Abstract
Objectives
We aimed to determine whether parameters associated with adipose tissue (adipocyte density and the circulating concentrations of markers of adipose tissue pathology) predict cardiovascular risk (CVR) modification after metabolic surgery (MS).
Methods
We performed a case–control study of patients with morbid obesity who were candidates for MS. CVR was defined using flow-mediated dilation (FMD) and carotid intima media thickness (CIMT), which were measured during the 9 months following MS. Subgroups of CVR reduction were defined using the following cut-offs: CIMT 10% and/or a two-fold increase in FMD.
Results
We studied 40 patients with morbid obesity (mean age 44.5 years, 75% women, mean body mass index 46.4 kg/m2) and high prevalences of the metabolically unhealthy obesity phenotype, hypertension, and diabetes mellitus. A significant reduction in CVR was associated with lower vascular endothelial growth factor-A concentration (6.20 vs. 1.59 pg/mL, respectively), low adipocyte density in visceral adipose tissue (100 vs. 80 cells/field), low infiltration with CD68+ cells (18 vs. 8 cells/field) and higher concentrations of lipid peroxidation markers and malondialdehyde (313.7 vs. 405.7 ng/mL).
Conclusion
The characteristics of adipose tissue and the circulating concentrations of markers of adipose pathology might represent useful predictors of the reduction in CVR following MS.
Clinical trial registration number: NCT0356198 (https://clinicaltrials.gov)
Keywords: Metabolic surgery, cardiovascular risk, adipose tissue, morbid obesity, vascular endothelial growth factor A, adipocyte density, malondialdehyde
Introduction
Metabolic surgery (MS) causes weight loss and may ameliorate obesity-related co-morbidities, such as type 2 diabetes mellitus (t2DM), dyslipidaemia, non-alcoholic fatty liver disease, high blood pressure, obstructive sleep apnoea/hypopnoea and/or cardiovascular risk (CVR).1,2 However, the metabolic benefits of MS differ for each patient. Therefore, it is desirable to be able to predict the probability of a reduction in metabolic risk. Parameters such as age, body mass index (BMI), the duration of t2DM, the presence of vascular complications, glycated haemoglobin (HbA1c), and C-peptide have been used to predict the remission of t2DM after MS.3–7
It has previously been shown that age and sex predict the reduction in CVR in patients undergoing MS,8–11 but the precise mechanisms responsible for this remain unclear, and no study to date has been specifically designed to identify potential predictors of CVR reduction following MS.
The CVR associated with obesity is thought to be related to low-grade inflammation and vascular damage induced by oxidative stress. In addition, adipose tissue dysfunction, fat distribution and adipocyte characteristics have been shown to play key roles in the cardiometabolic risk phenotypes known as metabolically unhealthy obesity (MUO) and metabolically healthy obesity (MHO).12,13 It has been hypothesized that intrinsic processes in adipose tissue may be involved in the reduction in CVR that occurs in individuals with obesity following MS. Therefore, markers of such processes may represent predictors of the likely clinical efficacy of MS in individual patients. In the present study, we aimed to determine whether such adipose-related factors would predict the reduction in CVR of patients that undergo MS.
Methods
Study design and setting
We performed a case-control study in the Bariatric Surgery and Clinical Research Departments of the Centro Médico Nacional “20 de Noviembre”, ISSSTE in Mexico City, between January 2016 and December 2018. The recruitment period was from January to September 2016 and the participants were followed up after MS in 2016 and 2017. Data collection and analysis were performed between 2016 and 2018.
Characteristics of and measurements made in the participants
The eligible patients were those who had morbid obesity (BMI ≥40 kg/m2 or BMI ≥35 kg/m2, with obesity-related health conditions, such as t2DM, hypertension, or obstructive sleep apnoea/hypopnoea), and were therefore candidates for MS. All of the participants underwent endoscopic screening for Helicobacter pylori and investigations for endocrine abnormalities and/or psychiatric evaluations to exclude concomitant disorders that might have reduced compliance with therapy following MS. The patients did not undergo weight-reducing therapy during the 6 months prior to enrolment. The exclusion criteria were a second bariatric surgery, inflammatory disease, severe renal and/or hepatic disease, active malignancy, pregnancy, and a history of cardiovascular disease (self-reported or diagnostic evidence of ischemic heart disease, coronary artery disease, myocardial structural abnormality, or cardiac intervention, or treatment for any of these conditions).
A case was defined as a participant who showed a reduction in carotid intima-media thickness (CIMT) of ≥10% and/or a ≥2-fold increase in flow mediated dilation (FMD) during a 9-month follow-up period following MS, and controls were defined as participants who showed a CIMT reduction of <10% and/or a <2-fold increase in FMD, as previously described.10,13 These parameters reflect the magnitude of the changes in endothelial dysfunction and subclinical atherogenesis following MS. The following parameters were retrospectively measured: the circulating adiponectin, leptin tumour necrosis factor (TNF)-α, interleukin (IL)-1β, nitric oxide, malondialdehyde (MDA), vascular cell adhesion molecule (VCAM)-1, soluble intercellular adhesion molecule (sICAM)-1 and vascular endothelial growth factor (VEGF)-A concentrations; adipose tissue area; adipocyte size; and the level of infiltration of adipose tissue with CD45+ and/or CD68+ cells. The interactions of adipose tissue-related parameters with the demographic and anthropometric characteristics of the participants were also evaluated.
