Abstract
The inbred mouse strain C57BL/6 has been widely used as a background strain for spontaneous and induced mutations. Developed in the 1930s, the C57BL/6 strain diverged into two major groups in the 1950s, namely, C57BL/6J and C57BL/6N, and more than 20 substrains have been established from them worldwide. We previously reported genetic differences among C57BL/6 substrains in 2009 and 2015. Since then, dozens of reports have been published on phenotypic differences in behavioral, neurological, cardiovascular, and metabolic traits. Substrains need to be chosen according to the purpose of the study because phenotypic differences might affect the experimental results. In this paper, we review recent reports of phenotypic and genetic differences among C57BL/6 substrains, focus our attention on the proper use of C57BL/6 and other inbred strains in the era of genome editing, and provide the life science research community wider knowledge about this subject.
Keywords: C57BL/6, genetic difference, phenotypic difference, single nucleotide polymorphism (SNP), substrain
Origin of the C57BL/6 Mice
The inbred mouse strain C57BL/6 has a long history. It was derived from the C57BL line, which was established in 1921 by Dr. C.C. Little, who mated littermates female 57 and male 52, which were obtained from the stock of Ms. A.E. Lathrop. This line was separated into sublines 10 and 6 in 1937, thereby establishing the C57BL/10 and C57BL/6 strains, respectively [1]. The C57BL/6 strain was introduced to the Jackson Laboratory (Bar Harbor, ME, USA) in 1948, and in 1951, the mice were sent from the Jackson Laboratory to the National Institutes of Health (NIH; Bethesda, MD, USA); thereafter, the strain was separately maintained at both institutions [2, 3]. Subsequently, the Jackson Laboratory’s C57BL/6J and NIH’s N strains became the major lineages of C57BL/6 mice, with a large number of substrains having since been derived from each [4, 5]. At present, seven C57BL/6 substrains are available from domestic vendors in Japan, and 11 are offered by overseas vendors (Supplementary Table 1). Various substrains have been maintained for many years within individual laboratories in Japan, including the National Institute of Genetics (Ms; Mishima, Japan) and the National Institute of Radiological Sciences (Nrs; Chiba, Japan), all of which are substrains of C57BL/6J or C57BL/6N (Fig. 1). The C57BL/6J strain was the first line for which the mouse genome was sequenced [8], and it continues to be used as a representative inbred mouse strain in various life science fields. Through community effort, a highly germline-competent embryonic stem cell line was established from the C57BL/6NTac strain [9], and it has been used as a background strain in the International Knockout Mouse Project and the International Mouse Phenotyping Consortium [10,11,12,13].
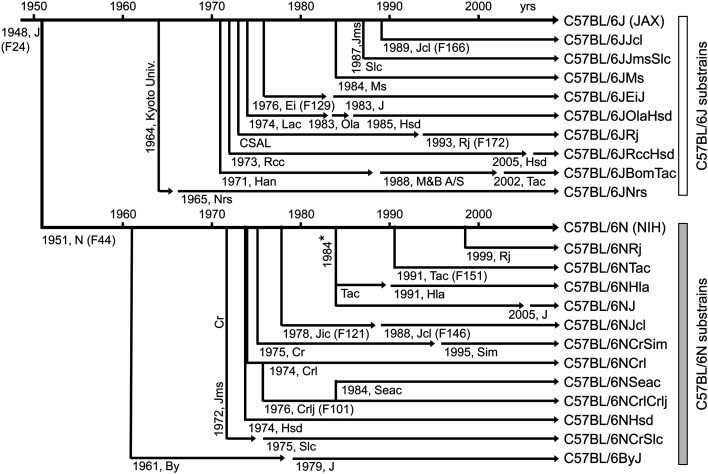
Fig. 1.
Genealogy of the C57BL/6 substrains. Dates and relationships are derived from the vendors’ websites and Mekada et al. (2009 and 2015) [6, 7]. Abbreviations: J, The Jackson Laboratory (JAX); Jcl, CLEA Japan, Inc.; Jms, Institute of Medical Science, Japan; Slc, Japan SLC, Inc.; Ms, National Institute of Genetics; Ei, Dr. Eva M. Eicher; Lac, Laboratory Animal Centre (MRC Carshalton Lab Animal Centre); Ola, Olac, Ltd.; Hsd, Harlan Sprague-Dawley, Inc.; CSAL, Centre de Sélection des Animaux de Laboratoire; Rj, Centre D’Elevage R. Janvier; Rcc, RCC Ltd.; Han, Zentralinstitut fur Versuchstierzucht, Hannover; M&B A/S, Taconic’s Bomholtgard, Denmark, facility; Tac, Taconic Biosciences, Inc.; Nrs, National Institute for Quantum and of Radiological Science and Technology; N, National Institutes of Health; Hla, Hilltop Lab Animals, Inc.; Jic, Central Institute for Experimental Animals, Japan; Cr, NCI, DCTD Animal Production Program; Sim, Simonsen Laboratories, Inc.; Crl, Charles River Laboratories; Crlj, Charles River Laboratories Japan; Seac, Seac Yoshitomi, Ltd. (Kyodo Co., Ltd.); By, Dr. Donald W. Bailey. *Derived from embryos frozen in 1984.
We analyzed the substrain status of 2,939 genetically modified C57BL/6 mice deposited at the RIKEN BioResource Research Center (BRC) (Fig. 2). Each substrain was further subdivided into substrains supplied by several vendors. In addition, over 600 strains (21% of the total) had a mixed genetic background of C57BL6/J and C57BL6/N or a genetic background of uncertain C57BL/6 substrain. We interviewed several scientists who either developed or deposited these strains and found that they had purchased and used the mice without knowing about the substrain distinctions or had not kept detailed breeding records. Similar occurrences have been reported elsewhere. Over 70% of the genetically engineered strains generated at German and Austrian institutions had a mixed genetic background [14]. A survey of Finnish institutions revealed that 39.5% of researchers did not consider the importance of differences among substrains and that 26% did not know which C57BL/6 substrain they had used [15]. Furthermore, 58% of the studies that were published in Diabetes during 2010–2014 and involved genetically modified mice provided incomplete descriptions of the background strains [16]. Despite the reported non-negligible phenotypic differences among C57BL/6 substrains, there seems to be little awareness of the importance of substrain selection within the research community.
Fig. 2.
Summary of a survey on the use of C57BL/6 substrains in Japan. According to information provided by the developer scientists of the 2,939 mouse strains deposited at RIKEN BRC, 49% were crossed with C57BL/6J substrains, including C57BL/6JJcl, C57BL/6JJmsSlc, and C57BL/6J, and 30% were crossed with C57BL/6N substrains, including C57BL/6NJcl, C57BL/6NCrSlc, C57BL/6NCrlCrlj (including C57BL/6NCrl in 35 strains), and C57BL/6NTac. Another 13% were crossed with C57BL/6 substrains of mixed background, and 8% were crossed with uncertain C57BL/6 substrains.
Phenotypic Differences Among C57BL/6 Substrains Caused by Identified Genetic Variants
To date, dozens of papers have reported various phenotypic differences among C57BL/6 substrains (Table 1). This information is classified according to the phenotype terms defined in the Mammalian Phenotype Ontology [116] and includes descriptions of phenotypes or phenotyping tests, the names of tested C57BL/6 substrains, and relevant references. Moreover, several of these phenotypic differences have been analyzed with the aim of identifying causative genes or genomic regions, as described below (summarized in Table 2).
Table 1. C57BL/6 substrains used for testing various phenotypes.
| Phenotype terms | Descriptions and/or tests | C57BL/6 substrains tested | Publication |
|---|---|---|---|
| Behavior/neurological phenotype | Aggression, light-dark box, nest-building | C57BL/6JNmg (Nijmegen Univ.), C57BL/6JKun (Nijmegen Univ.) | [17] |
| Alcohol preference | C57BL/6J (JAX), C57BL/6NCrSim (Simonsen Lab) | [18] | |
| C57BL/6J (JAX), C57BL/6NCrl (CRL) | [19] | ||
| C57BL/6J (JAX), C57BL/6J (Univ. of Albeta) | [20] | ||
| C57BL/6J (JAX), C57BL/6NCrl (CRL) | [21] | ||
| Alcohol preference and innate immune response | C57BL/6J (JAX), C57BL/6NJ (JAX) | [22] | |
| Behavioral response to amphetamine | C57BL/6J (JAX), C57BL/6By (JAX) | [23] | |
| Conditioned place preference | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [24] | |
| Corticosterone sensitivity | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [25] | |
| Degree of cuprizone-induced binge-like eating reduction | C57BL/6J (JAX), C57BL/6NJ (JAX) | [26] | |
| Degree of naloxone-induced conditioned place aversion | C57BL/6J (JAX), C57BL/6NJ (JAX) | [27] | |
| Eight-arm radial maze, elevated plus maze, open field, rotarod, Porsolt forced swim, prepulse inhibition, wire hang | C57BL/6J (JAX), C57BL/6NCrlCrlj (CRL Japan), C57BL/6NCrSlc (Japan SLC) | [28] | |
| Elevated plus maze, fear conditioning, light-dark box, prepulse inhibition, open field, social approach, acoustic startle | C57BL/6J (JAX), C57BL/6JRccHsd (Envigo), C57BL/6JRj (Janvier Labs), C57BL/6NCrl (CRL), C57BL/6NHsd (Envigo), C57BL/6NRj (Janvier Labs) | [15] | |
| Elevated plus maze, hole board, light–dark box | C57BL/6JOlaHsd (Envigo), C57BL/6NCrl (CRL) | [29] | |
| Fear conditioning | C57BL/6JIco (CRL), C57BL/6NCrl (CRL) | [30] | |
| C57BL/6J (Hsd) (Envigo), C57BL/6NCrl (CRL) | [31] | ||
| C57BL/6J (JAX), C57BL/6JOlaHsd (Envigo), C57BL/6NCrl (CRL) | [32] | ||
| C57BL/6JOlaHsd (Envigo), C57BL/6NCrl (CRL) | [33] | ||
| C57BL/6JOlaHsd (Envigo), C57BL/6NCrlBR (CRL) | [34] | ||
| Fear conditioning, hot plate, rotarod, tail withdrawal | C57BL/6J (JAX), C57BL/6NCrl (CRL), C57BL/6NHsd (Envigo), C57BL/6NTac (TAC) | [35] | |
| Fear conditioning, Morris water maze, open field, rotarod | C57BL/6J (JAX), C57BL/6NJ (JAX) | [36] | |
| Food consumption in conditioned site preference | C57BL/6J (JAX), C57BL/6NJ (JAX) | [37] | |
| High-fat diet-induced mechanical sensitivity | C57BL/6J (JAX), C57BL/6NCrl (CRL), C57BL/6NJ (JAX) | [38] | |
| Light-dark box | C57BL/6J (Envigo), C57BL/6ChR (CRL) | [39] | |
