Abstract
BACKGROUND:
Breast cancer is a preventable disease, using three secondary preventive methods of mammography, clinical breast examination (CBE), and breast self-examination (BSE) that can lead to early detection of breast cancer. This study was designed to assess breast cancer screening behavior and its associated factors in females employed in South Khorasan.
MATERIALS AND METHODS:
In this analytic-descriptive study, 2256 female personnel of governmental organizations were investigated in Birjand city in 2016–2017. The data collection tool was a three-part questionnaire: sociodemographic characteristics, knowledge about breast cancer screening methods plus women's performance, and stage of change regarding screening behaviors of mammography, CBE, and BSE. The data were analyzed by SPSS 16 and one-way analysis variance, Tukey's post hoc, and multiple logistic regression model statistical tests.
RESULTS:
The mean ± standard deviation score of knowledge of the women was 3.45 ± 1.5. There was a significant difference of the mean score of knowledge between the single and married (P = 0.03) and age group (P = 0.04). The stage action of mammography, CBE, and BSE was 6.8%, 12.3%, and 16.8%, respectively. Logistic regression model showed that variables such as age and family history of breast cancer were highly significant related to mammography and also CBE. Knowledge was also highly significant in mammography, CBE, and BSE. Education level in CBE, marital status in BSE and mammography, and job in BSE were also significant (P < 0.001).
CONCLUSIONS:
This study reveals insufficient knowledge of female workers about breast cancer and the negative influence of low knowledge on the practice of breast cancer screening behavior. Therefore, the establishment and maintenance of regular educational courses for female employees is essential.
Keywords: Breast cancer, breast self-examination, health behavior, mammography, screening, women's health
Introduction
Breast cancer is considered a chronic noncommunicable disease.[1] Nowadays, it has become a matter of serious public health in both developing and developed countries. Breast cancer is the most common female cancer worldwide and constitutes 23% of the total cancer cases and 14% of cancer death.[2]
Despite the reduction of breast cancer mortality rates in developed countries, the incidence has increased in developing countries like Iran (from 19% to 21.4%). Health-care professionals believed that the majority of Iranian women with breast cancer are diagnosed at advanced stages of disease. Increasing the incidence of breast cancer and the difficulties in its treatment in advanced stages imposes a huge burden on health systems and creates a major challenge for health policymakers in developing countries.[3,4] It seems that the control of modifiable breast cancer risk factors such as long menstrual history, obesity after menopause, recent use of oral contraceptives, postmenopausal hormone therapy, never having children, or having the first children after age 30, and exposure to radiation can impact reducing the incidence of breast cancer.[5]
Breast cancer can be prevented by three secondary preventive methods of mammography, clinical breast examination (CBE), and breast self-examination (BSE) that can lead to early detection of breast cancer. BSE can be done anytime and anywhere at no cost. The American Cancer Society (ACS) recommends mammography in women aged 40 years and above annually and CBE every 3 years by health professionals in 20s and 30s lifelong and after the age of 40 years every year.[6,7] Although BSE practice as a screening test can increase the probability of detecting breast cancer at an early stage by women themselves, it is associated with an increased number of biopsies for benign breast lesions and increased physician visits. Furthermore, mammography and CBE facilitate early detection and treatment of breast cancer and thus reduce the mortality rate.[8]
Delay in diagnosis and treatment of cancer can be due to patient-mediated delay, practitioner, or hospital delay. The patients who delayed for more than 3 months presented lower 5-year survival rates than those with <3 months of delay. This emphasizes the importance of screening methods for the early detection of breast cancer. Socioeconomic and demographic factors, mainly marital status, education, and income, influence patient delay.[9]
The transtheoretical model (TTM) was applied to the dynamic, motivational, and cognitive processes of behavioral change to study health-promoting behaviors such as exercise, substance and alcohol abuse, stress management, AIDS prevention, colon and breast cancer screening, and mammography. It consists of different stages of precontemplation, contemplation, action, maintenance, and relapse that were first developed by Prochaska and Di Clementein 1983. Although the TTM model structurally is more complex than other models, it integrates a wide range of information and it is an instrument with the capacity for the design and management of both individual and community level health behavior change intervention programs.[10,11]
Therefore, since the transition between the stages of change is influenced by independent variables, we conducted this study to examine breast cancer screening behavior and its associated factors in female employees in South Khorasan.
