Abstract
Reproducibility in animal research is crucial for its reliance and translational relevance. The 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced model of inflammatory bowel disease (IBD) is widely used but inconsistently and incompletely characterized throughout the literature. This hinders comparisons between studies and influences the low rate of translation of effective preclinical molecules. The purpose of this study was to categorize TNBS-induced colitis, based on macroscopic and microscopic scoring systems, and to identify basic routine parameters that could anticipate those categories. We retrospectively analysed male Wistar Rattus norvegicus (n=28 for the control group and n=87 for the TNBS group) and categorized TNBS-induced colitis in three phenotypes: Mild, Moderate and Severe colitis, as for human IBD. Also, we showed that the time course of food intake and fecal excretion (but not body weight, fluid intake or welfare scores) could foresee those categories. So, routine evaluation of food intake and fecal excretion may guide researchers in planning their experiments, selecting the animals with the severity of colitis that better matches their aims, or applying early humane endpoints to animals that will not be used in the experiments. In conclusion, categorizing TNBS-induced colitis enhances the reproducibility of data gathered with this experimental model and strengths its translational relevance.
Keywords: 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis; animal model; categorization; inflammatory bowel disease
Introduction
Animal models are fundamental to characterize the pathophysiology of diseases, uncover drug targets and test novel therapeutic approaches in vivo. However, complete information about the protocol for induction of the animal model and/or animal welfare is frequently absent in scientific literature and can lead to experimental failure and misguided conclusions, compromising the quality of science itself [1, 2]. Also, insufficient and/or not fully reported information contributes to decrease the reproducibility of animal studies and partially justifies why molecules that have been successful in preclinical studies have such a low efficacy when tested in humans [2].
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, is a chronic inflammatory condition of the gastrointestinal tract which prevalence surpasses 0.3% in western countries [3]. This complex and costly disease has a negative impact on the health and quality of life of patients. Several drugs induce and maintain remission but are not effective in every patient and frequently cause adverse reactions [2]. Due to the complexity of IBD, appropriate animal models, and associated metrics of disease, are essential to study its multifactorial aetiology and pathophysiology, as well as to test the efficacy of new prevention and/or treatment strategies [1, 2, 4].
The TNBS (2,4,6-trinitrobenzene sulfonic acid) model is the most common chemically-induced IBD model in Rattus norvegicus due to its cost-effectiveness, easy induction technique, and broad tissue collection [1, 4,5,6]. Administration of TNBS to Rattus norvegicus causes typical symptoms of the human disease: absence of stool formation or the presence of diarrhea (sometimes bloody), weight loss and a general welfare decrease [4, 7, 8]. TNBS is rectally instilled as an ethanolic solution in which the alcohol is essential for disrupting the intestinal epithelial cell barrier, enabling TNBS to penetrate the bowel wall and to be recognized as a target by the host immune system [7, 9]. It has been reported that TNBS-induced disease activity in Rattus norvegicus depends on the ethanol/TNBS dosage, strain, age, weight and housing conditions of the animals [1, 4, 7]. Furthermore, several parameters are used to evaluate the activity of the disease, like the disease activity index (DAI), colon weight/length ratio, and macroscopic [10, 11] or microscopic scores [11, 12]. But these are not standardized across studies, prompting the urge to implement uniformed criteria to monitor experimental colitis. A recent survey revealed that scientific papers generally do not report how TNBS-induced animals were maintained or their clinical evaluation, including humane endpoints [1, 4, 13].
From our experience, the symptoms manifested by the animals differ despite induction and monitoring standardization or housing conditions, constituting an interference factor for data gathered from functional studies. Therefore, the main aim of the present study was to define 3 categories of colitis in TNBS-induced Rattus norvegicus, based on macroscopic or microscopic scoring systems, in order to increase data reproducibility. Furthermore, we aimed at evaluating whether the time-course of basic routine parameters used for monitoring the animals could be used to anticipate those categories. To enhance the translational relevance of the Rattus norvegicus TNBS-induced model of colitis, the number and criteria for defining the categories was chosen to resemble those defined for classifying the activity of human IBD (mild, moderate and severe colitis), which are determined by macroscopic evaluation during colonoscopy procedure, and further characterized by microscopic evaluation of colonic biopsies.
Materials and Methods
Animals
Animals were raised and maintained in the rodent animal house facility of ICBAS-UP (approved by the national competent authority: 024159/2017-DGAV). According to the EU Directive 2010/63 and the Portuguese DL 113/2013, the project was approved by the local (179/2017-ORBEA-ICBAS-UP) and national (003511/2018-DGAV) competent authorities, and the study was reported in accordance with the ARRIVE guidelines.
Male Wistar Han Rattus norvegicus from different litters [14], were housed in individually ventilated cages (GR900 sealsafe plus; Tecniplast, Milan, Italy) with proper bedding (Corncob ultra 12; Ultragene, Santa Comba Dão, Portugal), nesting (soft paper), enrichment material (polycarbonate/paper tunnels, seeds and sugar-free cereal flakes weekly), and ad libitum access to autoclaved tap water and laboratory rodent diet (4RF21, Mucedola S.r.l., Milan, Italy). Complete feed for rats maintenance included the following ingredients (listed in descending order of inclusion): wheat, maize, soybean meal extracted toasted, corn gluten feed, wheat straw, fishmeal, lucerne meal, mineral dicalcium phosphate, calcium carbonate, sodium chloride, whey powder, soybean oil and yeasts. Proximate analysis: moisture (12.00%); crude protein (18.50%); crude oil (3.00%); crude fibre (6.00%); ash (7%) and Nitrogen Free Extract (NFE, 53.30%). The facilities had controlled ventilation, temperature (20–24°C), relative humidity (40–70%), and regular lighting cycles (12/12h). Animals were housed with their littermates (2–3 per cage) until colitis induction, from when they were kept individually.