For the sample size calculation, we predicted that 35% of participants would show reductions in CVR, based on previous findings,14,15 and used an alpha of 0.05, which yielded a sample size of 14. This was consistent with the number of patients that undergo bariatric surgery during a year, who would also meet the inclusion criteria, but it was only associated with a power of 0.5. However, we considered that this statistical limitation would be outweighed by the potential relevance of the results.
The study protocol complied with the principles of the Declaration of Helsinki and was approved by the Comisiones de Investigación, Ética en Investigación y Bioseguridad en Investigación del Centro Médico Nacional “20 de Noviembre” (approval number 386.2013, 6 November 2013). Written informed consent was obtained from all the participants. The present study is part of the “CROP” study (Biomarkers Derived from Adipose Tissue Useful for the Diagnosis and Prognosis of Cardiovascular Risk in Obese Patients), which is registered at clinical-trials.gov (NCT03561987).
Demographic and anthropometric characteristics of the participants
The baseline demographic and clinical data, including age, sex, height, body mass, the presence of chronic diseases, such as t2DM and hypertension, and the use of medication were obtained from the medical records of the participants. BMI was calculated by dividing body mass by the square of height. Waist circumference was measured according to the World Health Organisation recommendations, between the lowest point of the last rib and the iliac crest.
Metabolic surgery
Sleeve gastrectomy or Roux-en-Y gastric bypass (RYGB) were performed according to the presence of co-morbidities, eating habits, and/or gastrointestinal anatomy. RYGB was preferred for participants with a larger number of such problems.
Measurement of plasma parameters
Blood samples were obtained after 8 hours of fasting by antecubital venepuncture. The samples were immediately centrifuged at 4200 × g for 5 minutes at room temperature to isolate plasma. A complete blood cell count and standard biochemical analyses were performed using an automated analyser (Sysmex XN-1000, Sysmex, Kobe, Japan). The plasma TNF-α, IL-1β, adiponectin, and leptin concentrations were determined using an immunomagnetic multiplexing assay (Milliplex MAP Human TH17 [HTH17MAG-14K] and MAP Human Adipocyte [HADCYMAG-61K] Magnetic Bead Panels, Merck Millipore, Billerica, MA, USA) and a MAGPIX System (40-072–EM, Millipore, Austin, Texas, USA), according to the manufacturer’s instructions. Nitric oxide was measured using a Nitric Oxide Assay Kit (Abcam, Cambridge UK; ab65328), and MDA was reacted with thiobarbituric acid at 95°C and the absorbance of the product was measured at 532 nm. VCAM-1 was measured using a Human VCAM1 ELISA Kit (Abcam; ab100661), sICAM-1 was measured using a Quantikine® Human ICAM-1/CD54 Allele-specific Immunoassay (R&D Systems Inc., Minneapolis, MN, USA), and VEGF-A was measured using a Human VEGF ELISA Kit (Abcam; ab100662).
Analysis of adipose tissue parameters
Fifteen grammes of visceral adipose tissue (VAT) were obtained from the omentum during MS. The tissue was fixed in formalin, paraffin-embedded and sectioned, and the sections were stained with toluidine blue. The adipocyte area was calculated as the mean area of each of the adipocytes contained within five 10× fields per sample. Images were captured using an Olympus CX31 microscope (Tokyo, Japan) and measurements were made using Image Pro-Plus v.5 (Media Cybernetics, Inc., Bethesda, MD, USA).
For immunofluorescence imaging of target protein expression, deparaffinised and rehydrated sections were blocked using albumin for 30 minutes at room temperature and then incubated with monoclonal FITC-conjugated anti-human CD45 antibody (Santa Cruz Biotechnology, Dallas, TX, USA; SC-1187; 1:100, 2 hours at 37°C) or monoclonal mouse anti-human CD68 antibody (Santa Cruz; SC-70761; 1:100, 2 hours at 37°C) and then with FITC-conjugated goat anti-mouse IgG (Santa Cruz; SC-2010; 1:1000, 2 hours). After the addition of 100 µL of Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA; H-1200-10), images were acquired using an inverted fluorescence microscope (Axiovert S100, Zeiss, Oberkochen, Germany).
Measurement of FMD and CIMT
FMD was measured during ultrasonographic imaging using a wireless ultrasound device (Hercules Freedom®, Shenzhen, China) according to the recommendations of the International Brachial Artery Reactivity Task Force.16 CIMT was evaluated using a 4.0 MHz probe (Koninklijke Philips N.V., Amsterdan, the Netherlands), according to the 2004 Mannheim Consensus.17 Briefly, with the patient in a supine position, primary transverse and longitudinal scans of the common carotid artery were performed, focusing on the posterior carotid wall at the carotid bifurcation and the common carotid artery. CIMT was measured approximately 1 cm from the bifurcation of the common carotid artery as the largest distance between the lumen–intima interface and the media–adventitia interface. The results are expressed as the mean of at least four measurements. The reproducibility of the measurements was validated by obtaining an acceptable correlation coefficient (>0.85) for inter-observer reliability.