| Locomotor activity and anxiety-like behavior during the postpartum period | C57BL/6J (JAX), C57BL/6JJcl (CLEA Japan) | [40] | |
| Locomotor response to cocaine | C57BL/6J (JAX), C57BL/6N (NCI) | [41] | |
| Metabolic and behavioral response to shortened 21-h day and high-fat diet | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [42] | |
| Morris water maze | C57BL/6J (JAX), C57BL/6NTac (TAC) | [43] | |
| Open field | C57BL/6J (JAX), C57BL/6NTac (TAC) | [44] | |
| Open field, rotarod | C57BL/6JBomTac (TAC), C57BL/6NCrljOri (Orient Bio), C57BL/6NTacSam (Samtako Bio) | [45] | |
| Prepulse inhibition | C57BL/6J (JAX), C57BL/6NHsd (Envigo) | [46] | |
| Response to formalin-induced pain | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [47] | |
| Response to circadian disruption and wheel-running access | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [48] | |
| Response to neuropathic pain | C57BL/6NCrl (CRL), C57BL/6NTac (TAC) | [49] | |
| Sensitivity to the convulsant pilocarpine | C57BL/6J (JAX), C57BL/6NJ (JAX) | [50] | |
| C57BL/6J (JAX), C57BL/6JOlaHsd (Envigo), C57BL/6NCrl (CRL), C57BL/6NHsd (Envigo) | [51] | ||
| Sensitivity to nicotine | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [52] | |
| Sensitivity to thermal nociception | C57BL/6J (JAX), C57BL/6NJ (JAX) | [53] | |
| Susceptibility to cocaine-induced seizure | C57BL/6J (JAX), C57BL/6ByJ (JAX) | [54] | |
| Susceptibility to pilocarpine-induced status epilepticus | C57BL/6JJcl (CLEA Japan), C57BL/6NJcl (CLEA Japan) | [55] | |
| Tail suspension | C57BL/6J (JAX), C57BL/6NHsd (Envigo) | [56] | |
| Cardiovascular system phenotype | Cardiac effects of tribromoethanol and ketamine-midazolam | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [57] |
| Cardiac functional and metabolic flux parameters | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [58] | |
| Echocardiographic and electrocardiographic values | C57BL/6J (HZM), C57BL/6N (HZM) | [59] | |
| Incidence of cardiac rupture after myocardial infarction | C57BL/6J (JAX), C57BL/6J (Hsd) (Envigo) | [60] | |
| Incidence of dilated cardiomyopathy | C57BL/6J (JAX), C57BL/6NTac (TAC) | [61] | |
| High-fat diet-induced vascular superoxide | C57BL/6J (JAX), C57BL/6NJ (JAX) | [62] | |
| Response to Ang II-dependent left ventricle remodeling | C57BL/6J (JAX), C57BL/6NJ (JAX) | [63] | |
| Response to left ventricular pressure overload | C57BL/6J (JAX), C57BL/6NCrl (CRL), C57BL/6NTac (TAC) | [64] | |
| Response to neonatal hypoxia/ischemia | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [65] | |
| Response to transverse aortic constriction stimulation | C57BL/6J (JAX), C57BL/6NTac (TAC) | [66] | |
| Salt sensitivity of blood pressure | C57BL/6J (JAX), C57BL/6NTac (TAC) | [67] | |
| Systolic blood pressure level | C57BL/6J (JAX), C57BL/6NJ (JAX) | [68] | |
| Vulnerability to neonatal hypoxia-ischemia | C57BL/6J (Saarland Univ.), C57BL/6N (Saarland Univ.) | [69] | |
| Cellular phenotype | Abnormal sperm head morphology | C57BL/6JBomTac (TAC), C57BL/6NTac (TAC) | [70] |
| Hydrogen peroxide-producing capacities of liver mitochondria | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [71] | |
| Mitochondrial redox abnormalities | C57BL/6J (JAX), C57BL/6JUnib (CEMIB) | [72] | |
| Embryonic phenotype | Incidence of fetal alcohol syndrome | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [73] |
| Patterns of alcohol-induced facial dysmorphology | C57BL/6J (JAX), C57BL/6NHsd (Envigo) | [74] | |
| Susceptibility to Porphyromonas gingivalis | C57BL/6J (JAX), C57BL/6NJ (JAX) | [75] | |
| Endocrine/exocrine gland phenotype | Susceptibility to cerulein-induced chronic pancreatitis | C57BL/6J (JAX), C57BL/6NHsd (Envigo) | [76] |
| Hearing/vestibular/ear phenotype | Susceptibility to aminoglycoside-induced ototoxicity | C57BL/6J (JAX), C57BL/6NHsd (Envigo) | [77] |
| Hematopoietic system phenotype | Sensitivity of hematopoietic stem cells to oxidative stress | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [78] |
| Homeostasis/metabolic phenotype | Clinical chemistry parameters | C57BL/6J (JAX), C57BL/6NTac (TAC) | [79] |
| Effects of oats on plasma cholesterol and lipoproteins | C57BL/6JBomTac (TAC), C57BL/6NCrl (CRL) | [80] | |
| Hematological and iron-related parameters | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [81] | |
| Insulin secretory response to high-fat diet | C57BL/6J (JAX), C57BL/6J (WEHI), C57BL/6NCrl (CRL), C57BL/6NHsd (Envigo), C57BL/6NJ (JAX), C57BL/6NTac (TAC) | [82] | |
| Response to intermittent hypoxia | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [83] | |
| Response to diet-induced obesity | C57BL/6JRj (Janvier Labs), C57BL/6NTac (TAC) | [84] | |
| C57BL/6JRj (Janvier Labs), C57BL/6NTac (TAC) | [85] | ||
| C57BL/6J (JAX), C57BL/6NJ (JAX) | [86] | ||
| Response to glucose-stimulated insulin secretion | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [87] | |
| Response to high-fat diet | C57BL/6J (JAX), C57BL/6NJ (JAX) | [88] | |
| C57BL/6J (JAX), C57BL/6JBomTac (TAC), C57BL/6JRj (Janvier Labs) | [89] | ||
| Skeletal and metabolic responses to high-fat diet | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [90] | |
| Immune system phenotype | Neutrophil recruitment in response to inflammatory stimuli | C57BL/6J (JAX), C57BL/6N (NCI) | [91] |
| Non-ecotropic ERV expression level | C57BL/6J (JAX), C57BL/6NJ (JAX) | [92] | |
| Susceptibility to influenza A virus | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [93] | |
| Susceptibility to Listeria monocytogenes | C57BL/6J (JAX), C57BL/6ByJ (JAX) | [94] | |
| Integument phenotype | Sensitivity to Aldara™‐induced psoriasiform dermatitis | C57BL/6JRj (Janvier Labs), C57BL/6NRj (Janvier Labs) | [95] |
| Liver/biliary system phenotype | Susceptibility to acetaminophen-induced liver injury | C57BL/6J (JAX), C57BL/6NCr (NIH) | [96] |
| Susceptibility to CCl4- and high-fat diet–induced non-alcoholic steatohepatitis | C57BL/6J (JAX), C57BL/6NCrSlc (Japan SLC) | [97] | |
| Neoplasm phenotype | Incidence of 1,2-dimethylhydrazine–induced colorectal tumors | C57BL/6J (JAX), C57BL/6N (NIH), C57BL/6Ha (Health Research Inc.) | [98] |
| Susceptibility to Ehrlich ascites carcinoma | C57BL/6J (Andreevka), C57BL/6N (Pushchino) | [99] | |
| Nervous system phenotype | Degree of stroke vulnerability | C57BL/6J (JAX), C57BL/6JEiJ (JAX), C57BL/6NCrl (CRL), C57BL/6NJ (JAX), C57BL/6NTac (TAC), C57BL/6ByJ (JAX) | [100] |
| Ectopic distribution of cerebrospinal fluid contacting neurons | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [101] | |
| Hippocampal mossy fiber morphology | C57BL/6JKun (Nijmegen Univ.), C57BL/6JNmg (Nijmegen Univ.) | [102] | |
| Hippocampal mossy fiber morphology, radial arm maze | C57BL/6JKun (Nijmegen Univ.), C57BL/6JNmg (Nijmegen Univ.) | [103] | |
| Prevalence of molecular-layer heteropia | C57BL/6J (JAX), C57BL/6NCrl (CRL), C57BL/6NCrSim (Simonsen Lab), C57BL/6NHla (Hilltop Lab.), C57BL/6NHsd (Envigo), C57BL/6NTac (TAC) | [104] | |
| Susceptibility to scopolamine-induced neuronal impairment | C57BL/6J (Dae Han Bio Link), C57BL/6N (Dae Han Bio Link) | [105] | |
| Renal/urinary system phenotype | Degree of glyoxylate-induced kidney crystal deposition | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [106] |
| Respiratory system phenotype | Airway responsiveness | C57BL/6J (JAX), C57BL/6NCrl (CRL), C57BL/6NTac (TAC), C57BL/6NHsd (Envigo), C57BL/6NCr (NIH) | [107] |
| Response to neonatal hyperoxia-induced lung injury | C57BL/6J, C57BL/6N | [108] | |
| Skeleton phenotype | Bone structure and unloading-induced bone loss in adolescents | C57BL/6J (HJAX), C57BL/6NJ (JAX) | [109] |
| Trabecular bone mass | C57BL/6J (JAX), C57BL/6JOlaHsd (Envigo), C57BL/6JRccHsd (Envigo) | [110] | |
| Vision/eye phenotype | Constriction during pupillary light response with aging | C57BL/6J, C57BL/6N | [111] |
| Ocular dominance plasticity | C57BL/6J (JAX), C57BL/6JOlaHsd (Envigo) | [112] | |
| Susceptibility to laser-induced choroidal neovascularization | C57BL/6J (JAX), C57BL/6NTac (TAC) | [113] | |
| Susceptibility to photoreceptor oxidative stress | C57BL/6J (JAX), C57BL/6NCrl (CRL) | [114] | |
| Comprehensive phenotype* | EMPReSSslim pipelines | C57BL/6J (JAX), C57BL/6NTac (TAC) | [115] |
The phenotype terms column basically follows the Mammalian Phenotype Ontology [116]. The parentheses to the right of the strain names indicate the vendor or facility where the mice were produced. JAX, The Jackson Laboratory; CRL, Charles River Laboratories; TAC, Taconic Biosciences; WEHI, Walter and Eliza Hall Institute; HZM, Helmholtz Zentrum München; CEMIB, Multidisciplinary Center for Biological Investigation on Laboratory Animal Science, The University of Campinas. Strains without parentheses are those for which information on the vendor or production facility was not included in the publication. *By comprehensive phenotype screening (substrain differences were identified in cardiovascular, hematopoietic, adipose tissue, behavioral/neurological, renal/urinary, skeletal, hematopoietic, vision/eye, and immune system phenotypes).
