Abtract
"Ecstasy" (MDMA) and related drugs are amphetamine derivatives that also have some of the pharmacological properties of mescaline. They have become popular with participants in "raves," because they enhance energy, endurance, sociability and sexual arousal. This vogue among teenagers and young adults, together with the widespread belief that "ecstasy" is a safe drug, has led to a thriving illicit traffic in it. But these drugs also have serious toxic effects, both acute and chronic, that resemble those previously seen with other amphetamines and are caused by an excess of the same sympathomimetic actions for which the drugs are valued by the users. Neurotoxicity to the serotonergic system in the brain can also cause permanent physical and psychiatric problems. A detailed review of the literature has revealed over 87 "ecstasy"-related fatalities, caused by hyperpyrexia, rhabdomyolysis, intravascular coagulopathy, hepatic necrosis, cardiac arrhythmias, cerebrovascular accidents, and drug-related accidents or suicide. The toxic or even fatal dose range overlaps the range of recreational dosage. The available evidence does not yet permit an accurate assessment of the size of the problem presented by the use of these drugs.
Ecstasy is the popular or “street” name for a substance identified chemically as N-methyl-3,4-methylenedioxy-amphetamine or 3,4-methylenedioxy-methamphetamine. The initial letters of the major portions of the latter name (Methylenedioxy-Methamphetamine) give rise to the acronym MDMA, by which this substance is commonly designated in the clinical and research literature. As the name implies, MDMA is a derivative of methamphetamine (known by such street names as “speed,” “crystal” and “meth” among others) and its parent compound amphetamine. A closely related compound, N-ethyl-3,4-methylene-dioxyamphetamine or MDEA, differs from MDMA only in having a 2-carbon ethyl group, rather than a 1-carbon methyl group, attached to the nitrogen atom of the amphetamine structure.
The name “ecstasy” is in fact used somewhat unselectively. In earlier years, the name was applied to 3,4-methylenedioxyamphetamine (MDA). MDEA is also sometimes called ecstasy by its vendors and users, but is more often referred to as Eve. The 3 compounds are closely similar in their chemistry and in their biological effects, so that the description of MDMA in the rest of this review also applies in the main to MDEA, and to a considerable extent to MDA.
Ecstasy differs from amphetamine and methamphetamine in one important respect. As shown in Fig. 1, it has a methylenedioxy (-O-CH2-O-) group attached to positions 3 and 4 of the aromatic ring of the amphetamine molecule (i.e., it is “ring-substituted”). In this respect, it resembles the structure of the hallucinogenic material mescaline. As a result, the pharmacological effects of MDMA (and MDEA) are a blend of those of the amphetamines and mescaline, as will be described in later sections of this review. This group of substances is frequently referred to as “designer drugs,” because, when illicit laboratories began to produce them for nonmedical use, the blend of amphetamine-like and mescaline-like effects was intentionally sought and could be achieved reliably by the appropriate design of the drug molecule.1 All of these substances resemble the natural neurotransmitters epinephrine (adrenaline) and dopamine (Fig. 1), and most of their biological actions and effects resemble those of epinephrine, dopamine and serotonin.
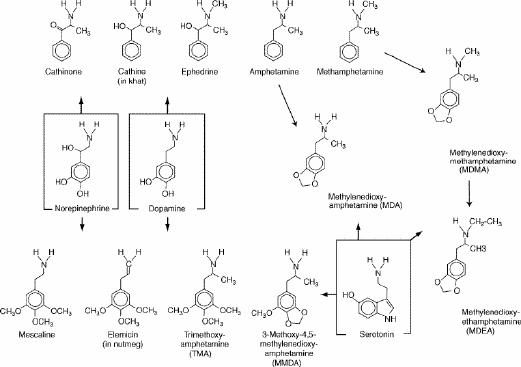
Fig. 1: Chemical structures of MDMA, MDEA and related drugs compared with those of the monoamine neurotransmitters. Arrows do not represent pathways of synthesis or metabolism; they merely indicate the closest resemblances of structure.
Like amphetamine, MDA and MDMA are completely synthetic substances that do not exist in nature. All 3 were first synthesized many decades ago: amphetamine in 1885,2 MDA in 1910 and MDMA in 1912.3 MDA was patented as a cough suppressant in 1956, as a tranquillizer in 1960 and as an appetite inhibitor in 1961, but was not marketed for any of these uses.1 The intention was apparently to market MDMA as an appetite inhibitor, but it was also never marketed and is now used only nonmedically. Amphetamine was in fact marketed for weight-reduction purposes among others, in the 1930s, though its sale was later sharply restricted because of its widespread abuse and the risk of dependence and other adverse effects.2 In 1985, the methylenedioxy and methoxy derivatives of the amphetamines were placed in Schedule 1 of the restricted drugs list in the United States,1 and they are classified similarly in Canada (Schedule III of the Controlled Drugs and Substances Act) and the United Kingdom (Class A under the Misuse of Drugs Act).
Routes of administration and dosage
Like the amphetamines, MDMA and its related compounds are amines that can exist either as free bases or as salts of various acids. The free bases are volatile and, indeed, amphetamine itself was first marketed in this form in an inhaler, for use as a nasal decongestant. Theoretically, MDMA and MDEA could also be used in this fashion, but the methylenedioxy group raises the boiling point of the free base so high that it is impossible to use it by sniffing the vapour.3 The salts, on the other hand, are not volatile but are quite soluble in water4 and can, therefore, be administered intravenously, orally or by “snorting” the powder.5,6 MDMA is almost always taken by mouth and is prepared as single-dose tablets for this purpose.
As sold illicitly in Europe and North America, MDMA is typically prepared in very professional-looking tablets stamped with a wide variety of symbols according to the whim or imagination of the maker (see illustration in Theune and colleagues7). However, the actual composition of the tablets varies greatly, with respect to both the drug(s) contained in them and the amounts. Several different laboratories have analyzed street samples sold in their localities, and have found that the drug sold as “ecstasy” may be MDMA, MDEA, MDA, PMA (para-methoxyamphetamine), MBDB (3,4-methylenedioxy-phenyl-N-methylbutanamine), ephedrine or varying mixtures of these,8,9,10 though the great majority consist of a single active drug.11 The typical dosage range of MDMA for recreational use varies from 50 mg to 150 mg,7,12 but the amount per tablet in different batches of tablets may vary 70-fold or more, from almost zero to well over 100 mg.9,13
The total amount consumed per occasion varies greatly among users. In Europe, North America and Australia, MDMA has been used primarily as a party drug rather than by solitary users.14 Its current popularity arises from its use at “raves,” the all-night dance parties at which it is taken to postpone fatigue and allow the user to dance energetically for hours on end. For this purpose, the most common dosage has been 1–2 tablets during the course of the party,15,16 but occasional case reports have indicated doses as high as 10 tablets in combination with other drugs,17,18,19 usually with toxic outcomes. In 2 fatal cases, the actual amount taken is uncertain because the victims ingested the drug unknowingly, their drinks having been “spiked” with it by friends playing a joke on them.20
Pharmacology of MDMA and related drugs
In order to understand the highly varied effects of these drugs, both desired and undesired, on the user, it is helpful to review briefly the basic pharmacology of the ring-substituted amphetamines, including their pharmacokinetics and their mechanism of action in the brain and other organs.
Pharmacokinetics
MDMA is readily absorbed from the intestinal tract and reaches its peak concentration in the plasma about 2 hours after oral administration.21,22 Doses of 50 mg, 75 mg and 125 mg to healthy human volunteers produced peak blood concentrations of 106 ng/mL, 131 ng/mL and 236 ng/mL respectively. These concentrations are quite low, because the drug passes readily into the tissues, and much of it is bound to tissue constituents. It is helpful to remember these peak concentrations for comparison with the levels found in patients who have suffered the serious and sometimes fatal adverse effects described later in this review.
The drug is broken down metabolically, mainly in the liver, where an enzyme designated CYP2D6 is chiefly responsible.23 However, several different enzymes are involved in its degradation,24 and some of these appear to be saturated at relatively low concentrations of the drug. Consequently, as the dose is increased and the higher-affinity enzymes are saturated, disproportionately large increases in blood and brain concentrations of the drug occur.25 Therefore, small increases in dosage may carry the risk of large increases in toxicity.
Elimination of the drug from the body is moderately slow, the half-life for MDMA disappearance from the blood being of the order of 8 hours.4,21,22 Because it takes about 5 half-lives (i.e., about 40 hours for MDMA) for over 95% of the drug to be cleared from the body, this may explain the persistence of troublesome after-effects for one or 2 days after use. Some of the metabolites of MDMA are still pharmacologically active, especially its first metabolite, MDA, so that the duration of action may be somewhat longer than the duration of MDMA itself in the body.
Pharmacodynamics
There is now an abundance of evidence, both experimental and clinical, that MDMA and the other ring-substituted amphetamine derivatives act by increasing the net release of the monoamine neurotransmitters (serotonin, noradrenaline and, to a smaller extent, dopamine) from their respective axon terminals.3,26,27 MDMA does not act by directly releasing serotonin but, rather, by binding to, and thus blocking, the transporter involved in its reuptake.3,28,29 Rats, trained to discriminate between the effects of saline and those of serotonin in an operant task, respond to MDMA as if it were serotonin.30 A similar, but weaker, action is also exerted on the reuptake of dopamine.31 The physiological effects of MDMA and MDA in mice are the same as those of amphetamine,32 which is known to act as a releaser of dopamine and noradrenaline. There is a small amount of experimental evidence that the net release of acetylcholine may also be increased by MDMA,33 but the importance of this effect in humans is unknown. It is clear, however, that the increase in the net release of serotonin (and possibly dopamine) is the major mechanism of action underlying the distinctive mental effects of MDMA, whereas the increased release of noradrenaline is mainly responsible for the physical effects that it shares with amphetamine.