Colitis induction
At 8–12 weeks of age, all animals were randomly assigned to control (n=28) or TNBS group (n=87). Controls were not subjected to any further manipulation. On day −1, animals ascribed to the TNBS group were individually housed and fasted overnight with ad libitum access to a 5% sucrose solution, to ensure the existence of as few fecal pellets as possible in the distal colon. On day 0, these animals were anesthetized ad effectum with isoflurane (Isoflo®; Esteve, Barcelona, Spain) and a 30% ethanolic solution of TNBS (20mg/ Rattus norvegicus; 250µL; Sigma-Aldrich Inc., St. Louis, MO, USA) was rectally instilled with a feeding needle (ST1 75-0285, Harvard Apparatus, Holliston, MA, USA) [9]. Since then, analgesia was provided with paracetamol (Paracetamol® Farmoz, Sintra, Portugal, approximately 500 mg/Kg, PO, daily throughout the entire protocol) and/or tramadol (20 mg/kg, SC, Tramadol®, Labesfal, Santiago de Besteiros, Portugal, 100 mg/2 ml) as needed. Metoclopramide (1 mg/Kg, SC, Metoclopramida Labesfal, Santiago de Besteiros, Portugal) was given to prevent obstipation in the first days after induction. Throughout this entire study we observed a 1/87 mortality rate and one other animal was sacrificed after considering humane endpoints and the category (severe colitis) in which it would fall into.
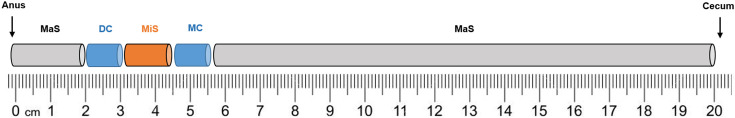
On day 7 or 8 after TNBS induction, animals were sacrificed by decapitation (small Decapitator, Harvard apparatus). The abdomen was opened, and the general appearance of the colon and surrounding tissues was observed. The colon with adjacent cecum was excised, the cecum was removed, and the colon gently cleaned of fecal content using Krebs-Henseleit solution (in mM: 118 NaCl, 4.8 KCl, 2.5 CaCl2.2H2O, 1.2 NaH2PO4.H2O, 1.2 MgSO4.7H2O, 25 NaHCO3, 0.02 Na2EDTA, 0.3 Ascorbic Acid and 11 glucose mono-hydrated). In a subset of control (n=4) and TNBS-induced (n=9) Rattus norvegicus, the colon was weighted (g) and measured (cm). Colonic perimeter was also assessed in a subset of controls (n=8) and TNBS-induced animals (n=16). Then, 1 cm-long segments of the middle colon (MC) and distal colon (DC) were collected (Fig. 1) for a functional ongoing study of our group. The rest of the colon was opened longitudinally to measure colonic perimeter (cm) and to allow evaluation of macroscopic and microscopic inflammatory damage (Fig. 1).
Fig. 1.
Portions of the colon used throughout the protocol to attribute the Macroscopic Score (MaS) and Microscopic Score (MiS). Distal Colon (DC) and Middle Colon (MC).
Evaluation of macroscopic inflammatory damage
Macroscopic inflammatory damage was quantified by observation of the mucosa and, according to the features described in Table 1, attribution of a Macroscopic score (MaS) that ranged from 0 to 12 points. We assessed the partial MaS of the MC and DC portions (Fig. 1) and then calculated the total MaS as the average of partial MaS.
Table 1. Criteria to assess Macroscopic Score (MaS) (Adapted from [10, 11]).
| Parameter | Score |
|---|---|
| Adhesions between the colon and the adjacent organs | 0 – absent |
| 1 - mild/focal | |
| 2 - moderate/zonal | |
| 3 - severe/diffuse | |
| Colon thickness | 0 - normal |
| 1 - mild increase | |
| 2 - moderate increase | |
| 3 - marked increased | |
| Mucosal edema/hyperemia | 0 - absent |
| 1 - mild | |
| 2 - moderate | |
| 3 - severe | |
| Mucosal ulcers | 0 - absent |
| 1 - single | |
| 2 - at one site | |
| 3 - at more sites | |
Evaluation of microscopic inflammatory damage
Microscopic inflammatory damage was evaluated in a 1.5 cm-length full thickness segment of the colon (between DC and MC; Fig. 1) of a subset of control (n=8) and TNBS-induced animals (n=17). For that, the segment was fixed in 10% buffered formalin, embedded in paraffin wax, and cut in 3µm full thickness sections that were stained with haematoxylin and eosin. A veterinary pathologist, who was blind to the experiment, applied a Microscopic score (MaS) that ranged from 0 to 10 points, according to criteria previously defined (Table 2) [11].
Table 2. Criteria to assess Microscopic Score (MiS) (Adapted from [11]).
| Parameter | Score |
|---|---|
| Mucosal damage | 0 - absent |
| 1 - mild | |
| 2 - moderate | |
| 3 - severe | |
| Inflammatory infiltrate | 0 - absent |
| 1 - mild | |
| 2 - moderate | |
| 3 - severe | |
| Number of layers infiltrated | 0 - none |
| 1 - one | |
| 2 - two | |
| 3 - three or more | |
| Mucosal edema | 0 - absent |
| 1 - present | |
Categorization of TNBS-induced colitis
To converge with the categorization of human IBD [15], we categorized TNBS-induced Rattus norvegicus in three categories that were defined by the MaS. As so, TNBS-induced Rattus norvegicus presenting MaS=[0–4[ were categorized as having Mild colitis, TNBS-induced Rattus norvegicus with MaS=[4–8[ were categorized as having Moderate colitis, and those TNBS-induced Rattus norvegicus with MaS=[8–12] were categorized as having Severe colitis.
Considering MiS, we defined TNBS-induced Rattus norvegicus presenting MiS=[0–4[ as having Mild colitis, TNBS-induced Rattus norvegicus with MiS=[4–7[ as having Moderate colitis, and those TNBS-induced Rattus norvegicus with MiS=[7–10] as having Severe colitis.
Monitoring of TNBS-induced colitis
All animals were daily monitored assuring the proper surveillance/application of humane endpoints. The following parameters were daily registered: body weight, food and fluid intake and the number of fecal pellets. Moreover, two scores were applied, every day, by cage-side assessment: general welfare/clinical signs (Table 3) and real-time Rattus norvegicus Grimace scale for facial expression of pain/discomfort [13, 16] (Table 4).