Table 2. Genetic or genomic structural variations affecting phenotypes among C57BL/6 substrains.
| Strain name | Reported genetic or genomic variations | Affected phenotypes |
|---|---|---|
| C57BL/6 (JAX), C57BL/6JJcl (CLEA Japan), C57BL/6JJmsSlc (Japan SLC), C57BL/6JMs (National Institute of Genetics) | 17,814 bp deletion between exons 6 and 12, which corresponds to exons 7–11 of the Nnt gene [117, 118]. | Abnormal glucose tolerance and impaired insulin secretion |
| C57BL/6JOlaHsd (Envigo) | 365 kb spontaneous deletion of chromosome 6, including the Snca and Mmrn1 genes [119, 120]. | No significant effects on brain structure or composition, but may affect motor neuron degeneration and trabecular bone mass |
| All C57BL/6N substrains, including C57BL/6ByJ (JAX) and C57BL/6JBy (JAX) | Single base deletion of the Crb1 gene, causing a frameshift and premature stop codon which truncates the transmembrane and cytoplasmic domain of the protein [121, 122]. | Typical lesions of rd8 retinal degeneration |
| C57BL/6NHsd (Envigo) | Genomic duplication of exons 28 and 29 and a frameshift mutation after exon 29 in the Dock2 gene [123, 124]. | Affected B cell signaling and immune tolerance |
| C57BL/6JBomTac (TAC) | 40 Mb-long deletion between 6.12/6.57 and 46.73/47.31 Mb on the Y chromosome long arm [125]. | Increased rate of sperm morphological abnormalities, unbalanced sex ratio, and dysregulation of several transcripts expressed in the testes |
The parentheses to the right of the strain names indicate the vendor or facility where the mice were produced. JAX, The Jackson Laboratory; TAC, Taconic Biosciences.
The C57BL/6J strain is missing the exon that encodes the nicotinamide nucleotide transhydrogenase (Nnt) gene, which plays an important role in glucose homeostasis and insulin secretion, and therefore has abnormal glucose tolerance and impaired insulin secretion [117, 118, 126]. A study using mitogen-activated protein kinase 9 (Mapk9) knockout mice demonstrated that susceptibility to acetaminophen-induced liver injury was influenced by the presence or absence of the Nnt gene defect carried by C57BL/6 mice [127]. The Nnt gene mutation in the C57BL/6J strain is a prominent phenotype-associated mutation in C57BL/6 substrains that affects protein expression in the mitochondria of many tissues and consequently many different metabolic traits [62, 72, 78, 82, 88, 106, 128]. The Nnt gene deletion status differs among C57BL/6J substrains, and the Nnt gene defect likely originated in C57BL/6J mice at the Jackson Laboratory after 1976 (Fig. 3).
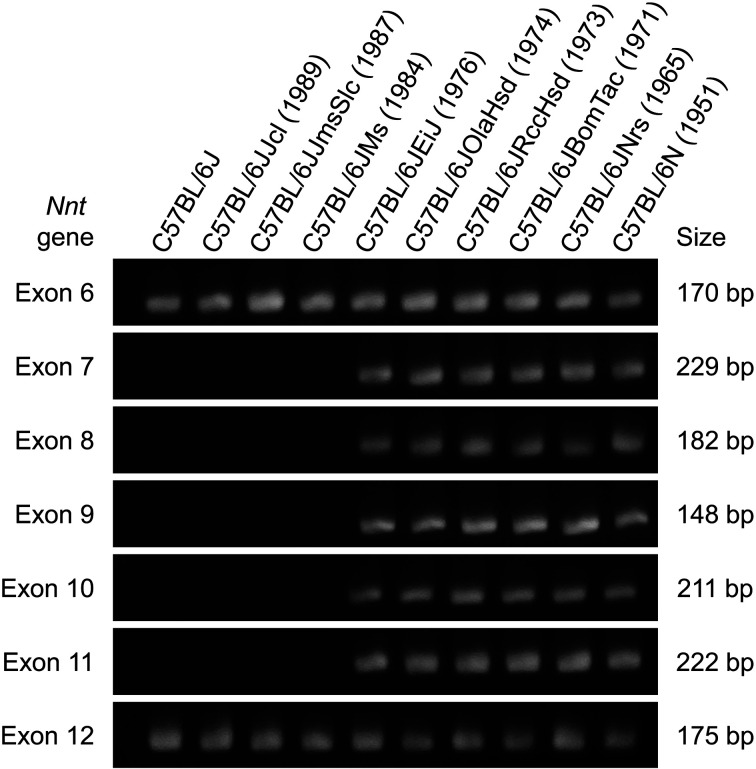
Fig. 3.
The deletion of exons 7–11 in the Nnt gene of C57BL/6J substrains. Genomic polymerase chain reaction (PCR) analysis of exons 6–12 in the Nnt gene showed the absence of PCR products from exons 7–11 in the C57BL/6J, C57BL/6JJcl, C57BL/6JJmsSlc, and C57BL/6JMs substrains. The deletion of the Nnt gene occurred in the C57BL/6J colony of the Jackson Laboratory between 1976 and 1984. The branching years of each strain from the original C57BL/6J strain are given in parentheses. PCR and gel electrophoresis procedures were performed according to the protocol described in Mekada et al. (2009) [6]. PCR product size data are from Huang et al. (2006) [117].
C57BL/6JOlaHsd mice are known to lack a 365 kb genomic region that includes the synuclein, alpha (Snca) and the nearby multimerin 1 (Mmrn1) gene [119, 120]. SNCA aggregates in the nervous system of individuals with Parkinson’s disease [129]. Loss of the Snca gene in C57BL/6JOlaHsd mice does not appear to contribute to prion disease-mediated synaptotoxicity or neurodegeneration but may affect motor neuron degeneration [130,131,132,133]. In addition, the C57BL/6JOlaHsd strain has a low trabecular bone mass, which has been shown to be associated with the lack of Snca and Mmrn1 genes, suggesting a role for these genes in bone metabolism [110]. Deletion of this genomic region is specific to the C57BL/6JOlaHsd strain; the mutation has not been identified in C57BL/6JRccHsd, C57BL/6J, C57BL/6NCrl, and C57BL/6NHsd strains [119]. Notably, we confirmed that the genotype is normal in other C57BL/6J and C57BL/6N substrains available in Japan (Supplementary Fig. 1). Furthermore, partial deletion of a chromosomal region was recently observed in C57BL/6JBomTac mice. In this strain, approximately 40 Mb of the long arm of the Y chromosome is deleted, and an increased rate of sperm morphological abnormalities, unbalanced sex ratio, and deregulation of several transcripts expressed in the testes have also been observed [70, 125].
Similarly, genetic mutations affecting physiological functions have been reported in C57BL/6N substrains. A nonsense mutation (retinal degeneration 8, rd8) that converts the amino acid codon of the crumbs family member 1, photoreceptor morphogenesis associated (Crb1) gene into a termination codon occurs in C57BL/6N substrains [121, 122]. Affected mice present with the typical lesions of rd8, which are detected by fundoscopy and histopathology at as early as 6 weeks of age. Although this mutation is seen in C57BL/6N substrains, C57BL/6J substrains have the wild-type genotype [121].
C57BL/6NHsd mice have a copy-number variant in the dedicator of cytokinesis 2 (Dock2) gene, which has been shown to affect B cell signaling and immune tolerance, but this is not found in other C57BL/6 substrains [123, 134]. The dysfunction of this gene has likely contributed to the negative outcomes of experiments using the sialic acid acetylesterase (Siae) knockout mice with an unclear C57BL/6 origin. Although the Siae gene was initially thought to be involved in B cell development and signal transduction, it was found that congenic mice generated by backcrossing to the C57BL6J strain did not reproduce the same negative outcomes. After much research, the cause was identified as a mutation in the Dock2 gene of the C57BL/6NHsd strain [134].
Other Phenotypic Differences Among C57BL/6 Substrains
In addition to the abovementioned cases in which the causative genes were identified, phenotypic differences have been reported among the substrains used to generate the C57BL/6 congenic strain. Greater improvement in terms of seizure severity and viability was achieved in Dravet syndrome model mice with knockout of the sodium channel, voltage-gated, type I, alpha (Scn1a) gene generated on a C57BL/6NJ versus C57BL/6J background [135]. Obvious phenotypic differences have been demonstrated between C57BL/6J and C57BL/6N substrains, as shown in studies on knockout of the coagulation factor VIII (F8) and fatty acid binding protein 1, liver (Fabp1) genes, suggesting the presence of genetic factors that differentially modify phenotypes between the substrains [136, 137]. It has also been demonstrated that tumor cell rejection in Eµ-TCL1 transgenic mice, a model of chronic lymphocytic leukemia, is associated with differences between the C57BL/6 substrains, suggesting that acute immune rejection may be mediated by antigens that differ between C57BL/6J and C57BL/6N mice [138]. In addition, C57BL/6J-XYPOS congenic mice, which have a Y chromosome derived from Mus musculus poschiavinus on a C57BL/6J genetic background, form ovotestes or ovaries [139, 140]. Changing from a C57BL/6J to C57BL/6N background increases the likelihood of testis-forming individuals, suggesting that genetic differences involved in the regulation of the sex determining region of Chr Y (Sry) and the SRY (sex determining region Y)-box 9 (Sox9) gene expression, which play an important role in testicular differentiation, exist between the C57BL/6J and N substrains [141, 142].
Differences in prevalence of sexual dimorphism among C57BL/6 substrains should also be considered. Significant variation in brain injuries has been reported among C57BL/6 substrains [69, 143]. In mouse stroke, male and female C57BL/6N mice show similar infarct sizes, although female C57BL/6J mice tend to have smaller infarcts [100]. Such substrain-specific sexual dimorphisms have also been identified in analyses of behavioral characteristics, serum biochemistry, and immune function [36, 79, 115]. Therefore, in studies of the effects of genetic modifications on sex differences, heterogeneity in substrain backgrounds would likely complicate the interpretation of experimental results. Thus, the phenotypic differences among C57BL/6 substrains are quite numerous, and they should be reported more in the future.
Genetic Polymorphisms in C57BL/6J Substrains
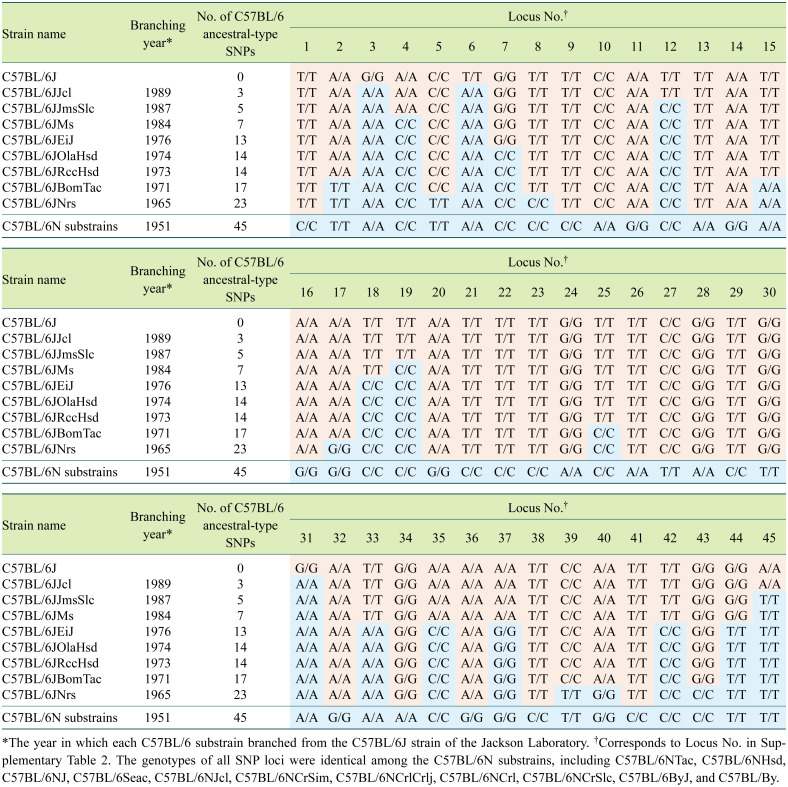
Single nucleotide polymorphisms (SNPs) are the most widely used genetic markers in genomes and are useful for uncovering genetic differences between genetically similar lineages, including substrains. In laboratory mouse strains, SNP databases have been developed by mapping sequence data from other inbred lines using the Jackson Laboratory’s C57BL/6J strain as a reference [144,145,146,147,148,149]. SNPs that distinguish each C57BL/6J substrain can be extracted from candidates specific to the C57BL/6J strain in SNP databases as well as by identifying the genotypes of candidate SNP loci for each C57BL/6J substrain [6, 150,151,152]. In Table 3, genotypes of C57BL/6J substrains and the C57BL/6N strain at 45 SNP loci are shown in chronological order with C57BL/6N at the bottom and eight C57BL/6J substrains and the current C57BL/6J strain at the top. This table clearly indicates that the ancestral-type SNPs (shaded in blue) of C57BL/6J and N (as represented by the C57BL/6N strain) have decreased and been replaced by the C57BL/6J-type SNPs at the 23 loci (shaded in red) since 1951. The SNPs are considered to have occurred and accumulated in the original C57BL/6J strain. Since the SNP genotype pattern of each substrain branched at different years is distinct, these SNP loci genotypes can be used to distinguish every C57BL/6J substrain available in the research community.
