Abstract
Dysregulation of microRNA-425 (miR-425) has been reported in several human cancers. However, the role of miR-425 in human cervical cancer via modulation of RAB2B expression is still unclear. This study was therefore designed to examine the expression and decipher the role of miR-425 in cervical cancer. The qRT-PCR was used for expression analysis. MTT and EdU assays were used for the determination of cell viability and proliferation, respectively. Annexin V/PI staining was used to detect apoptosis. Wound healing and transwell assays were used to monitor cell migration and invasion. Western blotting was used for protein expression analysis. The in vivo study was performed in xenografted mice model. The results of the present study revealed miR-425 to be significantly (P = 0.032) down-regulated in cervical cancer tissues and cell lines. Additionally, low expression of miR-425 was associated with significantly (P = 0.035) lower survival rate of the cervical cancer patients. Overexpression of miR-425 resulted in significant (P = 0.024) decline of cervical cancer cell proliferation via induction of apoptosis. The induction of apoptosis was associated with up-regulation of Bax and down-regulation of Bcl-2. Besides, the migration and invasion of cancer cells significantly (P < 0.01) decreased under miR-425 overexpression. Additionally, miR-425 could inhibit the growth of xenografted tumors in vivo. In silico analysis and dual luciferase assay revealed RAB2B as the direct target of miR-425 in cervical cancer. RAB2B was found to be significantly (P < 0.05) up-regulated in cervical cancer tissues and cell lines and miR-425 overexpression suppressed the expression of RAB2B. Additionally, silencing of RAB2B could suppress the growth of cervical cancer cells but its overexpression could rescue the tumor-suppressive effects of miR-425. Taken together, the results revealed the tumor-suppressive roe of miR-425 and point towards its therapeutic potential in the management of cervical cancer.
Keywords: cervical cancer, micro-RNA, RAB2B, apoptosis, xenograft mice, metastasis
Introduction
Human cervical cancer is one of the most prominent health disorders among women.1 The disease is considered as the most prevalent gynecological malignancy.2 Cervical cancer causes significant female mortality worldwide.3 As per the current reports, the disease has been reported to affect more than half a million females annually and results in more than 0.25 million annual deaths throughout the world.4 Moreover, the disease is believed to show increasing trend in terms of prevalence rates and percent mortality. The over-all 5-year survival rate of cervical cancer is still less than 50% despite the recent innovations in the therapeutic modalities. Thus, there is a huge demand to look for the alternate treatment procedures against the malignancy of cervical cancer.5 Researchers are exploring the utility of micro-RNAs (miRs) for their cancer regulatory role. The miRs are described as an endogenous class of short-length non-coding RNA entities which suppress the expression of majority of protein coding genes at translational level.6 The miRs interact with the untranslated regions of mRNA transcripts in sequence complementarity mode to directly repress their translation or target them for their degradation.7 The miRs have been proved to regulate vital biological and physiological processes in eukaryotes.8 The irregular expression profiles of miRs are associated with human disorders including cancer.9 They regulate the hallmarks of human tumorigenesis including proliferation and metastasis.10 Several miRs were shown to be involved in growth and proliferation of cervical cancer.11 MicroRNA-425 (miR-425) regulates the pathogenesis of human cancers like hepatocellular cancer, gastric cancer and colorectal cancer to name a few.12–14 In gastric cancer miR-425 regulates invasion and metastasis of gastric cancer.15 In yet another study, miR-425 has been shown to control the development of prostate cancer by targeting forkhead box J3.16 Zhu et al.,17 found that miR-425 to be downregulated in nasopharyngeal carcinoma and regulates tumor cell viability and invasion. It was previously suggested to act as the potential prognostic biomarker in cervical cancer.18 In yet another study miR-425 has been shown to target AIFM1 to suppress the cervical cancer tumorigenesis.19 However, the effects of miR-425 on cervical cancer via modulation of Ras-related protein Rab-2B (RAB2B) expression on cervical cancer proliferation, migration and invasion has not been studied. Additionally, the effect of miR-425 on the xenografted tumor growth has not been studied. The RaB2B is one of the important members of the Ras-like small GTPase superfamily.20 RAB2B harbors an evolutionarily conserved GTP binding domain and a variable N- and C-terminal domains.21 RAB2B acts as a proto-oncogene has been shown to be dysregulated in several human tumors.22
In the present study miR-425 was shown to be significantly downregulated in cervical cancer. The miR-425 was found to be negatively regulate the cervical cancer tumorigenesis and metastasis via post-transcriptional suppression of RAB2B. Collectively, the present study unrevealed the therapeutic implications of miR-425/RAB2B axis in human cervical cancer.