Table 3. General welfare/clinical signs score sheet.
| General welfare/Clinical signs | Score |
|---|---|
| Normal posture and activity | 0 |
| Reduced grooming and activity. Dehydration < 5%a) | 1 |
| Reduced grooming. Hypokinesia. CDR. Dehydration 5–8%b) | 2 |
| Piloerection. Hypokinesia. Kyphosis. CDR. Dehydration > 8%c) | 3d) |
| Inactive. Kyphosis. Non-responsive | 4e) |
a) Dehydration <5%: normal skin tent, membrane mucous and eyes; b) Dehydration between 5–8%: moderate skin tent and dry membrane mucous; c) Dehydration >8%: severe skin tent, dry membrane mucous and sunken eyes; d) Call for veterinarian assistance, with eventual withdraw from study (human endpoints); e) Immediate euthanasia (anaesthetic overdose). CDR=chromodacryorrhoea.
Table 4. Grimace Scale (Adapted from [16]).
| Not present | Moderately present | Obviously present | ||
|---|---|---|---|---|
| Orbital tightening | 0 | 1 | 2 | |
| • Closing of the eyelid (narrowing of orbital area) | ||||
| • A wrinkle may be visible around the eye | ||||
| Nose/Cheek flattening | 0 | 1 | 2 | |
| • Flattening and elongation of the bridge of the nose | ||||
| • Flattening of the cheeks | ||||
| Ear changes | 0 | 1 | 2 | |
| • Ears curl inwards and angled forward | ||||
| • Space between the ears increases | ||||
| Whisker change | 0 | 1 | 2 | |
| • Whiskers stiffen and angle along the face | ||||
| • Whiskers may “clump” together | ||||
| • Whiskers lose their natural “downward” curve | ||||
Statistical analysis
Using power analysis to calculate sample size [17], only 1-3 Rattus norvegicus per group would be necessary to detect differences between MaS or MiS categories in 95% (β of 0.05) of cases with an α value of 0.05 (“Sample Size Calculation” free software). However, we analysed a larger sample since we could share the TNBS-induced animals that were ascribed to functional ex vivo protocols of our group.
All data are mean (SEM) or median (25–75% interval) and, when stated, n refers to the number of experimental animals. Statistical analysis was performed using GraphPad Prism 7, by Mann-Whitney test or Kruskal-Wallis followed by Dunn’s multiples comparisons test, or by regular or Brown-Forsythe and Welch one-way ANOVA followed by Tukey’s or Dunnett’s multiple comparisons test, as appropriate. Also, the Chi-square test was used to compare the % of rats that were allocated to each category. Spearman’s correlation was used to estimate correlation between MaS and MiS of each animal and between average food intake or fecal excretion and MaS or MiS. Generally, a P<0.05 was considered statistically significant, unless other criteria is defined.
Results
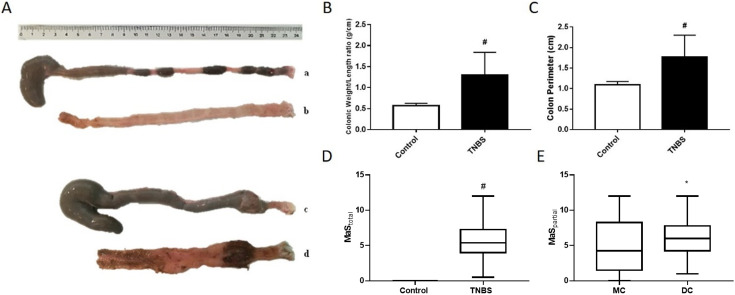
The colon of control animals was generally filled with ovoid-shaped fecal pellets (Fig. 2Aa), whereas that of TNBS-induced Rattus norvegicus was filled by pasty stools (Fig. 2Ac) and occasionally adhered to other organs, such as the small intestine or seminal vesicles. We frequently observed (mainly on day1 and day2) perineal muco-sanguineous secretions, that sometimes evolved to loosen stools, and less often to diarrhea. Abdominal liquid or blood was never observed. TNBS-induced animals had a higher colon weight/length ratio (Fig. 2B) and a higher colonic perimeter (Fig. 2C) than controls. Moreover, the mucosa of TNBS-induced Rattus norvegicus was usually thicker than that of controls (Figs. 2Ab and 2Ad), with a variable number of hyperaemic and ulcered areas, that were more abundant and deeper on the DC (Fig. 2Ad). Considering the previous reported criteria, TNBS-induced Rattus norvegicus had higher MaS than controls (Fig. 2D) and the DC had higher MaS than the MC (Figs. 2Ad and 2E).
Fig. 2.
(A) Representative photographs of intact cecum-colon and colonic mucosa of control (Aa and Ab, respectively) and TNBS-induced rats (Ac and Ad, respectively), with the same scale. (B) Colon weight/length ratio (g/cm) of control and TNBS-induced rats. (C) Colonic perimeter (cm) of control and TNBS-induced rats. (D) Total Macroscopic Score (MaS; median[25–75% interval]) of colonic mucosa of control and TNBS-induced rats. (E) MaS of Middle Colon (MC) and Distal Colon (DC) of TNBS-treated rats (median[25–75% interval]). TNBS-induced animals, n=87; Control animals, n=28. *P<0.05, #P<0.0001.
Categorization of the TNBS-induced model of colitis in Rattus norvegicus
From the 87 TNBS-induced Rattus norvegicus, 21 (24%) were found to develop Mild colitis (MaS=[0–4[), 50 (58%) developed Moderate colitis (MaS=[4–8[), and 16 (18%) developed Severe colitis (MaS=[8–12]). The distribution of cases between categories of TNBS-induced colitis was statistically different (P<0.0001), with most of the TNBS-induced animals developing moderate colitis.
