Abstract
Pulmonary endarterectomy is the treatment of choice for patients with operable chronic thromboembolic pulmonary hypertension (CTEPH) as it is potentially curative. In expert centers that conduct > 50 pulmonary endarterectomy procedures per year, peri- and post-surgical mortality rates are very low and long-term outcomes are excellent, with three-year post-operative survival of > 80%. Therapeutic decisions in CTEPH are based largely on the location of the arterial obstruction, with pulmonary endarterectomy for obstructions in main, lobar, and segmental vessels, and balloon pulmonary angioplasty and medical therapy for small-vessel disease. Medical therapy is also an option for patients with persistent/recurrent pulmonary hypertension after pulmonary endarterectomy or balloon pulmonary angioplasty. With increasing surgical experience and improvements in instruments and procedures, an increasing number of patients are now considered operable who would previously have been inoperable, including some patients with subsegmental disease. At our University (University of California San Diego), around 200 pulmonary endarterectomy procedures are performed every year and several advances have been developed, including resection of more distal disease, availability of pulmonary endarterectomy to patients previously considered to be at too high risk for surgery, improved management of post-pulmonary endarterectomy complications, and minimally invasive pulmonary endarterectomy. Pulmonary endarterectomy can be combined with other treatment modalities, including balloon pulmonary angioplasty, medical therapy for persistent/recurrent pulmonary hypertension after pulmonary endarterectomy, and medical therapy or balloon pulmonary angioplasty as bridging therapy before surgery. Data on these combinations are, however, limited. Combination treatment should therefore be considered on an individual patient basis. In the future, however, multimodal therapy with pulmonary endarterectomy, balloon pulmonary angioplasty, and/or medical therapy is likely to be an important treatment option for many patients.
Keywords: pulmonary endarterectomy, chronic thromboembolic pulmonary hypertension, surgical outcomes
Introduction
There are now several treatment options available for patients with chronic thromboembolic pulmonary hypertension (CTEPH).1,2 For patients with surgically accessible disease, pulmonary endarterectomy (PEA) is the standard of care as it is potentially curative. For patients with inoperable CTEPH, percutaneous treatment with balloon pulmonary angioplasty (BPA) is an emerging option, and the soluble guanylate cyclase stimulator riociguat is licensed for the treatment of patients with inoperable CTEPH and those with persistent/recurrent CTEPH after PEA. In addition, other pulmonary arterial hypertension-specific medical therapies (endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, and prostanoids) are widely used off-label to treat CTEPH. Regardless of operability status and choice of therapy, all patients with CTEPH should receive lifelong anticoagulation.
Around 0.9 PEA procedures per million population are performed annually in the USA, and around 1.7 per million population in Europe,3 representing a steady increase over the past decade as surgical expertise has improved and the number of expert centers has increased worldwide. Here we discuss the role of PEA in the management of CTEPH, with a focus on our experience at the University of California San Diego (UCSD) and describe the available literature on combinations of PEA with other CTEPH treatment modalities.
Role of PEA in current CTEPH management
