Abstract
Introduction
The Food and Drug Administration issued an advanced notice of proposed rulemaking for setting a product standard for nicotine levels in cigarettes, with an emphasis on minimally or non-addicting very low nicotine content (VLNC).
Methods
A 33 week, two-arm, double-blind randomized trial conducted in Hershey, Pennsylvania, USA and Washington, DC, USA included adult daily cigarette smokers (≥5 cigarettes per day) with less than a college degree, and who had no plans to quit within the next six months. Participants were randomized to either reduced nicotine content (RNC) study cigarettes tapered every three weeks to a final VLNC (0.2 mg/cigarette) for six weeks or to usual nicotine content (UNC) study cigarettes (11.6 mg/cigarette). Outcomes included acceptability of study cigarettes measured by attrition (primary outcome), compliance, reduction in cigarette dependence and tobacco biomarkers, and post-intervention cessation.
Results
The RNC (n = 122) versus UNC (n = 123) group had higher attrition (adjusted Hazard Ratio 3.4; 95% confidence interval [CI] 1.99 to 5.81). At the end of the intervention, cotinine levels were 50% lower in the RNC group (mean group difference −137 ng/mL; 95% CI −172, −102). The RNC group smoked fewer CPD (−4.1; 95% CI −6.44, −1.75) and had lower carbon monoxide levels (−4.0 ppm; 95% CI −7.7, −0.4). Forty seven percent (29/62) of the RNC group were biochemically-confirmed compliant with smoking VLNC cigarettes (mean cotinine = 8.9 ng/ml). At three month follow-up, only compliant VLNC smokers quit with an assisted quit attempt (N = 6/22, 27%).
Conclusions
This study supports a VLNC standard in cigarettes.
Implications
Differential dropout and noncompliance indicate some smokers had difficulty transitioning to cigarettes with reduced nicotine. These smokers will benefit from supplemental nicotine in medicinal or noncombustible tobacco products if a nicotine reduction standard is established. Other smokers successfully transitioned to very low nicotine content cigarettes exclusively and substantially reduced their exposure to nicotine.
Introduction
The Family Smoking Prevention and Tobacco Control Act enacted by the U.S. Congress in 2009 granted the Food and Drug Administration (FDA) the authority to regulate tobacco products to protect public health.1 The Act allows the FDA to create tobacco product standards on tobacco constituents, including reducing the nicotine content in cigarettes in order to reduce their addictiveness. The FDA issued an advanced notice of proposed rulemaking in March 2018 to collect information that would inform setting a product standard for nicotine levels in combustible cigarettes, with an emphasis on non-addicting levels of nicotine (~0.2 mg/cigarette). The policy could be implemented immediately or progressively over time. A standard would be considered within a comprehensive nicotine policy framework2 addressing the risks and benefits of nicotine regulation in cigarettes within a marketplace that includes noncombustible nicotine delivery systems and medicinal nicotine.
Previous clinical studies of smokers switching to research cigarettes with reduced nicotine content found reductions in mean levels of nicotine exposure and biomarkers of tobacco smoke toxicants, with minimal adverse health effects.3–10 While encouraging, the development of a nicotine standard in cigarettes needs to consider the distribution of successful and unsuccessful responses in reducing nicotine, specifically to minimally or non-addicting levels.11
The current trial was conducted to test a progressive reduction of nicotine content in cigarettes to minimally addicting levels, but in response to the advanced notice of proposed rulemaking and updated comprehensive nicotine policy framework in 2017,2 we modified the analytical plan to emphasize the level of attrition when switching to progressively reduced nicotine cigarettes over an extended period of time, which is considered a measure of their acceptability.12 We hypothesized that attrition will be higher within the experimental group receiving reduced nicotine cigarettes. Determining the extent to which the population of smokers can achieve this reduction to non-addicting levels will help determine the feasibility of this standard and the degree to which alternative forms of nicotine delivery from less harmful products need to be provided to smokers who still want or need nicotine. Such data will also help anticipate potential black market demands for fully nicotinized cigarettes.
The inclusion criteria of the current trial were designed to be representative of smokers with low socioeconomic status (SES), defined as less than a bachelor's degree, to understand the safety and efficacy of a reduced nicotine standard in this population. Cigarette smoking is more prevalent among the unemployed, less educated, and other economically disadvantaged populations.13 These smokers experience higher nicotine dependence,14 lower motivation to quit,15 and higher rates of disease.16
Our study examined the gradual tapering of nicotine among the same smokers over an extended period to capture the stability of responses at each dose and allow smokers to acclimate themselves to lower nicotine doses before smoking a minimally addictive cigarette with very low nicotine content (VLNC). Specifically, the VLNC cigarettes smoked during the last six weeks of the randomized phase of the trial contained approximately 98% less nicotine than commercially available cigarettes, a level considered to be minimally or non-addictive.17
Methods
Study Protocol
A two-site, two-arm, double-blind, parallel-group, randomized clinical trial was conducted at the Penn State College of Medicine (Hershey, PA) and George Washington University (Washington, DC) between 2015 and 2018. Participants were recruited using print/radio advertisements, flyers/posters, social media, direct mailings, and word of mouth. Further details of clinical trial recruitment strategies targeted to smokers in disadvantaged communities were previously described.18,19 Participants were screened by phone for initial eligibility and scheduled for their first study visit to determine final eligibility and obtain informed consent. Eligibility included adult cigarette smokers aged 18–65, who smoked at least five cigarettes per day for the past year, and had no plans to quit in the next six months and no quit attempt in the past month. Additional inclusion criteria included having less than a bachelor's degree or 16 years of education, ability to read and write in English, plans to live in the local area, and the ability to receive phone calls during the study. Exclusions included current pregnancy, a plan to become pregnant, or nursing; systolic blood pressure ≥160 mmHg; an unstable or significant medical condition; non-cigarette nicotine delivery product or marijuana use in the past week; use of smoking cessation medication in the past month; difficulty providing blood samples; regular use (daily or almost daily) of illegal drugs; inpatient treatment for substance abuse or mental health condition in the past six months; alcohol abuse hindering study participation (based on investigator discretion); other members of the household participating in a study using research cigarettes, major surgery planned; or other factors that may affect adherence or pose a health risk to the participant.