Macroscopic evaluation of TNBS-induced Rattus norvegicus
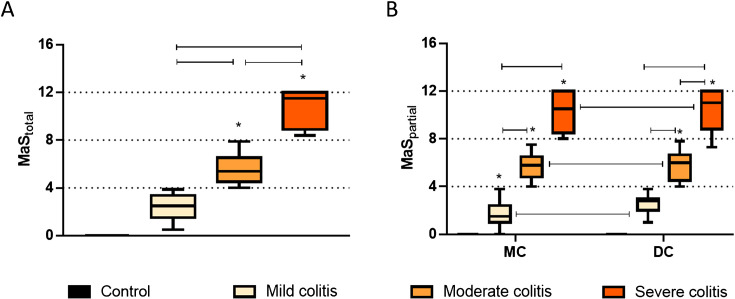
As can be seen in Fig. 3A, the total MaS calculated for TNBS-induced rats was higher than that of controls, which also scored 0, and increased progressively with colitis severity (Mild colitis < Moderate colitis < Severe colitis). This was also observed when we analysed partial MaS, for MC and DC (Fig. 3B). Moreover, the partial MaS of the DC was higher than that of the correspondent MC in TNBS-induced rats independently of colitis severity (Fig. 3B).
Fig. 3.
(A) Total Macroscopic Score (MaStotal) of TNBS-induced rats with Mild colitis, Moderate colitis and Severe colitis. (B) Partial Macroscopic Score (MaSpartial) of the Middle Colon (MC) and Distal Colon (DC) of TNBS-induced rats with Mild colitis, Moderate colitis and Severe colitis. TNBS-induced animals, n=87; Control animals, n=28. Results are median[25–75% interval]; *P<0.0001 vs controls.
Microscopic Evaluation of TNBS-induced rats
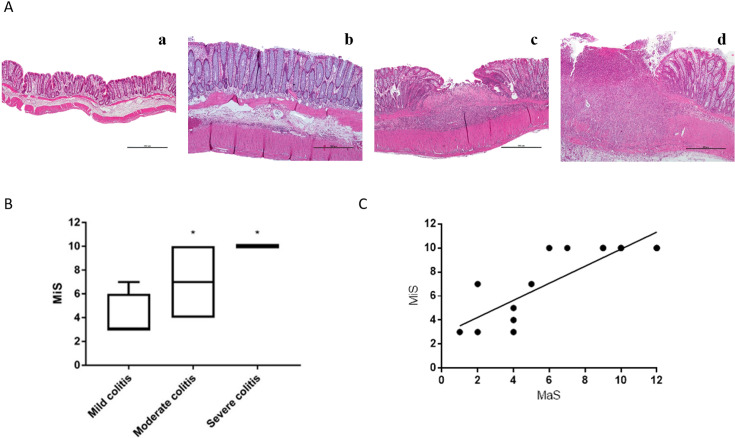
Microscopic evaluation distinguished control rats (Fig. 4Aa) and TNBS-induced Rattus norvegicus with Mild colitis (Fig. 4Ab), Moderate colitis (Fig. 4Ac) or Severe colitis (Fig. 4Ad). Colonic microscopic damage in TNBS-induced Rattus norvegicus with Mild colitis was characterized by mucosal thickening with proliferation of glandular units and Goblet cells hyperplasia, along with discrete diffuse inflammatory infiltrate (mononuclear cells, neutrophils and eosinophils, as previously observed [18]) confined to the mucosa. Multifocal lymphoid aggregates were occasionally observed in the lamina propria (Fig. 4Ab). Differently, colonic samples from TNBS-induced Rattus norvegicus with Moderate colitis showed multifocal erosive/ulcerative lesions, moderate to extensive inflammatory infiltrate, spreading from the mucosa to the submucosa, but spearing the muscular layers. Areas of mucosal fibrosis and signs of re-epithelization were also observed (Fig. 4Ac). Finally, TNBS-induced animals that evolved with Severe colitis had colonic microscopic lesions characterized by marked distortion of the architectural arrangements, with extensive and deep ulceration reaching the muscular layers and, sometimes, the serosa. A massive inflammatory infiltrate was observed involving all the layers of the colonic wall (Fig. 4Ad). As evidenced in Fig. 4B, the median rank of MiS progressively increased with colitis severity: Mild colitis < Moderate colitis < Severe colitis and was significantly higher in TNBS-induced Rattus norvegicus with Severe colitis than in TNBS-induced Rattus norvegicus with Mild colitis. There was a significant positive correlation (r=0.7057, P<0.0001; Fig. 4C) between the MaS and the MiS of each TNBS-induced animal.
Fig. 4.
(A) Representative photographs of H&E-stained distal colon cross-sections from Control rats (Aa) and of TNBS-induced rats with (Ab) Mild colitis, (Ac) Moderate colitis and (Ad) Severe colitis; scale bars, 500 µm. (B) Microscopic score (MiS) of TNBS-induced rats with Mild, Moderate and Severe colitis. Results are median[25–75% interval]. (C) Correlation between MaS and MiS of each TNBS-induced animal; TNBS-induced animals, n=87; Control animals, n=28. *P<0.05 vs Mild colitis group.
Monitoring of physiological parameters of TNBS-induced rats
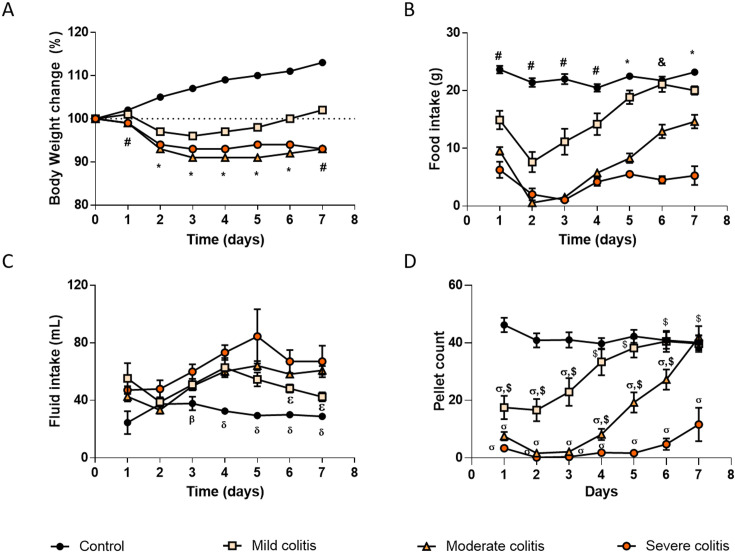
The body weight of all TNBS-induced Rattus norvegicus decreased in the first three days of the protocol and continued to be lower than that of controls until the end of the protocol (Fig. 5A). Then, TNBS-induced Rattus norvegicus with Mild colitis started to recover their body weight by day4 and, by the end of the protocol, their weight was already higher than their initial body weight (Fig. 5A). Differently, TNBS-induced Rattus norvegicus with Moderate colitis and Severe colitis lost 7.1% and 7.0% (respectively) of their initial body weight along the protocol (Fig. 5A). The area under the curve (AUC) for the body weight was different among the four experimental groups, the ranking order being: Control > Mild colitis > Severe colitis > Moderate colitis (750.5 ± 0.10; 690.0 ± 0.10; 653.5 ± 0.06 and 663.5 ± 0.06%.day respectively, P<0.0001).