Materials and Methods
This cross-sectional descriptive-analytic study was conducted on 2256 female employees of governmental agencies of Birjand city (capital of South Khorasan Province, Iran) in 2016–2017. The sampling method was a census. To increase the cooperation of organizations with this research project, a letter from the governorate was sent to all government offices to participate in a breast cancer screening project. In the first phase of the project, we requested each organization to introduce a trusted and interested woman as the correspondence for the project. Then, the correspondences of all departments were invited and made aware of the purpose of the research project. At the end of the session, we gave them the questionnaires to distribute to their colleagues. In total, 43 organizations participated in this project. Of the 2,500 questionnaires submitted to the correspondence of the organizations, 2256 questionnaires were completed in full (response rate = 90.24%).
The data collection tool was a three-part questionnaire. The first section contains sociodemographic characteristics (age, education, marital status, marriage age, first gestational age, number of children, lactation and menopause status, family income, and insurance).
The second part of the questionnaire included questions about the history of difficulty and problems in breasts, the history of breast cancer in the first-grade family, the history of referral for mammography or ultrasound, the status of information on breast cancer and its diagnostic methods, and the source of information and knowledge questions about breast cancer screening methods (8 questions), with a score of 1 for a correct answer and 0 for a wrong response. Knowledge scores were categorized to three levels of ≤4 as weak, 5–6 moderate, and ≥7 as good knowledge. To determine the content and face validity of knowledge questions, an expert's panel including 10 experts (three health educationists, two radiologists, one pathologist, two gynecologists, and two epidemiologists) was employed. The questionnaire was given to these faculty members and was revised based on their comments. Then, the modified questionnaire was completed by 25 females who were not included in the sample and its Cronbach's alpha was obtained to be 0.86.
The third section of the questionnaire consisted of three questions about women's performance regarding screening behaviors of mammography, CBE, and BSE. Furthermore, to evaluate breast cancer screening behavior stages of change, we used the Rakowski Change Stages Questionnaire.[12] This scale has 5 questions related to breast cancer screening behavior (mammography, CBE, and BSE), which requires a person to choose one of the five options as a stage of behavior changes (precontemplation, contemplation, preparation, action, maintenance, and relapse). The participants in this study were informed that all identifying information would be kept confidential.
The collected data were analyzed by the Statistical Package for the Social Sciences (SPSS version 16, Chicago, IL, USA) and were reported as mean ± standard deviation or as numbers and percentages for quantitative variables. At first, the normal distribution of the data was assessed by running the Kolmogorov–Smirnov test. The one-way analysis of variance and Tukey's post hoc statistical tests were used to compare knowledge score mean in terms of demographic variables. Odds ratios (ORs) and 95% confidence levels (CIs) were calculated using multiple logistic regressions to estimate relative factors of mammography, CBE, and BSE screening behaviors. P < 0.05 was considered statistically significant for all statistical tests.
Results
This study was conducted on 2256 female employees in Birjand executive agencies, with a mean age of 38.5 ± 7.5 years and an age of marriage of 23.3 ± 3.7 years. The majority of them were university graduates (87.1%) and 88.5% were married. As many as 94.5% were not menopausal and only 5.5% were menopausal. In terms of income level, 23.4% reported good, 67.6% moderate, and 9% weak.
Regarding the status of breast cancer screening behavior, 18.6% of women had a history of mammograms, 20.1% had a CBE, and 50.5% had a history of BSE. The results of the stages of screening behavior change of stage mammography, CBE, and BSE were 6.8%, 12.3%, and 16.8% women in the action stage, respectively. The frequency distribution of change stages according to the screening behavior is presented in Figure 1.
Figure 1.
