Abstract
Background
Chronic obstructive pulmonary disease (COPD) is often accompanied by pulmonary infection, inflammatory responses, decreased immunity, and decreased lung function. The relationships among the pulmonary inflammation index (PII), lung function, and immunity in COPD patients with pulmonary infection remain unclear.
Methods
This retrospective observational study enrolled 234 participants (patients with COPD and pulmonary infection, patients with COPD without pulmonary infection, and healthy individuals) from January 2017 to December 2019.
Results
Levels of interleukin (IL)-6 were lower and levels of IL-8 were higher in patients with COPD and pulmonary infection. Levels of white blood cells (WBCs), C-reactive protein (CRP), IL-6, IL-8, tumor necrosis factor (TNF)-α and CD8+ cells were higher, while levels of CD3+ and CD4+ cells, the CD4+/CD8+ ratio, forced expiratory volume in 1 s (FEV1), FEV1 % predicted (FEV1%pred), and FEV1/forced vital capacity (FVC) (FEV1%FVC) were lower in patients with COPD and pulmonary infection. Levels of WBCs, CRP, IL-6, IL-8, and TNF-α were negatively associated with FEV1, FEV1%pred and FEV1%FVC.
Conclusions
Patients with COPD and pulmonary infection have high PIIs, decreased immunity, and poor lung function. PII is closely related to lung function and may represent a useful biomarker for the assessment of patients with COPD and pulmonary infection.
Keywords: Pulmonary infection, chronic obstructive pulmonary disease, inflammatory index, immune function, lung function, biomarker
Background
Chronic obstructive pulmonary disease (COPD) is a heterogeneous chronic inflammatory disease characterized by persistent airflow limitations that are progressively aggravated.1 COPD imposes a large economic burden as well as high mortality and morbidity.2,3 COPD was the sixth most common cause of death globally in 1990 and was predicted to become the third most common by 2020.4,5 Decreased immune function in patients with COPD can lead to bacterial infections. Therefore, many patients with COPD develop pulmonary infections that are associated with significant inflammatory responses and hypoxia. Pulmonary infections in patients with COPD make treatment challenging and also severely impair lung function. High expression levels of inflammatory factors and diminished immune function in patients with COPD are associated with poor prognosis.6,7 However, there have been few reports on the relationships between inflammatory indices, lung function, and immune function in these patients. Changes in inflammatory factors in patients with COPD and pulmonary infection play an essential role in diagnosis, treatment, and prognosis determination. Analysis of correlations between inflammatory factor levels, lung function, and inflammatory indices may help in evaluating treatment of patients. In this study, we investigated levels of white blood cells (WBCs), C-reactive protein (CRP), interleukin (IL)-6, tumor necrosis factor (TNF)-α, CD3+ cells, CD4+ cells, and CD8+ cells, as well as the CD4+/CD8+ ratio, as biomarkers for diagnosis, treatment, and prognosis determination of patients with COPD and pulmonary infection. These data may provide a reference for clinicians to assess the treatment and prognosis of patients with COPD and lung infection during the early stages of disease.
Methods
Study participants
This was a single-center retrospective observational study conducted at a tertiary hospital between January 2017 and December 2019. All participants were enrolled at the Affiliated Hospital of Yangzhou University. Patients with COPD and pulmonary infection were enrolled if imaging results showed obvious pneumonia lesions. Patients with COPD but without obvious pneumonia lesions were enrolled for comparison. In addition, healthy individuals undergoing routine physical examination in our hospital were enrolled as controls. The absence of pre-existing medical conditions in healthy individuals was confirmed by taking their history. According to Anthonisen's criteria, all COPD patients were of type one and had increased difficulty breathing, increased sputum production, and purulent sputum.
Inclusion criteria were as follows: (1) diagnosis of COPD according to the diagnostic criteria for COPD in the 2017 Global Initiative for Chronic Obstructive Lung Disease guidelines; (2) COPD consistent with lung function test results and imaging findings of pulmonary emphysema; (3) for patients with COPD and pulmonary infection, imaging results showing obvious pneumonia lesions; and (4) patient and/or family member signature of informed consent.