PEA is the treatment of choice for CTEPH, and surgical mortality rates are low, particularly in centers that conduct a large number of such procedures.4 The proportion of patients with CTEPH considered inoperable has varied from 10% to 50%.5–7 Reasons for inoperability include the presence of distal pulmonary artery obstructions not accessible to surgery, imbalance between increased pulmonary vascular resistance (PVR), and the number of accessible occlusions (which suggests the presence of microvascular disease), and old age and comorbid conditions that make the patient unsuitable for surgery.6–8 Elevated PVR (>1500 dyn·s·cm−5) alone is not a contraindication to surgery; in fact there is no higher limit of PVR which may make a patient inoperable, as long as there is a corresponding degree of obstructive disease. In some patients, severely elevated PVR in combination with other risk factors may render a patient inoperable. Furthermore, some patients with operable disease choose not to undergo surgery. Experience suggests that the number of patients considered inoperable may be overestimated due to some patients being incorrectly diagnosed as having CTEPH.5
Treatment guidelines recommend that patients with suspected CTEPH are referred to expert centers for confirmation of diagnosis and treatment, including PEA.4 An expert center is defined as one with a high annual volume of PEA procedures (>50/year) and surgical mortality < 5%, and the ability to perform segmental endarterectomy.9 In addition, expert centers should be capable of evaluating the need for other established treatment modalities by individual patients and offering any that are deemed necessary.2 All expert centers must be able to call on a multidisciplinary team for evaluation and management of CTEPH, including a surgeon experienced with PEA, a pulmonary hypertension (PH) specialist, a BPA interventionist, and a CTEPH-trained radiologist.2 It should be noted that some patients initially considered inoperable go on to have surgery after a second opinion at an expert center.1
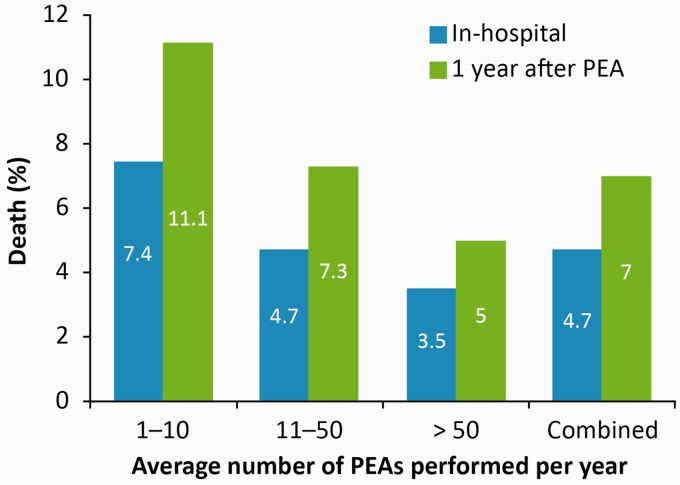
Patients with CTEPH who undergo PEA generally experience immediate improvement in CTEPH symptoms, right ventricular function, and exercise capacity, with normalization or near-normalization of hemodynamic parameters.6,9–11 As noted above, in-hospital mortality rates are low (<5%) when surgery is conducted in high-volume expert centers (Fig. 1),6,9,12 with mortality rates of 3% and 7% reported at 3 and 12 months, respectively.6,10 Even in patients with distal disease, in whom surgery is more challenging, in-hospital mortality rates of <10% are reported.11 Long-term results of PEA are also excellent, with improvements in both survival and quality of life compared with patients who do not undergo PEA,13–15 and no negative impact of circulatory arrest on cognitive function.16 Indeed, registries have reported 3-year post-operative survival in CTEPH of 83–89%,8,13–15 compared with ∼70% in patients who do not undergo PEA.8,13,15 After PEA, however, up to 51% of patients develop persistent PH,6,10,14,17 although definitions of persistent/recurrent PH varied between registries. For example, some used an mPAP-based definition (> 25 mmHg or ≥ 25 mmHg),6,14 while others used a combination of mPAP > 25 mmHg and PVR > 240 dyn·s·cm−5.10,17 Importantly, the prevalence of persistent/recurrent PH after PEA is underestimated,5 highlighting the importance of long-term follow-up after PEA. Some patients with persistent/recurrent CTEPH, however, will remain asymptomatic despite having elevated pulmonary pressures, and may not require treatment. It should also be noted that recurrent PH after successful PEA is a distinct but rare condition caused by a further thrombotic episode.9
Fig. 1.
Survival rates after PEA increase with the experience of the surgical center. Data from 26 European centers and one Canadian center.6 PEA: pulmonary endarterectomy.