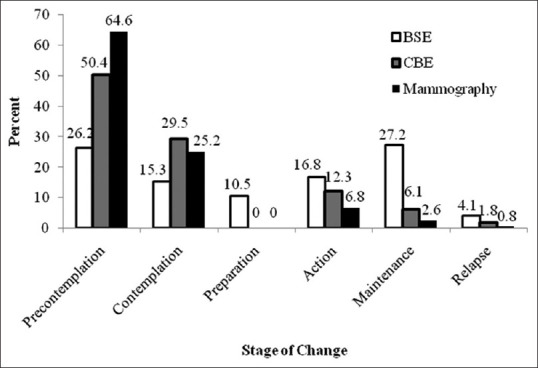
Frequency distribution of breast cancer screening behavior stage of change in 2256 female employees of South Khorasan, Iran
The mean score of knowledge in the women studied was 3.54 ± 1.5 out of 8 points. Figure 2 illustrates the knowledge levels. The main sources of awareness were the Internet (36.8%), book (34.5%), and TV (34.4%).
Figure 2.
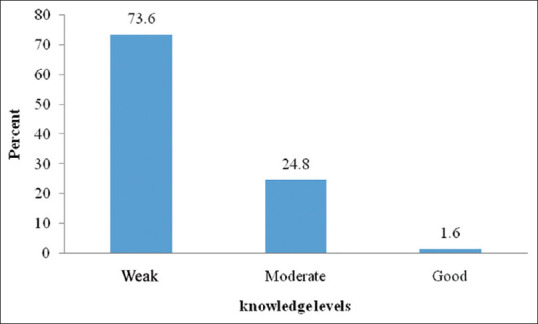
Frequency of female knowledge levels about breast cancer screening in 2256 female employees of South Khorasan, Iran
There was no significant difference in the mean score of knowledge on the level of education, but in the married women, it was significantly higher than the singles, and at the age of 50 and above, it was significantly higher than other age groups [Table 1]. The mean score of knowledge in women who performed mammography was significantly higher than those without mammogram history (3.79 ± 1.4, 3.48 ± 1.5, P < 0.001). There was a significant increase in women with a history of CBE as compared to women without a CBE history (3.84 ± 1.5, 3.46 ± 1.5, P < 0.001). Furthermore, the mean score of knowledge in women with a history of BSE was significantly higher than the women without a previous history (3.97 ± 1.4, 3.09 ± 1.4, P < 0.001).
Table 1.
Comparison of knowledge score mean in terms of demographic variables in 2256 female employees of South Khorasan, Iran
| Variables | n | Mean±SD | ANOVA test/Tukey’s post hoc |
|---|---|---|---|
| Education level | |||
| Elementary | 32 | 3.37±1.6 | P=0.73 |
| High school | 42 | 3.74±1.6 | |
| Diploma | 216 | 3.56±1.5 | |
| University | 1966 | 3.53±1.5 | |
| Marital status | |||
| Married# | 1996 | 3.56±1.5 | P=0.03* |
| Single## | 225 | 3.34±1.7 | |
| Divorced and widow### | 35 | 3.17±1.6 | |
| Age (year) | |||
| ≤29a | 254 | 3.52±1.5 | P=0.04* |
| 30-39b | 1064 | 3.58±1.5 | |
| 40-49c | 672 | 3.6±1.45 | |
| ≤50d | 216 | 3.28±1.6 |
*P<0.05 is significant, #,## ,### P=0.03, a,bP=0.04, c,dP=0.03. SD=Standard deviation, ANOVA=Analysis of variance
Multiple logistic regression models indicated that the chance of mammography and CBE in women without a family history of breast cancer was significantly less than those with a family history (Mamo: OR, 0.28; 95% CI, 0.15–0.54 and CBE: OR, 0.38; 95% CI, 0.24–0.62). For every 10 years increasing in age, the chance of mammography and CBE was 1.5 times and 0.5 times, respectively; with increasing in the knowledge score, the chance of mammography, CBE, and BSE was significantly increased (Mamo: OR, 1.28; 95% CI, 1.06–1.35 and CBE: OR, 1.22; 95% CI, 1.13–1.32 and BSE: OR, 1.43; 95% CI, 1.34–1.53). Women with marriage history had a significantly positive chance in mammography and BSE (Mamo: OR, 2.23; 95% CI, 1–5.02 and BSE: OR, 1.58; 95% CI, 1.18–2.11); the chance of mammography in health workers was significantly more than other employees (OR, 2.73; 95% CI, 1.74–4.29), and the chance of BSE in health workers was significantly less than other workers (OR, 0.42; 95% CI, 0.34–0.51). Furthermore, women with university education had less chance of CBE (OR, 0.56; 95% CI, 0.39–0.80) [Table 2].