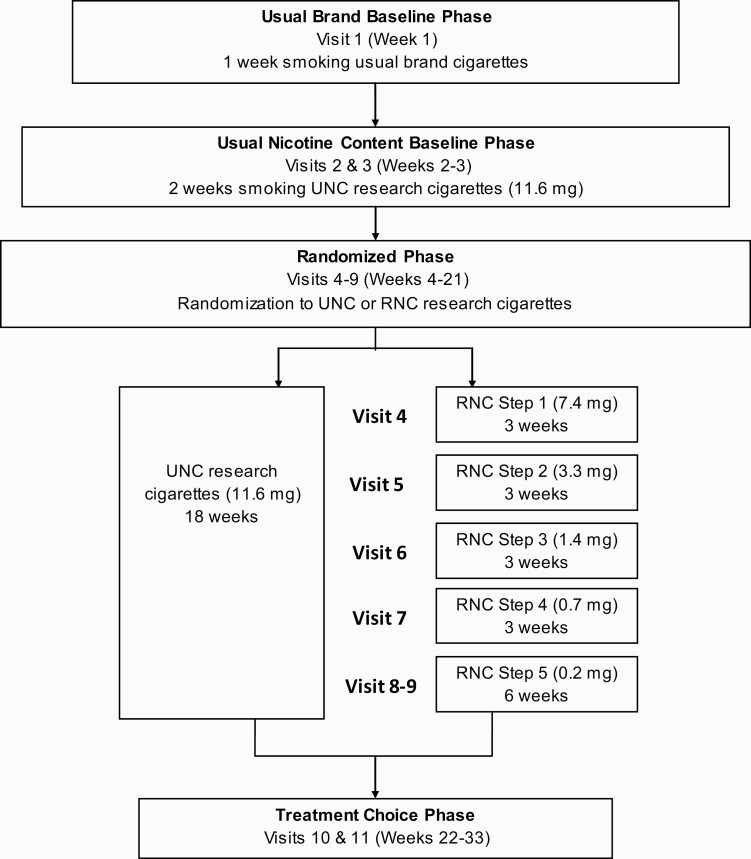
The study consisted of four phases (Usual Brand Baseline, Usual Nicotine Content Baseline, Randomized, and Treatment Choice) with 11 clinical visits at the study centers over 33 weeks (Figure 1). Usual Brand Baseline included smoking their usual brand cigarettes for one week. Usual Nicotine Content Baseline included two weeks of smoking usual nicotine content (UNC) study cigarettes (nicotine content approx. 11.6 mg/cigarette). In the 18 week Randomized phase, participants were randomized to the control arm in which they continued on the UNC study cigarettes, or the intervention arm to receive progressively reduced nicotine content (RNC) study cigarettes. The nicotine content in the RNC cigarettes was gradually reduced every three weeks from 7.4 to 0.2 mg/cigarette (VLNC). Participants received the VLNC cigarettes for another three weeks (a total of six weeks). During the Treatment Choice phase (12 weeks) participants chose to (1) continue to smoke study cigarettes at the same dose as their last visit (11.6 or 0.2 mg/cigarette), (2) return to their usual brand cigarettes (at their own cost), or (3) make a quit attempt with brief behavioral counseling and FDA-approved oral nicotine replacement therapy (NRT) (2 mg gum or lozenge). Those who chose to make a quit attempt received a six-day supply of study cigarettes (at the same dose as their last visit) and returned to the study center on the seventh day having abstained from smoking for a 24 hours period to assess nicotine withdrawal symptoms and receive NRT free of cost (if desired). All participants completed two study visits during the Treatment Choice phase of the study. Additional optional phone calls and study visits were provided to those who chose to quit to monitor and receive NRT and provide counseling. Participants received up to $1000 for their participation in the trial. Further details of the trial have been published.20 The study was approved by the institutional review board at each study site and registered at clinicaltrials.gov (NCT01928719).
Figure 1.
Study flow diagram. RNC = reduced nicotine content; UNC = usual nicotine content Due to limited availability of SPECTRUM UNC menthol cigarettes, 51 menthol smokers used comparable nicotine level SPECTRUM menthol cigarettes during Usual Nicotine Content Baseline and continued using this dose if they were randomized to the UNC group. Nicotine contents are based on an estimated 0.7 g tobacco content per cigarette and nicotine concentrations (mg/g) from Richter et al.21
Study Cigarettes
This trial used SPECTRUM research cigarettes (22nd Century Group, Inc.), obtained from the National Institute of Drug Abuse's Drug Supply Program. Their physical characteristics, chemical profiles, and pharmacokinetics are well characterized.21–23 During visits, participants received a supply of study cigarettes equal to 150% of their baseline cigarettes per day (CPD) to account for any potential changes in cigarette consumption or rescheduling of study visits. Participants received one cigarette flavor (menthol or non-menthol) based on their preference. All study cigarettes were provided free of charge. Participants were asked to return all study cigarette packs (empty, opened, unopened) to the study site at each visit, and to not use any other nicotine-containing products during the trial. While the use of other products was discouraged, participants were systematically asked to report the use of any non-study products during study contacts. Participants were instructed to keep a daily cigarette log to record study and non-study cigarettes smoked.
