Abstract
We analyzed the utility for a clot waveform analysis (CWA) of small tissue factor induced FIX activation (sTF/FIXa) assay in patients with major orthopedic surgery (including total hip arthroplasty [THA] and total knee arthroplasty [TKA]) receiving edoxaban for the prevention of venous thromboembolism (VTE). The sTF/FIXa assay using recombinant human TF in platelet-rich plasma (PRP) and platelet-poor plasma (PPP) was performed using a CWA in the above patients to monitor the efficacy of edoxaban administration. Of 147 patients (109 THA and 38 TKA), 21 exhibited deep vein thrombosis (DVT), and 15 had massive bleeding. Increased peak heights of the CWA-sTF/FIX were observed in almost patients after surgery and prolonged peak heights of the CWA-sTF/FIX were observed in almost patients treated with edoxaban. The peak heights and times of the CWA-sTF/FIX were significantly higher and shorter, respectively, in PRP than in PPP. There were no significant differences in parameters of the CWA-sTF/FIXa between the patients with and without DVT or between those with and without massive bleeding. The peak time of CWA-sTF/FIXa were significantly longer in TKA patients than in THA patients on day 1 after surgery. The second derivative peak height of the CWA-sTF/FIXa was significantly lower in TKA patients than in THA patients on day 4. The CWA-sTF/FIX reflected hemostatic abnormalities after surgery and the administration of edoxaban, and the results were better in PRP than PPP. Further studies separately analyzing the THA and TKA subgroups should be conducted.
Keywords: sTF/FIXa, APTT, CWA, edoxaban, orthopedic surgery
Introduction
Major orthopaedic surgery, such as total hip arthroplasty (THA) and total knee arthroplasty (TKA), carries a high risk of venous thromboembolism (VTE),1,2 including pulmonary embolism (PE) and deep vein thrombosis (DVT). PE caused by postoperative DVT3,4 has a sudden onset and can be fatal. Most patients who undergo a major orthopedic surgery, are preventively treated with low-molecular-weight heparin (LMWH),5 fondaparinux6 and direct oral anticoagulants (DOACs),7,8 such as apixaban, edoxaban and rivaroxaban. Their association with massive bleeding sometimes results in the withdrawal of these drugs,9 although they are generally considered to have a low frequency of massive bleeding. These drugs are usually difficult to monitor by routine coagulation tests via activated partial thromboplastin time (APTT) or prothrombin time (PT). Although anti-Xa assays have recently been developed to monitor LMWH and DOACs,9-11 these assays are not routine and are more expensive to conduct than measurements of APTT or PT.
A clot waveform analysis (CWA)12 evaluating the APTT (APTT-CWA) was previously reported to be useful for diagnosing disseminated intravascular coagulation (DIC)13 and measuring the levels of FVIII.14 Automatic optical coagulation analyzers using a CWA have been developed, describing the first and second derivative peaks (DPs) correspond to the velocity and acceleration, respectively.12 Thus, a CWA can contribute to the differential diagnosis of bleeding disorders and help monitoring supplementary treatments or anticoagulant therapies.15-18
The physiological blood coagulation system starts after small amounts of tissue factor (TF) and activated clotting factor VII (FVIIa) activate clotting factor Ⅸ (FⅨ) on the phospholipids (PLs) of the platelet membrane. Routine assays, such as measurement of the APTT and PT cannot sufficiently reflect the activation of FⅨ by a small amount of TF (sTF). Furthermore, the APTT and PT cannot reflect the effect of platelets on blood coagulation as accurately as thromboelastography.19 Previous studies20,21 have shown that a CWA using sTF without commercial PLs (CWA-sTF/FIXa) activated FⅨ (FIXa), but not FX, and a shorter peak time and higher peak height of the CWA-sTF/FIX were observed in platelet-rich plasma (PRP) than in platelet-poor plasma (PPP). Furthermore, the peak time and height depended on the platelet count in PRP.21
In the present study, the results of the sTF-induced FIX activation assay in PRP using a CWA (CWA-sTF/FIXa) were compared with those of the routine CWA-APTT in PPP in major orthopedic surgery patients treated with edoxaban.
Materials and Method
Patients
One hundred and forty-seven patients (109 women and 38 men; mean age, 67.2 years (standard deviation ±10.6 years) presenting for THA and TKA at Mie University Hospital from February 1, 2019, to April 31, 2020, and receiving edoxaban 30 mg (Daiichi-Sankyo, Tokyo, Japan) once daily for VTE prophylaxis were enrolled in the study.