Table 2.
Multiple logistic regression analysis predicting breast cancer screening behaviors in 2256 female employees of South Khorasan, Iran
| Predictors | Mammography | CBE | BSE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| 25% | 97.5% | 25% | 97.5% | 25% | 97.5% | ||||
| Age* | 1.15b | 1.11 | 1.19 | 1.05b | 1.03 | 1.07 | 1.01 | 0.99 | 1.03 |
| Knowledge | 1.20b | 1.06 | 1.35 | 1.22b | 1.13 | 1.32 | 1.43b | 1.34 | 1.53 |
| Job (employee, health workersa) | 2.73b | 1.74 | 4.29 | 1.22 | 0.95 | 1.56 | 0.42b | 0.34 | 0.51 |
| Family history of breast cancer (yes, noa) | 0.28b | 0.15 | 0.55 | 0.38b | 0.24 | 0.62 | 0.68 | 0.42 | 1.10 |
| Education (university, othersa) | 0.80 | 0.52 | 1.22 | 0.56b | 0.39 | 0.80 | 0.90 | 0.67 | 1.20 |
| Married (married, singlea) | 2.23b | 0.99 | 5.02 | 1.34 | 0.91 | 1.97 | 1.58b | 1.18 | 2.11 |
aReference category, bP≤0.001, *In mammography population age >40. CBE=Clinical Breast Examination, BSE=Breast self-examination, OR=Odds ratio, CI=Confidence interval
Discussion
The purpose of this study was to investigate breast cancer screening behavior and its associated factors in female employees in South Khorasan.
Our findings on the action stage of respective mammography, CBE, and BSE behavior of 6.8%, 12.3%, and 16.8% are lower than the study reported by Musugire among 226 nurses and midwives working at King Faisal Hospital[13] and higher than the study conducted in Addis Ababa.[14] However, our results are in agreement with Salmani et al.[15] A possible explanation for this result can be a significant association of environmental and socioeconomic factors including cost, accessibility, inaccurate knowledge about breast examination, or no knowledge about practicing BSE correctly, and also married status. Several studies have shown that low cultural perceptions and social norms can be barriers to breast cancer screening.[16,17,18]
The low level of knowledge found in our study is similar to Shiryazd et al. on 438 women referring to health centers of Yazd city.[19] Our findings on knowledge of female employees are in contrast with the study by Kotepui et al. on female personnel, lecturers and laboratory scientists, and general officers that had a significantly higher knowledge about breast cancer screening.[20] The difference may be due to the study population of higher educated female employees in the female personnel of Walailak University in Nakhon Si Thammarat, Thailand, than South Khorasan.
Our study indicated that age ≥50 and marital status significantly influenced the knowledge of breast cancer. Our result is in accordance with the study by Cruz-Castillo et al.[21] and is not compatible with Haghighi et al.'s study.[22] Our explanation is that older women do not believe that screening is a beneficial and essential preventive method for diagnosis in the earliest stages of breast cancer.
Marital status significantly influenced the knowledge of breast cancer as the married women had a greater OR of repeating mammography and BSE compared to single women. Our findings in this respect are in agreement with Abeje et al., Farhadifar et al., and Hanske et al.[14,23,24] but are contrary to the findings of Fouladi et al. on 380 women aged 30 years and above who had referred to health-care centers and Yıldırım and Özaydın on 1271 women aged between 40 and 69 years residing in Moda/İstanbul.[25,26] A possible explanation may be that married women benefit from the economic, emotional, and psychological support of their husbands and children, whereas divorced/widowed women lack such support. Since family member and the spouse are considered as the most important components of the social networks, therefore positive influence of marriage on their partner health behavior may play an important role in creating positive subjective norms to encourage mammography and BSE.[9] In addition, age at first full-term birth and the duration of breastfeeding are factors that have been proven for substantial influence on the incidence of breast cancer, whereas single women have no experience of childbirth or breastfeeding.[27]
In this study, the main source of awareness was the Internet search and media. Because of the limited access to these sources that have important roles in increasing knowledge and practice of breast cancer, routine proper counseling by health-care providers is recommended to improve breast cancer knowledge.