Exclusion criteria were as follows: (1) other pulmonary diseases (e.g., pulmonary embolism, bronchiectasis, pulmonary interstitial disease, tuberculosis, or lung neoplasm); (2) other systemic severe disease (e.g., heart failure, liver disease, or kidney disease); (3) other primary infectious or immune diseases; (4) cardiovascular and cerebrovascular diseases; (5) malignant tumors; (6) mental illness; or (7) incomplete clinical data.
This study was conducted according to the guidelines laid out in the Declaration of Helsinki. The study was approved by the Ethics Committee of the Medical Research, The Affiliated Hospital of Yangzhou University (Yangzhou, Jiangsu, China; approval number: 2017YKL-03; approval date: 1 January 2017). Written informed consent was obtained from each participant or their family before enrollment in the study and for publication of this manuscript.
Assessment of immune indices and lung function
Fasting venous blood (2 mL) was collected for detection of inflammatory factors such as WBCs, CRP, IL-6, and IL-8 the morning after admission. Levels of immune factors were quantitated by enzyme-linked immunosorbent assay (ELISA) using kits from BD Biosciences (San Jose, CA, USA) according to the manufacturer’s instructions. WBC counts and CRP levels were assessed using a BC-5390 hematology analyzer (Mindray, Shenzhen, China). Levels of IL-6, IL-8 and TNF-α were quantitated using ELISA. Expression of cell surface markers was assessed by flow cytometry. Lung function was measured using a CHESTAC-8900 pulmonary function instrument (CHEST M.I., Inc., Tokyo, Japan). Contradictions for pulmonary function examination included active hemoptysis, active pulmonary tuberculosis, pneumothorax without thoracic drainage, myocardial infarction, pulmonary embolism, hemangioma of the chest, upper abdomen, or skull, and recent eye surgery.
Statistical analysis
Statistical analysis was performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). G*Power 3.18 was used to calculate the minimum sample size required for the study. Assuming a significance level for two-sided tests of α = 0.05 and a confidence interval of 95%, the minimum number of cases required was 111 when the odds ratio was 1.5. Count data were expressed as counts and percentages; differences between groups were assessed using the chi-square test. Continuous data were expressed as mean ± standard deviation. One way analysis of variance (ANOVA) was used for comparison among three independent, normally distributed and homogeneous variables. Pearson’s method was used to evaluate correlations between variables. A two-sided value of P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 234 individuals were enrolled at the Affiliated Hospital of Yangzhou University: 78 patients with COPD and pulmonary infection, 78 patients with COPD but without obvious pneumonia lesions, and 78 healthy individuals undergoing routine physical examination. No significant differences in sex, age, or smoking status were observed among the three groups (Table 1).
Table 1.
Comparison of baseline data between the three groups.
| Patients with COPD and pulmonary infection | Patients with COPD without pulmonary infection | Healthy controls | χ2 | P | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 40 | 43 | 47 | 0.328 | >0.05 |
| Female | 38 | 35 | 31 | ||
| Age (years)a | 67.33 ± 6.11 | 67.74 ± 6.16 | 66.69 ± 6.60 | 0.552 | 0.577 |
| Smoking | |||||
| Yes | 46 | 43 | 35 | 2.538 | >0.05 |
| No | 32 | 35 | 39 | ||
aMean ± standard deviation.
COPD, chronic obstructive pulmonary disease.
Comparison of plasma inflammatory indices
Levels of WBCs, CRP, IL-6, IL-8, and TNF-α were highest among patients with COPD and pulmonary infections (Table 2). Levels of inflammatory indexes level differed significantly among the three groups of patients as shown by Student's t-tests and chi-square tests (P < 0.001).
Table 2.