Screening for DVT was performed by a whole-leg compression ultrasound examination using the standardized ultrasound criteria for venous non-compressibility before the operation, and on Days 4 and 14 after the operation.22 The study protocol was approved by the Human Ethics Review Committee of the Mie University School of Medicine, and a signed consent form was obtained from each subject. This study was faithfully carried out in accordance with the principles of the Declaration of Helsinki. Blood was drawn in the morning and 1 h after drug intake on Day 1.
Anti-Xa Activity
The anti-Xa activity was measured prospectively 1 h after drug intake on Day 1. The anti-Xa activity of edoxaban was measured using HemosIL® Liquid Heparin (Instrumentation Laboratory; Bedford, MA, USA) on ACL-TOP® (Instrumentation Laboratory).
The APTT Assay
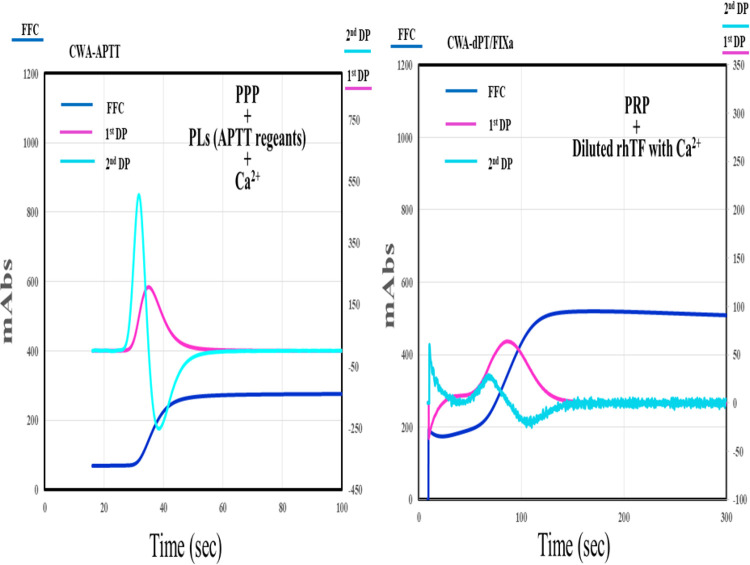
The APTT-CWA was performed using APTT-SP®, which uses silica as an activator of FXII and synthetic PLs (Instrumentation Laboratory) and PL, with an ACL-TOP® system (Instrumentation Laboratory) as previously reported.12,16 The CWA-sTF/FIXa was performed using PRP and 2,000-fold diluted HemosIL RecombiPlasTin 2G (TF concentration <0.1 pg/ml; Instrumentation Laboratory). Three types of curves are shown on the monitor of this system.12 One curve shows the changes in the absorbance observed while measuring the APTT, corresponding to fibrin formation (FF). The second (1st DP) corresponds to the coagulation velocity, and the third (2nd DP) corresponds to the coagulation acceleration. The height and time of the 1st DP, 2nd DP and FF are called the 1st DPH and 1st DPT, 2nd DPH and 2nd DPT, and FFH and FFT, respectively (Figure 1). PRP was prepared by centrifugation at 900 rpm for 15 minutes (platelet count, 40 × 1010/L), and PPP was prepared by centrifugation at 3,000 rpm for 15 minutes (platelet count, <0.5 × 1010/L).21
Figure 1.
A clot waveform analysis of APTT and sTF/FIXa in healthy volunteers. CWA indicates clot waveform analysis; sTF/FIXa, small amount of tissue factor induced FIX activation; APTT, activated partial thromboplastin time; PRP, platelet rich plasma; PPP, platelet poor plasma; navy blue line, fibrin formation curve; pink line, 1st derivative curve; light blue line, 2nd derivative curve; PLs, phospholipids; rhTF, recombinant human tissue factor.
Statistical Analyses
The data were expressed as the median (25-75th percentile). Differences between groups were examined for significance using the Mann-Whitney U test. P-values of ≤0.05 were considered to indicate statistical significance. The correlation was analyzed by Spearman’s rank correlation coefficient. A receiver operating curve analysis was performed to determine the cutoff value, sensitivity, and odds ratio.
All statistical analyses were performed using the Stat Flex software program (version 6. Artec Co Ltd, Osaka, Japan).