Fig. 5.
Time course of physiological parameters during the experimental period. (A) Body weight change from day 0 (%), (B) food intake (g), (C) fluid intake (ml) and (D) number of fecal pellets, of control rats and of TNBS-induced rats with Mild colitis, Moderate colitis and Severe colitis. TNBS-induced animals, n=87; Control animals, n=28. *P<0.05 all groups are different between them for the same day; #P<0.05 all groups are different except TNBS-induced rats with Moderate vs TNBS-induced rats with Severe colitis, for the same day; &P<0.05 all groups are different except controls vs TNBS-induced rats with Mild colitis, for the same day; βP<0.05 Control rats vs TNBS-induced rats with Severe colitis, for the same day; δP<0.05 Control rats vs all other groups, for the same day; εP<0.05 TNBS-induced rats with Mild colitis vs TNBS-induced rats with Moderate colitis, for the same day; ơP<0.05 vs control group, for the same day; $P<0.05 vs TNBS-induced rats with Severe colitis, for the same day.
Overall food intake (evaluated by the AUC) was also different between the experimental groups, the ranking order being Control > Mild colitis > Moderate colitis>Severe colitis (Fig. 5B) (131.6 ± 5.17; 90.38 ± 13.10; 41.13 ± 9.04; 23.00 ± 5.94 g.day, respectively, P<0.0001). Moreover, the time-course profile was distinct for every experimental group. Control animals maintained a quite constant food intake along the protocol (average 22 g/day). All TNBS-induced Rattus norvegicus already showed a progressively lower food intake than controls in the first two days after induction (Fig. 5B). After that, TNBS-induced Rattus norvegicus with Mild colitis started to regain food intake that normalized by day6 (Fig. 5B). The decrease in food intake was more marked in TNBS-induced animals with Moderate and Severe colitis, which also showed a very low food intake on day3. These two experimental groups showed a slight increase in food intake by day4 that was then kept low in the Severe colitis group until the end of the protocol. Differently, TNBS-induced Rattus norvegicus with Moderate colitis continued to recover food intake until the end of the protocol (Fig. 5B).
Considering fluid intake, the AUC in TNBS-induced Rattus norvegicus that developed Mild or Moderate colitis was similar to that of controls and this was lower than that of TNBS-induced Rattus norvegicus that developed Severe colitis (Control = 193.9 ± 26.28 ml.day; Mild colitis = 304.2 ± 41.39 ml.day; Moderate colitis = 317.4 ± 33.25 ml.day; Severe colitis = 389.7 ± 67.21 ml.day; P=0.0207 for Control vs Severe colitis; P>0.05 for other comparisons). TNBS-induced Rattus norvegicus with Severe colitis drank more than controls from day3 to the end of the protocol as did TNBS-induced Rattus norvegicus with Mild or Moderate colitis from day4 onwards (Fig. 5C). The fluid intake of the Severe colitis group was the highest although it was not statistically different from that of the Moderate colitis group (Fig. 5C). Fluid intake in the Mild colitis group was similar to that of Moderate and Severe groups until day5, but then it decreased although it did not normalize until the end of the protocol (Fig. 5C).
Last, we quantified the excretion of fecal pellets and noticed a very characteristic time-course profile for each experimental group. Controls kept a relatively stable fecal excretion (average 42 pellets/day). In the first days after TNBS induction, the excretion of fecal pellets was markedly lower in all TNBS-induced Rattus norvegicus than in controls, particularly in the Moderate and Severe colitis groups (Fig. 5D). TNBS-induced animals with Severe colitis maintained a low fecal excretion along the entire protocol (always lower than that of controls and of the Mild colitis group), with a slight improvement by day7 (Fig. 5D). TNBS-induced Rattus norvegicus with Mild colitis started to recover fecal excretion on day3 and normalized it from day4 onwards (Fig. 5D) while TNBS-induced Rattus norvegicus with Moderate colitis started to recover fecal excretion by day4 (differentiating themselves from the Severe colitis group) and normalized it at the end of the protocol (day7) (Fig. 5D). The AUC analysis revealed that TNBS-induced Rattus norvegicus excreted less fecal pellets than controls in a colitis severity-dependent ranking: Control ≅ Mild colitis ≥ Moderate colitis ≥ Severe colitis (247.9 ± 22.70; 180.3 ± 31.77; 83.14 ± 30.58; 16.33 ± 12.17 pellets.day, respectively, P<0.0001) (Fig. 5D). Also pertinent, even though the stools of TNBS-induced Rattus norvegicus were mushier and more elongated than those of controls, diarrhea was rarely present.