Comparison of plasma inflammatory indices in the three groups.
| Patients with COPD and pulmonary infection | Patients with COPD without pulmonary infection | Healthy controls | F | P | |
|---|---|---|---|---|---|
| WBC (109/L) | 17.34 ± 4.79 | 13.9 ± 4.21 | 6.41 ± 1.73 | 166.87 | <0.001 |
| CRP (mg/L) | 50.43 ± 11.10 | 40.68 ± 10.78 | 14.28 ± 10.12 | 239.32 | <0.001 |
| IL-6 (pg/mL) | 17.65 ± 3.09 | 13.90 ± 4.21 | 5.76 ± 1.51 | 483.57 | <0.001 |
| IL-8 (pg/mL) | 72.23 ± 3.74 | 61.52 ± 3.59 | 52.01 ± 3.66 | 593.53 | <0.001 |
| TNF-α (pg/mL) | 65.22 ± 7.88 | 54.89 ± 673 | 37.71 ± 6.80 | 296.80 | <0.001 |
COPD, chronic obstructive pulmonary disease; WBC, white blood cell; CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
Comparison of immune indices
As shown in Table 3, levels of immune indices were significantly different among the three groups (P < 0.001). Levels of CD3+ cells, levels of CD4+ cells, and the CD4+/CD8+ ratio gradually increased in the three groups. CD8+ cell levels gradually decreased in the three groups. Levels of immune indices differed significantly among the three groups according to the Student's t-test (P < 0.001).
Table 3.
Comparison of immune indices in the three groups.
| Patients with COPD and pulmonary infection | Patients with COPD without pulmonary infection | Healthy controls | F | P | ||
|---|---|---|---|---|---|---|
| CD3+ cells (%) | 55.03 ± 7.06 | 65.5 ± 7.21 | 70.7 ± 7.03 | 99.38 | <0.001 | |
| CD4+ cells (%) | 23.79 ± 3.10 | 30.70 ± 3.30 | 32.61 ± 3.18 | 164.96 | <0.001 | |
| CD8+ cells (%) | 31.81 ± 4.08 | 29.33 ± 4.06 | 22.62 ± 4.36 | 101.25 | <0.001 | |
| CD4+/CD8+ cell ratio | 1.15 ± 0.43 | 1.46 ± 0.30 | 1.84 ± 0.38 | 65.28 | <0.001 |
Data are shown as mean ± standard deviation.
COPD, chronic obstructive pulmonary disease.
Comparison of pulmonary function indicators
Table 4 shows the results of one-way ANOVA comparing lung function in the three groups. Lung function indices [forced expiratory volume in 1 s (FEV1), FEV1 % predicted (FEV1%pred) and FEV1/forced vital capacity (FVC) (FEV1%FVC)] were much lower in patients with COPD and pulmonary infection than in patients with COPD without pulmonary infection. Lung function indices were much lower in patients with COPD without pulmonary infection than in healthy individuals.
Table 4.
Comparison of pulmonary function in the three groups.
| Patients with COPD and pulmonary infection | Patients with COPD without pulmonary infection | Healthy controls | F | P | |
|---|---|---|---|---|---|
| FEV1 (L) | 1.22 ± 0.41 | 1.77 ± 0.42 | 3.04 ± 0.51 | 338.56 | <0.001 |
| FEV%pred (%) | 49.98 ± 13.41 | 57.68 ± 10.27 | 85.50 ± 10.05 | 215.59 | <0.001 |
| FEV1%FVC (%) | 48.54 ± 14.01 | 57.68 ± 10.27 | 85.50 ± 10.05 | 215.19 | <0.001 |
Data are shown as mean ± standard deviation.
FEV1, forced expiratory volume in 1 s; FEV1%pred, FEV1 % predicted; FEV1%FVC, FEV1/forced vital capacity.