Materials and methods
Clinical tissues
At total of 30 malignant cervical tissues and 30 normal adjacent tissue specimens were collected at the time of surgical resection at Linyi central hospital, Linyi, Shandong province, 276400, China. Only those patients who had not received any therapy prior to surgery were included in the study. Additionally, the patients provided informed consent for inclusion in the study. After excision, the tissues were immediately frozen in liquid nitrogen and for long term storage at −80°C temperature was used. The characteristics of the cervical cancer patients are listed in Table 1. All the standard ethical guidelines were followed, and the study was approved by the research ethics committee of the Linyi central hospital under approval number LCH/778/3A/2019.
Table 1.
Characteristics of the cervical patients used in the study.
| Characteristics | Cervical cancer patients (n = 30) |
|---|---|
| Age | |
| <50 | 17 |
| ⩾50 | 13 |
| Tumor size | |
| <4 | 18 |
| ⩾4 | 12 |
| TNM stage | |
| I–II | 11 |
| III | 19 |
| Lymph node metastasis | |
| Yes | 21 |
| No | 9 |
| Differentiation | |
| Poor | 6 |
| Moderate + Well | 24 |
Cell lines
Four different cervical cancer cell lines (HeLa, siHa, DoTc2 and C-33 A) and a normal cervical epithelial cell line, NCE were purchased from ATCC, USA. DMEM culture medium (Gibco) supplemented with 10% FBS was used for culturing the cell lines at 37°C with 5% CO2/95% air.
Transfection
The transfection constructs miR-425 mimics (over-expression), its negative control (miR-NC), si-RAB2B (silencing) along with negative control (si-NC) were designed and purchased from GenePharma company, China. pcDNA3.1 over-expression vector was used for generating the pcDNA-RAB2B construct (over-expression) and vector alone was used as negative control. The cell transfection was performed with the help of Lipofectamine 2000 reagent (Thermo Fisher Scientific) through manufacturer protocol.
RNA isolation and RT-PCR
Using TRIzol reagent (Thermo Fisher Scientific), total RNA was extracted from the cell lines and tissue samples. Approximately, 2.5 µg of total RNA was used for cDNA synthesis with the help of RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). SYBR Green PCR mix (Thermo Fisher Scientific) was used for performing the RT-PCR. The gene expression was quantified using 2-ddCt method. Human GADPH and U6 snRNA were used as internal control for expression analysis of RAB2B and miR-425 respectively. The primers used in this study are listed in Table 2.
Table 2.
List of primers used in the study.
| Primer | Direction | Sequence |
|---|---|---|
| RAB2B | Forward | 5′- GGTCCG GGA AGT CCATACTC -3′ |
| Reverse | 5′- GGCTGGAACCGCTTATCT GT -3′ | |
| miR-425 | Forward | 5- TGCGGAATGACACGATCACTCCCG-3′ |
| Reverse | 5′- CCAGTGCAGGGTCCGAGGT-3 -3′ | |
| U6 | Forward | 5′- ACG AATTGC GTG TCATCCT -3′ |
| Reverse | 5′- ACGAATTTG CGT GTCATC CT -3′ | |
| GADPH | Forward | 5′- AAG CCTGCCGGTGACTAA C -3′ |
| Reverse | 5′- GCATCACCCGGAGGAGAA AT -3′ |
Cell viability assay
The assessment of viability of transfected cancer cells was made through 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay. The transfected cells were seeded at 0.5 × 104 cells/density in the 96-well plate and cultured for 12, 24, 48, and 96 h at 37°C. Around 20 μL MTT was added to each sample and the incubation was extended for additional 4 h at 37°C. Thereafter, 200 μL dimethyl sulfoxide (DMSO) was added per sample to dissolve the formazan product. Using spectrophotometer (Olympus Corporation), optical density was recorded for each sample at 570 nm.