Results
One hundred and forty-seven orthopedic patients (109 THA and 38 TKA cases) treated with edoxaban (Daiichi-Sankyo, Tokyo, Japan) and intermittent pneumatic compression for DVT prophylaxis were enrolled in this study. These patients received 30 mg of edoxaban by oral administration once a day for 14 days beginning more than 2 hours after the discontinuation of lumbar anesthesia. Of these 147 patients, 21 exhibited DVT, and 15 had massive bleeding, defined as a reduction in the hemoglobin level by ≥2 g/dl compared with that at Day 1 or more than 4 units of packed red blood cell transfusion.10
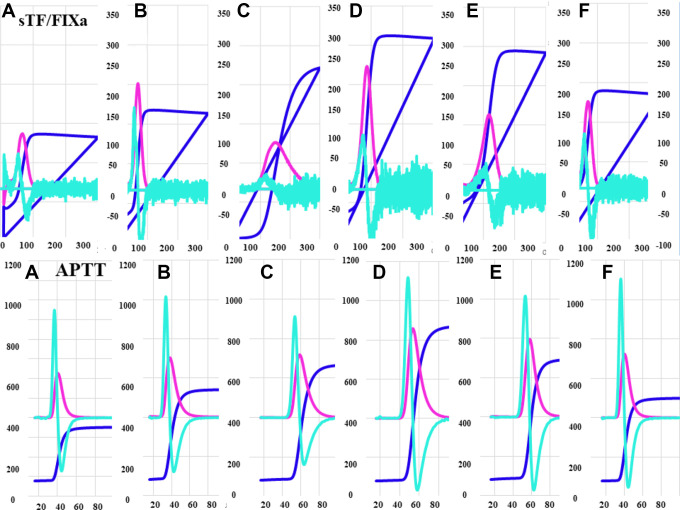
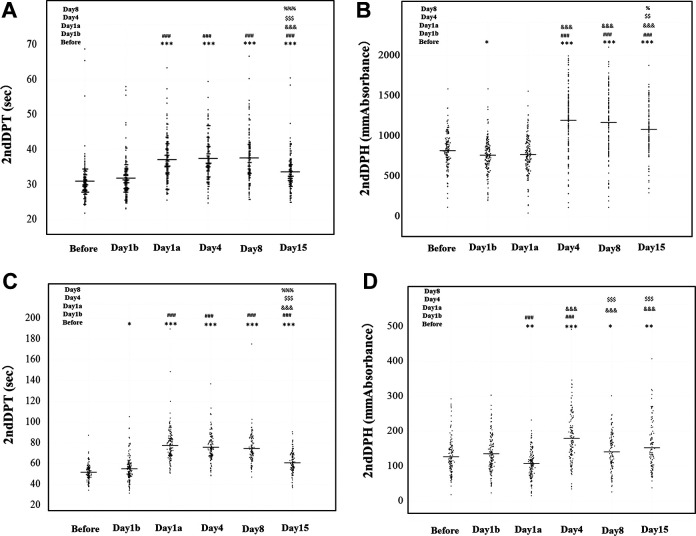
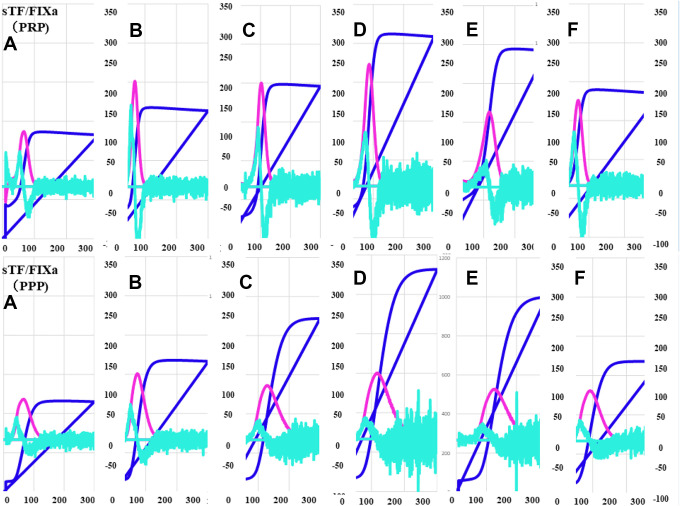
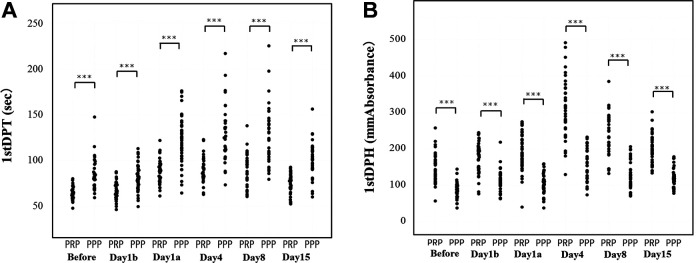
The typical patterns of the CWA-APTT and CWA-sTF/FIXa in these patients before the operation, and before and after the administration of edoxaban (day 1, day 4, day 8 and day 15) are shown in Figures 2 to 4. The 3 peak heights of the CWA-APTT and CWA-sTF/FIXa were significantly higher on days 4, 8 and 15 than before the operation, and the 1st DPH of the CWA-sTF/FIXa was significantly higher on day 1 than before the operation. The 3 peak time of the CWA-APTT and CWA-sTF/FIXa were significantly longer on days 1a, 4, 8 and 15 than before the operation (Figure 2 and Table 1). The 2nd DPT of the CWA-APTT and CWA-sTF/FIXa were significantly longer on days 1a, 4, 8 and 15 than on day 1b. The 2nd DPH of the CWA-APTT was significantly higher on days 4, 8 and 15 than on day 1b. The 2nd DPH of the CWA-sTF/FIXa was significantly lower on day 1a but significantly higher on day 4 than on day 1b (Figure 3). The coefficients of variation (CV) of the CWA-APTT and -sTF/FIXa were 55.6 s and 21.7 s, respectively, in 2nd DPT, 53.6 