Ultimately, therapeutic decisions in CTEPH are made according to the location of the arterial obstruction, with PEA for obstructions in larger vessels, BPA when the obstruction is in smaller vessels inaccessible to PEA, and medical therapy for obstructions not amenable to either intervention (Fig. 2). As surgeons gain more experience with PEA, and instruments and procedures improve, the distal limits of operability are becoming refined, leading to a greater percentage of patients being considered operable.3,11 For example, data from > 300 PEA operations at an Italian expert center show similar in-hospital mortality in patients with distal disease as in those with more proximal disease, with significant, sustained improvements in hemodynamic, echocardiographic, and functional parameters.11 PEA also plays a role in the management of chronic thromboembolic disease (CTED), a condition in which pulmonary thromboembolic occlusions are present without PH at rest, but with similar symptoms to CTEPH. Data on PEA in patients with CTED are limited, although small-scale studies (n = 23–42) showed hemodynamic and clinical improvements, with one-year survival of 95% and improvement in quality of life.18–20
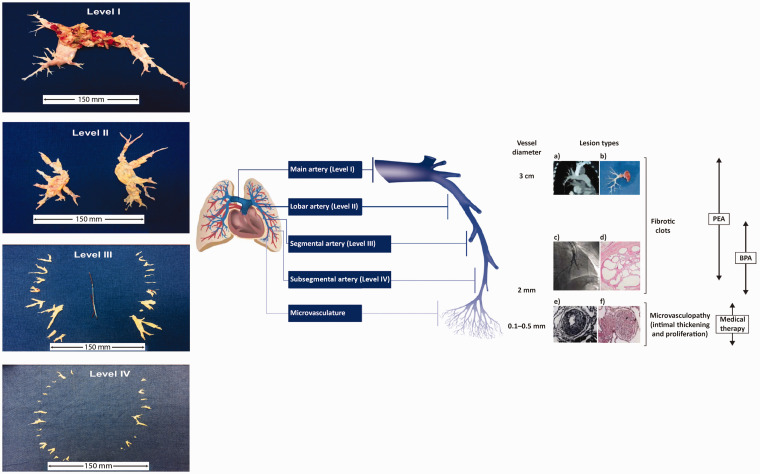
Fig. 2.
The management options for CTEPH target pathogenic manifestations in different parts of the pulmonary vascular bed. (a) Computed tomography scan of a pulmonary artery. (b) Organized fibrotic material removed during PEA. (c) Selective pulmonary angiogram of segmental and subsegmental pulmonary arteries, showing irregular vessel contour and occlusion, typical of CTEPH. (d) Microscopic examination showing a luminal filling defect with recanalized chronic thrombus (web lesion) and no evidence of vasculopathy in the subsegmental artery. (e) Intimal fibromuscular proliferation. (f) Plexiform lesion and vessel occlusion due to vasculopathy and proliferation. Adapted from Madani et al.5 [part (e) from Moser and Bloor33] and Madani.3 A schematic representation of a pulmonary artery is shown (note that vessel diameter is not to scale). PEA is used to remove thromboembolic lesions primarily in the proximal main artery (diameter of ∼1–3 cm), and lobar and segmental arteries;9,34 in expert surgical centers, lesions in distally located mid-segmental and subsegmental branches can be targeted by PEA,9 down to vessels of 2 mm in diameter. BPA mainly targets distal lesions in the segmental and subsegmental vasculature, down to small pulmonary arteries of 2–5 mm in diameter. Medical therapy targets microvasculopathy, including intimal thickening and fibromuscular proliferation, in vessels of 0.1–0.5 mm in diameter.35 Typical surgical specimens based on the most proximal level of obstruction are shown. The scale is in cm.
BPA: balloon pulmonary angioplasty; CTEPH: chronic thromboembolic pulmonary hypertension; PEA: pulmonary endarterectomy.
The UCSD PEA experience
Around 200 PEA procedures per year are conducted at UCSD, where multidisciplinary teams for management of CTEPH consist of a PEA surgeon, a pulmonary vascular medicine specialist, an intervention cardiologist, and an imaging specialist. All CTEPH diagnoses are confirmed using ventilation−perfusion scan and computed tomography pulmonary angiography,4 and patient selection for PEA is typically based on: severity of CTEPH symptoms, PH, and right heart dysfunction; extent and level of obstruction based on high-quality imaging techniques; correlation of PH severity with degree of obstruction; comorbidities; technical challenges for the procedure; and the risk:benefit ratio, based on the patient’s expectations of surgery and acceptance of risk.3 Surgical procedures and techniques used at UCSD include median sternotomy, cardiopulmonary bypass, circulatory arrest, plane identification, and complete bilateral endarterectomy.3 A live PEA procedure during the UCSD CTEPH Symposium, November 15--16 2019, highlighted aspects such as perfusion to the right heart, cross-clamping, and vessel inspection to ensure no residual disease as the goal.
Recent advances in management of CTEPH at UCSD include resection of more distal disease, availability of PEA to patients previously considered to be at too high risk for surgery, improved management of post-PEA complications, and minimally invasive PEA. To assist with treatment decisions, the UCSD team have developed an intraoperative classification of CTEPH based on the location of the fibrotic thromboembolic material (Fig. 2):1,5
Level 0: no evidence of CTEPH
Level I: in the main arteries
Level II: starting in the lobar branches
Level III: starting in the segmental branches
Level IV: only in subsegmental branches.