FMD and CIMT were measured at baseline and 9 months after MS, and the magnitude of the changes were calculated. The participants were allocated to one of two groups, on the basis of a 10% increase in CIMT and/or a 2-fold increase in FMD, in line with published data.15,18
Study endpoints
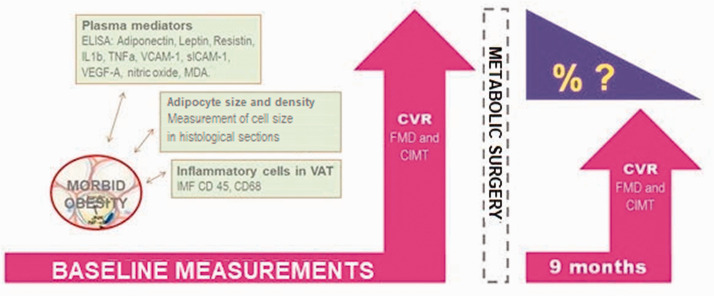
The primary endpoints were the a) adipose tissue parameters and b) circulating concentrations of adipose-related mediators that were associated with a significant reduction in endothelial dysfunction (≥2-fold increase in FMD) and/or subclinical atherogenesis (a reduction in CIMT of ≥10%) following MS. A summary of the study design is provided in Figure 1.
Figure 1.
Study experimental approach. Schematic description of the primary endpoint: 1) adipose tissue components (measurement of cell size in histological sections; and infiltration of inflammatory cells, evaluated by immunofluorescence [IMF] of CD45+, CD68+ cells); and 2) related soluble pro-inflammatory and pro-oxidant mediators, as evaluated by immunoassays. As well as their putative association with the dynamic reduction of cardiovascular risk (CVR) after metabolic surgery, as estimated by change of endothelial dysfunction (measured by flow mediated dilation [FMD]) and subclinical atherogenesis (measured by carotid intima media thickness [CIMT]).
Statistical analysis
The normality of the datasets was checked using the Shapiro–Wilks test. Continuous variables are expressed as medians and interquartile ranges (IQRs) and categorical data as n (%). Two-tailed, Mann–Whitney U and non-paired t-tests were used to analyse the data, as appropriate. Multivariate regression analysis was performed to identify potential confounders and their interactions. Statistical analyses were performed using Prism v. 6.0 (GraphPad, San Diego, CA, USA) and SPSS v. 20 (IBM Corp., Armonk, NY, USA). Statistical significance was accepted if p < 0.05.
Results
Forty-three patients met the selection criteria, but two were excluded because of the withdrawal of informed consent and one because of late surgical complications of the MS. Therefore, the final sample comprised 40 candidates for MS. Their mean age was 44.5 years, 75% were women, and their mean BMI was 46.4 kg/m2. They had high prevalences of hypertension, t2DM, and a MUO phenotype. Their clinical and demographic characteristics are shown in Table 1. The surgical intervention performed was sleeve gastrectomy (n = 14, 35%) or Roux-en-Y Gastric Bypass (RYGB n = 26, 65%). The biochemical profiles of the participants were typical of insulin resistance and high cardiometabolic risk, with high plasma concentrations of insulin, HbA1c, and low-density lipoprotein-cholesterol (LDL-c), as shown in Table 1.
Table 1.
Clinical and demographic characteristics of the participants (n=40)
| Characteristic | |
|---|---|
| Age (years) | 44 (39, 53) |
| Women | 30 (75) |
| Body mass (kg) | 123.5 (110.8, 140.0) |
| Height (cm) | 163.0 (158.3, 170.0) |
| BMI (kg/m2) | 44.9 (40.6, 50.9) |
| SAD (cm) | 133.0 (122.0, 144.0) |
| Comorbidity | |
| HBP | 18 (45.0) |
| HBP + t2DM | 6 (15.0) |
| HBP + Dyslip + t2DM | 4 (10.0) |
| Dyslip | 2 (5.0) |
| t2DM | 1 (2.5) |
| None | 9 (22.5) |
| Metabolic Phenotype | |
| MHO | 14 (35.0) |
| MUO | 26 (65.0) |
| Fasting glucose (mmol/L) | 5.55 (5.22, 6.24) |
| Triglycerides (mmol/L) | 1.34 (0.95, 1.80) |
| Total cholesterol (mmol/L) | 4.55 (3.70, 5.22) |
| HDL-c (mmol/L) | 1.07 (0.88, 1.33) |
| LDL-c (mmol/L) | 2.69 (2.26, 3.40) |
| AST (U/L) | 27.5 (21.5, 36.0) |
| ALT (U/L) | 31.0 (22.5, 46.5) |
| Insulin (mUI/L) | 24.7 (19.4, 38.9) |
| HbA1c (%) | 5.9 (5.6, 6.4) |
Categorical data are shown as n (%) and continuous data as median (IQR).
BMI, body mass index; SAD, sagittal abdominal diameter; HBP, high blood pressure; t2DM, type 2 diabetes mellitus; Dyslip, dyslipidaemia; MHO, metabolically healthy obese; MUO, metabolically unhealthy obese; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HbA1c, glycated haemoglobin.