Randomization and Blinding
Participants were randomized using a computer-generated randomization sequence (1:1, a block size of six, stratified by study site and flavor) created by the study statistician. The randomization assignment was housed within a Cigarette Management System24 and maintained within the Investigational Drug Pharmacy at each site, where the study cigarettes were dispensed and managed under pharmacy-controlled protocols. The researchers and participants were blinded to the randomized allocation throughout the trial.
Outcome Measures
The primary outcome was study attrition (ie dropout) (defined as both withdrawal and loss to follow-up) during the Randomized phase. Plasma cotinine was analyzed using an immunoassay kit (Calbiotech, El Cajon, CA). Exhaled carbon monoxide [CO] (smoke exposure biomarker) was collected using the piCO+ Smokerlyzer (coVita, Haddonfield, NJ). See the Supplementary Appendix for additional biomarker methods and outcomes (eg 1-hydroxypyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), glutathione, 8-isoprostanes, cortisol). Total (non-study + study) CPD at each visit was calculated as a previous six-day average. Dependence measures included the Fagerström Test for Cigarette Dependence (FTCD) [1–10],25 Penn State Cigarette Dependence Index [0–20],26 and the Hooked on Nicotine Checklist [0–10].27 Withdrawal symptoms and smoking urges were assessed using the Minnesota Nicotine Withdrawal Scale [0–32]28 and Questionnaire on Smoking Urges-Brief [10–70].29 Quit status was assessed at Visits 10/11 and defined by CO level <10 ppm (parts per million) and self-reported smoking abstinence on the day of the visit plus the prior six days.
Self-reported noncompliance during the Randomized phase was defined as any non-study cigarette use on the date of the visit and the previous six days or any other nicotine-containing product use since the prior visit. Biochemical compliance was assessed in the RNC group at the final Randomized phase visit (Visit 9) using the Benowitz et al.30 method [V9 cotinine/CPD]/[V3 cotinine/CPD] with adjustment for environmental tobacco smoke (cotinine values subtracted by 15 ng/ml).31 A compliance ratio value of ≤0.075 was considered fully compliant, >0.075 to <1 partially compliant, and ≥1 noncompliant with VLNC cigarette use.
The safety monitoring protocol and data for adverse events and other health measures (eg blood pressure, pulse, weight, alcohol intake, respiratory health status) are outlined in the Supplementary Appendix.
Statistical Analysis
To address the primary study endpoint, a time-to-event analysis assessed the amount of time each participant remained in the study from randomization (Visit 3), to the end of the Randomized phase completion (Visit 9), withdrawal, or loss to follow-up. A Kaplan–Meier curve was generated to illustrate the dropout rate by study group throughout the Randomized phase. Univariate Cox proportional hazards regression models were used to examine the associations between each potential dropout predictor. If the predictor was significant at the 0.10 level, it was included in a multivariable Cox proportional hazards regression model to examine the effects of multiple predictors jointly. Estimated hazard ratios (HR) and corresponding 95% confidence intervals (CI) were presented. Two-way interactions between the predictors were explored, but all were removed due to non-significance.
Linear mixed-effects models for repeated measures were used to analyze nicotine exposure, dependence, and all other continuous outcome measures. An unstructured covariance structure was assumed for all models, except in the event of non-convergence, the first order autoregressive (AR(1)) structure was assumed. Profile plots were generated to visualize the trajectories of outcome measures between groups over time. Unadjusted linear mixed models were used to evaluate the main effects of discrete time (Visits 4–9), group (RNC vs. UNC group), and the time-by-group interaction, while adjusting for the baseline (Visit 3) measure of the outcome. Known confounders (eg sex, site, and menthol flavor) were consistently included in the adjusted models, while other covariates were included if their individual association with the outcome was significant (p value < 0.10). Estimated least-squares mean differences and corresponding 95% confidence intervals (CI) were reported at each clinic visit.
To explore possible effect modification of cigarette menthol flavor on progressive nicotine content reduction and other markers of smoke exposure, the menthol-by-group interaction was evaluated in the linear mixed models. The three-way interaction of menthol-by-group-by-time was not statistically significant in any of the models and therefore removed to further analyze the menthol-by-group two-way interaction term.
Study data were collected and managed using REDCap (Research Electronic Data Capture) hosted at the Penn State College of Medicine.32 Analyses were conducted by the Penn State TCORS Biostatistics and Database Management Core using statistical software SAS Version 9.4 (SAS Institute, Cary, NC) and R programming language Version 3.5.1 (R Foundation). All tests were two-sided at the 0.05 significance level, except during model selection where 0.10 was considered significant.
Sample Size Calculation
Sample size calculation was based on the secondary outcome, plasma cotinine concentration (measured as ng/ml). A total sample size of 280 participants (70 per group*2 cigarette groups*2 races[black and white]) would enable detection of a mean cotinine difference of 68 ng/ml as observed in the Benowitz et al. trial3 with at least 90% power.