Correlation between inflammatory indices and lung function indicators
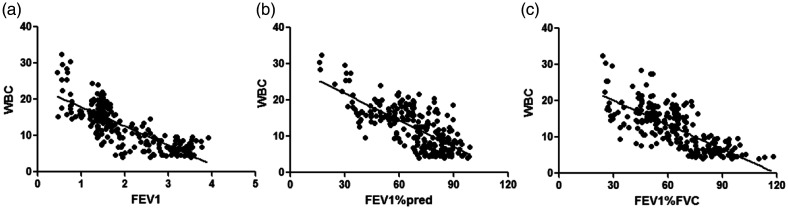
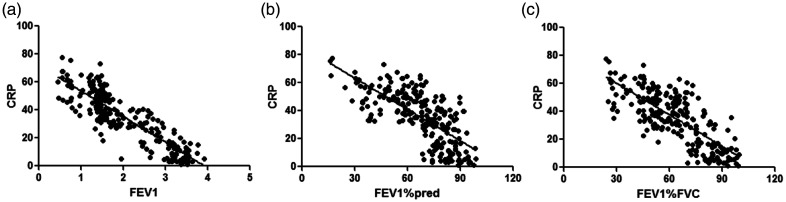
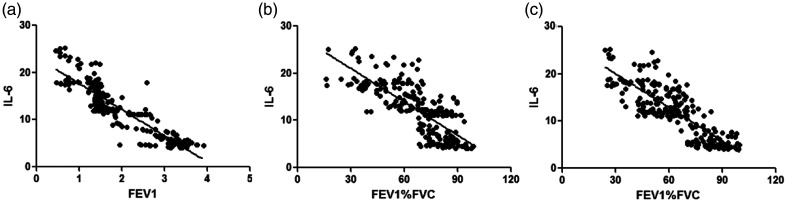
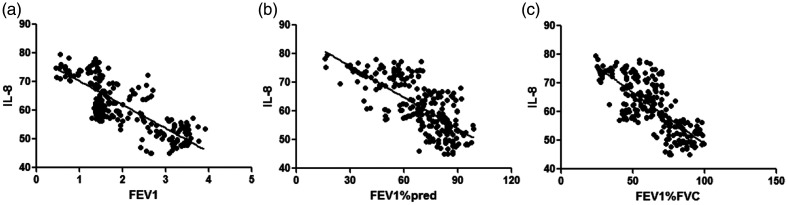
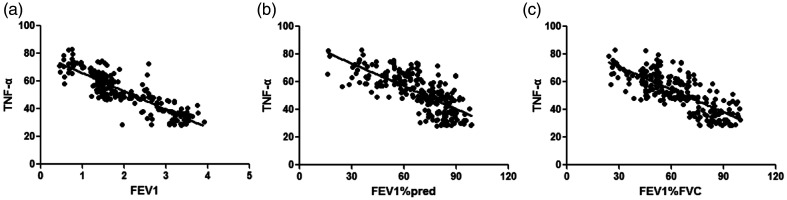
WBC counts were negatively correlated with FEV1 (r = −0.792), FEV1%pred (r = −0.701), and FEV1%FVC (r = −0.732) (all P < 0.001, Table 5 and Figure 1). CRP levels were negatively correlated with FEV1 (r = −0.873), FEV1%pred (r = −0.705), and FEV1/FVC (r = −0.766) (all P < 0.001, Figure 2). IL-6 levels were negatively correlated with FEV1 (r = −0.902), FEV1%pred (r = −0.753), and FEV1%FVC (r = −0.821) (all P < 0.001, Figure 3). IL-8 levels were negatively correlated with FEV1 (r = −0.807), FEV1%pred (r = −0.701), and FEV1%FVC (r = −0.747) (all P < 0.001, Figure 4). TNF-α levels were negatively correlated with FEV1 (r = −0.871), FEV1%pred (r = −0.717), and FEV1%FVC (r = −0.762) (all P < 0.001, Figure 5).
Table 5.
Correlation between inflammatory cytokine levels and pulmonary function indicators.
|
FEV1 |
FEV1%pred |
FEV1%FVC |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| WBC | −0.792 | <0.001 | −0.701 | <0.001 | −0.732 | <0.001 |
| CRP | −0.873 | <0.001 | −0.705 | <0.001 | −0.766 | <0.001 |
| IL-6 | −0.902 | <0.001 | −0.753 | <0.001 | −0.821 | <0.001 |
| IL-8 | −0.807 | <0.001 | −0.701 | <0.001 | −0.747 | <0.001 |
| TNF-α | −0.871 | <0.001 | −0.717 | <0.001 | −0.762 | <0.001 |
FEV1, forced expiratory volume in 1 s; FEV1%pred, FEV1 % predicted; FEV1%FVC, FEV1/forced vital capacity; WBC, white blood cell; CRP, C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
Figure 1.