MDMA and its related compounds are generally produced as racemic mixtures, but the stereoisomers differ from each other in several important respects. For example, S(+)-MDMA is more potent than R(-)-MDMA in producing the distinctive subjective effects that are characteristic of ecstasy.34,35 Some (but not all) studies suggest that the R(-) isomer has stronger mescaline-like or lysergic acid diethylamide (LSD)-like distinctive properties, whereas the S(+) isomer is more amphetamine-like.36 The S(+) and R(-) isomers of both MDA and MDMA differ in their dose–response curves for changes in serotonergic function and neurotoxicity.37 The MDMA isomers also differ in the rate at which they are converted to their corresponding MDA metabolites.38 It is, therefore, possible that some of the striking interindividual differences in the intensity, time course and toxicity of the effects of ecstasy may be related to individual differences in the metabolic handling of the isomers.
Effects on the user
The reported effects of MDMA vary according to the dose and the frequency and duration of use. In general, the effects desired by most users are those produced by low doses on single occasions. It is, therefore, convenient to describe the effects separately for acute (single-occasion) and chronic (long-term) use and, within each category, to describe separately the mental and the physical effects. A third category of effect, consisting of the serious or fatal toxicity seen at higher doses or in abnormally sensitive individuals, will be described separately.
Acute effects
Desired effects
The desired effects for which MDMA is used are closely similar to those that account for the continuing popularity of the other amphetamines. Physically, it produces a marked increase in wakefulness, endurance and sense of energy, sexual arousal, and postponement of fatigue and sleepiness.15,39,40 The accompanying psychological effects are described as a sense of euphoria, well-being, sharpened sensory perception, greater sociability, extraversion, heightened sense of closeness to other people, and greater tolerance of their views and feelings.13,39,40,41
The latter effects have given rise to the claim that MDMA represents a distinct class of drugs designated “empathogens” or “entactogens”10,40,42,43 that may be of potential value as an aid in psychotherapy.1,3,44,45 Similar claims were made earlier for MDA, LSD and other hallucinogens but, despite claims of success in noncontrolled trials with MDA,1 no lasting benefit was found in a 10-year follow-up study of patients treated with LSD.46 No comparable study has been conducted on patients treated with MDMA, and the recent clinical literature contains almost no reference to its use in psychotherapy. However, it is not possible to say whether this is because of disappointment with the results, or because of difficulty obtaining the drug since its change in legal status.
Undesired effects
Like the amphetamines, MDMA also has adverse effects on many physical functions, even when taken in moderate doses for the recreational purposes described earlier.47 Because the basic action of the amphetamines involves increased arousal and alertness, it is usually accompanied by an increase in tension, which is manifested as muscular tension, jaw clenching, tooth grinding (bruxism) and constant restless movement of the legs.3,13,15,41,43 The increased muscle activity, together with a direct action of the drug on the thermoregulatory system in the brain,48 leads to an increase in body temperature. Stiffness and pain in the lower-back and limb muscles are very common complaints during the first 2–3 days after the use of MDMA. Headache, nausea, loss of appetite, blurred vision, dry mouth and insomnia are other commonly reported physical symptoms during the drug experience and immediately afterwards. Heart rate and blood pressure, which are usually elevated during the drug experience, tend to fluctuate more widely than normal during the following days.
Undesired psychological acute effects commonly reported during the drug experience similarly represent an exaggeration of the effects for which the drug is taken. The increased arousal, if carried to excess, is converted into hyperactivity, flight of ideas (with a resulting inability to focus one's thoughts in a sustained and useful manner) and insomnia. Related complaints often include mild hallucinations, depersonalization (a feeling of separation of the self from the body), anxiety, agitation, and bizarre or reckless behaviour.13,15,41,43,49,50 Occasionally these symptoms lead to panic attacks,51,52,53,54 delirium55,56 or even brief psychotic episodes51,54,57 that usually (but not always) resolve rapidly when the drug action wears off. The day or 2 after drug use, the most common mental or mood complaints are difficulty concentrating, depression, anxiety and fatigue.39,41 These symptoms strongly resemble, in miniature, the “crash” that is typically seen as a withdrawal reaction after the prolonged euphoria or manic state produced by heavy use of amphetamine, cocaine or other central nervous system stimulants.2,58 Despite these complaints, the majority of users find the overall balance of the experience positive rather than negative but, with frequent repetition of the experience, the negative effects tend to become more prominent and the beneficial or pleasurable ones less so.41,44
Long-term or residual effects
Serotonin neurotoxicity
Apart from the small number of people who have reported improvement or resolution of emotional or personality problems after the use of MDMA in psychotherapy, the long-term effects are virtually all adverse ones. They are all thought to arise from a neurotoxic action of the methylenedioxy derivatives of the amphetamines.
The ability of MDMA to increase the concentration of serotonin in the synapse probably underlies its production of improved mood and of sensory alterations. However, at higher doses the massive release of serotonin not only gives rise to acute psychotic symptoms (as described earlier) but also causes chemical damage to the cells that released it.
This damage has been clearly demonstrated in animal experiments with MDMA and related drugs.59,60,61,62 Chemical and microscopic studies have shown reduced serotonin content of the brain, decreased numbers of identifiable serotonin-containing neurons and serotonin transporter molecules, and numerous degenerating serotonergic axons and axon terminals in the brains of animals treated with MDMA. Although there are conflicting theories concerning the mechanism of this neurotoxicity,4,27,63 it is clearly related to the excessive metabolic activity and neurotransmitter release in the serotonergic and, possibly, the dopaminergic neurons.
In humans, there has been only one postmortem study of changes in the levels of serotonin and its main metabolite in the brain of a single long-term MDMA user.64 The levels were reduced by 50%–80% in different regions of the brain, in comparison with those in the brains of controls who had not used MDMA, whereas the dopamine levels were unaltered. However, several types of experimental study in living humans have provided indirect evidence of serotonin neurotoxicity.65
· The levels of serotonin metabolites in the cerebrospinal fluid reflect the amount of release during neuronal activity in the brain.
· MRI or proton magnetic resonance spectroscopy can provide estimates of the numbers of intact neurons in different parts of the brain.
· Labelled compounds with high affinity and selectivity for serotonergic neurons, for the reuptake transporter or for the postsynaptic serotonin receptors are administered to the subjects. Techniques such as positron emission tomography (PET) or single photon emission computed tomography (SPECT) are then used to detect the locations and amounts of the labelled compounds in the brain.
· Drugs that are known to stimulate serotonergic pathways in the brain are administered, and the endocrine responses to the released serotonin (changes in blood levels of prolactin and cortisol) are measured.
Such studies permit either estimates or measurements of the numbers of functionally intact serotonin-releasing cells or serotonin-responsive cells in the living subject.
By these means, it has been demonstrated that the brains of long-term MDMA users, when examined while free of the drug, have abnormally low levels of serotonin and its metabolites in the cerebrospinal fluid,66 reduced numbers of serotonin transporter molecules,67,68,69 increased numbers of glial cells,70 and altered patterns of glucose metabolism and blood flow in certain parts of the brain.71,72 During the acute action of MDMA, SPECT studies show a downregulation of serotonin receptors (an adaptive response to the increased release of serotonin) in the cerebral cortex,73 but in long-term users in the drug-free state there is upregulation of receptors (an adaptive response to the decrease in serotonin release).74 Electroencephalographic studies indicate a decrease of bilateral symmetry of the wave patterns and frequencies in MDMA users, similar to the changes seen in aging and dementia,75 and a change in response to auditory stimuli that was seen only in MDMA users and not in matched groups of cannabis users and nonusers of any drugs.76 The prolactin and cortisol responses to stimulation of the serotonin system were reduced in the MDMA users, the changes persisting for up to a year or more after the last use of MDMA.77,78,79
A major limitation of these studies is that, even if they demonstrate decreased numbers of serotonin cells and reduced serotonin system function in the brains of MDMA users, they cannot prove that the MDMA use caused the changes. The alterations in serotonin function might have been present before the drug use began, they might even have contributed to the start of drug use or they might be purely coincidental.80 However, several studies have shown that the degree of change in serotonin function is proportional to the duration and intensity of the preceding use of MDMA, and this is more compatible with the MDMA use being the cause rather than the consequence of impaired serotonin function.
Long-term psychiatric problems
It has been suggested that the demonstrated neurotoxic effects of MDMA on the serotonin system may be the possible cause of a variety of mental and behavioural problems that outlast the actual drug experience by months or years. These problems are quite varied in nature, but they all involve functions in which serotonin is known to play an important role. Among such persisting problems described in the literature are the following:
· impairment of memory, both verbal and visual, with the degree of impairment being roughly proportional to the intensity of the preceding MDMA use81 and not seen in matched groups of polydrug users who had not taken MDMA.16,82,83 The memory deficit was correlated with changes in SPECT measurement of serotonin function.74 In one striking case, long-lasting memory deficits, associated with bilateral brain changes in the MRI image, followed a single exposure to MDMA;84
· impairment of decision-making (“executive function”), information processing, logical reasoning and simple problem solving;16,49,53,83,85,86,87
· greater impulsivity and lack of self-control;49,53,83,88,89
· panic attacks occurring repeatedly when the person is not under the influence of the drug, even after many months of abstinence;52,53,54
· recurrent paranoia, hallucinations, depersonalization, flashbacks and even psychotic episodes, occurring some time after the individual has stopped using MDMA;39,51,89,90,91,92,93,94,95
· severe depression, which is sometimes resistant to any treatment other than selective serotonin reuptake inhibitors39,49,51,53,54,91,93,96 and occasionally accompanied by suicidal thoughts.