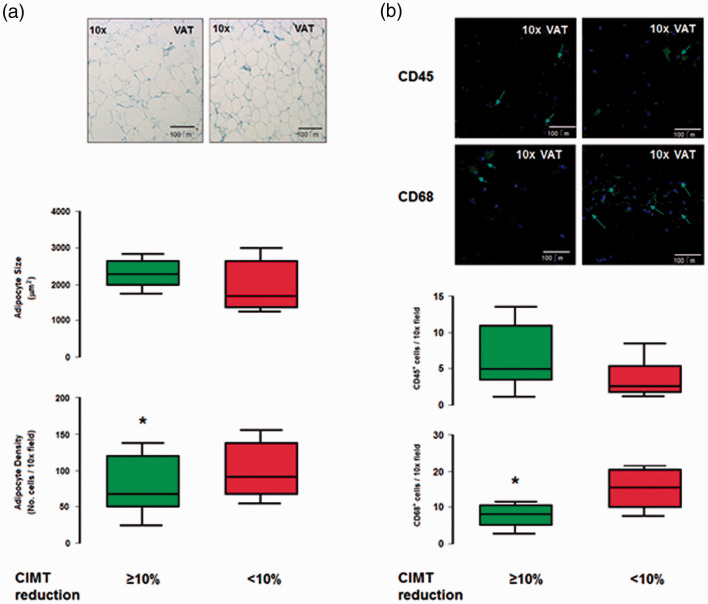
The participants were allocated to two groups according to their changes in CIMT and FMD during the 9 months following bariatric surgery (a reduction of CIMT ≥ or <10%, and ≥ or < a 2-fold increase in FMD) and the two groups were compared, as shown in Table 2. Relative youth was associated with the larger change in CIMT, and lower triglyceride concentration and higher alanine aminotransferase (ALT) activity were associated with the larger change in FMD (all p < 0.05). In addition, there was a lower VEGF-A concentration (6.20 [1.22, 19.23] vs. 1.59 [1.01, 2.66] pg/mL; p < 0.01), lower VAT adipocyte density (100 [70, 130] vs. 80 [50, 120] cells/field; p < 0.05), lower infiltration with CD68+ cells (18 [10, 21] vs. 8 [6, 10] cells/field; p < 0.05), and a higher MDA concentration (313.7 [202.5, 458.2] vs. 405.7 [260.3, 668.1] ng/mL; p < 0.05) in the group with the larger reduction in CIMT (Table 3 and Figure 2); whereas smaller VAT adipocytes alone were associated with the larger increase in FMD (Table 3).
Table 2.
Characteristics of participants who showed larger or smaller changes in cardiovascular risk (n=40)
| Characteristic |
Reduction in CIMT |
Increase in FMD |
||
|---|---|---|---|---|
| ≥10% (n = 28) | <10% (n = 12) | ≥2-fold (n = 25) | <2-fold (n = 15) | |
| Age (years) | 41.0 (29.7, 45.5)* | 48.5 (41.3, 53.2) | 42.0 (35.7, 54.2) | 47.0 (41.0, 53.0) |
| Men | 6.0 (21.4) | 4.0 (33.3) | 7.0 (28.0) | 4.0 (26.7) |
| Body mass (kg) | 125.0 (114.5, 136.6) | 123.0 (106.0, 144.0) | 125.5 (109.0, 142.5) | 123.0 (117.0, 140.0) |
| Height (cm) | 166.5 (160.0, 176.3) | 161.0 (155.5, 168.5) | 163.5 (158.5, 170.3) | 161.0 (155.5, 169.0) |
| BMI (kg/m2) | 43.5 (38.8, 49.3) | 45.1 (41.3, 52.4) | 44.4 (40.8, 48.1) | 47.6 (41.4, 52.2) |
| SAD (cm) | 133.0 (124.5, 137.5) | 132.5 (122.0, 144.6) | 133.0 (121.5, 137.5) | 135.0 (124.5, 146.5) |
| Comorbidity | ||||
| HBP | 13 (46.4) | 5 (41.7) | 10 (40.0) | 7 (46.6) |
| HBP + t2DM | 4 (14.3) | 2 (16.7) | 3 (12.0) | 4 (26.6) |
| HBP + Dyslip + t2DM | 3 (10.7) | 1 (8.3) | 3 (12.0) | 1 (6.7) |
| Dyslip | 1 (3.6) | 1 (8.3) | 1 (4.0) | 1 (6.7) |
| t2DM | 1 (3.6) | 0 (0) | 0 (0.0) | 1 (6.7) |
| None | 6 (21.4) | 3 (25.0) | 8 (32.0) | 1 (6.7) |
| Metabolic Phenotype | ||||
| MHO | 10 (35.7) | 4 (33.3) | 10 (40.0) | 6 (40.0) |
| MUO | 18 (64.3) | 8 (66.7) | 15 (60.0) | 9 (60.0) |
| Glucose (mmol/L) | 5.91 (5.22, 6.46) | 5.53 (5.15, 6.07) | 5.44 (4.94, 6.44) | 5.61 (5.25, 6.05) |
| Triglycerides (mmol/L) | 1.39 (0.87, 1.91) | 1.32 (0.96, 2.17) | 1.27 (0.93, 1.64)* | 1.79 (1.01, 2.62) |
| Cholesterol (mmol/L) | 4.90 (3.96, 5.31) | 4.46 (3.57, 5.11) | 4.45 (3.60, 5.10) | 4.90 (4.23, 5.45) |
| HDL-c (mmol/L) | 1.00 (0.87, 1.32) | 1.07 (0.86, 1.34) | 1.09 (0.91, 1.36) | 0.91 (0.82, 1.28) |
| LDL-c (mmol/L) | 3.09 (2.28, 3.59) | 2.65 (2.25, 3.34) | 2.71 (2.26, 3.41) | 2.81 (2.32, 3.40) |
| AST (U/L) | 25.5 (19.8, 32.0) | 28.5 (23.0, 36.5) | 30.0 (23.0, 40.0) | 26.5 (20.0, 29.0) |