Results
Sample Characteristics
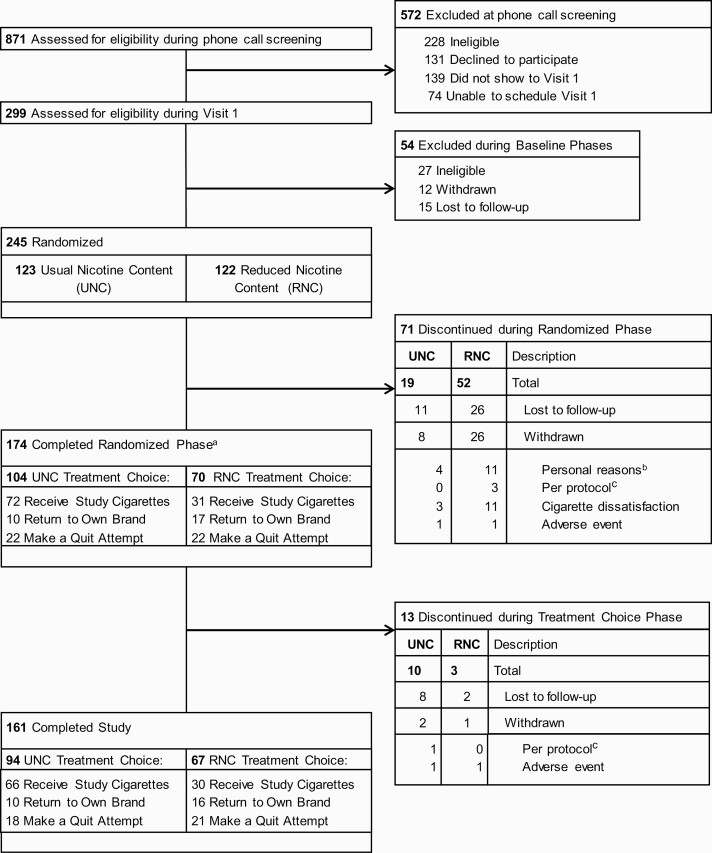
Of 280 participants enrolled, 245 (88%) were randomized (UNC, n = 123 and RNC, n = 122) [Figure 2 for CONSORT diagram]. Randomized participants were 48% male, 32% black, 55% unemployed, and 62% received a high school diploma/GED or less (Table 1 and Supplementary Table 1). Approximately 28% had incomes at the poverty level (<$20,000), and 76% were below the 2016 U.S. median household income (<$~60,000). The mean age was 44.9 (SD = 11.4) and the mean number of years reported smoking cigarettes daily was 27.8 (SD = 11.8). Approximately 69% smoked menthol cigarettes and the mean baseline CPD was 19.6 (SD = 9.3).
Figure 2.
CONSORT diagram. a31 (n = 16 RNC group; n = 15 UNC group) participants were limited to choices of returning to own brand or making a quit attempt due to limited study cigarette inventory. bPersonal reasons include schedule/transportation issues (n = 9), self-reported quit or active quit attempt (n = 3), or unrelated health issues (n = 3). cPer protocol reasons include pregnancy (n = 1), participant behavior (n = 1), or PI decision to withdraw (n = 2).
Table 1.
Demographic and Smoking Characteristics of Randomized Participants
| Overall (N = 245) | Usual nicotine content group (N = 123) | Reduced nicotine content group (N = 122) | |
|---|---|---|---|
| Usual brand baseline | |||
| aAge (years) | 44.9 (11.4) | 45.1 (10.8) | 44.7 (12.1) |
| aGender (male) | 117 (47.8) | 51 (41.5) | 66 (54.1) |
| aRace (n = 243) | |||
| White | 153 (62.4) | 82 (67.2) | 71 (58.7) |
| Black | 78 (31.8) | 35 (28.7) | 43 (35.5) |
| Other | 12 (4.8) | 5 (4.1) | 7 (5.7) |
| aHispanic ethnicity | 6 (2.4) | 2 (1.6) | 4 (3.3) |
| aEducation | |||
| Less than a HS degree | 40 (16.3) | 25 (20.3) | 15 (12.3) |
| HS degree or GED equivalent | 112 (45.7) | 57 (46.3) | 55 (45.1) |
| More than a HS degreeb | 93 (38) | 41 (33.3) | 52 (42.6) |
| aHousehold incomec (n = 184) | |||
| $0–19 999 | 68 (37) | 30 (31.2) | 38 (43.2) |
| $20 000–59 999 | 72 (39.1) | 41 (42.7) | 31 (35.2) |
| $60 000+ | 44 (23.9) | 25 (26) | 19 (21.6) |
| Cigarettes/day | 19.6 (9.3) | 19.8 (10.2) | 19.5 (8.4) |
| Exhaled carbon monoxide | 30 (15) | 30 (15) | 29 (15) |
| Plasma cotinine (ng/mL)d | 274 (151) | 266 (157) | 282 (145) |
| Median (Range) | 253 (3–812) | 237 (3–812) | 261 (24–730) |
Continuous measures reported as mean (standard deviation); Categorical measures reported as frequency (column percent).
HS= high school; GED= general education diploma.
aMeasure incorporated from PhenX Toolkit33 version October 5, 2015.
bLess than a Bachelor's degree required for inclusion.
cTotal Family (Household) Income includes the participant's income + income of all family members living in the participant's household (before taxes for the last calendar year).
dValues < Limit of Quantification (LOQ = 4.3) were coded as 3 (LOQ/sqrt(2)).