The same problems of interpretation arise in these cases as mentioned earlier in relation to studies of serotonin neurotoxicity in humans, namely, the difficulty of deciding whether the alterations found in patients with chronic use of MDMA were the cause of the drug use, the result of it or coincidental.97 However, the affected functions are known to depend on a serotonin mechanism; the degree of at least some of the functional disturbances is proportional to the degree of loss of serotonin cells; and it would be extremely difficult to propose any rational explanation for how so diverse a set of functional disturbances could all cause the same desire to use MDMA. Therefore, logic supports the view that these disturbances are indeed residual consequences of the drug use.
Residual physical problems
As is the case for psychiatric problems, there are a number of physical problems that either appear after drug use is over, or that begin during the period of drug use but persist long afterwards. Among these are the following:
· tooth grinding; the jaw clenching and tooth grinding (bruxism) that were described earlier as acute effects of MDMA often persist during periods of nonuse41,43,91 and result in significant wearing down of the back teeth.98,99
· muscle aches and pains; the same tendency to increased muscle tension and spasm that is responsible for the jaw clenching is also seen in other muscles, especially in the lower back and neck.39,41,44
· circulatory system; the acute effects of MDMA on the circulatory system, as noted earlier, include elevation of the blood pressure, but longer-term residual effects tend to result in low blood pressure and poorer control of heart rate and blood pressure by the autonomic nervous system.54,100 Changes in the regional pattern of blood flow in the brain have been reported in regular users.73
· neurological lesions; the neurotoxicity described earlier has been held responsible for 2 unusual long-term problems in the nervous system. One case of parkinsonism101 and one of bilateral abducens paralysis102 have been attributed to damage to dopaminergic neurons.
Major physical toxicity
This section deals with very serious and potentially life-threatening physical problems that are clearly and directly attributable to the known pharmacological actions of the drug itself. There are 4 principal types of such serious toxicity: hepatic, cardiovascular, cerebral and hyperpyrexic. Each is described separately below, but these patterns of toxicity are not mutually exclusive, and in severe cases patients may have features of 2 or more types concurrently.
Hepatic toxicity
A high proportion of the case reports of serious MDMA toxicity include the observation that the patients were jaundiced. Various explanations have been offered for this effect, including the possibility of an allergic drug reaction, a toxic contaminant in the individual batch of drug, or a secondary effect of hyperpyrexia,103,104 which will be described later. However, the most probable explanation relates to the pathways of metabolism of the drug. As noted earlier, MDMA and related drugs are largely metabolized in the liver by the cytochrome P450 variety designated CYP2D6.23 The immediate product of this reaction is then processed further by other enzymes into a variety of secondary products, some of which are highly reactive with glutathione. A marked decrease in the level of free glutathione permits a series of biochemical changes (massive influx of calcium, oxidative change in the cell-membrane lipids, and so on) that result in cell death.104,105,106
The clinical picture in such cases is varied. On the whole, it is relatively mild, resembling a viral hepatitis, with jaundice, an enlarged tender liver, an increased bleeding tendency, raised liver enzyme levels in the blood and a biopsy picture of acute hepatitis that is not in any sense diagnostic of MDMA toxicity. Spontaneous recovery usually occurs over a period of a few weeks to many months, but in chronic users of MDMA there may be repeated attacks of hepatitis.103,104,107,108,109,110,111,112 Several authors have suggested that in any case of repeated acute hepatitis in a young person, the use of MDMA should be suspected as a possible cause. Andreu and colleagues103 found that in their hospital “ecstasy” was the second most common cause of liver injury in patients under the age of 25 years.
The picture can be much more severe, however, progressing rapidly to a fulminating liver failure that proves fatal unless the patient is fortunate enough to receive a liver transplant.107,113,114 At intermediate grades of severity, there may be a prolonged course with slow recovery of liver function103 but possible permanent fibrosis of the liver.115 In one series of 7 cases,114 one patient died, one recovered after a liver transplant and 5 recovered spontaneously. The largest series of ecstasy-related transplants reviewed so far is described by Brauer and colleagues,116 who found 9 cases in the literature plus one of their own; of these, 4 died after the transplant while 6 survived, having either fully or partially recovered. A newer and perhaps less drastic procedure is auxiliary liver transplantation, in which the recipient's own liver is left in place but a donor liver is inserted as well, in order to carry out the necessary liver functions while the recipient's own liver gradually recovers, at which time the auxiliary liver is removed.117,118 At least 3 patients with “ecstasy”-induced acute liver failure have been treated in this way.
Cardiovascular toxicity
As noted earlier, MDMA and related drugs increase the net release not only of serotonin, but also of noradrenaline and dopamine. It is especially the noradrenaline that is responsible for most of the serious adverse effects on the cardiovascular system. These effects consist of 2 basic types: hypertension, with a consequent risk of ruptured blood vessels and internal hemorrhage, and tachycardia, with a consequently increased cardiac workload, and a resulting risk of heart failure. Both types have been described in the clinical literature about “ecstasy.”
· Major intracranial hemorrhages have been reported.20,119,120,121 These probably result from rupture of vessels that are already weakened by congenital anomaly or pre-existing disease, when the added burden of drug-induced hypertension is imposed upon them.
· Petechial hemorrhages have been observed in the brain9 and in various other organs as incidental observations at autopsy in many of the fatal cases to be described in the next section of this review. This type of hemorrhage affects the small vessels that are intrinsically weaker than the larger ones and does not require pre-existing damage to the vessel walls.
· Retinal hemorrhage has been observed122 at autopsy.
· Damage to blood vessel walls, and resulting intravascular thrombosis, may possibly be linked to reported cases of cerebral infarct.123,124,125
· Serious disturbances of cardiac rhythm were observed in a number of cases.9,114,126
Many of the fatal cases described later in this review had pulmonary edema, which is a sign of heart failure.
Cerebral toxicity
One of the consequences of the use of “ecstasy” at raves is profuse sweating as a result of both the vigorous physical activity and the pharmacological action of the drug on the thermoregulatory mechanism. Large amounts of sodium can be lost in sweat, and if the dancers drink large amounts of water in order to avoid overheating, the result is frequently hemodilution and resulting hyponatremia. An additional mechanism that can contribute to the same result is inappropriate secretion of the pituitary antidiuretic hormone, leading to retention of water by the kidneys,127,128 but in most cases it is probably caused by excessive water intake following profuse sweating. This leads to passage of water from the blood into the tissues, including the brain.129,130 This has 2 serious consequences: initiation of epilepsy-like seizures and compression of the brain stem and cerebellum downward toward the foramen magnum, which can lead to fatal disruption of respiration or circulation. Many such cases have been reported.7,9,56,131,132,133,134,135,136 Theune and colleagues7 state that seizures are among the most frequently encountered neurological problems arising from the use of “ecstasy.” There has even been one report of a 13-month-old child who suffered severe seizures, hypertension and fever, after ingesting a capsule of MDMA that he had found on the floor in his home.137
Hyperpyrexic pattern of toxicity
This pattern, which is perhaps the most dangerous form of toxicity induced by “ecstasy,” has become increasingly frequent since the adoption of MDMA by participants in raves. As noted earlier, the combination of the drug action, intense physical activity and a hot environment contribute to this increase. Even an increase of a few degrees in environmental temperature causes marked increases in body temperature and serotonin toxicity in the brains of rats treated with MDMA, but not in those treated with a physiological salt solution.138 In most human cases, this does not have serious consequences, but in a significant proportion it does lead to life-threatening or fatal effects on various tissues and organs of the body.139
The pattern of these changes closely resembles that seen in severe heatstroke.140,141 It is not specific to MDMA having been clearly described in cases of toxicity caused by amphetamine,140 methamphetamine and phenmetrazine,141 and MDEA.142 In the most severe cases, the marked elevation of body temperature initiates a series of interrelated effects that can differ in their relative prominence in different cases.143,144
· Rhabdomyolysis: the intense muscular activity that contributes to the production of heat also causes severe damage to the muscle tissue itself, including marked swelling and edema, loss of microscopic structural features of normal muscle, inflammatory cell infiltration, breakdown of the muscle cell membranes and leakage of the intracellular contents into the bloodstream, and finally necrosis.145 Even when recovery occurs, the necessary surgical removal of large amounts of necrotic muscle tissue may leave the patient with some residual disability when walking or moving in other ways.146,147
· Myoglobinuria and renal failure: one of the normal muscle constituents that leaks into the circulation as a result of rhabdomyolysis is myoglobin. This protein is taken up by the kidney and excreted in the urine, but it is also toxic to the kidney itself. Renal failure is, therefore, an end result of the muscle damage and may be severe enough to require hemodialysis to prevent death from uremia.148
· Liver damage: the high fever, or the metabolic disturbances resulting from it, can give rise to varying degrees of liver damage, which is usually a secondary feature but can aggravate the primary hepatic toxicity of the drug when the 2 conditions occur in the same patient.113
· Disseminated intravascular coagulopathy: for reasons that are not yet entirely understood, the high fever can trigger widespread clotting of blood within the blood vessels, causing obstruction of many small blood vessels throughout the body, with resulting microinfarcts. Because fibrinogen, platelets and other clotting factors are used up in the process, the remaining blood loses the ability to clot normally, so that hemorrhages can then occur.149,150
The treatment of the hyperpyrexic pattern of toxicity has been mainly symptomatic, an essential part being early recognition of the problem and rapid cooling of the body by ice-water sponging, intravenous infusion of chilled saline solution, gastric and bladder lavage with cooled fluids, and general supportive care.151 However, it has been proposed that dantrolene, which is a drug used to stop the intense muscle contractures in malignant hyperthermia, should also be useful in the hyperthermic type of MDMA toxicity.152,153 Numerous cases have now been treated in this way, some with rapid and dramatic results,142,144,153,154,155 even when the clinical picture suggested the likelihood of a fatal outcome.154,156 Early reviewers questioned the value of dantrolene,157 but the accumulated experience since then suggests that its use may be a life-saving measure.