Cell proliferation assay
The proliferative ability of transfected cells was deduced using 5-ethynyl-2′-deoxyuridine (EdU) assay. The transfected cancer cells were stained with EdU labeling solution with the help of EdU labeling/detection kit (RiboBio, China), as per the manufacturer guidelines. The cells were fixed with 70% ethanol and then incubated with glycine, PBS washed, treated with anti-EdU solution followed by their permeation with 0.5% Triton X-100 in PBS. 0.5% DAPI was used for staining the nuclei of cells and fluorescent microscope was used for visualizing the cells.
Apoptosis assays
The apoptosis of cancer cells was detected using acridine orange/ethidium bromide (AO/EB), 4′,6-diamidino-2-phenylindole (DAPI) staining methods and flow cytometric analysis. The transfected cells grew at 37°C for 24 h. The cells were fixed using 4% paraformaldehyde and then stained with AO/EB dual staining mix or DAPI solution and finally examined under fluorescence microscope. Annexin V-FITC/PI apoptosis staining/detection kit (Abycam) was used to analyze the apoptosis of transfected cancer cells through flow cytometry following the manufacturer guidelines.
Western blot analysis
Total cell lysates were obtained from the transfected cervical cancer cells using Laemmli S.D.S reducing buffer (2% S.D.S, 50 mM Tris–HCl, and 10% glycerol; pH 6.8) treatment. The protein lysates were separated on 8%–10% polyacrylamide gel and blotted to PVDF membrane. Antibodies against Bax, Bcl-2, MMP-2, MMP-9, RAB2B (Abycam) and β-actin (Sigma-Aldrich) were used to treat PVDF membranes. The membranes were treated with horseradish peroxidase-conjugated secondary antibodies and specific protein bands were detected using Chemiluminescence Substrate Kit (Thermo Fisher Scientific).
Wound-healing assay
The migration of transfected cancer cells was deduced through wound-healing method. Cells were cultured in 6-well plate to reach confluence. Afterwards, the scratch was drawn using pipette tip and the plate was incubated for 24 h at 37°C. Finally, the scratch width was observed and compared with initial scratch width. Photographs were obtained using light microscope at 0 h and after 24 h of scratch wound.
Transwell chamber invasion assay
Transwell chamber assay was used to determine cell invasion with the help of transwell chambers (4.5 µm pore size, Corning, USA). The transfected cells were re-suspended in DMEM culture medium and added to upper chamber while the lower chamber received only DMEM medium with 10% FBS. The transwell chamber plate was incubated at 37°C for 24 h after which, the cells invading the lower chamber were fixed with methanol, stained using 0.1% crystal violet, photographed, and manually counted using a microscope.
Xenograft tumor study
All animal experimental procedures were approved by the research ethics committee of Linyi central hospital under approval number LCH/778/3A/2019. A total of 5 × 106 DoTc2 cells in 100 µL PBS at logarithmic phase were injected subcutaneously into the right flank of 6-week-old BALB/c-nude mice. When solid tumors were established negative control (miR-NC) or miR-425 mimics were injected into the flanks of the mice and monitored every 3 days. The mice were sacrificed after 21 days under sodium pentobarbital anesthesia and xenografted tumors were excised and weighed. The expression of ki67 and caspase-3 (cleaved) proteins were determined from the tumor tissues through immuno-histochemical analysis.
In silico analysis and luciferase reporter assay
Online bioinformatics was performed using TargetScan Human 7.2 (http://www.targetscan.org/vert_72/) database. For performing the interaction assay, the wild-type or mutant 3′-UTR of RAB2B was cloned into pGL3 luciferase reporter vector. The reporter construct was co-transfected into DoTc2 cancer cells miR-425 mimics or miR-NC. The luciferase activity was determined after 48 h incubation at 37°C using Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
All experiments were performed with three biological replicates. The data is presented as mean ± standard deviation (SD). Statistical significance was determined using Student’s t-test or one-way ANOVA performed using GraphPad Prism 7.0 software. Differences were taken to be statistically significant at P < 0.05.