s and 22.6 s in 1st DPT, and 50.0 s and 22.1 s in FFT. The peak heights of 1st DP and 2nd DP of the CWA-sTF/FIXa were significantly higher in PRP than in PPP, and the peak times of 1st DP and 2nd DP of the CWA-sTF/FIXa were significantly shorter in PRP than in PPP (Figures 4 and 5 and Table 1).
Figure 2.
A clot waveform analysis for sTF/FIXa or APTT in comparison between sTF/FIXa and APTT: (A) before operation; (B) day 1 after operation; (C) 1 h after administration of edoxaban on day 1; (D) day 4 after operation; (E) day 8 after operation; (F) day 15 after operation. sTF/FIXa indicates small amount of tissue factor induced FIX activation; APTT, activated partial thromboplastin time; PRP, platelet rich plasma; PPP, platelet poor plasma; navy blue line, fibrin formation curve; pink line, 1st derivative curve; light blue line, 2nd derivative curve.
Figure 3.
A clot waveform analysis for APTT or sTF/FIXa: (A) 2nd derivative peak time (DPT) of APTT; (B) 2nd derivative peak height (DPH) of APTT; (C) 2nd DPT of sTF/FIXa; (D) 2nd DPH of sTF/FIX. ***, ###, &&&, $$$, %%% P < 0.001; **, ##, &&, $$, %% P < 0.01; *, #, &, $, % P < 0.05. APTT indicates activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor induced FIX activation.
Figure 4.
A clot wave analysis for sTF/FIXa in comparison between PRP and PPP: (A) before operation; (B) day 1 after operation; (C) 1 h after administration of edoxaban on day 1; (D) day 4 after operation; (E) day 8 after operation; (F) day 15 after operation. sTF/FIXa indicates small amount of tissue factor induced FIX activation; APTT, activated partial thromboplastin time; PRP, platelet-rich plasma; PPP, platelet-poor plasma; navy blue line, fibrin formation curve; pink line, 1st derivative curve; light blue line, 2nd derivative curve.
Table 1.