| ALT (U/L) | 25.0 (21.5, 47.0) | 34.5 (24.0, 49.0) | 42.0 (21.0, 61.0)* | 26.5 (22.5, 39.0) |
| Insulin (mIU/L) | 26.4 (13.2, 43.6) | 24.8 (21.2, 39.1) | 26.4 (18.4, 40.5) | 23.9 (18.5, 37.4) |
| HbA1c (%) | 5.9 (5.6, 6.6) | 5.9 (5.6, 6.5) | 5.9 (5.4, 6.5) | 5.9 (5.7, 6.2) |
| Risk factor for atherosclerosis | ||||
| CIMT | ||||
| Pre-surgery | 0.9 (0.8, 1.0)* | 0.7 (0.6, 0.8) | – | – |
| Post-surgery | 0.5 (0.4, 0.7)* | 0.8 (0.7, 1.0) | – | – |
| % age reduction | 33.7 (20.9, 64.6)* | −18.3 (−30.3, −6.48) | – | – |
| FMD | ||||
| Pre-surgery | – | – | 8.0 (4.2, 14.3)* | −3.7 (−17.6, 3.0) |
| Post-surgery | – | – | 34.6 (23.2, 40.0)* | 13.7 (4.0, 27.0) |
| % age increase | – | – | 243.6 (112.0, 587.5)* | −207.4 (−1,354.0, −60.1) |
Categorical data are shown as n (%) and continuous data as median (IQR).
CIMT, carotid intima-media thickness; FMD, flow-mediated dilation; BMI, body mass index; SAD, sagittal abdominal diameter; HBP, high blood pressure; t2DM, type 2 diabetes mellitus; Dyslip, dyslipidaemia; MHO, metabolically healthy obese; MUO, metabolically unhealthy obese; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HbA1c glycated haemoglobin, CIMT, carotid intima-media thickness; FMD, flow-mediated dilation. * p<0.05, two-tailed t-test.
Table 3.
Plasma concentrations of proinflammatory mediators and pro-oxidative factors in participants grouped according to their change in CIMT or FMD (n = 40)
| Pro-inflammatory and pro-oxidative mediators |
Reduction in CIMT |
Increase in FMD |
||
|---|---|---|---|---|
| ≥10% (n = 28) | <10% (n = 12) | ≥2-fold (n = 25) | <2-fold (n = 15) | |
| Adiponectin (pg/mL) | 25.5 (20.1, 29.1) | 27.9 (24.5, 28.8) | 26.6 (22.8, 28.8) | 25.6 (22.4, 27.1) |
| Leptin (ng/mL) | 37.8 (24.3, 52.6) | 43.0 (34.4, 56.2) | 42.1 (19.9, 52.6) | 41.8 (34.9, 52.9) |
| Resistin (ng/mL) | 7.2 (6.3, 8.2) | 7.5 (7.0, 8.1) | 7.3 (5.6, 8.2) | 7.5 (6.4, 8.0) |
| IL1β (pg/mL) | 28.3 (7.3, 63.5) | 31.2 (29.19, 34.31) | 28.7 (24.7, 49.6) | 27.4 (7.33, 35.18) |
| TNF-α (pg/mL) | 17.2 (7.3, 60.7) | 24.18 (15.76, 29.14) | 17.9 (4.5, 30.1) | 15.06 (7.33, 29.16) |
| VCAM-1 (ng/mL) | 0.97 (0.69, 1.14) | 0.83 (0.60, 1.25) | 0.83 (0.60, 1.10) | 0.98 (0.81, 1.16) |
| sICAM-1 (ng/mL) | 1.14 (0.78, 1.55) | 0.99 (0.73, 1.28) | 1.08 (0.75, 1.65) | 0.90 (0.71, 1.10) |
| VEGF-A (pg/mL) | 1.59 (1.01, 2.66)* | 6.20 (1.22, 19.23) | 1.69 (0.99, 4.60) | 1.80 (1.25, 13.09) |
| Nitric oxide (µmol/L) | 297.6 (205.5, 313.6) | 297.8 (278.3, 328.2) | 303.0 (292.5, 318.0) | 273.3 (204.6, 327.6) |
| MDA (µmol/L) | 405.7 (260.3, 668.1)* | 313.7 (202.5, 458.2) | 397.4 (208.3, 642.9) | 391.0 (260.3, 497.2) |
| AdipS VAT (µm2×10−3) | 2.1 (1.9, 2.5) | 1.6 (1.3, 2.5) | 1.8 (1.4, 2.2)* | 2.4 (1.9, 2.7) |
| AdipTA VAT (cm2) | 193.1 (153.7, 254.9) | 162.1 (130.6, 229.0) | 170.4 (135.8, 264.0) | 195.7 (148.8, 260.2) |
Categorical data are shown as n (%) and continuous data as median (IQR).