WBC counts were negatively correlated with (a) FEV1 (r = −0.792) (b) FEV1%pred (r = −0.701), and (c) FEV1%FVC (r = −0.732).
WBC, white blood cell; FEV1, forced expiratory volume in 1 s; FEV1%pred: FEV1 % predicted; FEV1%FVC: FEV1/forced vital capacity.
Figure 2.
CRP level was negatively correlated with (a) FEV1 (r = −0.873) (b) FEV1%pred (r = −0.705), and (c) FEV1%FVC (r = −0.766).
CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; FEV1%pred: FEV1 % predicted; FEV1%FVC: FEV1/forced vital capacity.
Figure 3.
IL-6 level was negatively correlated with (a) FEV1 (r = −0.902) (b) FEV1%pred (r = −0.753), and (c) FEV1%FVC (r = −0.821).
IL, interleukin; FEV1, forced expiratory volume in 1 s; FEV1%pred: FEV1 % predicted; FEV1%FVC: FEV1/forced vital capacity.
Figure 4.
IL-8 level was negatively correlated with (a) FEV1 (r = −0.807 (b) FEV1%pred (r = −0.701), and (c) FEV1%FVC (r = −0.747).
IL, interleukin; FEV1, forced expiratory volume in 1 s; FEV1%pred: FEV1 % predicted; FEV1%FVC: FEV1/forced vital capacity.
Figure 5.
TNF-α level was negatively correlated with (a) FEV1 (r = −0.871) (b) FEV1%pred (r = −0.717), and (c) FEV1%FVC (r = −0.762).
TNF, tumor necrosis factor; FEV1, forced expiratory volume in 1 s; FEV1%pred: FEV1 % predicted; FEV1%FVC: FEV1/forced vital capacity.
Discussion
COPD is a common chronic lung disease. COPD has a high incidence rate and mortality, seriously affects the lives and health of patients, and imposes a heavy economic burden. Smoking and inhaling harmful particles are risk factors for COPD.9 The pathological features of COPD include chronic inflammation of the airway, lung parenchyma, and pulmonary vessels. Inflammatory cells involved in COPD include macrophages, T lymphocytes, and neutrophils. Activated inflammatory cells release various inflammatory mediators including leukotriene B4, IL-6, IL-8, and TNF-α. Because of the long course of COPD, patients’ resistance to infection can decrease. Sputum secretion in the airways of COPD patients is increased, and sputum is difficult to discharge because of airway stenosis. Together, these factors promote pulmonary infection. Complication of COPD by pulmonary infection can further damage lung function and seriously affect prognosis.10
Levels of WBCs, CRP, IL-6, IL-8, and TNF-α levels were the highest in patients with COPD and pulmonary infection among the three groups. These results suggested that levels of inflammatory factors in patients with COPD and pulmonary infection were higher than in the other two groups, reflecting the severity of COPD. Pulmonary infection may directly affect lung function and the prognosis of patients with COPD. WBCs,11 CRP,21 IL-6,13 IL-814 and TNF-α15 are associated with inflammatory cells and the alveolar epithelium in patients with COPD; these factors are closely related to the level of infection, lung function, and severity of illness. Higher levels of inflammatory indicators in the blood are associated with more severe infection and more serious lung damage.