Table 3. Single nucleotide polymorphisms (SNPs) in C57BL/6J substrains.
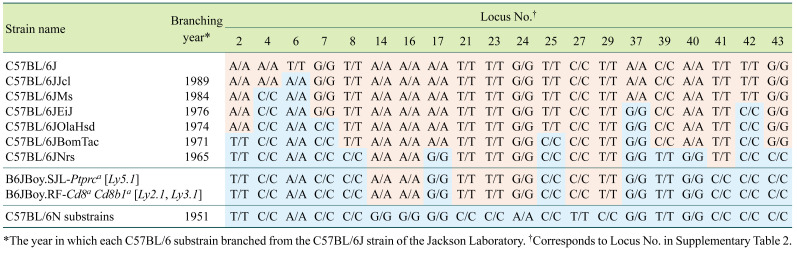
The lymphocyte antigen 5 (Ly5) congenic strain is a representative mouse strain derived from the C57BL/6J substrain. The lymphoid cell surface antigen produced by the protein tyrosine phosphatase, receptor type, C (Ptprc; Ly5/Cd45 gene) gene distinguishes between hematopoietic cells from donor and recipient mice; hence, this strain has been widely used as a cell marker for bone marrow transplantation studies [153, 154]. The C57BL/6-Ly5.1 congenic inbred strain (B6JBoy.SJL-Ptptca; RBRC00144) was established by Boyes et al. at Sloan–Kettering Institute through introduction of the Ly5.1 of the SJL/J strain into the C57BL/6JBoy strain background (Ly5.2 allele) by 23 serial backcrosses [155, 156]. The exact point at which the C57BL/6JBoy strain diverged from the C57BL/6J strain is not clear, but the literature suggests that divergence occurred in the 1970s, or possibly even earlier [157]. According to genetic background testing using C57BL/6J-specific SNP markers, the genetic background should be similar to that of older C57BL/6J substrains, which have an earlier divergence date (Table 4). Although C57BL/6-Ly5 antigen congenic mice are currently available from several vendors, a spontaneous mutation in the natural cytotoxicity triggering receptor 1 (Ncr1) locus and residual chromosomal fragments of the donor SJL/J strain, both of which affect immune response, have been reported [158, 159]. These effects must be considered to properly interpret the results of experiments involving these strains [158, 159].
Table 4. Genetic background of C57BL/6JBoy congenic strains.
Genetic Polymorphisms in C57BL/6N Substrains
In the C57BL/6N substrain, genotyping of the above C57BL/6J-specific SNP loci could not detect polymorphism as observed in C57BL/6J [6]. This is because the previous C57BL/6J-specific SNP information was obtained by mapping the abundant reference sequence data of C57BL/6J mice to the sequences of other strains. However, there was a lack of previous sequence data for C57BL/6N, and thus the detection of C57BL/6N strain-specific SNPs was infeasible. Later, in 2011, a re-sequencing analysis of the C57BL/6NJ strain was performed [160, 161], and highly accurate sequence information was published on the website of the Sanger Institute (Hinxton, United Kingdom; https://www.sanger.ac.uk/sanger/Mouse_SnpViewer/rel-1505). The results enabled a subsequent search for C57BL/6N strain SNPs and revealed SNP loci specific to the C57BL/6NJ strain and their genotypes in seventeen C57BL/6 substrains [7]. There were 86 polymorphic SNP loci at regular intervals on every chromosome, selected from approximately 1,400 C57BL6/NJ strain-specific SNP candidate loci. As results similar to those of the C57BL/6J group, the number of C57BL/6N-specific SNPs was correlated with the branching year and breeding history of each lineage (see Fig. 1 in Mekada et al. 2015 [7]). The previous information required for genotyping each SNP locus listed in Mekada et al. 2015 [7] was updated with additional primer information for allele-specific PCR, and it is listed in Supplementary Table 3.
Our research group continues to search for SNPs that can distinguish among various C57BL/6 substrains; such information is expected to improve our understanding of the genetic background of these strains. In addition to SNPs, DNA polymorphisms such as indels and short tandem repeats as well as structural variants, including C57BL/6-specific genetic variants found in Nnt and Snca genes, can be used to distinguish C57BL/6 strains in terms of genetic background [14, 115, 162]. These DNA polymorphism markers may be useful for comparing substrains and can also be applied to gene mapping involving the crossing of C57BL/6 substrains. A genetic screening model based on these principles would enable the mapping of polymorphic loci that contribute to the variability of a trait among strains with a largely homogenous background; this could lead to improvements in mapping resolution and aid in the selection of candidate genes. To date, numerous quantitative trait loci have been identified, including genes associated with susceptibility to heat nociception [53], diet-induced obesity [84], dominant obesity [163], circadian activity [164], feeding disorder [37], response to cocaine [41], defective neutrophil recruitment [91], and non-ectopic endogenous retroviral dysregulation [92]. With advances in genome sequencing technology, genomic information for inbred mice, including substrains, is being continually updated [165, 166]. It is expected that many new strain-specific haplotypes will be identified in the future.
Large-scale and Complex Resources
C57BL/6J substrains have been the most widely used background for many mutant strains, including genetically-modified lines. Another major lineage, the C57BL/6N substrain, has been used as the genetic background for embryonic stem cells in large-scale generation of knockouts [12]. As a result, many mutant mice derived from the C57BL/6J and C57BL/6N substrains are used worldwide, including in Japan. However, given that the substrains are widely considered interchangeable, significant phenotypic differences among substrains are often overlooked. The phenotypic differences among C57BL/6 substrains reported to date should be considered by researchers. For example, certain substrains should be used with caution in conditional knockout models because conditional knockout mice should be crossed with Cre and/or Flp recombinase mice. Combining recombinase strains with different genetic backgrounds might produce knockout animals with mixed genetic backgrounds, leading to unexpected phenotypic variations. When the genetic backgrounds of experimental mice are not identical, erroneous conclusions are likely to be drawn. If a suitable substrain cannot be used, wild-type littermates might serve as an appropriate control group. Recently, it has become clear that phenotypic differences among substrains are caused by a complex combination of genetic and microbial alterations [167, 168]. Use of wild-type littermates might standardize the microbiota, thereby facilitating investigation of the phenotype or function of specific genes that could be affected by the microbial community [169, 170].
Genome-edited Mice
Today, the rise in prominence of genome editing technologies has made it possible to directly generate various types of genetically modified mice, including gene knockout, conditional knockout, and gene knock-in, by using the fertilized eggs of inbred mice without the need for embryonic stem cells. At present, more than 50% of the strains deposited at the RIKEN BRC have been generated by genome editing using fertilized eggs from inbred strains, although not necessarily C57BL/6 mice. Just as there are C57BL/6 substrains, there are also substrains of other inbred lines, and as is the case with the C57BL/6 strain, an accumulation of genetic and phenotypic differences is to be expected. More recently, many mouse models have been generated for coronavirus research; the BALB/c mouse strain is known to be popular in the immunology field and susceptible to SARS-CoV-2 [171, 172], and it is increasingly being used as a background strain for COVID-19 research [173,174,175]. The BALB/c strain has a long history in the form of the above B6 mice [2, 3]. At least 16 BALB/c substrains, including BALB/cJ, BALB/cBy, and BALB/cAnN, available to the research community can be found by performing a PubMed research with the search term “BALB/c”. Furthermore, several papers have reported phenotypic variations in infection and immune responses [176,177,178]. Therefore, we should pay much more attention to the substrain status of BALB/c mice in research on COVID-19, which is increasing around the world.
Conclusion
Experimental data must be robust, reliable, and reproducible; only under these circumstances can science progress [179]. To ensure experimental reproducibility and minimize bias, researchers should recognize potential phenotypic differences among substrain mouse models, select appropriate substrains for their own research purposes, and share the full names of the substrains used with the scientific community. In the future, it is expected that journals will adopt a policy requiring a detailed description of the substrain and the breeding history of model mice used in research.
Supplementary
Acknowledgments
We are grateful to Masayo Kadota, Mayu Hirose, Ayumi Murakami, and the other members of the Experimental Animal Division, RIKEN BRC, for their devoted technical assistance. We also thank Ikuo Miura, Kuniya Abe, Toshihiko Shiroishi, Yuichi Obata, and Kazuo Moriwaki of RIKEN BRC for their valuable advice and support. This work was supported by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Grant Number 23700518) to K.M. The RIKEN BioResource Research Center has been a participant in the National BioResource Project (NBRP) organized by MEXT and designated as the core facility for mouse resources of the NBRP.