IL-1β interleukin 1β TNF-α tumour necrosis factor α; VCAM-1, vascular cell adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; VEGF-A, vascular endothelial growth factor-A; MDA, malondialdehyde; AdipS, adipocyte size; VAT, visceral adipose tissue; AdipTA, adipose tissue area, CIMT, carotid intima-media thickness; FMD, flow-mediated dilation. * p<0.05, two-tailed t-test.
Figure 2.
Adipocyte size, density and adipose tissue inflammatory infiltrate. The figure compares a. typical adipocyte morphology in VAT (toluidin blue, light microscopy); as well as cell surface size and density (whisker box below), between cases who developed 10% CIMT reduction or not. b. Immunofluorescent identification of CD45+ and CD68+ inflammatory cells infiltrating VAT, and whisker box below, comparing cases according 10% CIMT reduction. Two-tailed, U-Mann?Whitney and non-paired t-test were used as appropriate. (*) = p<0.05. Abbreviates: VAT, visceral adipose tissue; CIMT, carotid intima media thickness.
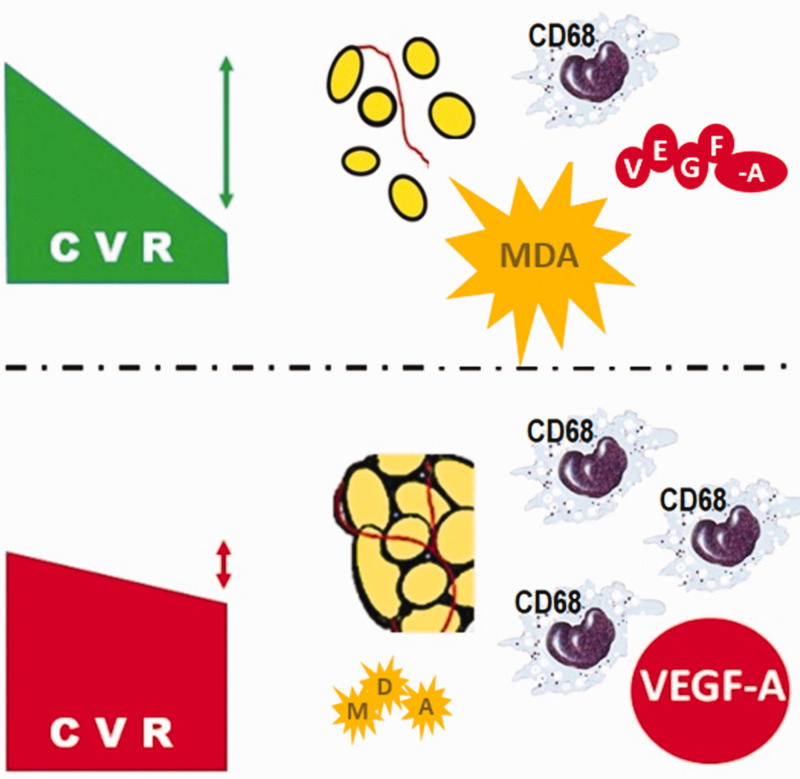
Figure 3.
(*) Adjusted by sex and type of surgery. Lower panel shows a proposed adipo-vascular dynamics model to explain effects of bariatric and metabolic surgery on cardiovascular risk, based on the interaction between adipocyte size, local infiltration of CD68+ cells and soluble vascular factors and mediators of oxidative stress, during reduction of cardiovascular risk.
Finally, the interactions between leptin concentration, VAT adipocyte size and/or VCAM-1 concentration were significantly and independently associated with the reduction in CIMT (Table 4), and these interactions also tended to be associated with the increase in FMD (Table 4).
Table 4.
Associations of the measured variables with the reduction in CIMT and the increase in FMD
|
≥10% CIMT reduction |
≥2-fold FMD increase |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Leptin | 4.5 (0.7–28.1) | 0.11 | 0.30 (0.05–1.5) | 0.15 |
| Leptin + Adipocyte size | 22.6 (1.3–381.2) | 0.03 | 0.28 (0.04–2.2) | 0.23 |
| Leptin + Adipocyte size + VCAM-1 | 23.7 (1.1–499.4) | 0.04 | 0.106 (0.01–1.64) | 0.10 |
| * Model 2 Adjusted | 33.2 (1.2–862.6) | 0.03 | 0.061 (0.01–2.83) | 0.15 |
The data are the results of multivariate regression analysis. (*) Adjusted by sex and type of surgery.
CIMT, carotid intima-media thickness; FMD, flow-mediated dilation; OR, odds ratio; CI, confidence interval; VCAM-1, vascular cell adhesion molecule 1.
Discussion
The principal findings of the present study were that the reduction in CVR following bariatric surgery could be predicted by adipocyte size, adipose tissue vascularization pattern, and the plasma concentrations of leptin and VEGF-A.