Miscellaneous toxicity
There have been occasional reports of types of toxicity other than those described earlier that have been attributed to the use of “ecstasy.” For example, administration of single small doses of MDMA, alone or in combination with alcohol, was reported to produce a transitory impairment of immune functions of lymphocytes examined in vitro.158 There is too little evidence to permit any conclusion about the possible significance of such effects in the living subject.
Fatalities due to ecstasy
All of the severe forms of toxicity described earlier have been capable of causing death. In addition, there have been deaths due to “ecstasy”-induced depression that was severe enough to cause suicide,159 or to pre-existing depression in which the drug was used as the means of suicide.160 There have also been several deaths due to accidents resulting from bizarre risk-taking behaviour while under the acute influence of the drug,161,162 or to motor vehicle accidents involving either drivers or pedestrians impaired by MDMA.114,162,163 The varied causes of death associated with “ecstasy” bear considerable resemblance to those seen with amphetamine164 and with PMA;165,166 the greater role of violence in amphetamine-related deaths may reflect a difference in the composition of the respective user populations.
As noted earlier in this review, the usual “recreational” dose of MDMA or MDEA produces blood levels in the range of 100–250 ng/mL, or 100–250 μg (0.1–0.25 mg) per litre. Most of the cases of serious toxicity or fatality have involved blood levels ranging from 0.5 mg/L to 10 mg/L, that is, up to 40 times higher than the usual recreational range. However, some have had levels as low as 0.11–0.55 mg/L, that is, overlapping the “normal” range and a little above it. This is an important point, because it demonstrates the degree to which the seriousness of the effects can be dependent on environmental factors other than the drug concentration.
All the fatal cases that have been located through a literature search are summarized in Appendix 1, which includes additional reference material167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182 (available on the CMAJ Web site at www.cma.ca/cmaj/vol-165/issue-7/ecstasyappendix.pdf). Most of these cases are associated with the use of MDMA, but a number are primarily associated with amphetamine, MDA, MDEA, MBDB and other amphetamine derivatives. They are included here to emphasize that the fatalities depend on mechanisms that are common to all the amphetamines and not specific to “ecstasy.” After eliminating, where possible, cases that are duplicates of the same cases already reported by other authors and cases involving only amphetamine or methamphetamine rather than the ring-substituted derivatives, the search indicates a total of 87 reported deaths involving “ecstasy” or related drugs, in which the primary cause of death appears to be as follows:
· cardiovascular, including cerebrovascular 8
· hepatic 4
· cerebral, including hyponatremia 9
· hyperpyrexic 30
· misadventure (suicide, accident) 14
· unknown — insufficient information 22
It must be emphasized that what is described in the literature cannot be regarded as complete, because not every physician who sees such cases will publish case reports about them. For example, Lora-Tamayo and colleagues163 present partial data on 16 fatalities involving MDMA, MDEA or MDA seen in a 2-year period in the Madrid laboratory of the Spanish National Institute of Toxicology, but none of these were reported in the international medical literature. White and colleagues151 state that there have been 12 deaths caused by “ecstasy” in Australia between 1995 and 1997, but only 6 of them have been described in the literature (the same 6 described in 3 different papers). In Ontario, there have been 13 “ecstasy”-related deaths reported to the office of the Chief Coroner in the past year (Dr. J. M. Cairns, Office of the Chief Coroner, Toronto, Ont.: personal communication, 2001), but these have not yet been reported in the literature, though a manuscript by Cairns and Kish is in preparation. It is, therefore, not possible to estimate the true incidence of serious or fatal toxicity due to MDMA and MDEA on the basis of published cases.
Addiction
There is no evidence at present to suggest that MDMA or MDEA are likely to give rise to a major problem of dependence as defined in the Diagnostic and Statistical Manual of Mental Disorders,183 or the International Statistical Classification of Diseases.184 Jansen185 describes 3 cases that appear to meet the diagnostic criteria for dependence, and Véléa and colleagues10 state that such cases do occur but give no details on their frequency or prevalence.
It has been claimed that dependence is unlikely to become a serious problem, because the decrease in pleasurable or rewarding effects if the drug is used too frequently, and the increase in frequency of unpleasant effects,41,44 would diminish the incentive to use the drug in a manner that could give rise to dependence. The same phenomenon occurs with the classical hallucinogens, which have not proven to give rise to dependence to nearly the same extent as alcohol, cannabis, benzodiazepines or opioids, for example. On the other hand, amphetamine and methamphetamine are at least as liable to produce dependence as cocaine.2,58 It may be that the methylenedioxy group on the phenyl ring in MDA, MDMA and MDEA makes them more like mescaline than like the parent amphetamines in this respect, but prudence suggests that it would be best to reserve judgement on this point. It was claimed that cocaine was not dependence producing when it began to reappear in the early 1980s, and it took years of growing experience to rediscover what had been known about cocaine dependence some 50 years earlier.186 The same claim was made for many years with respect to cannabis, but there is now abundant evidence that cannabis can, and does, give rise to dependence in a significant percentage of users.
It is clear that the use of “ecstasy” has increased greatly in recent years. Kirsch12 cites, for example, estimates of the number of doses produced by an illicit laboratory in the United States as growing from 10 000 a month in 1976, to 30 000 a month in 1984 and 500 000 a month in 1985. However, such figures cannot be regarded as accurate representations of the change in the extent of use in the population at large. The use of “ecstasy” is mainly confined to the late-adolescent and young-adult age groups. Among occasional users, novelty seeking appears to be an important motivating force.53 However, studies in the United Kingdom187 and in Norway188 found that the best predictor of drug use among young people was their preference for rave-style music; MDMA use seemed to be more closely linked to music preference and less to smoking and behaviour problems than was the case with amphetamine. Many of the “ecstasy” users seen by addiction services or hospital clinics are polydrug users,89,189 and this is also true of many of the other cases cited in the present review.
Comparison with other drugs
From the foregoing review, one must conclude that the whole group of amphetamines and related drugs strongly resemble each other and cocaine, at least with respect to their toxic effects.190 However, the question of the likelihood of addiction, as discussed earlier, cannot yet be answered and must await a longer accumulation of clinical experience.
On the other hand, there is a large difference between “ecstasy” and heroin and other opioids. The opioids have a clearly demonstrated dependence risk, but their intrinsic physical toxicity is considerably less dangerous than that of the amphetamine group.190 Apart from respiratory death due to overdose, most of the physical harm produced by heroin is caused by unsterile injection technique, needle swapping and intravenous use of preparations that are meant only for oral administration. In contrast, many of the serious effects of the amphetamines are directly due to the action of the drug itself, as described earlier. Moreover, the dose relationships are less clear for “ecstasy” than for heroin, in that there is a smaller separation between the usual dose and the toxic dose range for “ecstasy.” Some of the case histories reviewed earlier involved major toxicity following the consumption of only 1 or 2 tablets.
An attempt to calculate the relative death rates for “ecstasy” and for heroin in those aged 15–24 years in the United Kingdom191 simply underscores the lack of accurate data. The rates for deaths from accidents when driving, ecstasy, and heroin were as follows:
· driving accidents 1 per 10 000
· ecstasy between 0.2 and 5.3 per 10 000 (i.e., 27-fold range)
· heroin between 9.1 and 81.5 per 10 000 (i.e., 9-fold range).
These figures indicate that the death rate from heroin is higher, but that the precision of the estimate is much lower for ecstasy, which probably reflects a more heterogeneous user population, with fewer ecstasy users being as risk seeking as some of the heroin users.
Conclusions
This review of the literature indicates that ecstasy (MDMA) and related drugs are potentially dangerous, even in the doses typically used by participants at raves. Both the acute and the chronic effects can lead to serious and even fatal toxicity, the full extent of which cannot yet be estimated with accuracy. The variety of different adverse effects, including psychiatric, neurological, cardiovascular, hepatic, renal, thermoregulatory and even dental problems, indicates that patients with ecstasy-related difficulties may present in any part of the health care system and not only to emergency services. Because the main users are adolescents and young adults following the dictates of current drug fashion, physicians may need to be especially alert to such problems in an otherwise healthy population group.
Footnotes
This article has been peer reviewed.
Acknowledgements: I received an honorarium from the federal Department of Justice, Canada, for undertaking this review. I am indebted to M. Yvan Poulin of the Bureau Régional du Québec, Montréal, for arranging departmental support for this project and authorization for publication in the open literature.
Competing interests: None declared.
Correspondence to: Dr. H. Kalant, Department of Pharmacology, Medical Sciences Building, University of Toronto, Toronto ON M5S 1A8; fax 416 978-6395; harold.kalant@utoronto.ca
References
- 1.Climko RP, Roehrich H, Sweeney DR, Al-Razi J. Ecstacy [sic]: a review of MDMA and MDA. Int J Psychiatry Med 1987;16:359-72. [DOI] [PubMed]
- 2.Kalant OJ. The amphetamines — toxicity and addiction. 2nd ed. Toronto (Ont): University of Toronto Press; 1972.
