Abstract
Clarification of the criteria for managing animal health is essential to increase the reliability of experiments and ensure transparency in animal welfare. For experiments performed in space, there is no consensus on how to care for animals owing to technical issues, launch mass limitation, and human resources. Some biological processes in mammals, such as musculoskeletal or immune processes, are altered in the space environment, and mice in space can be used to simulate morbid states, such as senescence acceleration. Thus, there is a need to establish a novel evaluation method and evaluation criteria to monitor animal health. Here, we report a novel method to evaluate the health of mice in space through a video downlink in a series of space experiments using the Multiple Artificial-gravity Research System (MARS). This method was found to be more useful in evaluating animal health in space than observations and body weight changes of the same live mice following their return to Earth. We also developed criteria to evaluate health status via a video downlink. These criteria, with “Fur condition” and “Respiratory” as key items, provided information on the daily changes in the health status of mice and helped to identify malfunctions at an early stage. Our method and criteria led to the success of our missions, and they will help establish appropriate rules for space experiments in the future.
Keywords: health monitoring method, International Space Station (ISS) rodent mission, space flight
Introduction
Exposure to microgravity during space travel induces syndromes in astronauts that closely resemble those commonly observed in elderly and bedridden individuals [1,2,3] . Muscle weakness and atrophy typically occur in astronauts who stay on orbit for a long term. This condition, which is also common in elderly or bedridden individuals, termed as sarcopenia, occurs due to muscle misuse. To understand the mechanisms underlying these phenomena, animal experiments are needed in space. Several experiments on micro Gravity (µG) have been conducted using mice on orbit using the Space Shuttles, unmanned satellites “BION” and the International Space Station (ISS) [4,5,6,7,8], but such experiments have numerous constraints. For example, it is difficult to obtain data on body weight or food consumption due to the effects of microgravity. Moreover, it is difficult to develop test batteries by specialists due to a lack of human resources. Animal monitoring during space experiments in a manner similar to that performed on the ground has the following concerns: limited data traffic and communication time via satellite.
To ensure experimental reproducibility and animal welfare, experiments involving laboratory animals should be appropriately designed and transparently reported. It is particularly important to outline the criteria for monitoring animals. Comprehensive monitoring criteria for daily observations enable early detection of abnormalities, provide insights into phenotypes, and reduce the level of suffering. Accordingly, researchers can minimize data loss, improve the quality of their research, and consequently ensure experimental reproducibility.
With the establishment of guidelines [9,10,11], including detailed regulations of animal care management, appropriate management for ground-based animal experiments has been defined. On the ground, these guidelines help ensure that experiments are implemented under special environments or using genetically modified mice. Contrarily, there are no comprehensive criteria for the general assessment of rodents in space. To establish such criteria, it is not sufficient to simply apply guidelines used on the ground; there is a need to examine baseline variables while considering that microgravity is a specific environment associated with several constraints.
For animal experiments in space, the COSPAR Policy and Guideline for the Utilization and Care of Animals Used in Space Research was released in 2007, based on the principle of the 3Rs—Replacement, Reduction, and Refinement [12]. However, the policy does not include general criteria for animal care. Thus, it is necessary to establish new criteria for monitoring mice.
The Italian Space Agency (ASI) has developed animal habitation cage for use in experiments in the ISS [5, 7], and the cage comprises video camera systems for checking animal health. The Italian Mice Drawer System (MDS) housed six male mice individually for 90 days on the ISS (STS-128/129), 2009. During their stay on the ISS for 3 months, three mice died. Post-landing checking of the mice cage indicated that one of the mice died due to a failure of the food cassette system. With sustained and continuous video observation, such malfunctions may have been avoided. The Russian Bion-M1 biosatellite launched by the Institute for Biomedical Problems (IBMP, Russia) in 2013 accommodated male mice for a 30-day experiment [4]. Video visibility was compromised over time, as floating liquids and debris tended to obscure the camera lens and the general atmosphere of the animals. On the first day of the final week, none (0%) of the mice were clearly visible in the video records. In this milieu, new video systems for the observation of animals on-board are anticipated for future space missions.