The characteristics of the present study sample are comparable to those of the populations studied previously; therefore, our findings may be able to extrapolated to other similar populations.18,19
The findings that adipocyte size and leptin concentration are associated with the reduction in CVR following MS are consistent with those of previous studies, and are consistent with effects of adipose tissue on CVR in individuals who undergo bariatric surgery.10,13,20,21 However, variable effects of adipose tissue on CIMT have been identified,20 which may be explained by differences in the characteristics of the study population, the study design and the time period between MS and the measurement of CIMT.
A low plasma triglyceride concentration was also associated with a ≥2-fold increase in FMD following MS, which is consistent with the results of previous studies, which showed a relationship between the transendothelial transport of triglycerides and endothelial function.22,23 In addition, a high MDA concentration, reflecting oxidative stress, was associated with a ≥10% reduction in CIMT following bariatric surgery, and a similar result has been published with respect to diabetes remission following MS.24 Although oxidative stress is associated with vascular dysfunction, the high MDA and low VEGF-A concentrations were not associated with endothelial dysfunction (FMD), but instead subclinical atherogenesis; this may be explained by medium-term vascular damage induced by the higher oxygen consumption linked to the catabolic state induced by MS.
The present results suggest that the vascular density of adipose tissue and plasma VEGF-A concentration predict the reduction in CVR following bariatric surgery. The vascular pattern of adipose tissue and lipid metabolism may influence CVR through various mechanisms. First, inflammation in vascular tissues is considered to be a key pathophysiological factor in cardiometabolic risk in individuals with obesity25 and a close relationship between vascularization and adipose tissue has been demonstrated in experimental studies.26 Second, recent studies have shown that VEGF expression in adipose tissue and the pattern of vascularization are related to the adipose tissue dysfunction that is present in obesity and insulin resistance.27–29 Third, vascularization is linked to the degree of fat infiltration by inflammatory cells, which is associated with the release of proinflammatory mediators,30–32 such as TNF-α and IL-6, which may stimulate the hepatic production of C-reactive protein. Finally, high concentrations of adipose-derived free fatty acids in the portal circulation trigger the synthesis of very low-density lipoprotein, an increase in gluconeogenesis and lower clearance of insulin.10,32
Taken together, the present findings suggest a mechanistic model in which adipose tissue, intrinsic vascular pattern and angiogenic factors interact to modify CVR following MS (Table 4). Furthermore, these factors may represent useful biomarkers of the response to MS, as also suggested by the results of published meta-analyses.33,34
To our knowledge, this was the first study to use a longitudinal approach to study the impact of MS in patients with morbid obesity on CVR. However, some limitations of the study should be acknowledged. First, the study was relatively small and the participants were heterogeneous in their metabolic risk phenotype. Second, we did not compare the vascular patterns of subcutaneous and visceral adipose tissue.
Conclusion
Biomarkers such as adipocyte size, adipose tissue vascularization pattern, and the plasma concentrations of leptin and VEGF-A may predict the reduction in CVR following MS.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Alberto Melchor-López received a grant from Consejo Nacional de Ciencia y Tecnología (CONACYT; No. CVU 300012) during his PhD studies in the Programa de Maestría y Doctorado en Ciencias Médicas, Odontológicas y de la Salud – Universidad Nacional Autónoma de México. The authors also acknowledge support from grant SALUD-2015-1-262335 from Fondo Sectorial de Investigación en Salud, CONACYT, and the E015 Program at Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado.
ORCID iDs: Juan Antonio Suárez-Cuenca https://orcid.org/0000-0002-2098-5658
Gustavo De la Peña-Sosa https://orcid.org/0000-0002-6350-5845
Carlos Ramiro Zamora-Alemán https://orcid.org/0000-0002-0316-9552
References
- 1.Brolin RE, Kenler HA, Wilson AC, et al. Serum lipids after gastric bypass surgery for morbid obesity. Int J Obes 1990; 14: 939–950. [PubMed] [Google Scholar]
- 2.Brolin RE, Bradley LI, Wilson AC, et al. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000; 4: 464–469. [DOI] [PubMed] [Google Scholar]
- 3.Ahuja A, Tantia O, Chaudhuri T, et al. Predicting remission of diabetes post metabolic surgery: a comparison of ABCD, diarem, and DRS scores. Obes Surg 2018; 28: 2025–2031. [DOI] [PubMed] [Google Scholar]
- 4.Aigner F, Patsch JR. Markers of chronic inflammation and obesity: a prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes Relat Metab Disord 2002; 26: 659–662. [DOI] [PubMed] [Google Scholar]
- 5.Kopp HP, Kopp CW, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol 2003; 23: 1042–1047. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez LA, Pazos F, Berrazueta JR, et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab 2005; 90: 316–322. [DOI] [PubMed] [Google Scholar]