- 3.Shulgin AT. The background and chemistry of MDMA. J Psychoactive Drugs 1986; 18:291-304. [DOI] [PubMed]
- 4.Karch SB. Mescaline analogs (“designer drugs”). In: The pathology of drug abuse. 2nd ed. Boca Raton (Fla): CRC Press; 1986. p. 202-18.
- 5.Rohrig TP, Prouty RW. Tissue distribution of methylenedioxymethamphetamine. J Anal Toxicol 1992;16:52-3. [DOI] [PubMed]
- 6.Moore KA, Mozayani A, Fierro MF, Poklis A. Distribution of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) stereoisomers in a fatal poisoning. Forensic Sci Int 1996;83:111-19. [DOI] [PubMed]
- 7.Theune M, Esser W, Druschky KF, Interschick E, Patscheke H. Grand-mal-Serie nach Ecstasy-Einnahme. Nervenarzt 1999;70:1094-7. [DOI] [PubMed]
- 8.Carter N, Rutty GN, Milroy CM, Forrest AR. Deaths associated with MBDB use. Int J Legal Med 2000;113:168-70. [DOI] [PubMed]
- 9.Milroy CM, Clark JC, Forrest ARW. Pathology of deaths associated with “ecstasy” and “eve” misuse. J Clin Pathol 1996;49:149-53. [DOI] [PMC free article] [PubMed]
- 10.Véléa D, Hautefeuille M, Vazeille G, Lantran-Davoux C. Nouvelles drogues synthétiques empathogènes. Encéphale 1999;25:508-14. [PubMed]
- 11.Sondermann N, Kovar KA. Screening experiments of ecstasy street samples using near infrared spectroscopy. Forensic Sci Int 1999;106:147-56. [DOI] [PubMed]
- 12.Kirsch MM. “Ecstasy”. In: Designer drugs. Minneapolis (Minn): CompCare Publications; 1986. p. 75-97.
- 13.Sherlock K, Wolff K, Hay AW, Conner M. Analysis of illicit ecstasy tablets: implications for clinical management in the accident and emergency department. J Accid Emerg Med 1999;16:194-7. [DOI] [PMC free article] [PubMed]
- 14.Christopherson AS. Amphetamine designer drugs — an overview and epidemiology. Toxicol Lett 2000;112-3:127-31. [DOI] [PubMed]
- 15.Siegel RK. MDMA — nonmedical use and intoxication. J Psychoactive Drugs 1986; 18:349-54. [DOI] [PubMed]
- 16.Parrott AC, Lasky J. Ecstasy (MDMA) effects upon mood and cognition before, during, and after a Saturday night dance. Psychopharmacology 1998; 139:261-8. [DOI] [PubMed]
- 17.Dar KJ, McBrien ME. MDMA induced hyperthermia: report of a fatality and review of current therapy. Intensive Care Med 1996;22:995-6. [DOI] [PubMed]
- 18.Walubo A, Seger D. Fatal multi-organ failure after suicidal overdose with MDMA, ‘ecstasy’: case report and review of the literature. Hum Exp Toxicol 1999;18:119-25. [DOI] [PubMed]
- 19.Weinmann W, Bohnert M. Lethal monointoxication by overdosage of MDEA. Forensic Sci Int 1998;91:91-101. [DOI] [PubMed]
- 20.Harries DP, De Silva R. ‘Ecstasy’ and intracerebral haemorrhage. Scott Med J 1992; 37:150-2. [DOI] [PubMed]
- 21.Mas M, Farré M, de la Torre R, Roset PN, Ortuño J, Segura J, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylene-dioxymethamphetamine in humans. J Pharmacol Exp Ther 1999;290:136-45. [PubMed]
- 22.Verebey K, Alrazi J, Jaffe JH. The complications of ‘ecstasy’ (MDMA). JAMA 1988;259:1649-50. [DOI] [PubMed]
- 23.Wu D, Otton SV, Inaba T, Kalow W, Sellers EM. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol 1997;53:1605-12. [DOI] [PubMed]
- 24.Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (‘ecstasy’). Toxicol Lett 2000;112-3:133-42. [DOI] [PubMed]
- 25.de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, et al. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 2000;49:104-9. [DOI] [PMC free article] [PubMed]
- 26.Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”). Psychopharmacology (Berl) 1995;119(3):247-60. [DOI] [PubMed]
- 27.Huether G, Zhou D, Rüther E. Causes and consequences of the loss of serotonergic presynapses elicited by the consumption of 3,4-methylene-dioxymethamphetamine (MDMA, “ecstasy”) and its congeners. J Neural Transm 1997;104:771-94. [DOI] [PubMed]
- 28.Iravani MM, Asari D, Patel J, Wieczorek WJ, Kruk ZL. Direct effects of 3,4-methylenedioxymethamphetamine (MDMA) on serotonin or dopamine release and uptake in the caudate putamen, nucleus accumbens, substantia nigra pars reticulata, and the dorsal raphe nucleus slices. Synapse 2000;36:275-85. [DOI] [PubMed]
- 29.Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology 2000;22:513-21. [DOI] [PubMed]
- 30.Glennon RA, Young R. MDMA stimulus generalization to the 5-HT(1A) serotonin agonist 8-hydroxy-2-(di-n-propylamino)tetralin. Pharmacol Biochem Behav 2000;66:483-8. [DOI] [PubMed]
- 31.Liechti ME, Vollenweider FX. Acute psychological and physiological effects of MDMA (“ecstasy”) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol 2000;10:289-95. [DOI] [PubMed]
- 32.Gunn JA, Gurd MR, Sachs I. The action of some amines related to adrenaline: methoxy-phenylisopropylamines. J Physiol 1939;95:485-500. [DOI] [PMC free article] [PubMed]
- 33.Fischer HS, Zernig G, Schatz DS, Humpel C, Saria A. MDMA (‘ecstasy’) enhances basal acetylcholine release in brain slices of the rat striatum. Eur J Neurosci 2000;12:1385-90. [DOI] [PubMed]
- 34.Schechter MD. MDMA as a discriminative stimulus: isomeric comparisons. Pharmacol Biochem Behav 1987;27:41-4. [DOI] [PubMed]
- 35.Glennon RA, Young R, Dukat M, Cheng Y. Initial characterization of PMMA as a discriminative stimulus. Pharmacol Biochem Behav 1997;57:151-8. [DOI] [PubMed]
- 36.Baker LE, Taylor MM. Assessment of the MDA and MDMA optical isomers in a stimulant-hallucinogen discrimination. Pharmacol Biochem Behav 1997;57: 737-49. [DOI] [PubMed]
- 37.Johnson M, Letter AA, Merchant K, Hanson GR, Gibb JW. Effects of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine isomers on central serotonergic, dopaminergic and nigral neurotensin systems of the rat. J Pharmacol Exp Ther 1988;244:977-82. [PubMed]
- 38.Fitzgerald RL, Blanke RV, Rosecrans JA, Glennon RA. Stereochemistry of the metabolism of MDMA to MDA. Life Sci 1989;45:295-301. [DOI] [PubMed]
- 39.Cohen RS. Subjective reports on the effects of the MDMA (‘ecstasy’) experience in humans. Prog Neuropsychopharmacol Biol Psychiatry 1995;19:1137-45. [DOI] [PubMed]
- 40.Gouzoulis-Mayfrank E, Hermle L, Kovar KA, Sass H. Die Entaktogene “ecstasy” (MDMA), “eve” (MDEA) und andere ringsubstituirte Methamphetaminderivate. Eine neue Stoffklasse unter den illegalen Designerdrogen? Nervenarzt 1996;67:369-80. [PubMed]
- 41.Peroutka SJ, Newman H, Harris H. Subjective effects of 3,4-methylenedioxy-methamphetamine in recreational users. Neuropsychopharmacology 1988;1:273-7. [PubMed]
- 42.Nichols DE. Differences between the mechanisms of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs 1986;18:305-13. [DOI] [PubMed]
- 43.Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (“ecstasy”) in MDMA-naive healthy volunteers. Neuropsychopharmacology 1998;19:241-51. [DOI] [PubMed]
- 44.Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs 1986;18:319-27. [DOI] [PubMed]
- 45.Szukaj M. MDMA (“ecstasy”) — gefährliche Droge oder Psychotherapeutikum? Nervenarzt 1994;65:802-5. [PubMed]
- 46.McGlothlin WH, Arnold DO. LSD revisited. A 10-year follow-up of medical LSD use. Arch Gen Psychiatry 1971;24:35-49. [DOI] [PubMed]
- 47.Henry JA. Ecstasy and the dance of death. BMJ 1996;305:5-6. [DOI] [PMC free article] [PubMed]
- 48.Olson KR, Benowitz NL. Environmental and drug-induced hyperthermia. Pathophysiology, recognition and management. Emerg Med Clin North Am 1984; 2:459-74. [PubMed]
- 49.Bost RO. 3,4-Methylenedioxymethamphetamine (MDMA) and other substituted amphetamines. J Forensic Sci 1988;33:576-87. [PubMed]
- 50.Mørland J. Toxicity of drug abuse — amphetamine designer drugs (ecstasy): mental effects and consequences of single dose use. Toxicol Lett 2000;112-3: 147-52. [DOI] [PubMed]
- 51.McGuire PK, Cope H, Fahy TA. Diversity of psychopathology associated with use of 3,4-methylenedioxymethamphetamine (‘ecstasy’). Br J Psychiatry 1994;165:391-5. [DOI] [PubMed]