References
- 1.Russell ES. Genetic origins and some research used of C57BL/6, DBA/2, and B6D2F1 mice. In: Gibson DC, Adelman RC, Finch C. editors. Development of the rodent as a model system of aging. Bethesda: DHEW Publ No. (NIH) 79–161; 1978. pp. 37–44. [Google Scholar]
- 2.Festing MF. Inbred strains in biomedical research. London and Basingstoke: The Macmillan Press; 1979. [Google Scholar]
- 3.Festing MF. Origins and characteristics of inbred strains of mice. In: Lyon MF, Rastan S, Brown SDM, editors. Genetic variants and strains of the laboratory mouse. Oxford: Oxford University Press; 1996. pp. 1537–1576. [Google Scholar]
- 4.Altman PL, Kats DD. Inbred and genetically defined strains of laboratory animals, part 1 mouse and rat. Bethesda: Federation of American Societies for Experimental Biology; 1979. [Google Scholar]
- 5.Bailey DW. Sources of subline divergence and their relative importance for sublines of six major inbred strains of mice. In: Morse III HC. editor. Origins of inbred mice. New York: Academic Press; 1978. pp. 197–215. [Google Scholar]
- 6.Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009; 58: 141–149. doi: 10.1538/expanim.58.141 [DOI] [PubMed] [Google Scholar]
- 7.Mekada K, Hirose M, Murakami A, Yoshiki A. Development of SNP markers for C57BL/6N-derived mouse inbred strains. Exp Anim. 2015; 64: 91–100. doi: 10.1538/expanim.14-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, et al. Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002; 420: 520–562. doi: 10.1038/nature01262 [DOI] [PubMed] [Google Scholar]
- 9.Pettitt SJ, Liang Q, Rairdan XY, Moran JL, Prosser HM, Beier DR, et al. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat Methods. 2009; 6: 493–495. doi: 10.1038/nmeth.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley A, Anastassiadis K, Ayadi A, Battey JF, Bell C, Birling MC, et al. The mammalian gene function resource: the International Knockout Mouse Consortium. Mamm Genome. 2012; 23: 580–586. doi: 10.1007/s00335-012-9422-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown SD, Moore MW. The International Mouse Phenotyping Consortium. The International Mouse Phenotyping Consortium: past and future perspectives on mouse phenotyping. Mamm Genome. 2012; 23: 632–640. doi: 10.1007/s00335-012-9427-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins FS, Rossant J, Wurst W. International Mouse Knockout Consortium. A mouse for reasons. Cell. 2007; 128: 9–13. [DOI] [PubMed] [Google Scholar]
- 13.Meehan TF, Conte N, West DB, Jacobsen JO, Mason J, Warren J, et al. International Mouse Phenotyping Consortium. Disease model discovery from 3,328 gene knockouts by The International Mouse Phenotyping Consortium. Nat Genet. 2017; 49: 1231–1238. doi: 10.1038/ng.3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrowolski P, Fischer M, Naumann R. Novel insights into the genetic background of genetically modified mice. Transgenic Res. 2018; 27: 265–275. doi: 10.1007/s11248-018-0073-2 [DOI] [PubMed] [Google Scholar]
- 15.Åhlgren J, Voikar V. Experiments done in Black-6 mice: what does it mean? Lab Anim (NY). 2019; 48: 171–180. doi: 10.1038/s41684-019-0288-8 [DOI] [PubMed] [Google Scholar]
- 16.Fontaine DA, Davis DB. Attention to background strain is essential for metabolic research: C57BL/6 and the International Knockout Mouse Consortium. Diabetes. 2016; 65: 25–33. doi: 10.2337/db15-0982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sluyter F, Marican CC, Crusio WE. Further phenotypical characterisation of two substrains of C57BL/6J inbred mice differing by a spontaneous single-gene mutation. Behav Brain Res. 1999; 98: 39–43. doi: 10.1016/S0166-4328(98)00049-7 [DOI] [PubMed] [Google Scholar]
- 18.Blum K, Briggs AH, DeLallo L, Elston SF, Ochoa R. Whole brain methionine-enkephalin of ethanol-avoiding and ethanol-preferring C57BL mice. Experientia. 1982; 38: 1469–1470. doi: 10.1007/BF01955775 [DOI] [PubMed] [Google Scholar]
- 19.Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006; 40: 119–126. doi: 10.1016/j.alcohol.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poley W. Alcohol-preferring and alcohol-avoiding C57BL mice. Behav Genet. 1972; 2: 245–248. doi: 10.1007/BF01065693 [DOI] [PubMed] [Google Scholar]
- 21.Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007; 31: 1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x [DOI] [PubMed] [Google Scholar]
- 22.Warden AS, DaCosta A, Mason S, Blednov YA, Mayfield RD, Harris RA. Inbred substrain differences influence neuroimmune response and drinking behavior. Alcohol Clin Exp Res. 2020; 44: 1760–1768. doi: 10.1111/acer.14410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moisset B. Genetic analysis of the behavioral response to d-amphetamine in mice. Psychopharmacology (Berl). 1977; 53: 269–276. doi: 10.1007/BF00492362 [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro BS, Seidl SS, Habazettl E, Gruber BE, Bregolin T, Zernig G. Dyadic social interaction of C57BL/6 mice versus interaction with a toy mouse: conditioned place preference/aversion, substrain differences, and no development of a hierarchy. Behav Pharmacol. 2016; 27: 279–288. doi: 10.1097/FBP.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturm M, Becker A, Schroeder A, Bilkei-Gorzo A, Zimmer A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav. 2015; 14: 292–300. doi: 10.1111/gbb.12208 [DOI] [PubMed] [Google Scholar]
- 26.Babbs RK, Beierle JA, Yao EJ, Kelliher JC, Medeiros AR, Anandakumar J, et al. The effect of the demyelinating agent cuprizone on binge-like eating of sweetened palatable food in female and male C57BL/6 substrains. Appetite. 2020; 150: 104678. doi: 10.1016/j.appet.2020.104678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkpatrick SL, Bryant CD. Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Front Behav Neurosci. 2015; 8: 450. doi: 10.3389/fnbeh.2014.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labots M, Zheng X, Moattari G, Ohl F, van Lith HA. Effects of light regime and substrain on behavioral profiles of male C57BL/6 mice in three tests of unconditioned anxiety. J Neurogenet. 2016; 30: 306–315. doi: 10.1080/01677063.2016.1249868 [DOI] [PubMed] [Google Scholar]
- 30.Hager T, Jansen RF, Pieneman AW, Manivannan SN, Golani I, van der Sluis S, et al. Display of individuality in avoidance behavior and risk assessment of inbred mice. Front Behav Neurosci. 2014; 8: 314. doi: 10.3389/fnbeh.2014.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radulovic J, Kammermeier J, Spiess J. Generalization of fear responses in C57BL/6N mice subjected to one-trial foreground contextual fear conditioning. Behav Brain Res. 1998; 95: 179–189. doi: 10.1016/S0166-4328(98)00039-4 [DOI] [PubMed] [Google Scholar]
- 32.Siegmund A, Langnaese K, Wotjak CT. Differences in extinction of conditioned fear in C57BL/6 substrains are unrelated to expression of alpha-synuclein. Behav Brain Res. 2005; 157: 291–298. doi: 10.1016/j.bbr.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 33.Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007; 41: 848–860. doi: 10.1016/j.jpsychires.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 34.Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, et al. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav Brain Res. 1999; 104: 1–12. doi: 10.1016/S0166-4328(99)00047-9 [DOI] [PubMed] [Google Scholar]
- 35.Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008; 22: 315–331. doi: 10.1080/01677060802357388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashworth A, Bardgett ME, Fowler J, Garber H, Griffith M, Curran CP. Comparison of neurological function in males and females from two substrains of C57BL/6 Mice. Toxics. 2015; 3: 1–17. doi: 10.3390/toxics3010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkpatrick SL, Goldberg LR, Yazdani N, Babbs RK, Wu J, Reed ER, et al. Cytoplasmic FMR1-interacting protein 2 is a major genetic factor underlying binge eating. Biol Psychiatry. 2017; 81: 757–769. doi: 10.1016/j.biopsych.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper MA, O’Meara B, Jack MM, Elliot D, Lamb B, Khan ZW, et al. Intrinsic activity of C57BL/6 substrains associates with high-fat diet-induced mechanical sensitivity in mice. J Pain. 2018; 19: 1285–1295. doi: 10.1016/j.jpain.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Gaalen MM, Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res. 2000; 115: 95–106. doi: 10.1016/S0166-4328(00)00240-0 [DOI] [PubMed] [Google Scholar]
- 40.Shoji H, Miyakawa T. Increased depression-related behavior during the postpartum period in inbred BALB/c and C57BL/6 strains. Mol Brain. 2019; 12: 70. doi: 10.1186/s13041-019-0490-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, et al. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science. 2013; 342: 1508–1512. doi: 10.1126/science.1245503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maroni MJ, Capri KM, Arruda NL, Gelineau RR, Deane HV, Concepcion HA, et al. Substrain specific behavioral responses in male C57BL/6N and C57BL/6J mice to a shortened 21-hour day and high-fat diet. Chronobiol Int. 2020; 37: 809–823. doi: 10.1080/07420528.2020.1756840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clapcote SJ, Roder JC. Survey of embryonic stem cell line source strains in the water maze reveals superior reversal learning of 129S6/SvEvTac mice. Behav Brain Res. 2004; 152: 35–48. [DOI] [PubMed] [Google Scholar]
- 44.Bothe GWM, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005; 55: 326–334. [PubMed] [Google Scholar]
- 45.Kang M, Ryu HH, Lee YS. Comparisons of behavior and synaptic plasticity among three C57BL/6 substrains. Anim Cells Syst. 2015; 19: 181–187. doi: 10.1080/19768354.2015.1023830 [DOI] [Google Scholar]
- 46.Grottick AJ, Bagnol D, Phillips S, McDonald J, Behan DP, Chalmers DT, et al. Neurotransmission- and cellular stress-related gene expression associated with prepulse inhibition in mice. Brain Res Mol Brain Res. 2005; 139: 153–162. doi: 10.1016/j.molbrainres.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 47.Ulker E, Caillaud M, Patel T, White A, Rashid D, Alqasem M, et al. C57BL/6 substrain differences in formalin-induced pain-like behavioral responses. Behav Brain Res. 2020; 390: 112698. doi: 10.1016/j.bbr.2020.112698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capri KM, Maroni MJ, Deane HV, Concepcion HA, DeCourcey H, Logan RW, et al. Male C57BL6/N and C57BL6/J mice respond differently to constant light and running-wheel access. Front Behav Neurosci. 2019; 13: 268. doi: 10.3389/fnbeh.2019.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirabelli E, Ni L, Li L, Acioglu C, Heary RF, Elkabes S. Pathological pain processing in mouse models of multiple sclerosis and spinal cord injury: contribution of plasma membrane calcium ATPase 2 (PMCA2). J Neuroinflammation. 2019; 16: 207. doi: 10.1186/s12974-019-1585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bankstahl M, Müller CJ, Wilk E, Schughart K, Löscher W. Generation and characterization of pilocarpine-sensitive C57BL/6 mice as a model of temporal lobe epilepsy. Behav Brain Res. 2012; 230: 182–191. doi: 10.1016/j.bbr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 51.Müller CJ, Gröticke I, Hoffmann K, Schughart K, Löscher W. Differences in sensitivity to the convulsant pilocarpine in substrains and sublines of C57BL/6 mice. Genes Brain Behav. 2009; 8: 481–492. doi: 10.1111/j.1601-183X.2009.00490.x [DOI] [PubMed] [Google Scholar]
