 45.Standish RJ, et al. 2014. Resilience in ecology: abstraction, distraction, or where the action is? Biol. Conserv. 177, 43–51. ( 10.1016/j.biocon.2014.06.008) [DOI] [Google Scholar]
- 46.Jonsson M, Kaartinen R, Straub CS. 2017. Relationships between natural enemy diversity and biological control. Curr. Opin. Insect Sci. 20, 1–6. ( 10.1016/j.cois.2017.01.001) [DOI] [PubMed] [Google Scholar]
- 47.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holland JM, Bianchi FJ, Entling MH, Moonen AC, Smith BM, Jeanneret P. 2016. Structure, function and management of semi-natural habitats for conservation biological control: a review of European studies. Pest Manag. Sci. 72, 1638–1651. ( 10.1002/ps.4318) [DOI] [PubMed] [Google Scholar]
- 49.Suding KN, Hobbs RJ. 2009. Threshold models in restoration and conservation: a developing framework. Trends Ecol. Evol. 24, 271–279. ( 10.1016/j.tree.2008.11.012) [DOI] [PubMed] [Google Scholar]
- 50.Bengtsson J, et al. 2003. Reserves, resilience and dynamic landscapes. Ambio 32, 389–396. ( 10.1579/0044-7447-32.6.389) [DOI] [PubMed] [Google Scholar]
- 51.Feit B, et al. 2021. Data from: Landscape complexity promotes resilience of biological pest control to climate change. Dryad Digital Repository. ( 10.5061/dryad.np5hqbzrq) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Feit B, et al. 2021. Data from: Landscape complexity promotes resilience of biological pest control to climate change. Dryad Digital Repository. ( 10.5061/dryad.np5hqbzrq) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data used for calculation of predator-specific climate niches and climate resilience are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.np5hqbzrq [51].