- 52.Pallanti S, Mazzi D. MDMA (ecstasy) precipitation of panic disorder. Biol Psychiatry 1992;32:91-5. [DOI] [PubMed]
- 53.Schifano F. Potential human neurotoxicity of MDMA (‘ecstasy’): subjective self-reports, evidence from an Italian drug addiction centre and clinical case studies. Neuropsychobiology 2000;42:25-33. [DOI] [PubMed]
- 54.Thomasius R, Schmolke M, Kraus D. MDMA (“ecstasy”) — Konsum — ein Überblick zu psychiatrischen und medizinischen Folgen. Fortschr Neurol Psychiatr 1997;65:49-61. [DOI] [PubMed]
- 55.Alciati A, Scaramelli B, Fusi A, Butteri E, Cattaneo ML, Mellado C. Three cases of delirium after “ecstasy” ingestion. J Psychoactive Drugs 1999;31:167-70. [DOI] [PubMed]
- 56.Williams H, Dratcu L, Taylor R, Roberts M, Oyefeso A. “Saturday night fever”: ecstasy related problems in a London accident and emergency department. J Accid Emerg Med 1998;15:322-6. [DOI] [PMC free article] [PubMed]
- 57.McCann UD, Slate SO, Ricaurte GA. Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”). Drug Saf 1996;15:107-15. [DOI] [PubMed]
- 58.Gawin FH, Ellinwood EH Jr. Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med 1988;318:1173-82. [DOI] [PubMed]
- 59.Boot B, McGregor IS, Hall W. MDMA (ecstasy) neurotoxicity: assessing and communicating the risks. Lancet 2000;355:1818-21. [DOI] [PubMed]
- 60.Colado MI, Granados R, O'Shea E, Esteban B, Green AR. The acute effect in rats of 3,4-methylenedioxyethamphetamine (MDEA, “eve”) on body temperature and long-term degeneration of 5-HT neurons in brain: a comparison with MDMA (“ecstasy”). Pharmacol Toxicol 1999;84:261-6. [DOI] [PubMed]
- 61.Hegadoren KM, Baker GB, Bourin M. 3,4-Methylenedioxy analogues of amphetamine: defining the risks in humans. Neurosci Biobehav Rev 1999;23:539-53. [DOI] [PubMed]
- 62.Ricaurte GA, Yuan J, McCann UD. (+/-)3,4-Methylenedioxymethamphetamine (‘ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 2000;42:5-10. [DOI] [PubMed]
- 63.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 1998;32:117-31. [DOI] [PubMed]
- 64.Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS. Striatal serotonin is depleted in brain of a human MDMA (ecstasy) user. Neurology 2000;55:294-6. [DOI] [PubMed]
- 65.McCann UD, Eligulashvili V, Ricaurte GA. (+/-)3,4-Methylenedioxymeth-amphetamine (‘ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology 2000;42:11-6. [DOI] [PubMed]
- 66.McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after (+/-)3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”): a controlled study in humans. Neuropsychopharmacology 1994;10:129-38. [DOI] [PubMed]
- 67.McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“ecstasy”) on brain serotonin neurons in human beings. Lancet 1998;352:1433-7. [DOI] [PubMed]
- 68.Ricaurte GA, McCann UD, Szabo Z, Scheffel U. Toxicodynamics and long-term toxicity of the recreational drug, 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). Toxicol Lett 2000;112-3:143-6. [DOI] [PubMed]
- 69.Semple DM, Ebmeier KP, Glabus MF, O'Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry 1999;175:63-9. [DOI] [PubMed]
- 70.Chang L, Ernst T, Grob CS, Poland RE. Cerebral (1)H MRS alterations in recreational 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) users. J Magn Reson Imaging 1999;10:521-6. [DOI] [PubMed]
- 71.Chang L, Grob CS, Ernst T, Itti L, Mishkin FS, Jose-Melchor R, et al. Effect of ecstasy [3,4-methylenedioxymethamphetamine (MDMA)] on cerebral blood flow: a co-registered SPECT and MRI study. Psychiatry Res 2000;98:15-28. [DOI] [PubMed]
- 72.Obrocki J, Buchert R, Vaterlein O, Thomasius R, Beyer W, Schiemann T. Ecstasy — long-term effects on the human central nervous system revealed by positron emission tomography. Br J Psychiatry 1999;175:186-8. [DOI] [PubMed]
- 73.Reneman L, Habraken JB, Majoie CB, Booij J, den Heeten GJ. MDMA (“ecstasy”) and its association with cerebrovascular accidents: preliminary findings. AJNR Am J Neuroradiol 2000;21(6):1001-7. [PMC free article] [PubMed]
- 74.Reneman L, Booij J, Schmand B, van den Brink W, Gunning B. Memory disturbances in “ecstasy” users are correlated with an altered brain serotonin neurotransmission. Psychopharmacology 2000;148:322-4. [DOI] [PubMed]
- 75.Dafters RI, Duffy F, O'Donnell PJ, Bouquet C. Level of use of 3,4-methylenedioxymethamphetamine (MDMA or ecstasy) in humans correlates with EEG power and coherence. Psychopharmacology 1999;145:82-90. [DOI] [PubMed]
- 76.Tuchtenhagen F, Daumann J, Norra C, Gobbele R, Becker S, Pelz S, et al. High intensity dependence of auditory evoked dipole source activity indicates decreased serotonergic activity in abstinent ecstasy (MDMA) users. Neuropsychopharmacology 2000;22:608-17. [DOI] [PubMed]
- 77.Gerra G, Zaimovic A, Ferri M, Zambelli U, Timpano M, Neri E, et al. Long-lasting effects of (+/-)3,4-methylene-dioxymethamphetamine (ecstasy) on serotonin system function in humans. Biol Psychiatry 2000;47:127-36. [DOI] [PubMed]
- 78.Gerra G, Zaimovic A, Giucastro G, Maestri D, Monica C, Sartori R, et al. Serotonergic function after (+/-)3,4-methylenedioxy-metamphetamine (‘ecstasy’) in humans. Int Clin Psychopharmacol 1998;13:1-9. [DOI] [PubMed]
- 79.McCann UD, Eligulashvili V, Mertl M, Murphy DL, Ricaurte GA. Altered neuroendocrine and behavioral responses to m-chlorophenylpiperazine in 3,4-methylenedioxymethamphetamine (MDMA) users. Psychopharmacology 1999; 147: 56-65. [DOI] [PubMed]
- 80.Curran HV. Is MDMA (‘ecstasy’) neurotoxic in humans? An overview of evidence and of methodological problems in research. Neuropsychobiology 2000;42:34-41. [DOI] [PubMed]
- 81.Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“ecstasy”) users. Neurology 1998;51:1532-7. [DOI] [PubMed]
- 82.Morgan MJ. Memory deficits associated with recreational use of “ecstasy” (MDMA). Psychopharmacology 1999;141:30-6. [DOI] [PubMed]
- 83.Parrott AC. Human research on MDMA (3,4-methylenedioxymethamphetamine) neurotoxicity: cognitive and behavioural indices of change. Neuropsychobiology 2000;42:17-24. [DOI] [PubMed]
- 84.Spatt J, Glawar B, Mamoli B. A pure amnestic syndrome after MDMA (“ecstasy”) ingestion. J Neurol Neurosurg Psychiatry 1997;62:418-9. [DOI] [PMC free article] [PubMed]
- 85.Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, Pelz S, Becker S, Kunert HJ, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry 2000;68:719-25. [DOI] [PMC free article] [PubMed]
- 86.Kelly PA. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry 2000;68:690. [DOI] [PMC free article] [PubMed]
- 87.Wareing M, Fisk JE, Murphy PN. Working memory deficits in current and previous users of MDMA (‘ecstasy’). Br J Psychol 2000;91(Pt 2):181-8. [DOI] [PubMed]
- 88.Morgan MJ. Recreational use of “ecstasy” (MDMA) is associated with elevated impulsivity. Neuropsychopharmacology 1998;19:252-64. [DOI] [PubMed]
- 89.Parrott AC, Sisk E, Turner JJ. Psychobiological problems in heavy ‘ecstasy’ (MDMA) polydrug users. Drug Alcohol Depend 2000;60:105-10. [DOI] [PubMed]
- 90.Bone Pina I, Ramos Gorostiza P, Villalba Yilán P, Valle Fernandez J. Trastorno psicótico persistente inducido por consumo de extasis (MDMA). Actas Esp Psiquiatr 2000;28:61-5. [PubMed]
- 91.Cohen RS. Adverse symptomatology and suicide associated with the use of methylenedioxymethamphetamine (MDMA; “ecstasy”). Biol Psychiatry 1996;39:819-20. [DOI] [PubMed]
- 92.Creighton FJ, Black DL, Hyde CE. ‘Ecstasy’ psychosis and flashbacks. Br J Psychiatry 1991;159:713-5. [DOI] [PubMed]
- 93.McCann UD, Ricaurte GA. Lasting neuropsychiatric sequelae of (+-)methylenedioxymethamphetamine (‘ecstasy’) in recreational users. J Clin Psychopharmacol 1991;11:302-5. [PubMed]