Our study revealed that women who had previous mammography, BSE, and CBE behavior had significantly higher mean knowledge score than those who did not. It is not exactly clear whether breast cancer knowledge precedes cancer screening or, vice versa, previous screening behavior increases knowledge. Elobaid found a positive association between knowledge of breast cancer and screening uptake.[28] However, further studies are necessary to establish the direction of causality.
Age is considered as one of the most important risk factors for breast cancer. Several studies indicate that recommendation of screening behavior by doctors and health-care providers is the most important predictive factor in mammographic screening.[29,30] ACS recommends mammography in women aged 40 years and above; its implementation by physicians and health-care systems increases mammography behavior in women.[28,31]
It was found that knowledge about breast cancer is the common effective variable in BSE as well as in the practice of CBE. In our study, it was cleared that education level is an effective variable in CBE but not BSE. There are two reports on a higher level of education and higher knowledge score that are significant determinants of BSE and CBE practice that this can explain our result.[20,32]
The findings of our study revealed that the OR mammography practice was greater in women with a family history of breast cancer. This finding supports the results of previous studies that indicated the effect of family history on enhancing mammography behavior. Examining screening behaviors in women with a family history of breast cancer argues that this history increases their risk of breast cancer and induces greater worry leads to enhanced screening,[33,34] whereas other studies have reported that anxiety decreased the likelihood of mammography screening practice.[35,23]
This study had several limitations that are worth noting. This investigation is a cross-sectional survey and thus did not follow up the respondents. Another limitation was the lack of comparison between women who once or twice or even more times had screening behavior mammography, CBE, and BSE. Furthermore, breast cancer screening behavior barriers were not investigated in this research.
The present study has a number of strengths. First, the high participation rate increased the power of the results. Second, to our knowledge, this study is the first to report on screening for breast cancer behavior in female employees of the whole organizations of South Khorasan Province.
Conclusions
Our findings indicated that the rate of women in the action stage of mammography, CBE, and BSE behavior was low, and also, it revealed that the factors associated with mammography, CBE, and BSE practice in women including knowledge, level of education, age, family history of breast cancer, and marital status are important. Therefore, these factors must be considered for targeting and programming of efficient interventions on changeable barriers of screening. Breast cancer awareness promotion with collaboration among health-care providers and government and nongovernmental organizations for financial and social supports is recommended.
Financial support and sponsorship
This project has been sponsored by Birjand University of Medical Sciences (Project No: 937).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the Vice-Chancellor for Research and Technology of Birjand University of Medical Sciences for funding this study (Research project number: 937; Ethical Code: IR. bums. 1394.328) and all the female employees who participated in this study.