We, the Japan Aerospace Exploration Agency (JAXA), started a new project to assess the actual effects of microgravity or partial gravity on mice with the newly developed rodent experimental platform “MARS”—Multiple Artificial-gravity Research System [13]. Five space experiments (MHU1, MHU2, MHU3, MHU4, and MHU5) using the MARS were chosen by public invitation and conducted [13,14,15] . However, the opportunities for these missions are limited, and it is important to manage animal health for the reliability of experiments.
In terms of animal care, we adopted a system consisting of individual housing cages, each mounted with a video camera with an LED/IR source, and wipers inside the cage to clean the observation window. Single housing enables the identification of individual mouse with ease and observation of the whole body. Thus, we were able to confirm the status of each mouse without any loss of data due to unexpected interference by other mice and positional relationships. This method of individual, on-board observations enables video data for each mouse to be downlinked over successive days.
Herein, we present an overview of a novel method to evaluate the health condition of mice in space and provide a novel criterion for visual monitoring of animals in space. We also present the results of an observation experiment on orbit performed using this method. This method and criterion not only enhance the reliability of experiments but also provide the novel tool to carry out future unmanned operations in space.
Materials and Methods
Spaceflight mission
All data presented herein were obtained during two space experiments, namely, MHU-1 and MHU-3. These experiments were conducted on the ISS during the docking periods of space vehicles: SpaceX-9 (SPX9) and SpaceX-14 (SPX14) launched in 2016 and 2018, respectively.
In both experiments, mice were acclimatized to the food and nozzle in the same manner as in the habitat cage units (HCU) in the Space Station Processing Facility (SSPF) Science Annex at Kennedy Space Center (KSC), 3 weeks before launch. After acclimation, the mice were placed in the transportation cage unit (TCU) and launched by SpaceX. During the flight phase, the mice were housed in the HCUs. Food cartridge exchange, water supply, waste collection, and odor filter exchange, were performed approximately once per week. After days 36 and 28 of MHU-1 and MHU-3, live mice were retrieved on the ground. All mice were transported to Explora Biolabs and dissected. To compare data before launch and after retrieval, the body weight and health conditions of each mouse were determined at KSC and Explora Biolab, as detailed in Table 1. Detailed flight experimental timelines, and the configuration of the TCUs and HCUs has been described previously [13].
Table 1. Observational check items for each phase.
| Pre-flight |
Flight | Post-flight (After retrieval) |
|||
|---|---|---|---|---|---|
| Acclimation | Before launch | ||||
| <base item> | |||||
| Fur condition | ○ | ○ | ○ | ○ | |
| Respiratory | × | × | ○ | × | |
| <additional item> | |||||
| Ears | ○ | ○ | ○ | ○ | |
| Mouth | ○ | ○ | ○ | ○ | |
| Eye | ○ | ○ | ○ | ○ | |
| Nose | ○ | ○ | ○ | ○ | |
| Proctology | ○ | ○ | × | ○ | |
| Tail | ○ | ○ | ○ | ○ | |
| Limbs | ○ | ○ | ○ | ○ | |
| Whisker | ○ | ○ | × | ○ | |
| Teeth | × | ○ | × | ○ | |
| Abdominal tone | × | ○ | × | ○ | |
Animals
Male C57BL/6J mice (Stock #000664) were purchased from Jackson Laboratories (USA) and Charles River Laboratories (Japan) for MHU1 and MHU3, respectively. Both animal experiments were approved by the Institutional Animal Care and Use Committee of JAXA (protocol numbers 016-014 and 017-001), NASA (protocol numbers NAS-15-004-Y1 and FLT-17-112), and Explora Biolabs (EB15-010), and conducted according to the related guidelines and applicable laws in Japan and the USA .
Herein, we present the data of the two experiments, which were conducted during the docking periods of SPX9 and SPX14. Mice launched by SPX9 were numbered 1-1, 1-2, 1-3, 1-4, 1-5, and 1-6, and those by SPX14 were numbered 2-1, 2-2, 2-3, 2-4, 2-5, and 2-6. These mice were housed under microgravity conditions, and fed and supplied the same food and water: CRF-1 (Oriental Yeast Co., Ltd., Tokyo, Japan) and autoclaved tap water containing iodine (0.2 mg/L). Moreover, the interior components of the cage systems were the same, whereas the inner wall and nozzle design of the HCUs were improved [13].