- 52.Akinola LS, Mckiver B, Toma W, Zhu AZX, Tyndale RF, Kumar V, et al. C57BL/6 substrain differences in pharmacological effects after acute and repeated nicotine administration. Brain Sci. 2019; 9: 244. doi: 10.3390/brainsci9100244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryant CD, Bagdas D, Goldberg LR, Khalefa T, Reed ER, Kirkpatrick SL, et al. C57BL/6 substrain differences in inflammatory and neuropathic nociception and genetic mapping of a major quantitative trait locus underlying acute thermal nociception. Mol Pain. 2019; 15: 1744806918825046. doi: 10.1177/1744806918825046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henricks KK, Miner LL, Marley RJ. Differential cocaine sensitivity between two closely related substrains of C57BL mice. Psychopharmacology (Berl). 1997; 132: 161–168. doi: 10.1007/s002130050332 [DOI] [PubMed] [Google Scholar]
- 55.Otsuka S, Ohkido T, Itakura M, Watanabe S, Yamamori S, Iida Y, et al. Dual mechanisms of rapid expression of anxiety-related behavior in pilocarpine-treated epileptic mice. Epilepsy Res. 2016; 123: 55–67. doi: 10.1016/j.eplepsyres.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 56.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl). 2001; 155: 110–112. doi: 10.1007/s002130100687 [DOI] [PubMed] [Google Scholar]
- 57.Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr.Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002; 282: H2134–H2140. doi: 10.1152/ajpheart.00845.2001 [DOI] [PubMed] [Google Scholar]
- 58.Vaillant F, Lauzier B, Poirier I, Gélinas R, Rivard ME, Robillard Frayne I, et al. Mouse strain differences in metabolic fluxes and function of ex vivo working hearts. Am J Physiol Heart Circ Physiol. 2014; 306: H78–H87. doi: 10.1152/ajpheart.00465.2013 [DOI] [PubMed] [Google Scholar]
- 59.Moreth K, Fischer R, Fuchs H, Gailus-Durner V, Wurst W, Katus HA, et al. High-throughput phenotypic assessment of cardiac physiology in four commonly used inbred mouse strains. J Comp Physiol B. 2014; 184: 763–775. doi: 10.1007/s00360-014-0830-3 [DOI] [PubMed] [Google Scholar]
- 60.Deckx S, Carai P, Bateman J, Heymans S, Papageorgiou AP. Breeding strategy determines rupture incidence in post-infarct healing WARPing cardiovascular research. PLoS One. 2015; 10: e0139199. doi: 10.1371/journal.pone.0139199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams JL, Paudyal A, Awad S, Nicholson J, Grzesik D, Botta J, et al. Mylk3 null C57BL/6N mice develop cardiomyopathy, whereas Nnt null C57BL/6J mice do not. Life Sci Alliance. 2020; 3: e201900593. doi: 10.26508/lsa.201900593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vozenilek AE, Vetkoetter M, Green JM, Shen X, Traylor JG, Klein RL, et al. Absence of nicotinamide nucleotide transhydrogenase in C57BL/6J Mice exacerbates experimental atherosclerosis. J Vasc Res. 2018; 55: 98–110. doi: 10.1159/000486337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardin S, Scott-Boyer MP, Praktiknjo S, Jeidane S, Picard S, Reudelhuber TL, et al. Differences in cell-type-specific responses to angiotensin II explain cardiac remodeling differences in C57BL/6 mouse substrains. Hypertension. 2014; 64: 1040–1046. doi: 10.1161/HYPERTENSIONAHA.114.04067 [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Menendez L, Karamanlidis G, Kolwicz S, Tian R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am J Physiol Heart Circ Physiol. 2013; 305: H397–H402. doi: 10.1152/ajpheart.00088.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolf S, Mattheis A, Laufs U, Meier C, Tschernig T. Mitochondrial regulation of reactive oxygen species (ROS) production-Unexpected observations in early postnatal cerebral vasculature. J Chem Neuroanat. 2016; 74: 1–4. doi: 10.1016/j.jchemneu.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 66.Zi M, Stafford N, Prehar S, Baudoin F, Oceandy D, Wang X, et al. Cardiac hypertrophy or failure? - A systematic evaluation of the transverse aortic constriction model in C57BL/6NTac and C57BL/6J substrains. Curr Res Physiol. 2019; 1: 1–10. doi: 10.1016/j.crphys.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Combe R, Mudgett J, El Fertak L, Champy MF, Ayme-Dietrich E, Petit-Demoulière B, et al. How does circadian rhythm impact salt sensitivity of blood pressure in mice? a study in two close C57Bl/6 substrains. PLoS One. 2016; 11: e0153472. doi: 10.1371/journal.pone.0153472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leskov I, Neville A, Shen X, Pardue S, Kevil CG, Granger DN, et al. Nicotinamide nucleotide transhydrogenase activity impacts mitochondrial redox balance and the development of hypertension in mice. J Am Soc Hypertens. 2017; 11: 110–121. doi: 10.1016/j.jash.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 69.Wolf S, Hainz N, Beckmann A, Maack C, Menger MD, Tschernig T, et al. Brain damage resulting from postnatal hypoxic-ischemic brain injury is reduced in C57BL/6J mice as compared to C57BL/6N mice. Brain Res. 2016; 1650: 224–231. doi: 10.1016/j.brainres.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 70.MacBride MM, Navis A, Dasari A, Perez AV. Mild reproductive impact of a Y chromosome deletion on a C57BL/6J substrain. Mamm Genome. 2017; 28: 155–165. doi: 10.1007/s00335-017-9680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oldford C, Kuksal N, Gill R, Young A, Mailloux RJ. Estimation of the hydrogen peroxide producing capacities of liver and cardiac mitochondria isolated from C57BL/6N and C57BL/6J mice. Free Radic Biol Med. 2019; 135: 15–27. doi: 10.1016/j.freeradbiomed.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 72.Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HC, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med. 2013; 63: 446–456. doi: 10.1016/j.freeradbiomed.2013.05.049 [DOI] [PubMed] [Google Scholar]
- 73.Green ML, Singh AV, Zhang Y, Nemeth KA, Sulik KK, Knudsen TB. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev Dyn. 2007; 236: 613–631. doi: 10.1002/dvdy.21048 [DOI] [PubMed] [Google Scholar]
- 74.Anthony B, Vinci-Booher S, Wetherill L, Ward R, Goodlett C, Zhou FC. Alcohol-induced facial dysmorphology in C57BL/6 mouse models of fetal alcohol spectrum disorder. Alcohol. 2010; 44: 659–671. doi: 10.1016/j.alcohol.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer LA, Bittner-Eddy PD, Costalonga M. Fetal weight outcomes in C57BL/6J and C57BL/6NCrl mice after oral colonization with Porphyromonas gingivalis. Infect Immun. 2019; 87: e00280–e19. doi: 10.1128/IAI.00280-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ulmasov B, Oshima K, Rodriguez MG, Cox RD, Neuschwander-Tetri BA. Differences in the degree of cerulein-induced chronic pancreatitis in C57BL/6 mouse substrains lead to new insights in identification of potential risk factors in the development of chronic pancreatitis. Am J Pathol. 2013; 183: 692–708. doi: 10.1016/j.ajpath.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kendall A, Schacht J. Disparities in auditory physiology and pathology between C57BL/6J and C57BL/6N substrains. Hear Res. 2014; 318: 18–22. doi: 10.1016/j.heares.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morales-Hernández A, Martinat A, Chabot A, Kang G, McKinney-Freeman S. Elevated oxidative stress impairs hematopoietic progenitor function in C57BL/6 substrains. Stem Cell Reports. 2018; 11: 334–347. doi: 10.1016/j.stemcr.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Otto GP, Rathkolb B, Oestereicher MA, Lengger CJ, Moerth C, Micklich K, et al. Clinical chemistry reference intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ Mice (Mus musculus). J Am Assoc Lab Anim Sci. 2016; 55: 375–386. [PMC free article] [PubMed] [Google Scholar]
- 80.Andersson KE, Immerstrand T, Swärd K, Bergenståhl B, Lindholm MW, Oste R, et al. Effects of oats on plasma cholesterol and lipoproteins in C57BL/6 mice are substrain specific. Br J Nutr. 2010; 103: 513–521. doi: 10.1017/S000711450999211X [DOI] [PubMed] [Google Scholar]
- 81.Marques O, Neves J, Horvat NK, Colucci S, Guida C, Muckenthaler MU. Iron-related parameters are altered between C57BL/6N and C57BL/6J Mus musculus wild-type substrains. HemaSphere. 2019; 3: e304. doi: 10.1097/HS9.0000000000000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hull RL, Willard JR, Struck MD, Barrow BM, Brar GS, Andrikopoulos S, et al. High fat feeding unmasks variable insulin responses in male C57BL/6 mouse substrains. J Endocrinol. 2017; 233: 53–64. doi: 10.1530/JOE-16-0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ge MQ, Yeung SC, Mak JCW, Ip MSM. Differential metabolic and inflammatory responses to intermittent hypoxia in substrains of lean and obese C57BL/6 mice. Life Sci. 2019; 238: 116959. doi: 10.1016/j.lfs.2019.116959 [DOI] [PubMed] [Google Scholar]
- 84.Heiker JT, Kunath A, Kosacka J, Flehmig G, Knigge A, Kern M, et al. Identification of genetic loci associated with different responses to high-fat diet-induced obesity in C57BL/6N and C57BL/6J substrains. Physiol Genomics. 2014; 46: 377–384. doi: 10.1152/physiolgenomics.00014.2014 [DOI] [PubMed] [Google Scholar]