Results
miR-425 repression associates with and predicts cervical cancer survival
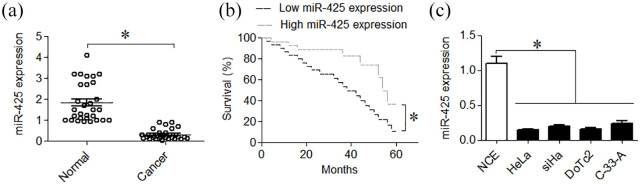
To verify the deregulation of miR-425 in cervical cancer, expression analysis of miR-425 was carried out using qRT-PCR. The miR-425 was found to be significantly (P = 0.032) down-regulated in cervical cancer tissues (Figure 1a). The Kaplan-Meier survival analysis performed using the blood samples, collected from cervical cancer patients from March 2012 to February 2014, indicated that lower transcript abundance of miR-425 is linked with significantly (P = 0.035) lower cervical cancer disease survival (Figure 1b). Moreover, the cervical cancer cell lines (HeLa, siHa, DoTc2 and C-33 A) exhibited significantly (P = 0.022) lower miR-425 expression than the normal cervical epithelial cell line (Figure 1c). The results suggest that cervical cancer is linked with miR-425 repression and that miR-425 acts as a biomarker of patient survival.
Figure 1.
miR-425 has considerable down-regulation in cervical cancer which predicts the disease survival. (a) qRT-PCR based expression analysis of miR-425 in cervical cancer and normal adjacent tissues. (b) Kaplan-Meier survival analysis of cervical cancer with reference to miR-425 expression. (c) qRT-PCR based expression analysis of miR-425 in cervical cancer cell lines (HeLa, siHa, DoTc2 and C-33 A) and normal cervical epithelial cell line, NCE. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05).
Overexpression of miR-425 reduces growth and proliferative viability of cervical cancer cells
To characterize the role of miR-425, miR-425 was over-expressed in DoTc2 cancer cells (Figure 2a). From the MTT assay results, the growth of DoTc2 cells over-expressing miR-425 was markedly inhibited (Figure 2b). The EdU staining assay revealed that the cervical cancer cells showed significantly (P = 0.024) lower proliferative viability under miR-425 up-regulation. The results are inclusive of negative growth regulatory role of miR-425 in cervical cancer highlighting its potential as viable therapeutic target against the same.
Figure 2.
miR-425 over-expression restricted the cancer cell growth and declined proliferative viability of cervical cancer cells. (a) qRT-PCR based expression analysis of miR-425 in DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC. (b) MTT assay for the analysis of viability of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC. (c) EdU assay for assessment of proliferative viability of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05).
Apoptosis was induced in cervical cancer cells by over-expressing miR-425
To study the underlying mechanism responsible for the miR-425 induced growth suppressive effects, the DoTc2 cells were subjected to AO/EB staining. The cancer cells were seen to exhibit nuclear deformation under miR-425 over-expression (Figure 3a). DAPI staining also indicated the similar apoptotic effects in cervical cancer cells over-expressing miR-425 (Figure 3b). Again, the flow cytometric analysis of DoTc2 cancer cells showed that miR-425 over-expression induced apoptosis in cancer cells and considerably higher proportion of apoptotic cells was observed under miR-425 over-expression (Figure 3c). The induction of cancer cell apoptosis in miR-425 overexpressing cells was found to be associated with upregulation of Bax and downregulation of Bcl-2 (Figure 3d). Evidently, the results suggest that miR-425 overexpression in cervical cancer cells induced apoptosis and declined the cell growth.
Figure 3.
miR-425 over-expression induced apoptosis in vitro in cervical cancer cells. (a) Analysis of nuclear morphology of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC using AO/EB staining (green colour depicts normal, yellow color early apoptotic and red color late apoptotic cells). (b) Analysis of nuclear morphology of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC using DAPI staining. (c) Analysis of apoptosis of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC using flow cytometry. (d) Western blotting of Bax and Bcl-2 from DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05).
miR-425 regulates the metastasis of cervical cancer cells
Whether miR-425 has any role in regulating the metastasis of cervical cancer, the migration and invasion of cancer cell was studied. The miR-425 over-expressing cervical cancer cells were found to possess significantly (P = 0.01) higher migratory ability in comparison to the negative control cells (Figure 4a). The transwell chamber method indicated that invasion of cervical cancer cells also decreased remarkably under miR-425 up-regulation (Figure 4b). Similarly, the expression of metalloproteinases, MMP-2 and MMP-9 was considerably reduced in cancer cells over-expressing miR-425 (Figure 4c). Together, the results indicate the negative role of miR-425 in regulating the cervical cancer metastasis highlighting its therapeutic value.