- 94.Williams H, Meagher D, Galligan P. M.D.M.A. (“ecstasy”); a case of possible drug-induced psychosis. Ir J Med Sci 1993;162:43-4. [DOI] [PubMed]
- 95.Wodarz N, Boning J. “Ecstasy”-induziertes psychotisches Depersonalisations-syndrom. Nervenarzt 1993;64:478-80. [PubMed]
- 96.Cohen RS, Cocores J. Neuropsychiatric manifestations following the use of 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”). Prog Neuropsychopharmacol Biol Psychiatry 1997;21:727-34. [DOI] [PubMed]
- 97.McGuire P. Long term psychiatric and cognitive effects of MDMA use. Toxicol Lett 2000;112-3:153-6. [DOI] [PubMed]
- 98.Milosevic A, Agrawal N, Redfearn P, Mair L. The occurrence of toothwear in users of ecstasy (3,4-methylenedioxymethamphetamine). Community Dent Oral Epidemiol 1999;27:283-7. [DOI] [PubMed]
- 99.Redfearn PJ, Agrawal N, Mair LH. An association between the regular use of 3,4-methylenedioxy-methamphetamine (“ecstasy”) and excessive wear of teeth. Addiction 1998;93:745-8. [DOI] [PubMed]
- 100.Brody S, Krause C, Veit R, Rau H. Cardiovascular autonomic dysregulation in users of MDMA (“ecstasy”). Psychopharmacology 1998;136:390-3. [DOI] [PubMed]
- 101.Mintzer S, Hickenbottom S, Gilman S. Parkinsonism after taking ecstasy. N Engl J Med 1999;340:1443. [DOI] [PubMed]
- 102.Schroeder B, Brieden S. Bilateral sixth nerve palsy associated with MDMA (“ecstasy”) abuse. Am J Ophthalmol 2000;129:408-9. [DOI] [PubMed]
- 103.Andreu V, Mas A, Bruguera M, Salmerón JM, Moreno V, Nogué S, et al. Ecstasy: a common cause of severe acute hepatotoxicity. J Hepatol 1998;29:394-7. [DOI] [PubMed]
- 104.Jones AL, Simpson KJ. Mechanisms and management of hepatotoxicity in ecstasy (MDMA) and amphetamine intoxications. Aliment Pharmacol Ther 1999; 13: 129-33. [DOI] [PubMed]
- 105.Beitia G, Cobreros A, Sainz L, Cenarruzabeitia E. 3,4-Methylenedioxy-methamphetamine (ecstasy)-induced hepatotoxicity: effect on cytosolic calcium signals in isolated hepatocytes. Liver 1999;19:234-41. [DOI] [PubMed]
- 106.Hiramatsu M, Kumagai Y, Unger SE, Cho AK. Metabolism of methylenedioxy-methamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther 1990;254: 521-7. [PubMed]
- 107.de Man RA, Wilson JH, Tjen HS. Acuut leverfalen door methyleendioxy-methamphetamine. Ned Tijdschr Geneesk 1993;137:727-9. [PubMed]
- 108.Dykhuizen RS, Brunt PW, Atkinson P, Simpson JG, Smith CC. Ecstasy induced hepatitis mimicking viral hepatitis. Gut 1995;36:939-41. [DOI] [PMC free article] [PubMed]
- 109.Fidler H, Dhillon A, Gertner D, Burroughs A. Chronic ecstasy (3,4-methylenedioxymethamphetamine) abuse: a recurrent and unpredictable cause of severe acute hepatitis. J Hepatol 1996;25:563-6. [DOI] [PubMed]
- 110.Gorard DA, Davies SE, Clark ML. Misuse of ecstasy. BMJ 1992;305:309. [DOI] [PMC free article] [PubMed]
- 111.Huarte Muniesa MP, Pueyo Royo AM. Hepatitis aguda tras consumo de extasis. Rev Esp Enferm Dig 1995;87:681-3. [PubMed]
- 112.Shearman JD, Chapman RWG, Satsangi J, Ryley NG, Weatherhead S. Misuse of ecstasy. BMJ 1992;305:309. [DOI] [PMC free article] [PubMed]
- 113.Ellis AJ, Wendon JA, Portmann B, Williams R. Acute liver damage and ecstasy ingestion. Gut 1996;38:454-8. [DOI] [PMC free article] [PubMed]
- 114.Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylene-dioxymethamphetamine (“ecstasy”). Lancet 1992;340:384-7. [DOI] [PubMed]
- 115.Khakoo SI, Coles CJ, Armstrong JS, Barry RE. Hepatotoxicity and accelerated fibrosis following 3,4-methylenedioxymethamphetamine (“ecstasy”) usage. J Clin Gastroenterol 1995;20:244-7. [DOI] [PubMed]
- 116.Brauer RB, Heidecke CD, Nathrath W, Beckurts KTE, Vorwald P, Zilker TR, et al. Liver transplantation for the treatment of fulminant hepatic failure induced by the ingestion of ecstasy. Transpl Int 1997;10:229-33. [DOI] [PubMed]
- 117.Chenard-Neu MP, Boudjema K, Bernuau J, Degott C, Belghiti J, Cherqui D et al. Auxiliary liver transplantation: regeneration of the native liver and outcome in 30 patients with fulminant hepatic failure — a multicenter European study. Hepatology 1996;23:1119-27. [DOI] [PubMed]
- 118.Hellinger A, Rauen U, de Groot H, Erhard J. Auxiliäre Lebertransplantation bei akuten Leberversagen nach Einnahme von 3,4-Methylendioxymetamphetamin (“ecstasy”). Dtsch Med Wochenschr 1997;122:716-20. [DOI] [PubMed]
- 119.Gledhill JA, Moore DF, Bell D, Henry JA. Subarachnoidal haemorrhage associated with MDMA abuse. J Neurol Neurosurg Psychiatry 1993;56:1036-7. [DOI] [PMC free article] [PubMed]
- 120.Hughes JC, McCabe M, Evans RJ. Intracranial haemorrhage associated with ingestion of ‘ecstasy’. Arch Emerg Med 1993;10:372-4. [DOI] [PMC free article] [PubMed]
- 121.Schlaeppi M, Prica A, de Torrente A. Hemorragie cérébrale et “ecstasy”. Schweiz Rundsch Med Prax 1999;88:568-72. [PubMed]
- 122.Jacks AS, Hykin PJ. Retinal haemorrhage caused by "ecstasy". Br J Ophthalmol 1998;82:842-3. [DOI] [PMC free article] [PubMed]
- 123.Manchanda S, Connolly MJ. Cerebral infarction in association with ecstasy abuse. Postgrad Med J 1993;69:874-5. [DOI] [PMC free article] [PubMed]
- 124.Roebroek RM, Korten JJ. Epileptische insulten, herseninfarct en rhabdomyolysis als complicaties van amfetamingebruik. Ned Tijdschr Geneesk 1996; 140:205-7. [PubMed]
- 125.Rothwell PM, Grant R. Cerebral venous sinus thrombosis induced by 'ecstasy'. J Neurol Neurosurg Psychiatry 1993;56:1035. [DOI] [PMC free article] [PubMed]
- 126.Suarez RV, Riemersma R. "Ecstasy" and sudden cardiac death. Am J Forensic Med Pathol 1988;9:339-41. [DOI] [PubMed]
- 127.Satchell SC, Connaughton M. Inappropriate antidiuretic hormone secretion and extreme rises in serum creatinine kinase following MDMA ingestion. Br J Hosp Med 1994;51:495. [PubMed]
- 128.Holden R, Jackson MA. Near-fatal hyponatraemic coma due to vasopressin over-secretion after "ecstasy" (3,4-MDMA). Lancet 1996;347:1052. [DOI] [PubMed]
- 129.Holmes SB, Banerjee AK, Alexander WD. Hyponatraemia and seizures after ecstasy use. Postgrad Med J 1999;75:32-3. [DOI] [PMC free article] [PubMed]
- 130.Wilkins B. Cerebral oedema after MDMA ("ecstasy") and unrestricted water intake: hyponatraemia must be treated with low water input. BMJ 1996;313: 689-90. [DOI] [PMC free article] [PubMed]
- 131.Kessel B. Hyponatraemia after ingestion of "ecstasy". BMJ 1994;308:414. [DOI] [PMC free article] [PubMed]
- 132.Magee C, Staunton H, Tormey W, Walshe JJ. Hyponatraemia, seizures and stupor associated with ecstasy ingestion in a female. Ir Med J 1998;91:178. [PubMed]
- 133.Matthai SM, Davidson DC, Sills JA, Alexandrou D. Cerebral oedema after ingestion of MDMA (“ecstasy”) and unrestricted intake of water. BMJ 1996; 313:689. [DOI] [PMC free article] [PubMed]
- 134.Maxwell DL, Polkey MI, Henry JA. Hyponatraemia and catatonic stupor after taking ecstasy. BMJ 1993;307:1399. [DOI] [PMC free article] [PubMed]
- 135.O'Connor A, Cluroe A, Couch R, Galler L, Lawrence J. Death from hyponatraemia-induced cerebral oedema associated with MDMA (“ecstasy”) use. N Z Med J 1999;112:255-6. [PubMed]
- 136.Parr MJA, Low HM, Botterill P. Hyponatraemia and death after “ecstasy” ingestion. Med J Aust 1997;166:136-7. [DOI] [PubMed]
- 137.Bedford Russell AR, Schwartz RH, Dawling S. Accidental ingestion of 'ecstasy' (3,4-methylenedioxymethylamphetamine). Arch Dis Child 1992;67: 1114-5. [DOI] [PMC free article] [PubMed]
- 138.Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 1998;18: 5086-94. [DOI] [PMC free article] [PubMed]
- 139.Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA ('ecstasy'). JAMA 1987;258:780-1. [PubMed]
- 140.Ginsberg MD, Hertzman M, Schmidt-Nowara WW. Amphetamine intoxication with coagulopathy, hyperthermia and reversible renal failure. A syndrome resembling heatstroke. Ann Intern Med 1970;73:81-5. [DOI] [PubMed]