- 85.Kern M, Knigge A, Heiker JT, Kosacka J, Stumvoll M, Kovacs P, et al. C57BL/6JRj mice are protected against diet induced obesity (DIO). Biochem Biophys Res Commun. 2012; 417: 717–720. doi: 10.1016/j.bbrc.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 86.Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring). 2010; 18: 1902–1905. doi: 10.1038/oby.2009.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fergusson G, Ethier M, Guévremont M, Chrétien C, Attané C, Joly E, et al. Defective insulin secretory response to intravenous glucose in C57Bl/6J compared to C57Bl/6N mice. Mol Metab. 2014; 3: 848–854. doi: 10.1016/j.molmet.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fisher-Wellman KH, Ryan TE, Smith CD, Gilliam LA, Lin CT, Reese LR, et al. A direct comparison of metabolic responses to high-fat diet in C57BL/6J and C57BL/6NJ mice. Diabetes. 2016; 65: 3249–3261. doi: 10.2337/db16-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siersbæk MS, Ditzel N, Hejbøl EK, Præstholm SM, Markussen LK, Avolio F, et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci Rep. 2020; 10: 14052. doi: 10.1038/s41598-020-70765-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rendina-Ruedy E, Hembree KD, Sasaki A, Davis MR, Lightfoot SA, Clarke SL, et al. A Comparative Study of the Metabolic and Skeletal Response of C57BL/6J and C57BL/6N Mice in a Diet-Induced Model of Type 2 Diabetes. J Nutr Metab. 2015; 2015: 758080. doi: 10.1155/2015/758080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ulland TK, Jain N, Hornick EE, Elliott EI, Clay GM, Sadler JJ, et al. Nlrp12 mutation causes C57BL/6J strain-specific defect in neutrophil recruitment. Nat Commun. 2016; 7: 13180. doi: 10.1038/ncomms13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Treger RS, Pope SD, Kong Y, Tokuyama M, Taura M, Iwasaki A. The lupus susceptibility locus Sgp3 encodes the suppressor of endogenous retrovirus expression SNERV. Immunity. 2019; 50: 334–347.e9. doi: 10.1016/j.immuni.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eisfeld AJ, Gasper DJ, Suresh M, Kawaoka Y. C57BL/6J and C57BL/6NJ mice are differentially susceptible to inflammation-associated disease caused by influenza A virus. Front Microbiol. 2019; 9: 3307. doi: 10.3389/fmicb.2018.03307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garifulin O, Qi Z, Shen H, Patnala S, Green MR, Boyartchuk V. Irf3 polymorphism alters induction of interferon beta in response to Listeria monocytogenes infection. PLoS Genet. 2007; 3: 1587–1597. doi: 10.1371/journal.pgen.0030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bezdek S, Hdnah A, Sezin T, Mousavi S, Zillikens D, Ibrahim S, et al. The genetic difference between C57Bl/6J and C57Bl/6N mice significantly impacts Aldara™-induced psoriasiform dermatitis. Exp Dermatol. 2017; 26: 349–351. doi: 10.1111/exd.13131 [DOI] [PubMed] [Google Scholar]
- 96.Duan L, Davis JS, Woolbright BL, Du K, Cahkraborty M, Weemhoff J, et al. Differential susceptibility to acetaminophen-induced liver injury in sub-strains of C57BL/6 mice: 6N versus 6J. Food Chem Toxicol. 2016; 98:(Pt B): 107–118. doi: 10.1016/j.fct.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawashita E, Ishihara K, Nomoto M, Taniguchi M, Akiba S. A comparative analysis of hepatic pathological phenotypes in C57BL/6J and C57BL/6N mouse strains in non-alcoholic steatohepatitis models. Sci Rep. 2019; 9: 204. doi: 10.1038/s41598-018-36862-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diwan BA, Blackman KE. Differential susceptibility of 3 sublines of C57BL/6 mice to the induction of colorectal tumors by 1,2-dimethylhydrazine. Cancer Lett. 1980; 9: 111–115. doi: 10.1016/0304-3835(80)90114-7 [DOI] [PubMed] [Google Scholar]
- 99.Kalish S, Lyamina S, Chausova S, Kochetova L, Malyshev Y, Manukhina E, et al. C57BL/6N mice are more resistant to Ehrlich ascites tumors than C57BL/6J mice: the tole of macrophage nitric oxide. Med Sci Monit Basic Res. 2015; 21: 235–240. doi: 10.12659/MSMBR.895555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao L, Mulligan MK, Nowak TS., Jr.Substrain- and sex-dependent differences in stroke vulnerability in C57BL/6 mice. J Cereb Blood Flow Metab. 2019; 39: 426–438. doi: 10.1177/0271678X17746174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tonelli Gombalová Z, Košuth J, Alexovič Matiašová A, Zrubáková J, Žežula I, Giallongo T, et al. Majority of cerebrospinal fluid-contacting neurons in the spinal cord of C57Bl/6N mice is present in ectopic position unlike in other studied experimental mice strains and mammalian species. J Comp Neurol. 2020; 528: 2523–2550. doi: 10.1002/cne.24909 [DOI] [PubMed] [Google Scholar]
- 102.Crusio WE, Schwegler H, van Abeelen JH. Behavioural and neuroanatomical divergence between two sublines of C57BL/6J inbred mice. Behav Brain Res. 1991; 42: 93–97. doi: 10.1016/S0166-4328(05)80043-9 [DOI] [PubMed] [Google Scholar]
- 103.Jamot L, Bertholet JY, Crusio WE. Neuroanatomical divergence between two substrains of C57BL/6J inbred mice entails differential radial-maze learning. Brain Res. 1994; 644: 352–356. doi: 10.1016/0006-8993(94)91703-5 [DOI] [PubMed] [Google Scholar]
- 104.Mangaru Z, Salem E, Sherman M, Van Dine SE, Bhambri A, Brumberg JC, et al. Neuronal migration defect of the developing cerebellar vermis in substrains of C57BL/6 mice: cytoarchitecture and prevalence of molecular layer heterotopia. Dev Neurosci. 2013; 35: 28–39. doi: 10.1159/000346368 [DOI] [PubMed] [Google Scholar]
- 105.Karthivashan G, Park SY, Kim JS, Cho DY, Ganesan P, Choi DK. Comparative studies on behavioral, cognitive and biomolecular profiling of ICR, C57BL/6 and its sub-strains suitable for scopolamine-induced amnesic models. Int J Mol Sci. 2017; 18: 1735. doi: 10.3390/ijms18081735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Usami M, Okada A, Taguchi K, Hamamoto S, Kohri K, Yasui T. Genetic differences in C57BL/6 mouse substrains affect kidney crystal deposition. Urolithiasis. 2018; 46: 515–522. doi: 10.1007/s00240-018-1040-3 [DOI] [PubMed] [Google Scholar]
- 107.Chang HY, Mitzner W, Watson J. Variation in airway responsiveness of male C57BL/6 mice from 5 vendors. J Am Assoc Lab Anim Sci. 2012; 51: 401–406. [PMC free article] [PubMed] [Google Scholar]
- 108.Will JP, Hirani D, Thielen F, Klein F, Vohlen C, Dinger K, et al. Strain-dependent effects on lung structure, matrix remodeling, and Stat3/Smad2 signaling in C57BL/6N and C57BL/6J mice after neonatal hyperoxia. Am J Physiol Regul Integr Comp Physiol. 2019; 317: R169–R181. doi: 10.1152/ajpregu.00286.2018 [DOI] [PubMed] [Google Scholar]
- 109.Sankaran JS, Varshney M, Judex S. Differences in bone structure and unloading-induced bone loss between C57BL/6N and C57BL/6J mice. Mamm Genome. 2017; 28: 476–486. doi: 10.1007/s00335-017-9717-4 [DOI] [PubMed] [Google Scholar]
- 110.Liron T, Raphael B, Hiram-Bab S, Bab IA, Gabet Y. Bone loss in C57BL/6J-OlaHsd mice, a substrain of C57BL/6J carrying mutated alpha-synuclein and multimerin-1 genes. J Cell Physiol. 2018; 233: 371–377. doi: 10.1002/jcp.25895 [DOI] [PubMed] [Google Scholar]
- 111.Banks G, Heise I, Starbuck B, Osborne T, Wisby L, Potter P, et al. Genetic background influences age-related decline in visual and nonvisual retinal responses, circadian rhythms, and sleep. Neurobiol Aging. 2015; 36: 380–393. doi: 10.1016/j.neurobiolaging.2014.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ranson A, Cheetham CE, Fox K, Sengpiel F. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc Natl Acad Sci USA. 2012; 109: 1311–1316. doi: 10.1073/pnas.1112204109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schnabolk G, Stauffer K, O’Quinn E, Coughlin B, Kunchithapautham K, Rohrer B. A comparative analysis of C57BL/6J and 6N substrains; chemokine/cytokine expression and susceptibility to laser-induced choroidal neovascularization. Exp Eye Res. 2014; 129: 18–23. doi: 10.1016/j.exer.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lajko M, Cardona HJ, Taylor JM, Farrow KN, Fawzi AA. Photoreceptor oxidative stress in hyperoxia-induced proliferative retinopathy accelerates rd8 degeneration. PLoS One. 2017; 12: e0180384. doi: 10.1371/journal.pone.0180384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013; 14: R82. doi: 10.1186/gb-2013-14-7-r82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith CL, Goldsmith CA, Eppig JT. The Mammalian Phenotype Ontology as a tool for annotating, analyzing and comparing phenotypic information. Genome Biol. 2005; 6: R7. doi: 10.1186/gb-2004-6-1-r7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006; 15: 1187–1194. doi: 10.1093/hmg/ddl034 [DOI] [PubMed] [Google Scholar]
- 118.Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005; 48: 675–686. doi: 10.1007/s00125-005-1680-z [DOI] [PubMed] [Google Scholar]
- 119.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001; 2: 11. doi: 10.1186/1471-2202-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Specht CG, Schoepfer R. Deletion of multimerin-1 in alpha-synuclein-deficient mice. Genomics. 2004; 83: 1176–1178. doi: 10.1016/j.ygeno.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 121.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012; 53: 2921–2927. doi: 10.1167/iovs.12-9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mehalow AK, Kameya S, Smith RS, Hawes NL, Denegre JM, Young JA, et al. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum Mol Genet. 2003; 12: 2179–2189. doi: 10.1093/hmg/ddg232 [DOI] [PubMed] [Google Scholar]
- 123.Mahajan VS, Demissie E, Mattoo H, Viswanadham V, Varki A, Morris R, et al. Striking immune phenotypes in diverse gene-targeted mice are driven by a copy number variant originating from a commercially available C57BL/6 strain. Cell Rep. 2016; 15: 1901–1909. doi: 10.1016/j.celrep.2016.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Purtha WE, Swiecki M, Colonna M, Diamond MS, Bhattacharya D. Spontaneous mutation of the Dock2 gene in Irf5-/- mice complicates interpretation of type I interferon production and antibody responses. Proc Natl Acad Sci USA. 2012; 109: E898–E904. doi: 10.1073/pnas.1118155109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fischer M, Kosyakova N, Liehr T, Dobrowolski P. Large deletion on the Y-chromosome long arm (Yq) of C57BL/6JBomTac inbred mice. Mamm Genome. 2017; 28: 31–37. doi: 10.1007/s00335-016-9669-0 [DOI] [PubMed] [Google Scholar]
- 126.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006; 55: 2153–2156. doi: 10.2337/db06-0358 [DOI] [PubMed] [Google Scholar]
- 127.Bourdi M, Davies JS, Pohl LR. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol. 2011; 24: 794–796. doi: 10.1021/tx200143x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao KNS, Shen X, Pardue S, Krzywanski DM. Nicotinamide nucleotide transhydrogenase (NNT) regulates mitochondrial ROS and endothelial dysfunction in response to angiotensin II. Redox Biol. 2020; 36: 101650. doi: 10.1016/j.redox.2020.101650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388: 839–840. doi: 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- 130.Asuni AA, Hilton K, Siskova Z, Lunnon K, Reynolds R, Perry VH, et al. Alpha-synuclein deficiency in the C57BL/6JOlaHsd strain does not modify disease progression in the ME7-model of prion disease. Neuroscience. 2010; 165: 662–674. doi: 10.1016/j.neuroscience.2009.10.047 [DOI] [PubMed] [Google Scholar]
- 131.Pelkonen A, Yavich L. Neuromuscular pathology in mice lacking alpha-synuclein. Neurosci Lett. 2011; 487: 350–353. doi: 10.1016/j.neulet.2010.10.054 [DOI] [PubMed] [Google Scholar]
- 132.Peña-Oliver Y, Buchman VL, Dalley JW, Robbins TW, Schumann G, Ripley TL, et al. Deletion of alpha-synuclein decreases impulsivity in mice. Genes Brain Behav. 2012; 11: 137–146. doi: 10.1111/j.1601-183X.2011.00758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peña-Oliver Y, Sanchez-Roige S, Stephens DN, Ripley TL. Alpha-synuclein deletion decreases motor impulsivity but does not affect risky decision making in a mouse Gambling Task. Psychopharmacology (Berl). 2014; 231: 2493–2506. doi: 10.1007/s00213-013-3416-y [DOI] [PubMed] [Google Scholar]