References
- 1.Ghodsi Z, Hojjatoleslami S. A survey about educational needs of breast cancer and BSE in Iranian women. Procedia Soc Behav Sci. 2012;46:2561–5. [Google Scholar]
- 2.Atuhairwe C, Amongin D, Agaba E, Mugarura S, Taremwa IM. The effect of knowledge on uptake of breast cancer prevention modalities among women in Kyadondo County, Uganda. BMC Public Health. 2018;18:279. doi: 10.1186/s12889-018-5183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadian M, Samah AA. Application of health behavior theories to breast cancer screening among Asian women. Asian Pac J Cancer Prev. 2013;14:4005–13. doi: 10.7314/apjcp.2013.14.7.4005. [DOI] [PubMed] [Google Scholar]
- 4.Shams M, Fayazbakhsh A, Safari M. A review of studies conducted on efficacy of health educational interventions to correct women's behavior in performing breast self-examination. Basic Clin Cancer Res. 2014;6:2–9. [Google Scholar]
- 5.Nindrea RD, Aryandono T, Lazuardi L. Breast cancer risk from modifiable and non-modifiable risk factors among women in Southeast Asia: A meta-analysis. Asian Pac J Cancer Prev. 2017;18:3201–6. doi: 10.22034/APJCP.2017.18.12.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opoku SY, Benwell M, Yarney J. Knowledge, attitudes, beliefs, behaviour and breast cancer screening practices in Ghana, West Africa. Pan Afr Med J. 2012;11:28. [PMC free article] [PubMed] [Google Scholar]
- 7.Farajzadegan Z, Fathollahi-Dehkordi F, Hematti S, Sirous R, Tavakoli N, Rouzbahani R. The transtheoretical model, health belief model, and breast cancer screening among Iranian women with a family history of breast cancer. J Res Med Sci. 2016;21:122. doi: 10.4103/1735-1995.193513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah TA, Guraya SS. Breast cancer screening programs: Review of merits, demerits, and recent recommendations practiced across the world. J Microsc Ultrastruct. 2017;5:59–69. doi: 10.1016/j.jmau.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghazali SM, Othman Z, Cheong KC, Hock LK, Wan Mahiyuddin WR, Kamaluddin MA, et al. Non-practice of breast self examination and marital status are associated with delayed presentation with breast cancer. Asian Pac J Cancer Prev. 2013;14:1141–5. doi: 10.7314/apjcp.2013.14.2.1141. [DOI] [PubMed] [Google Scholar]
- 10.Elkazeh E, Elsaay O. Applying the transtheoretical model of change and the health belief model to breast self-examination in females undergraduate students in Faculty of Nursing Tanta University. J Am Sci. 2012;8:804–14. [Google Scholar]
- 11.Moodi M, Rezaeian M, Mostafavi F, Sharifirad G. The study of mammography screening behavior based on stage of change model in Isfahanian women of age 40 and older: A population-based study. ZUMS J. 2013;21:24–35. [Google Scholar]
- 12.Rakowski W, Clark MA, Pearlman DN, Ehrich B, Rimer BK, Goldstein MG, et al. Integrating pros and cons for mammography and Pap testing: extending the construct of decisional balance to two behaviors. Prev Med. 1997;26:664–73. doi: 10.1006/pmed.1997.0188. [DOI] [PubMed] [Google Scholar]
- 13.Musugire V. Factors Influencing Self-Breast Cancer Screening Practrices among Nurses and Midwives Working at King Faisal Hospital. University of Rwanda; 2020. [Google Scholar]
- 14.Abeje S, Seme A, Tibelt A. Factors associated with breast cancer screening awareness and practices of women in Addis Ababa, Ethiopia. BMC Womens Health. 2019;19:4. doi: 10.1186/s12905-018-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmani F, Moodi M, Yousefi A, Norozi E. Healthy beliefs regarding breast cancer screening in iranian women health volunteers: A path analysis. Korean J Fam Med. 2020 doi: 10.4082/kjfm.20.0001. InPress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azaiza F, Cohen M, Awad M, Daoud F. Factors associated with low screening for breast cancer in the Palestinian Authority: Relations of availability, environmental barriers, and cancer-related fatalism. Cancer. 2010;116:4646–55. doi: 10.1002/cncr.25378. [DOI] [PubMed] [Google Scholar]
- 17.Shirzadi S, Allahverdipour H, Sharma M, Hasankhani H. Perceived barriers to mammography adoption among women in Iran: A qualitative study. Korean J Fam Med. 2020;41:20–7. doi: 10.4082/kjfm.18.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok C, Lee MJ, Lee CF. Breast cancer perceptions and screening behaviours among korean women in Australia. J Immigr Minor Health. 2020;22:126–33. doi: 10.1007/s10903-019-00876-8. [DOI] [PubMed] [Google Scholar]