Figure 4.
miR-425 over-expression minimized the cancer cell migration and invasion. (a) Analysis of migration of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC using wound-healing method. (b) Analysis of invasion of DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC using transwell-chamber assay. (c) Western blotting of MMP-2 and MMP-9 from DoTc2 cervical cancer cells transfected with miR-425 mimics or miR-NC. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05).
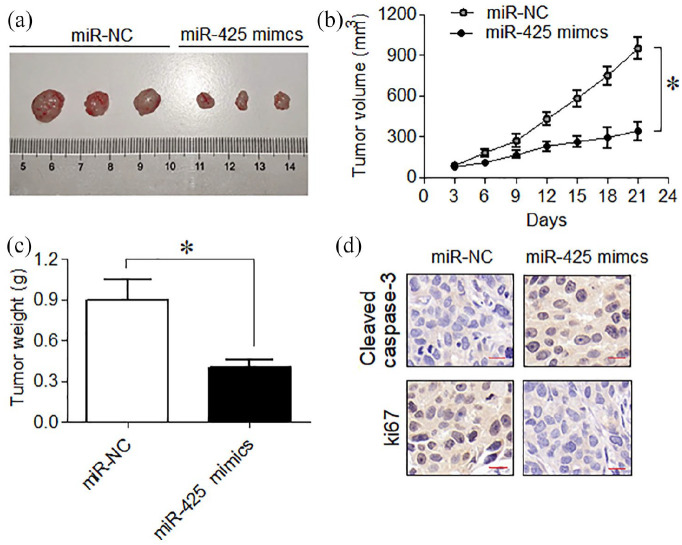
miR-425 negatively affected mice xenograft tumorigenesis
The animal xenograft models of cervical cancer were used for assessing the in vivo role of miR-425. The tumor xenografts were seen to exhibit significantly (P = 0.02) lower size under miR-425 over-expression (Figure 5a). The volume of xenograft tumors was also negatively affected when the animals were administered with miR-425 over-expression (Figure 5b). Finally, miR-425 up-regulation was deduced to restrict the average tumor weight in vivo (Figure 5c). The xenograft mice tumors expressed higher caspase-3 (cleaved) protein while the expression of ki67 proliferation marker protein was decreased significantly under miR-425 up-regulation (Figure 5d). In sum, the results show that miR-425 negatively regulates the cervical cancer tumorogenesis.
Figure 5.
miR-425 restricted in vivo xenograft tumor growth and proliferation. (a) Analysis of tumor size under administration of miR-425 mimics or miR-NC. (b) Analysis of tumor volume under administration of miR-425 mimics or miR-NC. (c) Average tumor weight under administration of miR-425 mimics or miR-NC. (d) Immune-histochemical fluorescence staining of caspase-3 (cleaved) and ki67 from mice tumors under administration of miR-425 mimics or miR-NC. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05).
RAB2B is the functional target of miR-425 in cervical cancer
The prediction of the molecular regulatory targets of miR-425 was made through online in silico analysis. RAB2B was predicted to be targeted by miR-425 through sequence specific interaction with its 3′-UTR (Figure 6a). The dual luciferase reporter assay confirmed this interaction (Figure 6b). As expected, the cervical cancer tissues and cell lines exhibited significantly (P = 0.031) higher transcripts of RAB2B corresponding to lower miR-425 expression (Figure 6c and d). The DoTc2 cancer cells over-expressing miR-425 were shown to express lower RAB2B protein levels in comparison to the negative control cells (Figure 6e). The silencing of RAB2B in cervical cancer cells declined the cell growth in a similar fashion as under miR-425 over-expression (Figure 6e). The anti-growth effects of miR-425 over-expression were shown to be minimized when RAB2B was up-regulated in cervical cancer cells (Figure 6f). Collectively, the results are indicative of RAB2B translational repression by miR-425 and suggest that miR-425 exerts its regulatory control via RAB2B in cervical cancer.