Randomized Phase Attrition
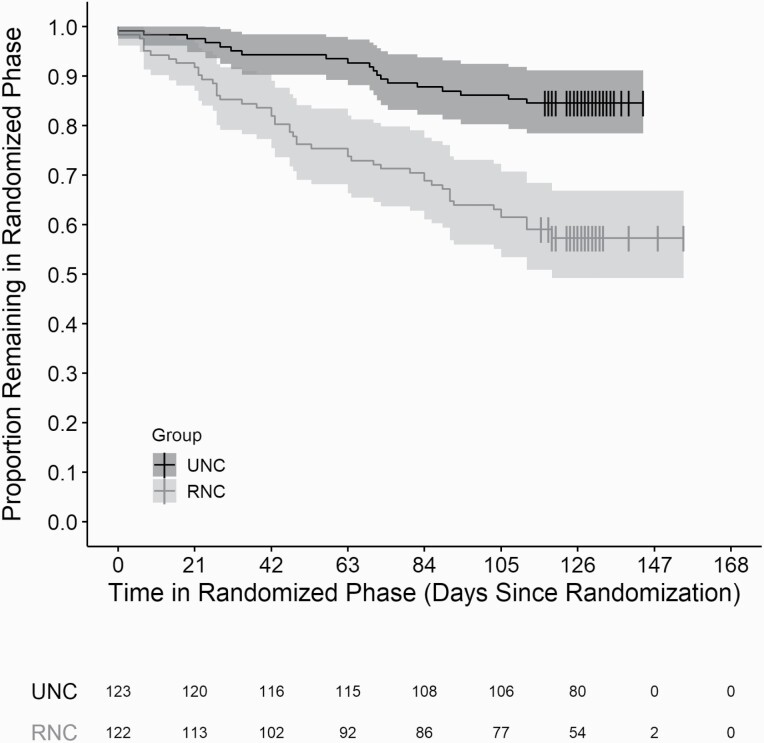
Differential attrition rates were observed after randomization (RNC group = 43% [52/122] vs. UNC group = 15% [19/123]). The RNC group was significantly more likely to dropout (either withdrawal or loss to follow-up) than the UNC group (HR 3.4; 95% CI 1.99 to 5.81; p < 0.001). In addition, age, body mass index, and employment status at the time of enrollment were associated with attrition (univariate models p < 0.10; Supplementary Table 2). In the final multivariable model, age, body mass index, and treatment group were significant (all p < 0.05) (Supplementary Table 2). A Kaplan–Meier curve is displayed in Figure 3.
Figure 3.
Kaplan–Meier curves. Graph shows Kaplan–Meier curves plotted by cigarette group strata, with corresponding confidence bands. The table below the plot identifies the number remaining in the randomized phase by cigarette group in three week increments. Vertical bars on the curves represent censoring, where participants completed the randomized phase of the study (Visit 9).
Smoking Behaviors and Biomarkers
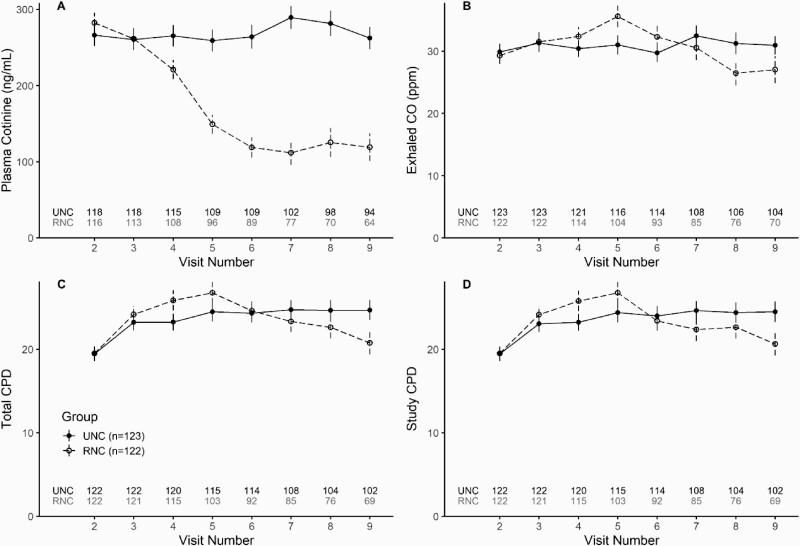
Significant reductions in CPD and exposure biomarkers were observed at the completion of the Randomized phase for the RNC group versus the UNC group (Figure 4). Plasma cotinine levels were nearly 50% lower among RNC smokers (Visit 9 mean difference [V9 MD], −137 ng/mL; 95% CI, −172, −102). Lower exhaled CO levels (V9 MD, −4.0ppm; 95% CI, −7.7, −0.4) and total CPD (V9 MD, −4.1; 95% CI, −6.44, −1.75) were observed in the RNC group. Menthol-by-group interactions were not significant and therefore not included in the models. See Supplementary Table 3 for outcome results for each visit.
Figure 4.
Cigarettes per day and exposure biomarkers. CO = Carbon Monoxide; PPM = parts per million; CPD = cigarettes per day. Graphs show means and standard errors of observed data. Visit 2 represents usual brand cigarettes and Visit 3 represents Usual Nicotine Content (UNC) cigarettes (11.6 mg/cigarette). UNC group received UNC cigarettes through Visit 9. Reduced Nicotine Content group received 7.4 mg/cigarette (Visit 3), 3.3 mg/cigarette (Visit 4), 1.4 mg/cigarette (Visit 5), 0.7 mg/cigarette (Visit 6), 0.2 mg/cigarette (Visit 7 and 8). Total CPD summarizes both study and non-study cigarettes. One outlier participant from the UNC group was removed in both CPD figures.
Levels of the tobacco carcinogen biomarker NNAL were lower in the RNC group at the end of the Randomized phase (V9 MD, −0.45; 95% CI, −0.74, −0.17). Total urinary 1-hydroxypyrene and oxidative stress measures (ie urinary 8-isoprostane and blood glutathione redox status [GSSP+2GSSG]/GSH), and peak cortisol levels were similar between the groups. Figures and results are presented in the Supplementary Appendix. Menthol-by-group interactions were non-significant in all models, except for NNAL where a menthol effect was significant in the UNC group (p = 0.02).