On-orbit observation
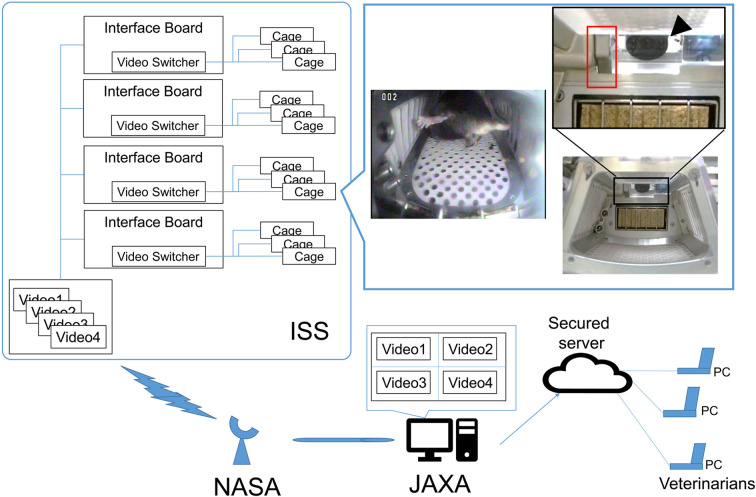
Video configuration: All video data were obtained using a camera attached to the HCU. Videos were captured at 30 frames per second (fps) and transmitted to the ground at a transfer rate of 6 Mbps after analog-to-digital conversion. Each camera included a wiper with a washer reservoir. Figure 1 provides details of this video data downlink system. Camera windows were washed at the discretion of the ground staff when the visibility of the subject image was poor. Detailed information regarding the cameras and a representative image of onboard habitation in an HCU have been published previously [13]. The cage image video was downlinked through the satellite communication line, such as K under Band. The video data were uploaded by ground staff to an online secured server and reviewed by JAXA attending veterinarians.
Fig. 1.
Overview of the observation system via downlink. Habitat cage unit (HCU) with a camera (arrowhead) and a wiper (red box) per cage. Movies of the wiper operation are shown in Supplementary Video 3. HCU cameras were connected to IFs. The IFs contained a switch limiting the view to one of three videos. Video switches could be controlled from the ground. While the video data downloaded via satellite, communications were projected on a monitor. Animal care staff then observed animal health, created a report for veterinarians, and uploaded the video to an online secured server, for the attending veterinarian.
Time constraints for observations
The communication time for on orbit observations is limited for the following reasons. First, we could not downlink all video data from HCUs simultaneously, because the camera was connected to video interface boards (IFs) (Fig. 1). Data from a maximum of four cages could be downlinked at the same time for observation. Second, the use of the communication line between ISS and JAXA was constrained. When using satellite communication, we had to consider the acquisition of signal (AOS) periods and the loss of signal (LOS). We could only downlink video data during the AOS period. Furthermore, when maintaining the HCUs during the daytime, the video line had to be disconnected. Therefore, depending on the day, the maximum available time for observation was 2 h per mouse per day.
Observation checklist items on orbit
In addition to time constraints, HCUs only have one camera due to the limited power and size capacity. Furthermore, cameras for each HCU were arranged in one direction, and the angle of the camera could not be changed. Therefore, ‘Fur condition’ and ‘Respiratory’ were set as base checklist items because these could be confirmed in a short time, regardless of the animals’ sleep state or location. Furthermore, both ‘Fur condition’ and ‘Respiratory’ are commonly observed to identify clinical problems and to determine the state of vital signs and physiological responses. For checklist items, additional six items were included: tail, limbs, ears, mouth, eyes, and nose. These additional checklist items were similar to those recorded during the pre-flight phase, except for proctology appearance. Proctology appearance and whisker was not checked on orbit as this was difficult to perform using videos. Proctology appearance was observed before launch and after retrieval; no changes were recorded.