sTF/FIX Assays Between PRP and PPP.a
| Pre | Day 1b | Day 1a | Day 4 | Day 8 | Day 15 | ||
|---|---|---|---|---|---|---|---|
| 2nd Derivative Peak Time (sec) | PRP | 51.8 (48.3-56.1) | 56.1 (49.9-60.6) | 76.9 (67.3-83.2)b | 73.6 (68.6-84.0)b | 77.5 (65.2-90.2)b | 63.9 (57.7-69.7)b |
| P | <0.01 | NS | <0.01 | <0.01 | <0.01 | <0.01 | |
| PPP | 58.6 (50.8-66.3) | 58.7 (49.3-67.4) | 88.5 (72.8-103.6)b | 89.3 (78.4-100.1)b | 95.5 (77.2-107.6)b | 73.4 (62.3-83.0)b | |
| 2nd Derivative Peak Height (mm Absorbance) | PRP | 114 (90.9-152) | 135 (104-176) | 104 (79.9-131) | 163 (111-211)b | 133 (94.2-161) | 148 (99.8-184) |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| PPP | 44.0 (34.0-62.4) | 58.2 (48.4-77.8)c | 35.9 (26.7-43.7)c | 49.4 (36.6-67.6) | 114 (94.9-167) | 107 (96.8-145) | |
| 1st Derivative Peak Time (sec) | PRP | 62.9 (58.7-68.6) | 65.4 (60.6-73.8) | 88.2 (77.8-96.3)b | 87.0 (81.5-107)b | 94.1 (78.5-103)b | 77.9 (69.6-84.3)b |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| PPP | 81.7 (72.7-92.5) | 81.1 (70.4-91.1) | 124 (104-138)b | 125 (111-143)b | 127 (104-144)b | 102 (91.1-112)b | |
| 1st Derivative Peak Height (mm Absorbance) | PRP | 143 (115-172) | 185 (144-203)c | 185 (158-228)b | 309 (256-367)b | 241 (200-283)b | 196 (164-225)b |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| PPP | 84.4 (68.7-100) | 113 (99.3-127)b | 107 (90.2-115)b | 154 (118-198)b | 121 (100-143)b | 118 (102-130)b | |
| Fibrin Formation Time (sec) | PRP | 61.3 (58.3-68.6) | 66.1 (60.6-74.5)d | 89.3 (78.6-96.0)b | 88.0 (82.1-102)b | 91.9 (78.7-99.2)b | 77.1 (68.5-82.4)b |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| PPP | 87.1 (76.1-96.1) | 85.8 (75.7-95.3) | 131 (112-146)b | 136 (117-154)b | 135 (112-153)b | 109 (96.2-116)b | |
| Fibrin Formation Height (mm Absorbance) | PRP | 339 (300-378) | 345 (297-369) | 418 (376-462)b | 675 (608-739)b | 578 (502-636)b | 501 (451-580)b |
| P | <0.001 | <0.05 | NS | NS | NS | <0.001 | |
| PPP | 250 (225-296) | 312 (264-345)b | 411 (381-480)b | 665 (620-722)b | 552 (473-613)b | 407 (343-494)b |
Abbreviations: sTF/FIX, small tissue factor induced FIX activation; PRP, platelet-rich plasma; PPP, platelet-poor plasma; NS, not significant.
a P values indicate the difference between PRP and PPP.
b P < 0.001 in comparison with “pre.”
c P < 0.01 in comparison with “pre.”
d P < 0.05 in comparison with “pre.”
Figure 5.
Clot waveform analysis for sTF/FIXa in PRP and PPP: (A) 1st derivative peak time (1st DPT); (B) 1st derivative peak height (1st DPH). sTF/FIXa indicates small amount of tissue factor induced FIX activation; PRP, platelet rich plasma; PPP, platelet poor plasma.
The correlation between the anti-Xa activity and the peak time and height of 1st DP and 2nd DP of the CWA-sTF/FIXa on days 1, 4 and 8 were low (Table 2). There were no significant differences in any parameters of the CWA-APTT and CWA-sTF/FIXa between these patients with and without DVT or between patients with and without massive bleeding. The CV (10.0 s in 2nd DPT, 8.8 s in 1st DPT, and 8.8 s in FFT) of the peak time of the CWA-sTF/FIXa was small in the patient with massive bleeding and the adequate cutoff value for massive bleeding was 86.3 s in 2nd DPT (sensitivity 92.9% and odds ratio, 4.33), 97.9 s in 1st DPT (sensitivity 92.9%; odds ratio, 5.06), and 98.3 s in FFT (sensitivity 92.9%; odds ratio, 5.05). Three peak time of the CWA-sTF/FIXa were significantly longer in TKA patients than in THA patients on day 1a. The 2nd DPH of the CWA-sTF/FIXa was significantly lower in TKA patients than in THA patients on day 4 (Table 3).
Table 2.
Correlation Between the Anti-Xa Activity and Parameters of sTF/FIXa.
| Day 1 | Day 4 | Day 8 | |
|---|---|---|---|
| 2nd DPT | r = 0.0182 (P = 0.8334) | r = −0.1003 (P = 0.2859) | r = 0.0900 (P = 0.4185) |
| 2nd DPH | r = −0.0828 (P = 0.3381) | r = 0.0406 (P = 0.6664) | r = −0.1385 (P = 0.2117) |
| 1st DPT | r = 0.0459 (P = 0.5942) | r = −0.0823 (P = 0.3801) | r = 0.1090 (P = 0.3237) |
| 1st DPH | r = −0.0793 (P = 0.3572) | r = 0.0631 (P = 0.5009) | r = −0.0364 (P = 0.7241) |
Abbreviations: DPT, derivative peak time; DPH, derivative peak height; sTF/FIX, small tissue factor induced FIX activation.