- 7.Vilarrasa N, Vendrell J, Sanchez-Santos R, et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, c-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol (Oxf) 2007; 67: 679–686. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Ruiz KL, Ferreira-Hermosillo A, Molina-Ayala MA, et al. Evaluation of cardiovascular risk factors in obesity before and after bariatric surgery. Rev Med Inst Mex Seguro Soc 2017; 55: 556–567. [PubMed] [Google Scholar]
- 9.Michalsky MP, Inge TH, Jenkins TM, et al. Cardiovascular Risk Factors after Adolescent Bariatric Surgery. Pediatrics 2018; 141: e20172485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kardassis D, Schönander M, Sjöström L, et al. Carotid artery remodelling in relation to body fat distribution, inflammation and sustained weight loss in obesity. J Intern Med 2014; 275: 534–543. [DOI] [PubMed] [Google Scholar]
- 11.Sturm W, Tschoner A, Engl J, et al. Effect of bariatric surgery on both functional and structural measures of premature atherosclerosis. Eur Heart J 2009; 30: 2038–2043. [DOI] [PubMed] [Google Scholar]
- 12.Bala C, Craciun AE, Hancu N. Updating the concept of metabolically healthy obesity. Acta Endocrinol (Buchar) 2016; 12: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupoli R, Nicola MD, Guidone C, et al. Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: a meta-analysis of literature studies. Int J Obesity 2016; 40: 395–402. [DOI] [PubMed] [Google Scholar]
- 14.Wei JH, Chou RH, Huang PH, et al. Metabolic surgery ameliorates cardiovascular risk in obese diabetic patients: Influence of different surgical procedures. Surg Obes Relat Dis 2018; 14: 1832–1840. [DOI] [PubMed] [Google Scholar]
- 15.Jonker FHW, Van Houten VAA, Wijngaarden LH, et al. Age-Related Effects of Bariatric Surgery on Early Atherosclerosis and Cardiovascular Risk Reduction. Obes Surg 2018; 28: 1040–1046. Erratum in: Obes Surg 2018; 28: 1809. [DOI] [PubMed] [Google Scholar]
- 16.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol 2002; 39: 257–265. [DOI] [PubMed] [Google Scholar]
- 17.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis 2004; 18: 346–349. [DOI] [PubMed] [Google Scholar]
- 18.Gluszewska A, Gryglewska B, Rewiuk K, et al. Arterial structure and function and its short- and long-term changes after bariatric surgery. J Physiol Pharmacol 2019; 70: 909–916. [DOI] [PubMed] [Google Scholar]
- 19.Boesing F, Moreira EA, Wilhelm-Filho D, et al. Roux-en-Y bypass gastroplasty: markers of oxidative stress 6 months after surgery. Obes Surg 2010; 20: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 20.Hanusch-Enserer U, Zorn G, Wojta J, et al. Non-conventional markers of atherosclerosis before and after gastric banding surgery. Eur Heart J 2009; 30: 1516–1524. [DOI] [PubMed] [Google Scholar]
- 21.Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance? Clin Sci (Lond) 2016; 130: 1603–1614. [DOI] [PubMed] [Google Scholar]
- 22.Hagberg CE, Falkevall A, Wang X, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 2010; 464: 917–921. [DOI] [PubMed] [Google Scholar]
- 23.Jang SY, Kim SM, Sung J, et al. Coronary artery calcium scores and cardiovascular risk factors in 31,545 asymptomatic Korean adults. Int J Cardiovasc Imaging 2016; 32: 139–145. [DOI] [PubMed] [Google Scholar]
- 24.Nosso G, Lupoli R, Saldalamacchia G, et al. Diabetes remission after bariatric surgery is characterized by high glycemic variability and high oxidative stress. Nutr Metab Cardiovasc Dis 2017; 27: 949–955. [DOI] [PubMed] [Google Scholar]
- 25.Lovren F, Teoh H, Verma S. Obesity and Atherosclerosis: Mechanistic Insights. Canadian J Cardiol 2015; 31: 177–183. [DOI] [PubMed] [Google Scholar]
- 26.Ledoux S, Queguiner I, Msika S, et al. Angiogenesis Associated with Visceral and Subcutaneous Adipose Tissue in Severe Human Obesity. Diabetes 2008; 57: 3247–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosanská L, Michalský D, Lacinová Z, et al. The Influence of Obesity and Different Fat Depots on Adipose Tissue Gene Expression and Protein Levels of Cell Adhesion Molecules. Physiol Res 2010; 59: 79–88. [DOI] [PubMed] [Google Scholar]
- 28.Ferri C, Desideri G, Valenti M, et al. Early Upregulation of Endothelial Adhesion Molecules in Obese Hypertensive Men. Hypertension 1999; 34: 568–573. [DOI] [PubMed] [Google Scholar]
- 29.Spencer M, Unal R, Zhu B, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 2011; 96: E1990–E1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer M, Yang L, Adu A, et al. Pioglitazone treatment reduces adipose tissue inflammation through reduction of mast cell and macrophage number and by improving vascularity. PLoS One 2014; 9: e102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Jemtel TH, Samson R, Milligan G, et al. Visceral Adipose Tissue Accumulation and Residual Cardiovascular Risk. Curr Hypertens Rep 2018; 20: 77. [DOI] [PubMed] [Google Scholar]
- 32.Suárez-Cuenca JA, Ruíz-Hernández AS, Mendoza-Castañeda AA, et al. Neutrophil-to-lymphocyte ratio and its relation with pro-inflammatory mediators, visceral adiposity and carotid intima-media thickness in population with obesity. Eur J Clin Invest 2019; 49: e13085. [DOI] [PubMed] [Google Scholar]
- 33.Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res 2012; 61: 789–807. [DOI] [PubMed] [Google Scholar]
- 34.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724–1737. [DOI] [PubMed] [Google Scholar]