- 141.Kendrick WC, Hull AR, Knochel JP. Rhabdomyolysis and shock after intravenous amphetamine administration. Ann Intern Med 1977;86:381-7. [DOI] [PubMed]
- 142.Tehan B, Hardern R, Bodenham A. Hyperthermia associated with 3,4- methylenedioxyethamphetamine ('eve'). Anaesthesia 1993;48:507-10. [DOI] [PubMed]
- 143.Demirkiran M, Jankovic J, Dean JM. Ecstasy intoxication: an overlap between serotonin syndrome and neuroleptic malignant syndrome. Clin Neuropharmacol 1996;19:157-64. [DOI] [PubMed]
- 144.Nimmo SM, Kennedy BW, Tullett WM, Blyth AS, Dougall JR. Drug-induced hyperthermia. Anaesthesia 1993;48:892-5. [DOI] [PubMed]
- 145.Behan WM, Madigan M, Clark BJ, Goldberg J, McLellan DR. Muscle changes in the neuroleptic malignant syndrome. J Clin Pathol 2000;53:223-7. [DOI] [PMC free article] [PubMed]
- 146.Ferrie R, Loveland RC. Bilateral gluteal compartment syndrome after 'ecstasy' hyperpyrexia. J R Soc Med 2000;93(5):260. [DOI] [PMC free article] [PubMed]
- 147.Screaton GR, Singer M, Cairns HS, Thrasher A, Sarner M, Cohen SL. Hyperpyrexia and rhabdomyolysis after MDMA (“ecstasy”) abuse. Lancet 1992; 339: 677-8. [DOI] [PubMed]
- 148.Fahal IH, Sallomi DF, Yaqoob M, Bell GM. Acute renal failure after ecstasy. BMJ 1992;305:29. [DOI] [PMC free article] [PubMed]
- 149.Chadwick IS, Curry PD, Linsley A, Freemont AJ, Doran B. Ecstasy, 3,4-methylenedioxymethamphetamine (MDMA), a fatality associated with coagulopathy and hyperthermia. J R Soc Med 1991;84(6):371. [DOI] [PMC free article] [PubMed]
- 150.Simpson DL, Rumack BH. Methylenedioxyamphetamine. Clinical description of overdose, death, and review of pharmacology. Arch Intern Med 1981; 141: 1507-9. [DOI] [PubMed]
- 151.White JM, Bochner F, Irvine RJ. The agony of “ecstasy”. Med J Aust 1997;66: 117-8. [DOI] [PubMed]
- 152.Denborough MA, Hopkinson KC. Dantrolene and “ecstasy”. Med J Aust 1997; 166: 165-6. [DOI] [PubMed]
- 153.Singarajah C, Lavies NG. An overdose of ecstasy. A role for dantrolene. Anaesthesia 1992;47:686-7. [DOI] [PubMed]
- 154.Logan AS, Stickle B, O'Keefe N, Hewitson H. Survival following 'ecstasy' ingestion with a peak temperature of 42°C. Anaesthesia 1993;48(11):1017-8. [PubMed]
- 155.Webb C, Williams V. Ecstasy intoxication: appreciation of complications and the role of dantrolene. Anaesthesia 1993;48:542-3. [DOI] [PubMed]
- 156.Hall AP, Lyburn ID, Spears FD, Riley B. An unusual case of ecstasy poisoning. Intensive Care Med 1996;22:670-1. [DOI] [PubMed]
- 157.Watson JD, Ferguson C, Hinds CJ, Skinner R, Coakley JH. Exertional heat stroke induced by amphetamine analogues: Does dantrolene have a place? Anaesthesia 1993;48:1057-60. [DOI] [PubMed]
- 158.Pacifici R, Zuccaro P, Farré M, Pichini S, Di Carlo S, Roset PN, et al. Immunomodulating properties of MDMA alone and in combination with alcohol: a pilot study. Life Sci 1999;65:PL309-16. [DOI] [PubMed]
- 159.Iwersen S, Schmoldt A. Two very different fatal cases associated with the use of methylenedioxyethylamphetamine (MDEA): Eve as deadly as Adam. J Toxicol Clin Toxicol 1996;34:241-4. [DOI] [PubMed]
- 160.Arimany J, Medallo J, Pujol A, Vingut A, Borondo JC, Valverde JL. Intentional overdose and death with 3,4-methylenedioxyethamphetamine (MDEA; “eve”): case report. Am J Forensic Med Pathol 1998;19:148-51. [DOI] [PubMed]
- 161.Dowling GP, McDonough ET III, Bost RO. 'Eve' and 'ecstasy' — a report of five deaths associated with the use of MDEA and MDMA. JAMA 1987;257: 1615-7. [DOI] [PubMed]
- 162.Hooft PJ, van de Voorde HP. Reckless behaviour related to the use of 3,4-methylenedioxymethamphetamine (ecstasy): apropos of a fatal accident during car-surfing. Int J Legal Med 1994;106:328-9. [DOI] [PubMed]
- 163.Lora-Tamayo C, Tena T, Rodriguez A. Amphetamine derivative related deaths. Forensic Sci Int 1997;85:149-57. [DOI] [PubMed]
- 164.Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. CMAJ 1975;112:299-304. [PMC free article] [PubMed]
- 165.Cimbura G. PMA deaths in Ontario. CMAJ 1974;110:1263-7. [PMC free article] [PubMed]
- 166.Felgate HE, Felgate PD, James RA, Sims DN, Vozzo DC. Recent para-methoxyamphetamine deaths. J Anal Toxicol 1998;22:169-72. [DOI] [PubMed]
- 167.Byard RW, Gilbert J, James R, Lokan RJ. Amphetamine derivative fatalities in South Australia — Is “ecstasy” the culprit? Am J Forensic Med Pathol 1998;19:261-5. [DOI] [PubMed]
- 168.James RA, Dinan AC. Hyperpyrexia associated with fatal paramethoxy-amphetamine abuse. Med Sci Law 1998;38:83-5. [DOI] [PubMed]
- 169.Campkin NTA, Davies UM. Another death from ecstasy. J R Soc Med 1992; 85:61. [PMC free article] [PubMed]
- 170.Cimbura G. 3,4-Methylenedioxyamphetamine: analytical and forensic aspects of fatal poisoning. J Forensic Sci 1972;17:329-33. [PubMed]
- 171.Coore JR. A fatal trip with ecstasy: a case of 3,4-methylenedioxymethamphetamine/3,4-methylenedioxyamphetamine toxicity. J R Soc Med 1996;89:51P-2P. [DOI] [PMC free article] [PubMed]
- 172.Cox DE, Williams KR. 'Adam' or 'Eve'? — A toxicological conundrum. Forensic Sci Int 1996;77:101-8. [DOI] [PubMed]
- 173.Crifasi J, Long C. Traffic fatality related to the use of methylenedioxy-methamphetamine. J Forensic Sci 1996;41:1082-4. [PubMed]
- 174.Fineschi V, Centini F, Mazzeo E, Turillazzi E. Adam (MDMA) and Eve (MDEA) misuse: an immunohistochemical study on three fatal cases. Forensic Sci Int 1999;104:65-74. [DOI] [PubMed]
- 175.Fineschi V, Masti A. Fatal poisoning by MDMA (ecstasy) and MDEA: a case report. Int J Legal Med 1996;108:272-5. [DOI] [PubMed]
- 176.Forrest AR, Galloway JH, Marsh ID, Strachan GA, Clark JC. A fatal overdose with 3,4-methylenedioxyamphetamine derivatives. Forensic Sci Int 1994;64:57-9. [DOI] [PubMed]
- 177.Henry JA, Hill IR. Fatal interaction between ritonavir and MDMA. Lancet 1998; 352: 1751-2. [DOI] [PubMed]
- 178.Lukaszewski T. 3,4-Methylenedioxyamphetamine overdose. Clin Toxicol 1979; 15: 405-9. [DOI] [PubMed]
- 179.Mueller PD, Korey WS. Death by “ecstasy”: The serotonin syndrome? Ann Emerg Med 1998;32:377-80. [DOI] [PubMed]
- 180.Poklis A, Mackell MA, Drake WK. Fatal intoxication from 3,4-methylene-dioxyamphetamine. J Forensic Sci 1979;24:70-5. [PubMed]
- 181.Reed D, Cravey RH, Sedgwick PR. A fatal case involving methylenedioxy-amphetamine. Clin Toxicol 1972;5:3-6. [DOI] [PubMed]
- 182.Squier MV, Hilton-Jones D, Series H. Death after ecstasy ingestion: neuropathological findings. J Neurol Neurosurg Psychiatry 1995;58:756. [DOI] [PMC free article] [PubMed]
- 183.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th rev ed. Washington: The Association; 1994.
- 184.International Statistical Classification of Diseases and Related Health Problems. 10th rev. Geneva: World Health Organization; 1992.
- 185.Jansen KL. Ecstasy (MDMA) dependence. Drug Alcohol Depend 1999;53:121-4. [DOI] [PubMed]
- 186.Kalant OJ. Maier's cocaine addiction (Der Kokainismus). English translation. Toronto (Ont): ARF Books; 1987.
- 187.Forsyth AJ, Barnard M, McKeganey NP. Musical preference as an indicator of adolescent drug use. Addiction 1997;92:1317-25. [PubMed]
- 188.Pedersen W, Skrondal A. Ecstasy and new patterns of drug use: a normal population study. Addiction 1999;94:1695-1706. [DOI] [PubMed]
- 189.Schifano F, Di Furia L, Forza G, Minicuci N, Bricolo R. MDMA ('ecstasy') consumption in the context of polydrug abuse: a report on 150 patients. Drug Alcohol Depend 1998;52:85-90. [DOI] [PubMed]
- 190.Vollenweider-Scherpenhuyzen MFI, Vollenweider FX. Notfälle bei Drogenmissbrauch. Internist 2000;41:886-98. [DOI] [PubMed]
- 191.Gore SM. Fatal uncertainty: death rate from use of ecstasy or heroin. Lancet 1999; 354:1265-6. [DOI] [PubMed]