Table 3.
sTF/FIX Assays Between THA and TKA.
| Pre | Day 1b | Day 1a | Day 4 | Day 8 | Day 15 | ||
|---|---|---|---|---|---|---|---|
| 2nd Derivative Peak Time (sec) | THA | 51.2 (46.8-55.2) | 54.2 (48.2-59.7) | 72.3 (67.7-84.4) | 74.1 (67.8-84.0) | 74.0 (66.3-85.2) | 58.7 (53.8-67.4) |
| P | NS | NS | <0.05 | NS | NS | NS | |
| TKA | 51.9 (48.2-56.3) | 56.5 (51.3-59.9) | 81.9 (73.0-87.5) | 73.7 (67.3-81.7) | 71.4 (66.6-77.8) | 60.9 (56.0-67.7) | |
| 2nd Derivative Peak Height (mm Absorbance) |
PRP | 115 (91.1-152) | 132 (101-174) | 109 (83.3-133) | 183 (140-239) | 137 (102-181) | 139 (101-203) |
| P | NS | NS | NS | <0.05 | NS | NS | |
| PPP | 120 (79.8-149) | 117 (88.9-117) | 101 (68.7-119) | 154 (101-194) | 128 (96.2-165) | 123 (93.0-160) | |
| 1st Derivative Peak Time (sec) | PRP | 62.2 (57.1-67.3) | 66.5 (59.4-73.0) | 85.1 (77.6-97.1) | 85.5 (80.4-128) | 85.4 (77.6-98.8) | 69.8 (64.8-81.1) |
| P | NS | NS | <0.05 | NS | NS | NS | |
| PPP | 63.5 (57.4-67.8) | 69.2 (63.1-75.1) | 92.0 (86.6-101) | 88.2 (78.7-99.4) | 84.0 (79.0-93.8) | 72.2 (67.3-79.6) | |
| 1st Derivative Peak Height (mm Absorbance) | PRP | 147 (117-178) | 182 (145-205) | 200 (160-235) | 330 (274-381) | 265 (209-300) | 214 (179-261) |
| P | NS | NS | NS | NS | NS | <0.001 | |
| PPP | 146 (118-170) | 168 (143-193) | 174 (151-218) | 288 (237-362) | 256 (221-279) | 197 (156-220) | |
| Fibrin Formation Time (sec) | PRP | 62.3 (57.9-67.1) | 67.3 (60.6-73.7) | 85.4 (77.7-97.9) | 86.9 (81.0-100) | 85.2 (76.2-97.7) | 69.4 (64.7-80.3) |
| P | NS | NS | <0.05 | NS | NS | NS | |
| PPP | 63.7 (57.7-68.3) | 71.0 (63.7-75.8) | 93.6 (87.3-103) | 89.4 (79.7-101) | 83.6 (80.0-91.1) | 71.5 (67.5-78.6) | |
| Fibrin Formation Height (mm Absorbance) | PRP | 322 (288-365) | 329 (286-377) | 427 (374-470) | 678 (611-739) | 582 (510-642) | 485 (417-549) |
| P | NS | NS | NS | NS | NS | <0.001 | |
| PPP | 322 (283-357) | 347 (300-372) | 414 (366-491) | 633 (585-715) | 551 (520-651) | 468 (394-568) |
Abbreviations: PRP, platelet-rich plasma; PPP, platelet-poor plasma; NS, not significant; THA, total hip arthroplasty; TKA, total knee arthroplasty; sTF/FIX, small tissue factor induced FIX activation.
Discussion
An CWA-APTT has recently been used in the diagnosis of DIC,13,18,23 hemophilia14,15,24 and monitoring anticoagulant therapy in orthopedic patients.12,17 Although the CWA-sTF/FIXa assay using PRP was developed for evaluating physiological coagulation, including platelets,21 the usefulness of sTF/FIXa remain unclear.