Figure 6.
RAB2B is the functional molecular target of miR-425 in cervical cancer. (a) TargetScan analysis for prediction of molecular target of miR-425. (b) Dual luciferase reporter assay for the interaction analysis of miR-425 with 3’-UTR of RAB2B. (c) qRT-PCR based expression analysis of miR-425 in cervical cancer and normal adjacent tissues. (d) qRT-PCR based expression analysis of miR-425 in cervical cancer cell lines (HeLa, siHa, DoTc2 and C-33 A) and normal cervical epithelial cell line, NCE. (e) Western blotting of RAB2B form DoTc2 cancer cells transfected with miR-425 mimics or miR-NC. (f) MTT assay for the analysis of viability of DoTc2 cervical cancer cells transfected with si-RAB2B or si-NC. (g) MTT assay for the analysis of viability of DoTc2 cervical cancer cells transfected with miR-425 mimics, miR-NC or miR-425 mimics plus pcDNA-RAB2B. The experiments were performed in triplicate and expressed as mean ± SD (*P < 0.05).
Discussion
The micro-RNAs (miRs) have received considerable attention during the recent years and the reports are emerging about their crucial involvement in maintaining the homeostasis of human body.23 Almost every cancerous malignancy is shown to be associated with the expressional irregularity of specific miRNA molecules for which the miRs are ranked as molecular prognostic tools of cancer.24 The miRs have been shown to play deciding roles in controlling the tumorigenic behavior of cancer cells.25 Many of the miRs were found to be involved in growth and propagation of cervical cancer.11,26 The latter is fatal gynecological disorder and accounts for tremendous mortality among human females.3 Many workers have suggested the utilization of miRNAs as effective therapeutic agents in combating the seriousness of cervical cancer.27 Besides, the miRs may aid in disease screening and prediction of clinical outcomes of treatment strategies against the cervical cancer.11 The results of our current work also support role of miR-425 in cervical cancer diagnosis and prediction of the cervical cancer patient survival. The miR-425 has been reported to exhibit tumor suppressive role in several types of cancer.12–14 Previous reports signified that miR-425 over-expression limits the cancer cell growth and proliferation by inducing apoptosis in cancer cells.13 Consistent with such findings, we found that cervical cancer cells proliferated at significantly lower rates under miR-425 over-expression due to the induction of apoptotic cell death. The Bax and Bcl-2 proteins are important markers of apoptosis.28 Apoptosis is associated with upregulation of Bax and downregulation of Bcl-2. These proteins are considered as important targets for the development efficient therapeutic agents.29 Consistently, the results of the present study revealed that miR-425 induced apoptosis was associated upregulation of Bax and depletion of Bcl-2. The metalloproteinases, which are involved in controlled re-modeling of extracellular matrix, have crucial involvement in cancer metastasis and their enhanced expression levels mark the spread of cancerous malignancy to lymph nodes.30,31 When miR-425 was over-expressed in cervical cancer cells, the cells showed limited migration and invasion together with decline in the expression of metalloproteinases which indicates the negative role of miR-425 in regulating cancer metastasis.24 This is also consistent with the results of Zhang et al.,15 wherein miR-425 was found to suppress the invasion and metastasis of human breast cancer. In vivo tumorigenesis was also significantly restricted under miR-425 over-expression. The miRs act at post-transcriptional level to repress the expression of specific target genes.7 Ras-related protein Rab-2B (RAB2B), belonging to GTPase super-family and involved in protein transportation, was shown to be functional molecular target of miR-425 in cervical cancer.32 RAB2B has established oncogenic role and its transcriptional up-regulation is one the key factors responsible for human tumorigenesis including cervical cancer.33 RAB2B was found to be over-expressed in cervical cancer due to miR-425 repression and its up-regulation was shown to enhance the cancer cell growth and proliferation. In conclusion, the study elaborated the role of miR-425/RAR2B regulatory axis in cervical cancer and the results suggest miR-425 to be a viable therapeutic target against the human cervical cancer as has been elucidated in human glioma.34 The main limitation of the study is that only one molecular target of miR-425 was studies in the present study. Another limitation of the present study is the small sample size, similar studies with large sample size need to be carried out. Finally, the present study revealed the role of miR-425 in human cervical cancer via modulation of RAB2B expression, more studies are required to unravel the other molecular mechanisms underlying the tumor-suppressive effects of miR-425.