Multiple studies have shown that lymphocyte subsets are closely related to pulmonary infection.16,17 Recently Su et al.18 found that lymphocyte subsets (CD4+ T cells and CD8+ T cells) in patients with coronavirus disease 2019 (COVID-19) differed significantly between mild and severe cases; the frequencies of these cells were closely related to the severity of COVID-19 pneumonia. Our results showed that CD3+ cell levels, CD4+ cell levels, and the CD4+/CD8+ ratio gradually increased in all three groups. By contrast, CD8+ cell levels progressively decreased in all three groups. These results showed that immune function in patients with COPD was significantly reduced, especially in patients with pulmonary infection. It has been reported that T lymphocyte abnormalities play a vital role in the pathogenesis of pulmonary infection.19 CD3+ cells represent all T cells, and can document total levels of cellular immunity in patients.20 CD4+ cells, also known as T-helper cells, can secrete lymphokines to enhance the immune response and induce relevant immune cells to produce synergistic anti-infective effects.21 CD8+ cells are cytotoxic T cells and play a negative role in the immune response. These cells can inhibit the functions of CD4+ cells and B cells as well as production of antibody.22,23
FEV1, FEV1%pred, and FEV1%FVC in patients with COPD and pulmonary infection were much lower than in patients with COPD without infection. Similarly, these parameters were lower in patients with COPD compared with healthy individuals. The lung tissues of patients with COPD and pulmonary infection promote the proliferation and metaplasia of bronchial mucosa,24 resulting in increased mucus secretion and sputum volume. The inflammatory reaction produces different degrees of stenosis of the bronchial lumen,25 eventually leading to incomplete bronchial blockage affecting ventilation function. Repeated infection of the respiratory tract can easily damage the alveolar interstitium, making the bronchial and surrounding lung parenchyma lose supporting attachment and weakening respiratory function. Acute exacerbation and repeated pulmonary infection lead to progressive decrease of lung function in patients with COPD.26 Thus, pulmonary infection can seriously reduce lung function in patients with COPD and increase the inflammatory index.
Correlation studies showed that inflammatory indices (WBCs, CRP, IL-6, IL-8, and TNF-α) were negatively correlated with FEV1, FEV1%pred, and FEV1%FVC. These results showed that PII might be associated with lung function in the three groups studied here. However, the specific mechanisms underlying the association between PII and pulmonary function need further study.
Several previous studies reported that levels of the inflammatory cytokine IL-6 increased along with increasing severity of COPD, and were correlated with decreasing quality of life and severity of hypoxia.27 Huang et al. found that plasma inflammatory cytokine levels were related to the severity of airway diseases and could be potential markers for evaluation of asthma, COPD, and asthma-COPD overlap syndrome.10 Our study had the additional benefit of examining healthy persons as a control group and monitoring more diverse inflammatory markers. These indicators are common and routinely examined in hospitals. Thus, our results have clinical significance and can help guide investigations of patients with COPD.
There were two major limitations of our study. First, it was a single-center study, and the sample size was small. Second, additional variables may be needed to better understand the role of inflammatory indices in patients with COPD and pulmonary infection.
Conclusions
The inflammatory response of patients with COPD and pulmonary infection is severe, and lung function and clinical prognosis are worse in these patients. Therefore, timely prevention and intervention strategies should be applied for patients with COPD to avoid pulmonary infection.
Footnotes
Authors’ contributions: JZ and FW, study design and made the decision to submit the article for publication; JZ and FJ, collection, analysis, and interpretation of data; JZ and FJ, conduct of experiments; JZ, FJ and YW, writing of the paper. All authors read and approved the manuscript.