Elevated peak heights of the CWA-APTT were observed in orthopedic patients12,17 and in the early phase of DIC.18 Prolonged peak times of the CWA-APTT were also observed in orthopedic patients12,17 and hemophilic patients.12,15 In the present study, elevated peak heights and prolonged peak times of the CWA-sTF/FIXa were also observed in orthopedic patients, suggesting that the CWA-sTF/FIXa may have a similar ability to monitor anticoagulant therapy in orthopedic patients. The anti-Xa activity is considered useful for monitoring direct oral anti-Xa inhibitors.25,26 Although the peak time of the CWA-APTT were weakly correlated with anti-Xa activity, those of the CWA-sTF/FIXa had a very low correlation with the anti-Xa activity, suggesting that CWA-sTF/FIXa cannot be used in place of the anti-Xa activity.
On comparing the CWA-APTT and CWA-sTF/FIXa, the 2nd DPH of the CWA-sTF/FIXa was found to be significantly lower on day 1a than on day 1b, whereas that of the CWA-APTT was not significantly lower on day 1a than on day 1b. This suggests that the reflection of hemostatic abnormalities induced by the administration edoxaban may be stronger in the CWA-sTF/FIXa than in the CWA-APTT. As the CV values in the CWA-sTF/FIXa were lower than those in the CWA-APTT, the CWA-sTF/FIXa was suggested to be superior to CWA-APTT. In addition, as the CWA-sTF/FIXa uses PRP, the CWA-sTF/FIXa may reflect platelet counts.21 These findings suggest that the CWA-sTF/FIXa may be sensitive for bleeding tendency.
Although there were no significant differences in the parameters of the CWA-sTF/FIXa between the DVT and non-DVT groups or between the groups with and without major bleeding in the present study, CWA-sTF/FIXa showed a cutoff value with high sensitivity for massive bleeding. The cutoff value of the CWA-APTT was also reported previously.9 These findings indicate the possible application of CWA-sTF/FIXa in the monitoring of anticoagulant therapy in postoperative patients. In addition, as the results of the CWA-sTF/FIXa were more prominent in PRP in PPP, the combination of edoxaban and aspirin may be useful as prophylaxis for DVT.27
The present study was associated with some limitations including the relatively small study population and the fact that it contained both THA and TKA patients. There were differences in the pharmacokinetics, hemostatic ability and inflammatory reactions after surgery in each patient. The peak time and height of the CWA-sTF/FIXa were longer and lower, respectively, in TKA patients than THA patients, suggesting that hemostatic abnormalities may be stronger in TKA patients than in THA patients. Further studies separately analyzing the THA and TKA subgroups should be conducted.
In conclusion, the CWA-sTF/FIXa using PRP is useful for evaluating hemostatic abnormalities after surgery and the administration edoxaban. Further study should be separately analyzed in THA and TKA subgroups. Further studies separately analyzing the THA and TKA subgroups should be conducted.
Acknowledgment
The authors thank Mrs. Yumi Sakano and Hiroko Nisii for performing the CWA-APTT, CWA-sTF/FIXa and anti-Xa assay.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labour and Welfare of Japan and IL Japan Co. Ltd.
ORCID iD: Hideo Wada  https://orcid.org/0000-0001-9021-8633
https://orcid.org/0000-0001-9021-8633
References
- 1. Geerts WH, Heit JA, Clagett P, et al. Prevention of venous thromboembolism. Chest. 2001;119(1 Suppl 1):S132–S175. [DOI] [PubMed] [Google Scholar]
- 2. Piovella F, Wang CJ, Lu H, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3(12):2664–2670. [DOI] [PubMed] [Google Scholar]
- 3. Mearns ES, Coleman CI, Patel D, et al. Index clinical manifestation of venous thromboembolism predicts early recurrence type and frequency: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;13(6):1043–1052. [DOI] [PubMed] [Google Scholar]
- 4. Robert-Ebadi H, Righini M. Management of distal deep vein thrombosis. Thromb Res. 2017;149:48–55. [DOI] [PubMed] [Google Scholar]
- 5. Huang W, Goldberg RJ, Cohen AT, et al. Declining long-term risk of adverse events after first-time community-presenting venous thromboembolism: the population-based Worcester VTE Study (1999 to 2009). Thromb Res. 2015;135(6):1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikejiri M, Wada H, Yamaguchi T, et al. Comparison of three different anti-Xa assays in major orthopedic surgery patients treated with fondaparinux. Int J Hematol. 2016;103(5):554-559. [DOI] [PubMed] [Google Scholar]