Conclusion
The results of current study are supportive that miR-425 exhibits transcriptional down-regulation in cervical cancer and the latter is one of the crucial factors responsible for the malignant behavior of cervical cancer cells. The miR-425 was shown to exert tumor-suppressive effects on cervical cancer via post-transcriptional suppression of RAB2B protein. Taken together, miR-425 might be utilized as potential prognostic biomarker and therapeutic target against the human cervical cancer.
Footnotes
Animal welfare: This study followed the international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation. All experiments were carried out according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all animals were treated in strict accordance with protocols approved by the Institutional Animal Care and Use Committee of Linyi Central Hospital. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: All animal experiments were approved by the research ethics committee of the Linyi central hospital under approval number LCH/778/3A/2019
Patient consent: Written informed consent was obtained from all subjects before the study
ORCID iD: Yue Tian  https://orcid.org/0000-0003-0673-4980
https://orcid.org/0000-0003-0673-4980
Data availability statement: The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1. Vu M, Yu J, Awolude OA, et al. (2018) Cervical cancer worldwide. Current Problems in Cancer 42(5): 457–465. [DOI] [PubMed] [Google Scholar]
- 2. Cohen PA, Jhingran A, Oaknin A, et al. (2019) Cervical cancer. The Lancet 393(10167): 169–182. [DOI] [PubMed] [Google Scholar]
- 3. Arbyn M, Weiderpass E, Bruni L, et al. (2020) Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. The Lancet Global Health 8(2): e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torre LA, Bray F, Siegel RL, et al. (2015) Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 65(2): 87–108. [DOI] [PubMed] [Google Scholar]
- 5. Wright JD, Chen L, Tergas AI, et al. (2015) Population-level trends in relative survival for cervical cancer. American Journal of Obstetrics and Gynecology 213(5): 670.e1–670.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvarajan S, Vijayaraghavan J, Bobby Z, et al. (2019) Micro RNAs-A review. Journal of Evolution of Medical and Dental Sciences 2019; 8(38): 2918–2924. [Google Scholar]
- 7. Masi LN, Serdan TD, Levada-Pires AC, et al. (2016) Regulation of gene expression by exercise-related micrornas. Cellular Physiology and Biochemistry 2016; 39(6): 2381–2397. [DOI] [PubMed] [Google Scholar]
- 8. O’Connell RM, Rao DS, Baltimore D. (2012) microRNA regulation of inflammatory responses. Annual Review of Immunology 30: 295–312. [DOI] [PubMed] [Google Scholar]
- 9. Hata A, Kashima R. (2016) Dysregulation of microRNA biogenesis machinery in cancer. Critical Reviews in Biochemistry and Molecular Biology 2016; 51(3): 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu P, Tang H, Chen B, et al. (2015) miR-26a suppresses tumour proliferation and metastasis by targeting metadherin in triple negative breast cancer. Cancer Letters 357(1): 384–392. [DOI] [PubMed] [Google Scholar]
- 11. Hu X, Schwarz JK, Lewis JS, et al. (2010) A microRNA expression signature for cervical cancer prognosis. Cancer Research 70(4):1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fang F, Song T, Zhang T. (2017) MiR-425-5p promotes invasion and metastasis of hepatocellular carcinoma cells through SCAI-mediated dysregulation of multiple signaling pathways. Oncotarget 8(19): 31745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng WZ, Ma R, Wang F, et al. (2014) Role of miR-191/425 cluster in tumorigenesis and diagnosis of gastric cancer. International Journal of Molecular Sciences 15(3): 4031–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Hu X, Miao X, et al. (2016) MicroRNA-425-5p regulates chemoresistance in colorectal cancer cells via regulation of programmed cell death 10. Journal of Cellular and Molecular Medicine 20(2): 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Z, Li Y, Fan L, et al. (2015) microRNA-425-5p is upregulated in human gastric cancer and contributes to invasion and metastasis in vitro and in vivo. Experimental and Therapeutic Medicine 9(5): 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang JY, Su XP, Li YN, et al. (2019) MicroRNA-425-5p promotes the development of prostate cancer via targeting forkhead box J3. European Review for Medical and Pharmacological Sciences 23(2): 547–554. [DOI] [PubMed] [Google Scholar]