Cigarette Dependence
When examining the RNC group according to the FTCD specified categories that define minimal cigarette dependence (ie FTCD score of 1–2), 5 of 66 smokers (8%) with a baseline (Visit 3) FTCD score of >3 achieved an FTCD score of 1–2 at the end of the Randomized phase. UNC and RNC groups showed small differences in dependence measures including FTCD (V9 MD, −0.77; 95% CI, −1.13, −0.42); Penn State Cigarette Dependence Index (V9 MD, −1.02; 95% CI, −1.64, −0.4); and Hooked on Nicotine Checklist (V9 MD, −0.32; 95% CI, −0.8, 0.17). RNC smokers reported lower smoking urges based on the Questionnaire on Smoking Urges-Brief (V9 MD, −4.71; 95% CI, −8.41, −1.01). No differences were observed for withdrawal symptoms using the Minnesota Nicotine Withdrawal Scale (V9 MD, −0.09; 95% CI, −1.73, 1.55). All results are presented in Supplementary Figure 1 and Supplementary Table 5.
Self-reported Compliance
Self-reported noncompliance during at least one clinic visit was reported among 78 participants (UNC group, n = 43; RNC group, n = 35). Most noncompliant participants reported using non-study cigarettes. Six participants (UNC group, n = 2; RNC group, n = 4) reported using other nicotine-containing products (ie cigar, e-cigarette, or smokeless tobacco).
Biochemical Compliance
Out of the 62 participants assessed for biochemical compliance when smoking VLNC cigarettes at the end of the Randomized phase (n = 8 missing data for assessment), 47% (n = 29) were fully compliant, 34% (n = 21) were partially compliant, and 19% (n = 12) were noncompliant. Cotinine levels by compliance status and group are presented in Supplementary Figure 5.
Adverse Events
Safety measures and adverse event information are presented in Supplementary Tables 6–9 and Supplementary Figure 4.
Treatment Choice and Cessation
At Visit 9, 44% (n = 31/70) of the RNC group and 69% (n = 72/104) of the UNC group chose to continue to receive study cigarettes, 24% (n = 17/70) and 10% (n = 10/104) chose to return to their own brand, and 31% (n = 22/70) and 21% (n = 22/104) chose to make a quit attempt, respectively (chi-square p = 0.003). Among all randomized participants (dropouts were assumed to be smoking), quit rates biochemically confirmed by CO were 9% (n = 11/122) versus 3% (n = 4/123) at one month (Visit 10) [Fisher exact p = 0.07] and 7% (n = 9/122) versus 4% (n = 5/123) at three months (Visit 11) [Fisher exact p = 0.29] for the RNC and UNC groups, respectively. Among participants who chose to quit, the quit rate at one month was 36% (n = 8/22) for the RNC group and 18% (n = 4/22) [Fisher exact p = 0.31] for the UNC group and the three month quit rate was 27% (n = 6/22) for the RNC group and the quit rate for the UNC group remained the same [Fisher exact p = 0.72]. Participants in the RNC group were more likely to quit if they were determined to be biochemically compliant with the use of VLNC cigarettes (n = 7/22 compliers vs. 0/22 non-compliers quit at Visit 10 [Fisher exact p < 0.01]; n=6/22 compliers vs. 0/22 non-compliers quit at Visit 11 [Fisher exact p < 0.01]).
Discussion
In this trial of progressively reduced nicotine cigarettes where smokers were tapered to a six week regimen of minimally addictive cigarettes, the attrition rate was 3.4 fold higher in the RNC group compared to the UNC group. The higher attrition rate in the RNC group was consistent with our hypothesis. Study attrition is an important outcome in clinical trials of psychoactive drugs as a measure of their acceptability.34,35 While subjective ratings of RNC cigarettes have been assessed,19,36,37 study attrition as an indication of actual RNC cigarette use in randomized trials is perhaps the most objective measure of their acceptability.12 The larger attrition rate in the RNC group indicates some smokers were unwilling or unable to comply with smoking RNC cigarettes.
Although attrition was not formally evaluated in short-term (four–six weeks) gradual and immediate nicotine reduction trials, completion rates were similar between experimental and control groups.7,9 Longer term trials can measure more sustained ability to smoke reduced nicotine cigarettes. In a 20-week randomized trial comparing gradual versus immediate nicotine reduction by Hatsukami et al.5 the completion rate was 81% in the gradual arm and 86% in the control arm. In the current trial, the attrition rate was significantly higher in the RNC group, even with the monetary and free cigarette incentives that might be appealing to a low SES population. Attrition was also associated with low body mass index (<18.5) and younger age (18–29 years) in our final models. A driver of higher attrition in the RNC group could have been dissatisfaction in the taste of the RNC cigarettes as previously noted.12 In this trial, 11 participants in the RNC group versus only three in the UNC group reported dissatisfaction in the study cigarettes as a reason for withdrawal.
Compliance among those who remained in the trial is another important measure of the acceptability of reduced nicotine cigarettes. Overall, 47% of RNC smokers who completed the Randomized phase were found to be biochemically compliant with using VLNC cigarettes. We compared cotinine levels over time among compliance groups to provide more information on nicotine reduction acclimation among participants (Supplementary Figure 5). All RNC smokers had similar levels of cotinine reduction after the first nicotine dose reduction, after which cotinine levels in RNC non-compliers declined at a slower rate compared to RNC compliers, suggesting participants started to supplement with nicotine-containing products. Thereafter, cotinine levels for RNC non-compliers began to steadily rise after the third nicotine dose reduction, signaling the use of more nicotine-containing than non-nicotine containing products. These results provide an indication of when the tapering in nicotine became the most challenging for smokers and when relapse to nicotine-containing products most likely occurred.
Another measure of determining the efficacy of reducing cigarette nicotine content to minimal levels is the effect on cigarette dependence. Very few RNC subjects reported lowering their FTCD scores to minimal levels despite smoking reduced, and even minimally addictive cigarettes. The other measures of dependence also remained similar between the groups. Subjects may be habituated to the behavior of smoking cigarettes and as a result, don't experience lower dependence even when nicotine is progressively reduced. On the other hand, traditional measures of dependence like the FTCD may not be sensitive enough to capture a change in dependence in the context of a reduced nicotine trial.