Availability of data and materials: All the data supporting the study findings is contained within the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Chinese National Nature Science Foundation (Grant 81903850) and the 2018 Science Research Foundation of the Affiliated Hospital of Yangzhou University (to J.Z.). The funding bodies had no role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
ORCID iD: Jun Zhou https://orcid.org/0000-0002-5777-2701
References
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 2.Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med 2013; 1: 173–183. [DOI] [PubMed] [Google Scholar]
- 3.Agusti AG, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 347–360. [DOI] [PubMed] [Google Scholar]
- 4.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta–analysis. J Glob Health 2015; 5: 020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 6.Tan DBA, Teo TH, Setiawan AM, et al. Increased CTLA-4 T cells may contribute to impaired T helper type 1 immune responses in patients with chronic obstructive pulmonary disease. Immunology 2017; 151: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polverino F, Seys LJ, Bracke KR, et al. B cells in chronic obstructive pulmonary disease: Moving to center stage. Am J Physiol Lung Cell Mol Physiol 2016; 311: L687–L695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Beha Res Methods 2009; 41: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Li M, Chen J, et al. Smoking status and gene susceptibility play important roles in the development of chronic obstructive pulmonary disease and lung function decline: A population-based prospective study. Medicine (Baltimore) 2017; 96: e7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AX, Lu LW, Liu WJ, et al. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-α levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome. Med Sci Monit 2016; 22: 2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie C, Wen Y, Zhao Y, et al . Clinical features of patients with bronchiectasis with comorbid chronic obstructive pulmonary disease in China. Med Sci Monit 2019; 25: 6805–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celli BR, Anderson JA, Brook R, et al. Serum biomarkers and outcomes in patients with moderate COPD: A substudy of the randomised SUMMIT trial. BMJ Open Respir Res 2019; 6: e000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubini A. Interleukin-6 and lung inflammation: Evidence for a causative role in inducing respiratory system resistance increments. Inflamm Allergy Drug Targets 2013; 12: 315–321. [DOI] [PubMed] [Google Scholar]
- 14.Nakamoto K, Watanabe M, Sada M, et al. Pseudomonas aeruginosa-derived flagellin stimulates IL-6 and IL-8 production in human bronchial epithelial cells: A potential mechanism for progression and exacerbation of COPD. Exp Lung Res 2019; 45: 255–266. [DOI] [PubMed] [Google Scholar]
- 15.Ruso S, Marco FM, Martínez-Carbonell JA, et al. Bacterial vaccines in chronic obstructive pulmonary disease: Effects on clinical outcomes and cytokine levels. APMIS 2015; 123: 556–561. [DOI] [PubMed] [Google Scholar]
- 16.Quan XQ, Xu C, Wang RC, et al. The relationship between Chlamydia pneumoniae infection and CD4/CD8 ratio, lymphocyte subsets in middle-aged and elderly individuals. Microb Pathog 2020; 1419: 104541. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Chen X, Yao W, et al. Dynamic distribution and clinical value of peripheral lymphocyte subsets in children with infectious mononucleosis. Indian J Pediatr 2021; 882: 113–119. [DOI] [PubMed] [Google Scholar]
- 18.Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol 2020; 189: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanguas A, Garasa S, Teijeira Á, et al. ICAM-1-LFA-1 dependent CD8+ T-lymphocyte aggregation in tumor tissue prevents recirculation to draining lymph nodes. Front Immunol 2018; 9: 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotliar C, Juncos L, Inserra F, et al. Local and systemic cellular immunity in early renal artery atherosclerosis. Clin J Am Soc Nephrol 2012; 7: 224–230. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Liu T, Yan Y, et al. The role of Th1/Th2 cytokines played in regulation of specific CD4 Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease. Scand J Immunol 2018; 88: e12674. [DOI] [PubMed] [Google Scholar]
- 22.Onion D, Isherwood M, Shridhar N, et al. Multicomponent analysis of the tumour microenvironment reveals low CD8 T cell number, low stromal caveolin-1 and high tenascin-C and their combination as significant prognostic markers in non-small cell lung cancer. Oncotarget 2018; 9: 1760–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg N, Punch C, Stein M, et al. When Occam's razor can fail – active mycobacteria infection and lung cancer: A case of neuroendocrine lung cancer diagnosed in the setting of refractory Mycobacterium avium-intracellulare. Clin Case Reps 2018; 6: 2156–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puchelle E, Zahm JM, Tournier JM, et al. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3: 726–733. [DOI] [PubMed] [Google Scholar]
- 25.Zeng YY, Hu WP, Zuo YH, et al. Altered serum levels of type I collagen turnover indicators accompanied by IL-6 and IL-8 release in stable COPD. Int J Chron Obstruct Pulmon Dis 2019; 14: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldi S, Jose PE, Bruschi C, et al. The mediating role of cytokine IL-6 on the relationship of FEV1 upon 6-minute walk distance in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014; 9: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghobadi H, Aslani MR, Hosseinian A, et al. The correlation of serum brain natriuretic peptide and interleukin-6 with quality of life using the chronic obstructive pulmonary disease assessment test. Med Princ Pract 2017; 26: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]