- 134.Wong SY, Coffre M, Ramanan D, Hines MJ, Gomez LE, Peters LA, et al. B cell defects observed in Nod2 knockout mice are a consequence of a Dock2 mutation frequently found in inbred strains. J Immunol. 2018; 201: 1442–1451. doi: 10.4049/jimmunol.1800014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang SK, Hawkins NA, Kearney JA. C57BL/6J and C57BL/6N substrains differentially influence phenotype severity in the Scn1a+/- mouse model of Dravet syndrome. Epilepsia Open. 2018; 4: 164–169. doi: 10.1002/epi4.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kopić A, Benamara K, Schuster M, Leidenmühler P, Bauer A, Glantschnig H, et al. Coagulation phenotype of wild-type mice on different genetic backgrounds. Lab Anim. 2019; 53: 43–52. doi: 10.1177/0023677218811059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Newberry EP, Kennedy S, Xie Y, Luo J, Jiang H, Ory DS, et al. Phenotypic divergence in two lines of L-Fabp-/- mice reflects substrain differences and environmental modifiers. Am J Physiol Gastrointest Liver Physiol. 2015; 309: G648–G661. doi: 10.1152/ajpgi.00170.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Öztürk S, Roessner PM, Schulze-Edinghausen L, Yazdanparast H, Kalter V, Lichter P, et al. Rejection of adoptively transferred Eµ-TCL1 chronic lymphocytic leukemia cells in C57BL/6 substrains or knockout mouse lines. Leukemia. 2019; 33: 1514–1539. doi: 10.1038/s41375-018-0332-5 [DOI] [PubMed] [Google Scholar]
- 139.Eicher EM, Shown EP, Washburn LL. Sex reversal in C57BL/6J-YPOS mice corrected by a Sry transgene. Philos Trans R Soc Lond B Biol Sci. 1995; 350: 263–268, discussion 268–269. doi: 10.1098/rstb.1995.0160 [DOI] [PubMed] [Google Scholar]
- 140.Eicher EM, Washburn LL, Whitney JB, 3rd, Morrow KE. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science. 1982; 217: 535–537. doi: 10.1126/science.7089579 [DOI] [PubMed] [Google Scholar]
- 141.Umemura Y, Miyamoto R, Hashimoto R, Kinoshita K, Omotehara T, Nagahara D, et al. Ontogenic and morphological study of gonadal formation in genetically-modified sex reversal XY(POS) mice. J Vet Med Sci. 2016; 77: 1587–1598. doi: 10.1292/jvms.15-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yokoyama T, Miura Y, Yamamoto A, Hasegawa C, Kawanishi K, Takada N, et al. Genetic differences between C57BL/6 substrains affect the process of testis differentiation in YPOS mice. J Vet Med Sci. 2019; 81: 608–611. doi: 10.1292/jvms.18-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nowak TS, Jr, Mulligan MK. Impact of C57BL/6 substrain on sex-dependent differences in mouse stroke models. Neurochem Int. 2019; 127: 12–21. doi: 10.1016/j.neuint.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 144.Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, et al. In silico mapping of complex disease-related traits in mice. Science. 2001; 292: 1915–1918. doi: 10.1126/science.1058889 [DOI] [PubMed] [Google Scholar]
- 145.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004; 14: 1806–1811. doi: 10.1101/gr.2825804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tsang S, Sun Z, Luke B, Stewart C, Lum N, Gregory M, et al. A comprehensive SNP-based genetic analysis of inbred mouse strains. Mamm Genome. 2005; 16: 476–480. doi: 10.1007/s00335-005-0001-7 [DOI] [PubMed] [Google Scholar]
- 147.Wade CM, Kulbokas EJ, 3rd, Kirby AW, Zody MC, Mullikin JC, Lander ES, et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002; 420: 574–578. doi: 10.1038/nature01252 [DOI] [PubMed] [Google Scholar]
- 148.Wiltshire T, Pletcher MT, Batalov S, Barnes SW, Tarantino LM, Cooke MP, et al. Genome-wide single-nucleotide polymorphism analysis defines haplotype patterns in mouse. Proc Natl Acad Sci USA. 2003; 100: 3380–3385. doi: 10.1073/pnas.0130101100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang J, Hunter KW, Gandolph M, Rowe WL, Finney RP, Kelley JM, et al. A high-resolution multistrain haplotype analysis of laboratory mouse genome reveals three distinctive genetic variation patterns. Genome Res. 2005; 15: 241–249. doi: 10.1101/gr.2901705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Petkov PM, Cassell MA, Sargent EE, Donnelly CJ, Robinson P, Crew V, et al. Development of a SNP genotyping panel for genetic monitoring of the laboratory mouse. Genomics. 2004; 83: 902–911. doi: 10.1016/j.ygeno.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 151.Wakana S, Suzuki T, Furuse T, Kobayashi K, Miura I, Kaneda H, et al. Introduction to the Japan Mouse Clinic at the RIKEN BioResource Center. Exp Anim. 2009; 58: 443–450. doi: 10.1538/expanim.58.443 [DOI] [PubMed] [Google Scholar]
- 152.Zurita E, Chagoyen M, Cantero M, Alonso R, González-Neira A, López-Jiménez A, et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011; 20: 481–489. doi: 10.1007/s11248-010-9403-8 [DOI] [PubMed] [Google Scholar]
- 153.Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017; 546: 421–425. doi: 10.1038/nature22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mise-Omata S, Alles N, Fukazawa T, Aoki K, Ohya K, Jimi E, et al. NF-κB RELA-deficient bone marrow macrophages fail to support bone formation and to maintain the hematopoietic niche after lethal irradiation and stem cell transplantation. Int Immunol. 2014; 26: 607–618. doi: 10.1093/intimm/dxu062 [DOI] [PubMed] [Google Scholar]
- 155.Shen FW, Saga Y, Litman G, Freeman G, Tung JS, Cantor H, et al. Cloning of Ly-5 cDNA. Proc Natl Acad Sci USA. 1985; 82: 7360–7363. doi: 10.1073/pnas.82.21.7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tung JS, Scheid MP, Pierotti MA, Hämmerling U, Boyse EA. Structural features and selective expression of three Ly-5+ cell-surface molecules. Immunogenetics. 1981; 14: 101–106. doi: 10.1007/BF00344303 [DOI] [PubMed] [Google Scholar]
- 157.Klein J. List of congenic lines of mice. I. Lines with differences at alloantigen loci. Transplantation. 1973; 15: 137–153. doi: 10.1097/00007890-197301000-00021 [DOI] [PubMed] [Google Scholar]
- 158.Chisolm DA, Cheng W, Colburn SA, Silva-Sanchez A, Meza-Perez S, Randall TD, et al. Defining genetic variation in widely used congenic and backcrossed mouse models reveals varied regulation of genes important for immune responses. Immunity. 2019; 51: 155–168.e5. doi: 10.1016/j.immuni.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jang Y, Gerbec ZJ, Won T, Choi B, Podsiad A, B Moore B, et al. Cutting edge: check your mice-a point mutation in the Ncr1 locus identified in CD45.1 congenic mice with consequences in mouse susceptibility to infection. J Immunol. 2018; 200: 1982–1987. doi: 10.4049/jimmunol.1701676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011; 477: 289–294. doi: 10.1038/nature10413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yalcin B, Wong K, Agam A, Goodson M, Keane TM, Gan X, et al. Sequence-based characterization of structural variation in the mouse genome. Nature. 2011; 477: 326–329. doi: 10.1038/nature10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Egan CM, Sridhar S, Wigler M, Hall IM. Recurrent DNA copy number variation in the laboratory mouse. Nat Genet. 2007; 39: 1384–1389. doi: 10.1038/ng.2007.19 [DOI] [PubMed] [Google Scholar]
- 163.Hossain MS, Asano F, Fujiyama T, Miyoshi C, Sato M, Ikkyu A, et al. Identification of mutations through dominant screening for obesity using C57BL/6 substrains. Sci Rep. 2016; 6: 32453. doi: 10.1038/srep32453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Corty RW, Kumar V, Tarantino LM, Takahashi JS, Valdar W. Mean-variance QTL mapping identifies novel QTL for circadian activity and exploratory behavior in mice. G3 (Bethesda). 2018; 8: 3783–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lilue J, Doran AG, Fiddes IT, Abrudan M, Armstrong J, Bennett R, et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nat Genet. 2018; 50: 1574–1583. doi: 10.1038/s41588-018-0223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sarsani VK, Raghupathy N, Fiddes IT, Armstrong J, Thibaud-Nissen F, Zinder O, et al. The genome of C57BL/6J “Eve”, the mother of the laboratory mouse genome reference strain. G3 (Bethesda). 2019; 9: 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang E, Kang S, Park H, Park S, Ji Y, Holzapfel WH. Differences in anxiety levels of various murine models in relation to the gut microbiota composition. Biomedicines. 2018; 6: 113. doi: 10.3390/biomedicines6040113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smoczek M, Vital M, Wedekind D, Basic M, Zschemisch NH, Pieper DH, et al. A combination of genetics and microbiota influences the severity of the obesity phenotype in diet-induced obesity. Sci Rep. 2020; 10: 6118. doi: 10.1038/s41598-020-63340-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stappenbeck TS, Virgin HW. Accounting for reciprocal host-microbiome interactions in experimental science. Nature. 2016; 534: 191–199. doi: 10.1038/nature18285 [DOI] [PubMed] [Google Scholar]
- 170.Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012; 209: 1445–1456. doi: 10.1084/jem.20120504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Roberts A, Paddock C, Vogel L, Butler E, Zaki S, Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005; 79: 5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wentworth DE, Gillim-Ross L, Espina N, Bernard KA. Mice susceptible to SARS coronavirus. Emerg Infect Dis. 2004; 10: 1293–1296. doi: 10.3201/eid1007.031119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dinnon KH, 3rd, Leist SR, Schäfer A, Edwards CE, Martinez DR, Montgomery SA, et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020; 586: 560–566. doi: 10.1038/s41586-020-2708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hassan AO, Case JB, Winkler ES, Thackray LB, Kafai NM, Bailey AL, et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020; 182: 744–753.e4. doi: 10.1016/j.cell.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weston S, Coleman CM, Haupt R, Logue J, Matthews K, Li Y, et al. Broad anti-coronaviral activity of Food and Drug Administration approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J Virol. 2020; e01218-20. doi: 10.1128/JVI.01218-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaushansky A, Austin LS, Mikolajczak SA, Lo FY, Miller JL, Douglass AN, et al. Susceptibility to Plasmodium yoelii preerythrocytic infection in BALB/c substrains is determined at the point of hepatocyte invasion. Infect Immun. 2015; 83: 39–47. doi: 10.1128/IAI.02230-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nicholson SM, Peterson JD, Miller SD, Wang K, Dal Canto MC, Melvold RW. BALB/c substrain differences in susceptibility to Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. 1994; 52: 19–24. doi: 10.1016/0165-5728(94)90157-0 [DOI] [PubMed] [Google Scholar]
- 178.Poyntz HC, Jones A, Jauregui R, Young W, Gestin A, Mooney A, et al. Genetic regulation of antibody responsiveness to immunization in substrains of BALB/c mice. Immunol Cell Biol. 2019; 97: 39–53. doi: 10.1111/imcb.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020; 18: e3000410. doi: 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.