These findings inform the FDA's comprehensive regulatory plan for tobacco and nicotine, which includes assessing the feasibility of nicotine reduction in cigarettes and the role of alternative, less harmful nicotine products.2 Our findings of differential attrition and noncompliance show that there were smokers unwilling or unable to smoke reduced nicotine cigarettes. These smokers may potentially benefit from alternative nicotine sources38,39 as well as novel approaches to increase the efficacy of NRT, such as more rapid nicotine delivery. In a study where participants were assigned to UNC or VLNC cigarettes while also having access to an open marketplace of alternative nicotine products, the VLNC smokers were more likely to use alternative products and further resulted in less toxicant exposure for those who were offered non-combusted products only.40 Alternative nicotine sources will need to be affordable in order to reduce incentives to purchase black market, fully nicotinized cigarettes.
There were clear benefits to smoking progressively reduced nicotine cigarettes. This included significant reductions in nicotine and smoke exposure (CPD and exhaled CO), without increased nicotine withdrawal symptoms or other adverse health effects. Exposure to the tobacco-specific carcinogen NNAL decreased in the RNC group, although other measures of toxicant exposure and oxidative stress from tobacco smoke remained the same between groups. The findings on tobacco smoke biomarkers are similar to other progressive nicotine reduction studies,3,7 and indicate a benefit of nicotine reduction in low SES smokers even if they are less willing or able to quit.
The most important goal in harm reduction from switching to reduced nicotine cigarettes is cessation.41 In this study, we evaluated quit attempts and quit success post-intervention as a follow up to smoking cigarettes with minimally or non-addicting nicotine levels. Among subjects who completed the trial, the RNC group was significantly more likely to choose to make a quit attempt. At the three month follow up visit, 7% (n = 9) of the RNC group achieved biochemically-validated abstinence with behavioral counseling and/or nicotine pharmacotherapy support. Further, only smokers who were compliant with smoking VLNC cigarettes achieved this abstinence. This supports setting a 0.2 mg nicotine level in cigarettes as a tobacco product standard. Although our study is one of few RNC cigarette trials to have validated smoking abstinence during follow up, others have shown self-reported quit attempts9 and higher quitting contemplation3 among VLNC smokers.
Strengths of the trial include the use of standardized research cigarettes, a low SES sample, inclusion of Black subjects, a sustained intervention over a relatively long period, and a post-intervention assessment of smoking status and quit behavior. Several limitations should be considered. Research cigarettes were given to participants at no cost which could have incentivized an increased frequency of use and/or study retention. By design, the study included smokers who had no plans to quit over a six month period. However, at the population level where some smokers have intentions to quit in the immediate future, the success of nicotine reduction might be even greater than that observed in the trial. UNC menthol cigarettes were no longer available towards the end of the study which prevented the choice to continue using UNC study cigarettes in the Treatment Choice phase for some menthol smokers. One cigarette brand (SPECTRUM) was used in this trial. RNC cigarettes in the marketplace may have different tastes or dimensions that affect smoking behavior. Sample sizes were not large in some subgroup analyses such as the quit rates in the RNC group of smokers who were VLNC compliers. The generalizability of the findings is limited by the inclusion and exclusion criteria. Although we did not exclude subjects with major psychiatric or mood disorders (except for recent inpatient hospitalization), potential subjects with these conditions were selectively recruited to a parallel study that required mood and anxiety disorders as an inclusion criteria.42 The findings cannot be generalized to dual users of tobacco products or women who are planning to become pregnant, are pregnant, or nursing. While there were no exclusions by race and ethnicity, the study included few Asians and Hispanics. Finally, it is possible that differential attrition might have been affected by the perceived benefits to stay in the trial, where the UNC group valued their cigarettes more because of their higher nicotine content.
This study supports a proposed policy of progressively reducing nicotine in cigarettes to minimally or non-addicting levels. While the specific amount of nicotine that is a threshold for addiction has not yet been identified; the current study supports a standard of 0.2 mg nicotine/cigarette. There were no significant adverse events attributed to the study cigarettes. Subjects switching to reduced nicotine cigarettes did not experience increased withdrawal symptoms during the trial, which is possibly related to the tapered nicotine reduction. Increased symptoms were observed in a trial with immediate reduction of nicotine although these were mild or temporary.43 Alternatively, smokers with high withdrawal symptoms may have dropped out of the study, negating a larger difference between groups. The RNC group substantially reduced their exposure to nicotine and NNAL. Successful one month and three month smoking cessation occurred among subjects who were able to switch to VLNC cigarettes. The differential dropout rate, inability for some smokers to smoke RNC and VLNC cigarettes exclusively over an extended period, and the overall lack of change from high or moderate to minimal cigarette dependence in many RNC smokers support the idea that concomitant intervention methods during nicotine reduction in commercial cigarettes are needed to help smokers transition to safer sources of nicotine. Further, sustained smoking cessation efforts will still be needed for smokers who are unable or unwilling to quit smoking very low nicotine cigarettes.
Supplementary Material
A Contributorship Form detailing each author's specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
The authors would like to acknowledge the Penn State Clinical and Translational Science Institute, Pennsylvania State University CTSA, and National Center for Advancing Translational Sciences (Grant numbers UL1TR000127, UL1TR002014) which funds the Clinical Research Center and the REDCap data management tools used in this study. The authors would also like to thank Rebecca Schorr, Abid Kazi, Jonathan McKinney, and Kayla Frederick for their lab support in the Biomarker Core lab.
Funding
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health and Center for Tobacco Products of the U.S. Food and Drug Administration (Grant numbers P50DA036107, P50DA036107-05S1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. The Penn State Cancer Institute provided funding for biomarker development to NT and LR. The funders had no role in the design or conduct of the study, interpretation of the data, or the preparation or decision to submit the manuscript for publication.