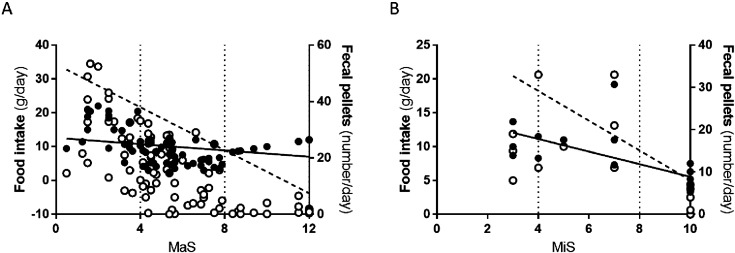
There was a significant inverse correlation between MaS and the average food intake (g/day; r=−0.2956, P=0.0054; Fig. 6A) as well as between MaS and the average excretion of fecal pellets (number/day; r=−0.7015, P<0.0001; Fig. 6A). Moreover, there was also a significant inverse correlation between MiS and the average food intake (g/day; r=−0,7769, P=0.0005; Fig. 6B) as well as between MiS and the average excretion of fecal pellets (number/day; r=−0.7466, P=0.0010; Fig. 6B). Based on these linear regressions, TNBS-induced Rattus norvegicus with Mild colitis should, on an average daily basis, eat more than 11 g/day and excrete more than 22 pellets/day; those with Moderate colitis would eat 9–11 g/day and excrete 9–22 pellets/day; and those with Severe colitis should eat less than 7 g/day and excrete less than 1 pellet/day.
Fig. 6.
Correlation between food intake and fecal pellets with MaS (A) and MiS (B). Black circles represent correlation between Food intake and MaS/MiS; White circles represent correlation between Fecal pellets and MaS/MiS.
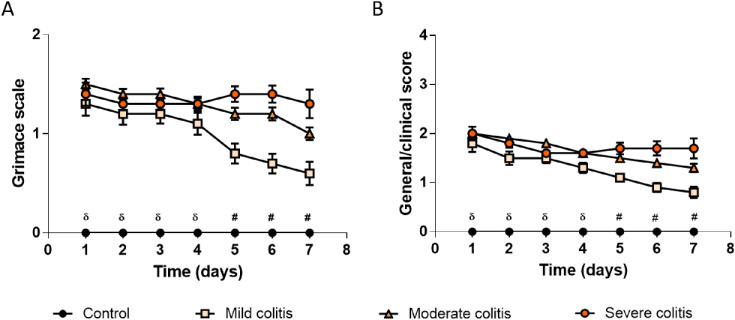
Monitoring of clinical signs of welfare of TNBS-induced Rattus norvegicus
TNBS-induced animals exhibited clinical signs and facial expressions of discomfort like orbital tightening, nose/cheek flattening, piloerection, chromodacryorrhoea, reduced grooming, dehydration or hypokinesia, which were never perceived in control Rattus norvegicus. Facial expression of discomfort (Fig. 7A) and general welfare/clinical signs (Fig. 7B) of TNBS-induced Rattus norvegicus with Mild colitis started to fade 5 days after colitis induction, while those of Rattus norvegicus from the Moderate and Severe colitis groups persisted. Controls always scored 0 points. This was also reflected by a higher AUC for all categories of TNBS-induced animals when compared with controls (scored 0) for both the Grimace scale (Mild colitis = 5.950 ± 0.834 points.day, Moderate colitis = 7.750 ± 0.7202 points.day, Severe colitis = 8.050 ± 0.5559 points.day; P<0.0001), and the general clinical signs score (Mild colitis = 7.60 ± 0.8941 points.day, Moderate colitis = 9.850 ± 0.9258 points.day, Severe colitis = 10.250 ± 0.7720 points.day; P<0.0001).
Fig. 7.
Welfare assessment of control and TNBS-induced rats. (A) Real-time Rat Grimace Scale by cage-side assessment and (B) General welfare/clinical signs scoring. Control group presented a score of zero, while rats with Mild colitis, Moderate colitis and Severe colitis presented a positive score. TNBS-induced animals, n=87; Control animals, n=28. δP<0.05 Control rats vs all other groups, for the same day; #P<0.05 all groups are different except TNBS-induced rats with Moderate vs TNBS-induced rats with Severe colitis, for the same day.
Discussion
This study is the first to propose three colitis categories to distinguish TNBS-induced Rattus norvegicus, based on a standardized macroscopic score that can be easily determined by any trained researcher, immediately after the animals’ euthanasia. This MaS-based categorization can help to improve reproducibility of data resulting from experimental colitis studies, inverting the tendency of failure during human clinical trials of molecules that were effective in animal preclinical testing (9 out of 10) [2]. Other studies on experimental IBD only use a disease activity index or other cumulative scores to determine the degree of the macroscopic damage [10] and to compare it between controls and TNBS-induced animals eventually treated with some innovative substances [11, 19]. These 3 categories were inspired by the colitis “disease status” activity categorization in human IBD (Mild colitis, Moderate colitis and Severe colitis) [20], crucial to choose the best therapeutic option for a particular patient [20]. To promote translational research, we based our categorization on a MaS that included some criteria also comprised in the Mayo score (the most frequently used macroscopic index in clinical trials), such as mucosal edema/hyperemia or the presence and extension of mucosal ulcers [15]. Further, we added parameters not feasible in the clinical setting but easy to assess in experimental models, like adhesions and colon thickness.
Also to promote translation, we performed a blind microscopic evaluation guided by similar criteria to those used in IBD patient biopsies (magnitude of the inflammatory infiltrate, affected layers, epithelial damage and the presence of ulcers) [21,22,23]. Histologic evaluation is one of the most cited technique to assess inflammatory damage in IBD models (present in 62.5% of articles on TNBS-induced colitis) [12, 13] but it is uncommon to categorize it. Interestingly, our MaS strongly correlated with MiS, despite the different anatomical localization of the samples sent to histological analysis (segment between the MC and DC) and those where the macroscopic evaluation was performed (the rest of the colon), that included less damaged areas than those sent for microscopic evaluation.
Our results also show that the mucosa of the DC was significantly more damaged by TNBS than that of the MC in the three categories of colitis severity, which is consistent with the distal-to-proximal inflammatory profile that is observed in human colitis, emphasising the translational relevance of this experimental model [20]. Additionally, this mucosal damage difference highlights the importance of clearly identifying the Rattus norvegicus colon sections used in each experimental protocol, in order to improve reproducibility.
We also aimed to identify basic routine parameters that could anticipate which colitis category each TNBS-induced Rattus norvegicus would fall into. Daily monitoring showed that the time-course of all the parameters (body weight, food and water intake, fecal pellets excretion, clinical signs and welfare) could differentiate control from TNBS-induced Rattus norvegicus. However, we uncovered that only the time-course of food intake and fecal excretion could distinguish between the three categories of TNBS-induced colitis. Even though our follow-up last only 7 days, which might be considered a small period to evaluate a chronic condition, it was sufficient to distinguish the time-course profile of the defined categories of colitis.