Under normal ‘Fur condition’ and ‘Respiratory’ conditions, as shown in Supplementary Video 1 and 2, the score was “0.” Gasping respiration, or slow/fast breathing, was assigned a score of “1.” When an abnormal “Fur condition” was observed, such as the presence of wounds, dermatitis, dull fur, or alopecia, the score was “1.” Wetted Fur was scored “1” when the wetted area covered more than half of the face or one-third of the back. Additional points were added to the base points when the tail, limbs, ears, mouth, eyes, and nose showed signs of abnormality, such as bleeding, wounds, and dirt. Representative normal status is shown in Supplementary Video 2. Detailed criteria are presented in Supplementary Table 1.
Data quality of downlinked video images from the ISS
As detailed above, “Fur condition” was set as a base checklist item. Therefore, before the space experiment, a ground-based biocompatibility test replicated on the orbit network connection was conducted to determine the quality of the video. From this, the amount of downlinked data was set as 6 Mbps. All video data used for observations were downlinked as planned during the flight phase. Although visibility from the camera windows was sometimes impaired by the presence of feces and urine, the wiper worked effectively to eliminate dirt. Moreover, the image quality was sufficient to observe animal status, including “Fur condition,” on orbit during habitation.
Results
Observation of on-board animal health condition through downlinked videos
During the flight phase, the health condition of each mouse was checked daily by veterinarians and animal care staff on the ground through downlinked videos. The health condition of each mouse was determined by observing the appearance, “Fur condition,” and “Respiratory.” The observations are summarized in Table 2.
Table 2. Observational findings in each experiment.
| SPX9 |
SPX14 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | 1-6 | 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | 2-6 | ||
| <base item> | |||||||||||||
| Fur | 0 | 0 | 0 | 4a | 9a | 5a | 0 | 0 | 0 | 0 | 0 | 0 | |
| Respiratory | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| <additional item> | |||||||||||||
| Ears | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mouth | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Eye | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tail | 0 | 0 | 0 | 2 b | 1b | 1 b | 0 | 0 | 0 | 1 b | 1 b | 0 | |
| Limbs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SUM | 0 | 0 | 0 | 6 | 10 | 6 | 0 | 0 | 0 | 1 | 1 | 0 | |
aWetted. bReddening, Kinking. Under normal health status, the score is “0.” When abnormal status was observed, the score is “1” or more. The criteria are stated in the Material and Methods.
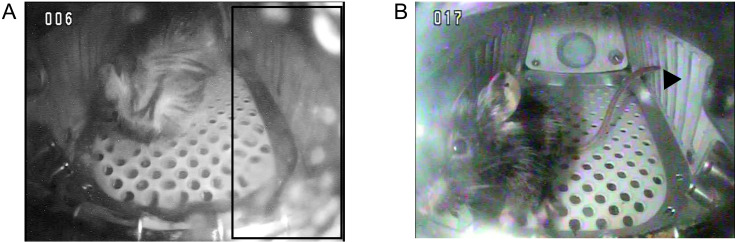
Five mice were scored higher than or equal to “1”: mice 1-4, 1-5, 1-6, 2-4, and 2-5. In mice 1-4, 1-5, and 1-6, which were launched by SPX9, two types of abnormalities were observed. First, the fur on the head and back appeared wetted over several days. Subsequently, other conditions, such as necrosis, were observed at the tip of the tails. These necroses were desquamated within a few days after cage replacement, and scab formation was observed. The scabs remained until the end of the flight experiment. A representative image is shown in Fig. 2 (mouse 1-5). These symptoms were caused by the malfunction of HCUs, because they were observed within a few days of water leakage.
Fig. 2.
Representative image of abnormalities exhibited by mouse 1-5. A, mouse head and back were wet. Water leaking from water nozzles. The boxed half of the observation window was covered with water. B, Scabs were observed on the tail tips (arrowhead). Scabs on the tail are also shown in Supplementary Video 4
Second, mouse 1-4 showed alopecia-like abnormalities, which were concluded to be partially wetted fur. As described below, “Fur condition” appeared differently depending on the lighting, making it difficult to distinguish alopecia.
One abnormality was observed in mice 2-4 and 2-5, which were launched by SPX14; this occurred because they were stacked in the door on day 1. This stacking caused tail reddening in mouse 2-4 and tail kinking in mouse 2-5. Tail reddening in mouse 2-4 resolved by day 2. Conversely, the tail of mouse 2-5 withered due to necrosis, and it was desquamated on day 24.