- 19.Shiryazdi SM, Kholasehzadeh G, Neamatzadeh H, Kargar S. Health beliefs and breast cancer screening behaviors among Iranian female health workers. Asian Pac J Cancer Prev. 2014;15:9817–22. doi: 10.7314/apjcp.2014.15.22.9817. [DOI] [PubMed] [Google Scholar]
- 20.Kotepui M, Piwkham D, Chupeerach C, Duangmano S. Knowledge, attitudes and practice of breast cancer screening among female personnel of Walailak University. Health Expect. 2015;18:3069–78. doi: 10.1111/hex.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Castillo AB, Hernández-Valero MA, Hovick SR, Campuzano-González ME, Karam-Calderón MA, Bustamante-Montes LP. A study on the knowledge, perception, and use of breast cancer screening methods and quality of care among women from central Mexico. J Cancer Educ. 2015;30:453–9. doi: 10.1007/s13187-014-0722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haghighi F, Portaghali P, Rafaty Javanbakht L, Ghanbarzadeh N, Hosseini SM. Knowledge, attitude, and practice of female teachers regarding breast cancer screening in Birjand. Mod Care J. 2012;9:146–55. [Google Scholar]
- 23.Farhadifar F, Taymoori P, Bahrami M, Zarea S. The relationship of social support concept and repeat mammography among Iranian women. BMC Womens Health. 2015;15:92. doi: 10.1186/s12905-015-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanske J, Meyer CP, Sammon JD, Choueiri TK, Menon M, Lipsitz SR, et al. The influence of marital status on the use of breast, cervical, and colorectal cancer screening. Prev Med. 2016;89:140–5. doi: 10.1016/j.ypmed.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Fouladi N, Pourfarzi F, Mazaheri E, Asl HA, Rezaie M, Amani F, et al. Beliefs and behaviors of breast cancer screening in women referring to health care centers in northwest Iran according to the champion health belief model scale. Asian Pac J Cancer Prev. 2013;14:6857–62. doi: 10.7314/apjcp.2013.14.11.6857. [DOI] [PubMed] [Google Scholar]
- 26.Yıldırım AD, Özaydın AN. Sources of breast cancer knowledge of women living in Moda/İstanbul and their attendance to breast cancer screening. J Breast Health. 2014;10:47–56. [Google Scholar]
- 27.Vishwakarma G, Ndetan H, Das DN, Gupta G, Suryavanshi M, Mehta A, et al. Reproductive factors and breast cancer risk: A meta-analysis of case-control studies in Indian women. South Asian J Cancer. 2019;8:80–4. doi: 10.4103/sajc.sajc_317_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elobaid YE, Aw TC, Grivna M, Nagelkerke N. Breast cancer screening awareness, knowledge, and practice among arab women in the United Arab Emirates: a cross-sectional survey. PLoS One. 2014;9:e105783. doi: 10.1371/journal.pone.0105783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson-Johnson LC, DeGroff A, Steele CB, Revels M, Smith JL, Justen E, et al. Mammography adherence: A qualitative study. J Womens Health (Larchmt) 2011;20:1887–94. doi: 10.1089/jwh.2010.2724. [DOI] [PubMed] [Google Scholar]
- 30.Tolma EL, Stoner JA, Li J, Kim Y, Engelman KK. Predictors of regular mammography use among American Indian women in Oklahoma: A cross-sectional study. BMC Womens Health. 2014;14:101. doi: 10.1186/1472-6874-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González P, Borrayo EA. The role of physician involvement in Latinas’ mammography screening adherence. Womens Health Issues. 2011;21:165–70. doi: 10.1016/j.whi.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Asmar M, Bechnak A, Fares J, Al Oweini D, Alrazim A, El Achkar A, et al. Knowledge, attitudes and practices regarding breast cancer amongst Lebanese females in Beirut. Asian Pac J Cancer Prev. 2018;19:625–31. doi: 10.22034/APJCP.2018.19.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haber G, Ahmed NU, Pekovic V. Family history of cancer and its association with breast cancer risk perception and repeat mammography. Am J Public Health. 2012;102:2322–9. doi: 10.2105/AJPH.2012.300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kırca N, Tuzcu A, Gözüm S. Breast cancer screening behaviors of first degree relatives of women receiving breast cancer treatment and the affecting factors. Eur J Breast Health. 2018;14:23–8. doi: 10.5152/ejbh.2017.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taymoori P, Berry T, Farhadifar F. Predicting mammography stage of adoption among Iranian women. J Educ Health Promot. 2012;1:13. doi: 10.4103/2277-9531.98571. [DOI] [PMC free article] [PubMed] [Google Scholar]