In control animals, food intake and fecal excretion were constant throughout the protocol. However, in TNBS-induced Rattus norvegicus, both parameters markedly decreased in the first 48h after induction, but then evolved differently between the three categories of colitis. Taken together the results obtained, we conclude that TNBS-induced Rattus norvegicus that start to recover food intake and fecal excretion by day 3 will probably evolve to Mild colitis, particularly if both parameters regain pre-induction levels by day 5. Differently, if TNBS-induced Rattus norvegicus start to recover only by day 4, the outcome will most probably be a Moderate colitis, with pre-induction levels of fecal excretion being attained by day 7. With a very different profile, TNBS-induced Rattus norvegicus that keep a very low food intake and fecal excretion (particularly until day5) will most probably develop the Severe colitis phenotype. Our data is not comparable to most studies since those usually do not evaluate physiological parameters daily, but every two days [24] or only at the beginning and at the end of the protocol [19]. Regarding fecal excretion, we quantified the number of fecal pellets while published data usually refers to stool consistency (varying between 0=normal to 4=diarrhea) and rectal bleeding (and/or fecal occult blood), merged in the quantification of DAI, an old but popular score to assess colitis [25,26,27]. Also, data on food intake is seldom reported in the literature [28]. Based on the inverse correlations found between average food intake or average fecal excretion and Mas or MiS, we could define cut off values for average food intake and average fecal excretion. However, we think that these cut offs should be interpreted with care, since the parameters that distinguished the colitis category were the time-course of food intake and fecal excretion and not the average daily values, which assume a regular intake/excretion of the animals. As so, we think it is crucial that researchers daily monitor TNBS-induced Rattus norvegicus to access their welfare, apply human endpoints (if needed) and anticipate their colitis category.
The time course of body weight and behaviour scores could only differentiate TNBS-induced Rattus norvegicus evolving to Mild colitis from those that would remain in the Moderate/Severe colitis categories. Also, TNBS-induced Rattus norvegicus that developed Mild colitis were the only TNBS-induced Rattus norvegicus that recovered their initial weight (by day 6). Body weight was not reported in 43.75% of the papers on TNBS-induced colitis [1]. As much, most publications report that while control animals gain weight along the protocols, TNBS-induced Rattus norvegicus lose weight particularly in the first 48h after induction and, then recover, though always maintaining a lower body weight than controls [11]. Furthermore, TNBS-induced Rattus norvegicus that developed Mild colitis were also the only ones that showed an improvement in both clinical signs and welfare scores, by day 5. Daily monitoring of general welfare/clinical signs and facial expression of pain/discomfort by real-time Rattus norvegicus Grimace scale was important to ensure humane endpoints. Our results are in agreement with others that highlight the relevance of the Rattus norvegicus Grimace scale [29,30], but assume limitations regarding the discrimination of subtle facial differences [31].
Water intake is also rarely reported [28] and data is controversial [25]. In our study, fluid intake was higher in TNBS-induced Rattus norvegicus independently of colitis category, from day4 onwards. We assume that the reported difference was a reflex of thirst, probably to avoid dehydration that follows fecal impaction and potential toxic megacolon [32]. Overall, to our knowledge, this is the first time that the time course of all these parameters was analysed in 3 different categories of TNBS-induced Rattus norvegicus, and so, it is not possible to compare our data with other observations.
In conclusion, our study can boost the quality of Rattus norvegicus TNBS-induced colitis-based research. First, it is possible to categorize Rattus norvegicus TNBS-induced colitis in three different categories: Mild, Moderate and Severe colitis, according to the MaS presented. This came up as a reliable tool since it correlated well with more laborious/skilled MiS, having the advantage of being easily incorporated in daily routine. This categorization resembles that used in human IBD, supporting data for translational research. Second, the time course of food intake and excretion of fecal pellets seem to anticipate colitis categorization ante mortem, allowing researchers to foresee the degree of colitis that better suits their investigation, reducing experimental variability, anticipating the feasibility of experimental protocols, and supporting the application of humane endpoints precociously. Finally, but not less important, our study increases awareness to the importance of discussing results gathered in comparable conditions, which should include the experimental colitis category of TNBS-induced Rattus norvegicus, in order to improve this animal model reproducibility.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia under the Partnership Agreement [UIDB 50006/2020 and SFRH/BD/145654/2019 to MFD]. Also, MM thanks Grupo de Estudos da Doença Inflamatória Intestinal for funding.