Comparison between on-board and post-flight observations
Observations were conducted during the post-flight phase for comparison. As shown in Table 3, five mice presented abnormalities on the ground: mice 1-4, 1-5, 1-6, 2-5, and 2-6. Conversely, on-orbit, five mice were scored higher than or equal to “1”: mice 1-4, 1-5, 1-6, 2-4, and 2-5.
Table 3. Observational findings after retrieval.
| SPX9 |
SPX14 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 1-2 | 1-3 | 1-4 | 1-5 | 1-6 | 2-1 | 2-2 | 2-3 | 2-4 | 2-5 | 2-6 | ||
| <base item> | |||||||||||||
| Fur | 0 | 0 | 0 | 0 | 1a | 1a,b | 0 | 0 | 0 | 0 | 0 | 1a | |
| <additional item> | |||||||||||||
| Ears | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mouth | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Eye | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Proctology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tail | 0 | 0 | 0 | 1c | 1c | 1c | 0 | 0 | 0 | 0 | 1c | 0 | |
| Limbs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Whisker | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Teeth condition | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Abdominal tone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SUM | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | |
aDull fur. bAlopecia. cScabs. Under normal health status, the score is “0.” When abnormal status was observed, the score is “1” or more. The criteria are stated in the Material and Methods.
Regarding tail condition, except for those of mouse 2-4, observations made on the ground were consistent with those made on orbit via the video data downlink system. Scabs on the tails of mice 1-4, 1-5, 1-6, and 2-5 were verified through observations on the ground. Tail reddening in mouse 2-4 could not be observed after landing; this was observed only on day 1 of the experiment and had resolved on orbit by day 2. Furthermore, we were unable to verify this during the post-flight phase.
On the ground, mice 1-5, 1-6, and 2-6 were found to have dull fur, and mouse 1-6 had alopecia; however, this was not detected in the flight phase. The dull fur of mice 1-5 and 1-6 were considered to have occurred during the returning phase because an additional malfunction, water leaking from cages 1-5 and 1-6 of the TCUs, occurred. The fur of mouse 2-6 was wetted during the last week of flight experiments; however, it was not scored because the area was less than half of the animal’s head. Similarly, mouse 1-4 was observed to have an alopecia-like condition on its head via the downlink. This was not verified during the post-flight observation. This alopecia-like condition remained until close to the end of the flight experiment.
Malfunction and improvement of hardware
The changes in the health condition of mice were observed within a few days of the malfunctions. Figure 3 shows that the malfunction was associated with their health condition. During flight periods, three kinds of malfunction were observed: food cartridge stacking, water leakage, and water depletion. Food cartridges were stacked on days 17 (1-3 cage), 19 (1-5 cage), and 20 (1-6 cage) during the docking period of SPX9. The surface of a food bar in the food cartridge was compared with that on the previous day to determine whether the animal had eaten food. Therefore, all these stackings were dealt on the following day by replacing food cartridges. Water leakage occurred on days 12 (1-5 cage), 15 (1-6 cage), 22 (1-4 cage), and 25 (1-5 cage). Furthermore, water leakage occurred in cages 1-5 and 1-6 of the TCU during the return phase. Approximately 80% of the malfunctions during the SPX9 period were caused by water leakages, and the leakages affected the mice more seriously than other malfunctions. A detailed analysis of this malfunction revealed that countermeasures against water leakage in the HCU and TCU were taken. The design of the cage and the associated materials were changed; subsequently, there were no water leakage malfunctions and no significant abnormalities. Therefore, the conditions of mice launched by SPX14 were more stable, except for water depletion, which occurred on day 22 (2-5 cage).
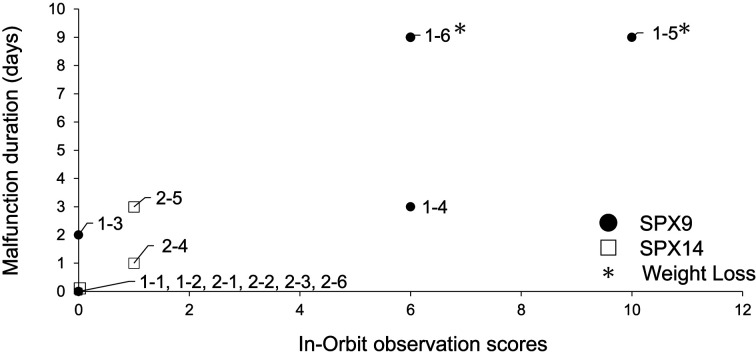
Fig. 3.