- 7. Schellings MW, Boonen K, Schmitz EM, et al. Determination of dabigatran and rivaroxaban by ultra-performance liquid chromatography-tandem mass spectrometry and coagulation assays after major orthopaedic surgery. Thromb Res. 2016;139:128–134. [DOI] [PubMed] [Google Scholar]
- 8. Feng W, Wu K, Liu Z, et al. Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: systemic review, traditional meta-analysis, dose-response meta-analysis and network meta-analysis. Thromb Res. 2015;136(6):1133–1144. [DOI] [PubMed] [Google Scholar]
- 9. Hasegawa M, Wada H, Wakabayashi H, et al. The relationships among hemostatic markers, the withdrawal of fondaparinux due to a reduction in hemoglobin and deep vein thrombosis in Japanese patients undergoing major orthopedic surgery. Clin Chim Acta. 2013;425:109–113. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida K, Wada H, Hasegawa M, et al. Increased fibrinolysis increases bleeding in orthopedic patients receiving prophylactic fondaparinux. Int J Hematol. 2012;95(2):160–166. [DOI] [PubMed] [Google Scholar]
- 11. Ota S, Wada H, Mastuda A, et al. Anti-Xa activity in VTE patients treated with fondaparinux. Clin Chim Acta. 2015;442:22–23. [DOI] [PubMed] [Google Scholar]
- 12. Wada H, Matsumoto T, Ohishi K, Shiraki K, Shimaoka M. Update on the clot waveform analysis. Clin Appl Thromb Hemost. 2020;26:1076029620912027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toh CH, Giles AR. Waveform analysis of clotting test optical profiles in the diagnosis and management of disseminated intravascular coagulation (DIC). Clin Lab Haematol. 2002;24(6):321–327. [DOI] [PubMed] [Google Scholar]
- 14. Shima M, Matsumoto T, Fukuda K, et al. The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII: C). Thromb Haemost. 2002;87(3):436–441. [PubMed] [Google Scholar]
- 15. Matsumoto T, Nogami K, Shima M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int J Hematol. 2017;105(2):174–183. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto T, Wada H, Fujimoto N, et al. An evaluation of the activated partial thromboplastin time waveform. Clin Appl Thromb Hemost. 2018;24(5):764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasegawa M, Wada H, Tone S, et al. Monitoring of hemostatic abnormalities in major orthopedic surgery patients treated with edoxaban by APTT waveform. Int J Lab Hematol. 2018;40(1):49–55. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki K, Wada H, Matsumoto T, et al. Usefulness of the APTT waveform for the diagnosis of DIC and prediction of the outcome or bleeding risk. Thromb J. 2019;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konstantinidi A, Sokou R, Parastatidou S, et al. Clinical application of thromboelastography/thromboelastometry (TEG/TEM) in the neonatal population: a narrative review. Semin Thromb Hemost. 2019;45(5):449–457. [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto T, Wada H, Toyoda H, Hirayama M, Yamashita Y, Katayama N. Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab: comment. J Thromb Haemost. 2018;16:1665–1666. [DOI] [PubMed] [Google Scholar]
- 21. Wada H, Shiraki K, Matsumoto T, Ohishi K, Shimpo H, Shimaoka M. Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb Res. 2020;193:146–153. [DOI] [PubMed] [Google Scholar]
- 22. Schellong SM, Schwarz T, Halbritter K, et al. Complete compression ultrasonography of the leg veins as a single test for the diagnosis of deep vein thrombosis. Thromb Haemost. 2003;89(2):228–234. [PubMed] [Google Scholar]
- 23. Matsumoto T, Wada H, Nishioka Y, et al. Frequency of abnormal biphasic aPTT clot waveforms in patients with underlying disorders associated with disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2006;12(2):185–192. [DOI] [PubMed] [Google Scholar]
- 24. Tokunaga N, Inoue C, Sakata T, et al. Usefulness of the second-derivative curve of activated partial thromboplastin time on the ACL-TOP coagulation analyzer for detecting factor deficiencies. Blood Coagul Fibrinolysis. 2016;27(4):474–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost. 2014;111(2):240–248. [DOI] [PubMed] [Google Scholar]
- 26. Gosselin RC, Francart SJ, Hawes EM, Moll S, Dager WE, Adcock DM. Heparin-calibrated chromogenic anti-Xa activity measurements in patients receiving rivaroxaban: can this test be used to quantify drug level? Ann Pharmacother. 2015;49(7):777–783. [DOI] [PubMed] [Google Scholar]
- 27. Wilson DG, Poole WE, Chauhan SK, Rogers BA. Systematic review of aspirin for thromboprophylaxis in modern elective total hip and knee arthroplasty. Bone Joint J. 2016;98-B(8):1056–1061. [DOI] [PubMed] [Google Scholar]