- 17. Zhu W, Ma Y, Zhuang X, et al. (2018) MicroRNA-425 is downregulated in nasopharyngeal carcinoma and regulates tumor cell viability and invasion by targeting hepatoma-derived growth factor. Oncology Letters 15(5): 6345–6351. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Sun L, Jiang R, Li J, et al. (2017) MicoRNA-425-5p is a potential prognostic biomarker for cervical cancer. Annals of Clinical Biochemistry 54(1): 127–133. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Yang Y, Liu R, et al. (2019) Downregulation of microRNA-425-5p suppresses cervical cancer tumorigenesis by targeting AIFM1. Experimental and Therapeutic Medicine 17(5): 4032–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukuda M, Kanno E, Ishibashi K, et al. (2008) Large scale screening for novel rab effectors reveals unexpected broad rab binding specificity. Molecular & Cellular Proteomics 7: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 21. Tisdale EJ, Bourne JR, Khosravi-Far R, et al. (1992) GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. Journal of Cell Biology 119: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Culine S, Honore N, Closson V, et al. (1994) A small GTP-binding protein is frequently overexpressed in peripheral blood mononuclear cells from patients with solid tumours. European Journal of Cancer 30: 670–674. [DOI] [PubMed] [Google Scholar]
- 23. Rayner KJ, Suárez Y, Dávalos A, et al. (2010) MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328(5985): 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singhal A, Jayaraman M, Dhanasekaran DN, et al. (2012) Molecular and serum markers in hepatocellular carcinoma: Predictive tools for prognosis and recurrence. Critical Reviews in Oncology/Hematology 82(2): 116–140. [DOI] [PubMed] [Google Scholar]
- 25. Wen KC, Sung PL, Yen MS. (2013) MicroRNAs regulate several functions of normal tissues and malignancies. Taiwanese Journal of Obstetrics and Gynecology 52(4): 465–469. [DOI] [PubMed] [Google Scholar]
- 26. Lee JW, Choi CH, Choi JJ, et al. (2008) Altered MicroRNA expression in cervical carcinomas. Clinical Cancer Research 14(9): 2535–2542. [DOI] [PubMed] [Google Scholar]
- 27. Pardini B, De Maria D, Francavilla A, et al. (2018) MicroRNAs as markers of progression in cervical cancer: A systematic review. BMC Cancer 18(1): 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams JM, Cory S. (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26(9): 1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams JM, Cory S. (2007) Bcl-2-regulated apoptosis: Mechanism and therapeutic potential. Current Opinion in Immunology 19(5): 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson C, Galis ZS. (2004) Matrix metalloproteinase-2 and− 9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arteriosclerosis, Thrombosis, and Vascular Biology 24(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 31. Dufour A, Zucker S, Sampson NS, et al. (2010) Role of matrix metalloproteinase-9 dimers in cell migration: Design of inhibitory peptides. Journal of Biological Chemistry 285(46): 35944–35956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ni X, Ma Y, Cheng H, et al. (2002) Molecular cloning and characterization of a novel human Rab (Rab2B) gene. Journal of Human Genetics 47(10): 0548–0551. [DOI] [PubMed] [Google Scholar]
- 33. Hu Y, Li Y, Huang Y, et al. (2020) METTL3 regulates the malignancy of cervical cancer via post-transcriptional regulation of RAB2B. European Journal of Pharmacology 879: 173134. [DOI] [PubMed] [Google Scholar]
- 34. Fan X, Zhang Q, Geng X, et al. (2019) MiR-425-5p/RAB2B promotes the proliferation, invasion and migration of glioma cells. Journal of Biomaterials and Tissue Engineering 9(10): 1317–1326. [Google Scholar]