Correlation between the duration of malfunction and the on-board observation score. The on-orbit observation score is shown in Table 2, and accumulates the days over which the mice showed abnormalities. The duration of the malfunction was the number of days that the malfunctions were observed. Animals with a change in body weight of more than 1 g between pre and post values are indicated with an asterisk.
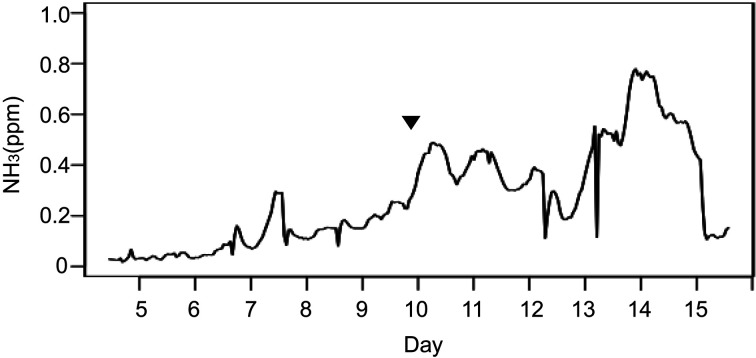
During the flight period, we monitored the carbon dioxide and ammonia concentrations, humidity, and temperature. HCUs drew air into each cage from the Japanese Experiment Module (JEM) module. During the flight period, there were no significant changes in the carbon dioxide concentration, humidity, or temperature. Although the ammonia concentration increased from day 10, as shown in Fig. 4, the concentration was below 1 ppm and close to the limit of detection. This stable concentration of ammonia, which was below the target threshold, could be attributed to the paper sheets set at the bottom and along the walls of the HCU, which were intended to minimize water overflow. This measure was effective in maintaining a suitable housing environment. Furthermore, gas concentrations were monitored using a sensor attached inside the rack, not inside each cage, and the concentration was decreased through dispersal. Although there was little change in this concentration, this method can help to detect abnormalities, because digitized data can be continuously monitored for 24 h.
Fig. 4.
Increasing concentration of ammonia gas after the first instance of water leakage. Wetted fur was observed on Day 10 (mouse 1-5), and is indicated with an arrowhead.
Discussion
Herein, we provide the preliminary results that summarize the changes in animal health conditions in an individual housing cage system with daily video monitoring in space. The results are important to “individual housing in space,” as stressed animals usually affect others in a group-housing cage.
Comprehensive evaluation through a flight experiment
Most mice either gained weight or maintained their pre-flight weight. The average weight changes in the mice launched by SPX9 and SPX14 between pre- to post-flight were −0.08 and 0.45 g per week, respectively. The average weight of mice launched by SPX9 appeared to decrease markedly, with a decrease of more than 1 g in mice 1-5 and 1-6. Except mice 1-5 and 1-6, the average weight gain was 0.1 g per week.
Notably, the mice launched by SPX14 gained weight at the same rate as those on the ground. For the flight experiment during the SPX14 period, the mice were housed on orbit from 16 to 20 weeks of age. At this age, the body weight of mice increase by 0.53 g per week, according to bodyweight information for C57BL/6J provided by Jackson Laboratory (Stock Number: 000664, 2019, https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664). Previous studies have shown that microgravity can decrease the rate of weight gain in mice [4, 8] . Contrary to the results of the previous studies, our study demonstrated that mice could maintain the same weight as on the ground by using our method.
Advantages of single housing
Single housing was considered to have contributed to the success of our study. Animal experiments on orbit encounter malfunctions; this has been reported previously [4, 5]. Single housing ensures that malfunctions occur only in the affected cage. Moreover, with several limitations, such as launch mass, human resources, and communication traffic, a simple analysis is beneficial for improving management efficiency. On the ground, even when mice are housed in a group, care staff can easily identify individuals through hands-on examination. Conversely, in space, the health conditions of all mice are checked within the constraints of communication traffic and time. Video images of individually housed mice could reduce the time taken to confirm identification, which could then be used to perform in-depth observations. Furthermore, individually housed mice are not at a risk of attack by other mice.