References
- 1.Bramhall M, Flórez-Vargas O, Stevens R, Brass A, Cruickshank S. Quality of methods reporting in animal models of colitis. Inflamm Bowel Dis. 2015; 21: 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeeff SB, Kunne C, Bouma G, de Vries RB, Te Velde AA. Actual Usage and Quality of Experimental Colitis Models in Preclinical Efficacy Testing: A Scoping Review. Inflamm Bowel Dis. 2016; 22: 1296–1305. doi: 10.1097/MIB.0000000000000758 [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390: 2769–2778. doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 4.Dothel G, Vasina V, Barbara G, De Ponti F. Animal models of chemically induced intestinal inflammation: predictivity and ethical issues. Pharmacol Ther. 2013; 139: 71–86. doi: 10.1016/j.pharmthera.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Ferri D, Costero AM, Gaviña P, Parra M, Merino V, Teruel AH, et al. Efficacy of budesonide-loaded mesoporous silica microparticles capped with a bulky azo derivative in rats with TNBS-induced colitis. Int J Pharm. 2019; 561: 93–101. doi: 10.1016/j.ijpharm.2019.02.030 [DOI] [PubMed] [Google Scholar]
- 6.Seoane-Viaño I, Gómez-Lado N, Lázare-Iglesias H, Barreiro-de Acosta M, Silva-Rodríguez J, Luzardo-Álvarez A, et al. Longitudinal PET/CT evaluation of TNBS-induced inflammatory bowel disease rat model. Int J Pharm. 2018; 549: 335–342. doi: 10.1016/j.ijpharm.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 7.Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, et al. The TNBS-induced colitis animal model: An overview. Ann Med Surg (Lond). 2016; 11: 9–15. doi: 10.1016/j.amsu.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi-Ahmadipour A, Rashidian A, Mirzaei MR, Farsinejad A, PourMohammadi-Nejad F, Ghazi-Khansari M, et al. Combination therapy of mesenchymal stromal cells and sulfasalazine attenuates trinitrobenzene sulfonic acid induced colitis in the rat: The S1P pathway. J Cell Physiol. 2019; 234: 11078–11091. doi: 10.1002/jcp.27944 [DOI] [PubMed] [Google Scholar]
- 9.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989; 96: 795–803. doi: 10.1016/0016-5085(89)90904-9 [DOI] [PubMed] [Google Scholar]
- 10.Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol. 1995; 269: G119–G125. [DOI] [PubMed] [Google Scholar]
- 11.Zizzo MG, Auteri M, Amato A, Caldara G, Nuzzo D, Di Carlo M, et al. Angiotensin II type II receptors and colonic dysmotility in 2,4-dinitrofluorobenzenesulfonic acid-induced colitis in rats. Neurogastroenterol Motil. 2017; 29: e13019. doi: 10.1111/nmo.13019 [DOI] [PubMed] [Google Scholar]
- 12.Brenna Ø, Furnes MW, Drozdov I, van Beelen Granlund A, Flatberg A, Sandvik AK, et al. Relevance of TNBS-colitis in rats: a methodological study with endoscopic, histologic and Transcriptomic [corrected] characterization and correlation to IBD. PLoS One. 2013; 8: e54543. doi: 10.1371/journal.pone.0054543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fentener van Vlissingen JM, Borrens M, Girod A, Lelovas P, Morrison F, Torres YS. The reporting of clinical signs in laboratory animals: FELASA Working Group Report. Lab Anim. 2015; 49: 267–283. doi: 10.1177/0023677215584249 [DOI] [PubMed] [Google Scholar]
- 14.Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013; 14: 37. doi: 10.1186/1471-2202-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohn’s Colitis. 2012; 6: 965–990. doi: 10.1016/j.crohns.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011; 7: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Festing MF. On determining sample size in experiments involving laboratory animals. Lab Anim. 2018; 52: 341–350. doi: 10.1177/0023677217738268 [DOI] [PubMed] [Google Scholar]
- 18.Zhao P, Guan H, Dong L, Luo J, Gong J. Role of mast cells and eosinophils in different stages of trinitrobenzenosulphonic acid-induced rat colitis. Int J Clin Exp Pathol. 2019; 12: 498–506. [PMC free article] [PubMed] [Google Scholar]
- 19.Petrella C, Giuli C, Broccardo M, Eutamene H, Cartier C, Leveque M, et al. Protective and worsening peripheral nociceptin/orphanin FQ receptor-mediated effect in a rat model of experimental colitis. Pharmacol Res. 2013; 70: 72–79. doi: 10.1016/j.phrs.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 20.Bernstein CN, Eliakim A, Fedail S, Fried M, Gearry R, Goh KL, et al. Review Team. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: Update August 2015. J Clin Gastroenterol. 2016; 50: 803–818. doi: 10.1097/MCG.0000000000000660 [DOI] [PubMed] [Google Scholar]
- 21.Jauregui-Amezaga A, Geerits A, Das Y, Lemmens B, Sagaert X, Bessissow T, et al. A Simplified Geboes Score for Ulcerative Colitis. J Crohn’s Colitis. 2017; 11: 305–313. [DOI] [PubMed] [Google Scholar]
- 22.Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, et al. European Society of Pathology (ESP)European Crohn’s and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohn’s Colitis. 2013; 7: 827–851. doi: 10.1016/j.crohns.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 23.Mohammed N, Subramanian V. Clinical relevance of endoscopic assessment of inflammation in ulcerative colitis: Can endoscopic evaluation predict outcomes? World J Gastroenterol. 2016; 22: 9324–9332. doi: 10.3748/wjg.v22.i42.9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah MK, Wan J, Janyaro H, Tahir AH, Cui L, Ding MX. Visceral Hypersensitivity Is Provoked by 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Ileitis in Rats. Front Pharmacol. 2016; 7: 214. doi: 10.3389/fphar.2016.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci. 2012; 7: 159–169. [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69: 238–249. [PubMed] [Google Scholar]
- 27.Cai Y, Liu W, Lin Y, Zhang S, Zou B, Xiao D, et al. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J Gastroenterol Hepatol. 2019; 34: 1554–1562. doi: 10.1111/jgh.14583 [DOI] [PubMed] [Google Scholar]
- 28.Pohlmann A, Tilling LC, Robinson A, Woolmer O, McCleary S, Kruidenier L, et al. Progression and variability of TNBS colitis-associated inflammation in rats assessed by contrast-enhanced and T2-weighted MRI. Inflamm Bowel Dis. 2009; 15: 534–545. doi: 10.1002/ibd.20800 [DOI] [PubMed] [Google Scholar]
- 29.Miller AL, Leach MC. The Mouse Grimace Scale: A Clinically Useful Tool? PLoS One. 2015; 10: e0136000. doi: 10.1371/journal.pone.0136000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperry MM, Yu YH, Welch RL, Granquist EJ, Winkelstein BA. Grading facial expression is a sensitive means to detect grimace differences in orofacial pain in a rat model. Sci Rep. 2018; 8: 13894. doi: 10.1038/s41598-018-32297-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung V, Zhang E, Pang DS. Real-time application of the Rat Grimace Scale as a welfare refinement in laboratory rats. Sci Rep. 2016; 6: 31667. doi: 10.1038/srep31667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onori L, Aggio A, D’Alo’ S, Muzi P, Cifone MG, Mellillo G, et al. Role of nitric oxide in the impairment of circular muscle contractility of distended, uninflamed mid-colon in TNBS-induced acute distal colitis in rats. World J Gastroenterol. 2005; 11: 5677–5684. doi: 10.3748/wjg.v11.i36.5677 [DOI] [PMC free article] [PubMed] [Google Scholar]