Declaration of Interests
JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience, and received a research grant and study product from Pfizer Inc. (not related to reduced nicotine cigarettes). There are no competing interests to declare for other authors.
References
- 1. Family Smoking Prevention and Tobacco Control Act of 2009. Pub. L. 111–31, 123 Stat. 1776 (June 22, 2009). [Google Scholar]
- 2. Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. [DOI] [PubMed] [Google Scholar]
- 3. Benowitz NL, Dains KM, Hall SM, et al. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomark Prev. 2012;21(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond D, O'Connor RJ. Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomark Prev. 2014;23(10):2032–2040. [DOI] [PubMed] [Google Scholar]
- 7. Mercincavage M, Souprountchouk V, Tang KZ, et al. A randomized controlled trial of progressively reduced nicotine content cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings. Cancer Epidemiol Biomark Prev. 2016;25(7):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P 3rd. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomark Prev. 2007;16(11):2479–2485. [DOI] [PubMed] [Google Scholar]
- 9. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hatsukami DK, Perkins KA, Lesage MG, et al. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19(5):e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mercincavage M, Wileyto EP, Saddleson ML, Lochbuehler K, Donny EC, Strasser AA. Attrition during a randomized controlled trial of reduced nicotine content cigarettes as a proxy for understanding acceptability of nicotine product standards. Addiction. 2017;112(6):1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang TW, Asman K, Gentzke AS, et al. Tobacco product use among adults - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. [DOI] [PubMed] [Google Scholar]
- 15. Reid JL, Hammond D, Boudreau C, Fong GT, Siahpush M, ITC Collaboration . Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12( suppl):S20–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 17. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 18. Horn K, Kuprewicz RM, Patterson K, et al. Clinical trial recruitment of adult African American smokers from economically disadvantaged urban communities. J Ethn Subst Abus. 2020;19(1):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veldheer S, Midya V, Lester C, et al. Acceptability of SPECTRUM research cigarettes among participants in trials of reduced nicotine content cigarettes. Tob Regul Sci. 2018;4(1):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krebs NM, Allen SI, Veldheer S, et al. Reduced nicotine content cigarettes in smokers of low socioeconomic status: study protocol for a randomized control trial. Trials. 2017;18(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richter P, Steven PR, Bravo R, et al. Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding YS, Richter P, Hearn B, et al. Chemical characterization of mainstream smoke from SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2017;3(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamens HM, Silva CP, Nye RT, et al. Pharmacokinetic profile of spectrum reduced nicotine cigarettes. Nicotine Tob Res. 2020;22(2):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kazi A, Fazzi A, Krebs NM, et al. Cigarette management system: an operating procedures guide to obtaining and managing investigational tobacco products for regulatory science research. Contemp Clin Trials Commun. 2018;11:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 26. Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015;17(2):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wellman RJ, DiFranza JR, Savageau JA, Godiwala S, Friedman K, Hazelton J. Measuring adults' oss of autonomy over nicotine use: the hooked on nicotine checklist. Nicotine Tob Res. 2005;7(1):157–161. [DOI] [PubMed] [Google Scholar]
- 28. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 29. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 30. Benowitz NL, Nardone N, Hatsukami DK, Donny EC. Biochemical estimation of noncompliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomark Prev. 2015;24(2):331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foulds J, Hobkirk A, Wasserman E, et al. Estimation of compliance with exclusive smoking of very low nicotine content cigarettes using plasma cotinine. Prev Med. 2018;117:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174(3):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rabinowitz J, Levine SZ, Barkai O, Davidov O. Dropout rates in randomized clinical trials of antipsychotics: a meta-analysis comparing first- and second-generation drugs and an examination of the role of trial design features. Schizophr Bull. 2009;35(4):775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berman ML, Glasser AM. Nicotine reduction in cigarettes: literature review and gap analysis. Nicotine Tob Res. 2019;21(Suppl 1):S133–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. M<?formattrackingstart -20?>ercincavage M, Saddleson ML, Gup E, Halstead A, Mays D, Strasser AA. Reduced nicotine content cigarette advertising: how false beliefs and subjective ratings affect smoking behavior. Drug Alcohol Depend. 2017;173:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Denlinger-Apte RL, Joel DL, Strasser AA, Donny EC. Low nicotine content descriptors reduce perceived health risks and positive cigarette ratings in participants using very low nicotine content cigarettes. Nicotine Tob Res. 2017;19(10):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benowitz NL. Comprehensive nicotine regulation to end the combustible tobacco epidemic. Ann Intern Med. 2017;167(10):736–737. [DOI] [PubMed] [Google Scholar]
- 39. Benowitz NL, Henningfield JE. Nicotine reduction strategy: state of the science and challenges to tobacco control policy and FDA tobacco product regulation. Prev Med. 2018;117:5–7. [DOI] [PubMed] [Google Scholar]
- 40. Hatsukami DK, Luo X, Dick L, et al. Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial. Addiction. 2017;112(1):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization Study Group on Tobacco Product Regulation. Global Nicotine Reduction Strategy. Geneva: World Health Organization; 2015. [Google Scholar]
- 42. Allen SI, Foulds J, Pachas GN, et al. A two-site, two-arm, 34-week, double-blind, parallel-group, randomized controlled trial of reduced nicotine cigarettes in smokers with mood and/or anxiety disorders: trial design and protocol. BMC Public Health. 2017;17(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dermody SS, McClernon FJ, Benowitz N, et al. Effects of reduced nicotine content cigarettes on individual withdrawal symptoms over time and during abstinence. Exp Clin Psychopharmacol. 2018;26(3):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.