Space experiments must be performed with limited resources; therefore, it is sometimes difficult to interrupt other operations and provide care immediately. Thus, when focused only on mouse management, single housing is clearly a better solution. Here, all mice launched to the ISS were retrieved alive and healthy after space missions; therefore, we could not check abnormal respiratory parameters. However, respiratory parameters were obtained under normal conditions, including respiration rate and breathing patterns. Therefore, the inclusion of video data in the management criteria will enable abnormalities to be readily identified. The observation of respiratory parameters would help detect the status of vitals, that is, the decision whether a mouse is anxious or not, more easily.
Mice are “social animals,” and some studies on social interactions require group-housed mice. Therefore, for these experiments, more complex technologies are needed to facilitate the identification of mice, such as the use of a multi-video system. Using 20 mice for behavioral analyses, the NASA reported that mice in space present behavior typical of mice on the ground [6]. All mice in the experiments were housed in a group and were not assessed individually, making it difficult to observe individual changes in health status. On the ground, video tracking analysis using video cameras and infrared beams have been developed [16], and the evaluation process has been automated. It is important for this analysis method that the distance between mice and the camera is constant [17]. However, on orbit, as the distance between the camera and mice is not constant because mice float, it is difficult to apply the method unaltered. We are currently developing a new method to analyze the mouse activities calculated from pixel intensity change of each video frame (Shimomura et al. in preparation). By combining this method and data presented here, it will be possible to automatically determine the observation criteria that are currently being judged by humans. Furthermore, global standards can be set, and the reproducibility of experiments will be improved.
Countermeasures and destress of animals in case of hardware malfunction and animal sign
The video downlink system is a powerful tool to observe animal health condition on-board without astronauts, but the system is just for observation. When ground staffs and veterinarian notice hardware malfunctions such as water leakage inside the HCU through downlinked videos, they can plan to accommodate the crew task to replace the HCU with a new one. For deciding on the crew task, by referencing the ammonia concentration with downlinked videos, the ground staff can identify the signs of abnormalities at an early stage and minimize animal stress.
Our last resort alternative on-board is euthanasia of the mouse if the ground veterinarian decides that there is no way to continue the experiment on-board. JAXA developed a new CO2 gas euthanasia system for mouse, and this system has already been checked on-board without mouse.
Conclusions and future perspectives
Muscle atrophy in astronauts is classified as secondary sarcopenia according to the Report of the European Working Group on Sarcopenia in Older People 2010 [1]. As secondary sarcopenia also occurs in bedridden and elderly individuals, understanding such conditions in astronauts may benefit patients on Earth, and may help identify physical or pharmacological therapies for sarcopenia in elderly individuals. Studies utilizing rodents in space are warranted to understand the molecular mechanisms and biological processes underlying these syndromes under special environments. Our method can be used to guarantee the reliability of animal conditions in space. The health status of mice can be linked to malfunctions, especially water leakage. Therefore, the early detection of leakage is key to maintain the health of onboard mice. Our results show the importance of checking the environments of animal cages, such as telemetry monitoring of the concentration of ammonia as a reference, so that water leakage can be detected reliably.
Technological advances are leading us to an exciting stage in space exploration. In the future, space exploration will expand to include the Moon and Mars. However, the launch mass of an experiment must be decreased as the mission becomes further away, further restricting experimental constraints. Therefore, unmanned operations, namely automation, should be utilized on orbit, especially for maintaining animal health. From the view of animal welfare and distress, the determination of appropriate endpoints and the treatment procedures must be set together with the development of an automated habitation system. Visual monitoring is one of the best ways to resolve them. The changes in skin conditions are useful for detecting hardware malfunctions and reflecting animal health, and respiration can be monitored clearly. Our results are reliable in determining and maintaining mice on orbit and are useful for further applications.
Supplementary
Acknowledgments
We thank Noriko Kajiwara, JAXA visiting veterinarian, Fumika Yamaguchi, Masumi Umehara, Ramona Bober, Autumn L. Cdebaca, and Rebecca A. Smith for support with animal care and ground experiments; Hiroe Kobayashi, Teruhiro Senkoji, Hiromi Sano, Yui Nakata, Hiromi Suzuki-Hashizume, Chie Matsuda, Natsuhiko Inoue, Ari Yamanaka, Eiji Ohta, Hideaki Hotta, Hatsumi Ishida, Mariko Shimizu, and members of the JEM operational team for research coordination; Hong Xin for landing site operational support; Sayaka Umemura, Laura Lewis, Charles E. Hopper, Jennifer J. Scott Williams, and Robert Kuczajda for international coordination.
References
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48: 61. doi: 10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucaro MA, Zahm AM, Risbud MV, Ayyaswamy PS, Mukundakrishnan K, Steinbeck MJ, et al. The effect of simulated microgravity on osteoblasts is independent of the induction of apoptosis. J Cell Biochem. 2007; 102: 483–495. doi: 10.1002/jcb.21310 [DOI] [PubMed] [Google Scholar]
- 3.Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol 1985. 2000; 89: 1046–1054. [DOI] [PubMed] [Google Scholar]
- 4.Andreev-Andrievskiy A, Popova A, Boyle R, Alberts J, Shenkman B, Vinogradova O, et al. Mice in Bion-M 1 space mission: training and selection. PLoS One. 2014; 9: e104830. doi: 10.1371/journal.pone.0104830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancedda R, Liu Y, Ruggiu A, Tavella S, Biticchi R, Santucci D, et al. The Mice Drawer System (MDS) experiment and the space endurance record-breaking mice. PLoS One. 2012; 7: e32243. doi: 10.1371/journal.pone.0032243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronca AE, Moyer EL, Talyansky Y, Lowe M, Padmanabhan S, Choi S, et al. Author Correction: Behavior of mice aboard the International Space Station. Sci Rep. 2019; 9: 10154. doi: 10.1038/s41598-019-45362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandonà D, Desaphy JF, Camerino GM, Bianchini E, Ciciliot S, Danieli-Betto D, et al. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS One. 2012; 7: e33232. doi: 10.1371/journal.pone.0033232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gridley DS, Mao XW, Stodieck LS, Ferguson VL, Bateman TA, Moldovan M, et al. Correction: Changes in Mouse Thymus and Spleen after Return from the STS-135 Mission in Space. PLoS One. 2013; 8: 8. doi: 10.1371/annotation/e66bdc4e-2409-4582-b163-7bc182db275e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Science Council of Japan. 2006. Guidelines for Proper Conduct of Animal Experiments.
- 10.Kagiyama N, Ikeda T, Nomura T. Japanese guidelines and regulations for scientific and ethical animal experimentation. In Vivo Models of Inflammation. 2006; 1: 187–191. [Google Scholar]
- 11.Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen; 1959. [Google Scholar]
- 12.Committee on Space Research. 2010. The COSPAR Policy and Guideline for the Utilization and Care of Animals Used in Space Research.
- 13.Shiba D, Mizuno H, Yumoto A, Shimomura M, Kobayashi H, Morita H, et al. Development of new experimental platform ‘MARS’-Multiple Artificial-gravity Research System-to elucidate the impacts of micro/partial gravity on mice. Sci Rep. 2017; 7: 10837. doi: 10.1038/s41598-017-10998-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda C, Kato T, Inoue-Suzuki S, Kikuchi J, Ohta T, Kagawa M, et al. Dietary intervention of mice using an improved Multiple Artificial-gravity Research System (MARS) under artificial 1 g. NPJ Microgravity. 2019; 5: 16. doi: 10.1038/s41526-019-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki T, Uruno A, Yumoto A, Taguchi K, Suzuki M, Harada N, et al. Nrf2 contributes to the weight gain of mice during space travel. Commun Biol. 2020; 3: 496. doi: 10.1038/s42003-020-01227-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhuang H, Garrote E, Mutch J, Yu X, Khilnani V, Poggio T, et al. Automated home-cage behavioural phenotyping of mice. Nat Commun. 2010; 1: 68. doi: 10.1038/ncomms1064 [DOI] [PubMed] [Google Scholar]
- 17.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999; 835: 18–26. doi: 10.1016/S0006-8993(98)01258-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.