Level IC signifies complete occlusion of one lung with total obstruction of the main right or left pulmonary artery. Clinical experience has shown that PEA in patients with Level III or IV disease is feasible and results in hemodynamic and clinical improvement. With improvements in surgical techniques, perioperative mortality has fallen from almost 20% in the early years to <2% at UCSD.1
The UCSD experience includes 42 patients who underwent repeat PEA, with an average time between surgeries of 7.2 years. Patients experienced significant hemodynamic improvement, with acceptable surgical mortality (2.3%).21 The following causes for recurrent PEA were identified in 39 of the 42 patients: anticoagulant treatment failure (n = 14 [warfarin, n = 9; direct oral anticoagulants, n = 4; low-molecular-weight heparins, n = 1]); anticoagulation non-compliance (n = 10); incomplete initial endarterectomy (n = 9); and discontinuation of anticoagulation for medical indications or bleeding (n = 6).
Based on advances in cardiac surgery, minimally invasive techniques for PEA have been developed at UCSD, with 15 patients having undergone the procedure to date.22,23 The procedure is performed through bilateral mini-anterior thoracotomies, with central or peripheral cannulation and no cross-clamp. Importantly, however, minimally invasive PEA is not appropriate for patients with unsuitable chest anatomy, obesity, or distal disease, or who are undergoing concomitant cardiac procedures. Compared with patients who underwent conventional PEA, those undergoing minimally invasive surgery were younger, and both circulatory arrest and length of stay were shorter with minimally invasive PEA. Furthermore, PVR and lung perfusion were improved to a similar extent with minimally invasive and conventional PEA, although larger studies are needed to confirm the utility of this novel technique.
Combining PEA with other treatment modalities
Data on combination options are limited, and it is therefore difficult to provide definitive guidelines. As a result, all combination treatment should be considered on an individual basis.
Combination PEA plus BPA
While most patients with CTEPH have bilateral disease, some patients have heterogeneous CTEPH, with operable disease on one side but distal, inoperable disease on the opposite side.24 There is therefore a rationale for combining unilateral PEA with BPA on the contralateral side. Case studies have demonstrated the feasibility of this combination, with improved hemodynamic parameters and functional class.24
BPA or riociguat after PEA for patients with persistent/recurrent CTEPH
In patients with persistent PH after PEA, there may be a role for BPA, and this was assessed in a pilot study (n = 20; mean of 7 months after PEA).25 The results showed that BPA was associated with improved exercise capacity and hemodynamic parameters in patients with persistent PH compared with patients who did not undergo BPA. More recently, BPA for treatment of persistent PH (mean of 28 months after PEA) was evaluated in 15 patients, showing improvement in exercise capacity and hemodynamic parameters.26
Available data suggest PEA combined with riociguat may also benefit patients. The CHEST-1 study of riociguat in patients with CTEPH included a subgroup of 72 patients who received riociguat for persistent/recurrent CTEPH after PEA.27 In these patients, riociguat improved exercise capacity, PVR, and other secondary endpoints, and the treatment effect was consistent with that in patients with inoperable CTEPH.27 The study was not, however, powered to detect differences between the two subgroups.
PEA after BPA
PEA after BPA is also possible, but challenging. In some patients, the plane of dissection can be distorted or obliterated during the BPA procedure. PEA relies on a careful and meticulous dissection of the intima and media, the correct identification of which is a key principle of this challenging operation. If, for any reason, this plane is disrupted during BPA (e.g. aggressive ballooning, or use of large size balloons), the two layers of the artery (intima and media) heal and seal, and the appropriate plane scars over time and disappears, making PEA in this plane impossible. In these circumstances, the plane of dissection has to be developed further and deeper, well into the media, making vessel wall injury, rupture, and/or disruption more likely. In rare occasions, when there is evidence of complete vessel wall disruption as a result of prior aggressive BPA, significant scarring of the remaining thromboembolic material and parenchymal lung tissue makes subsequent PEA extremely challenging and, in some cases, impossible.
Bridging therapy before PEA
Greater hemodynamic impairment, particularly increased PVR, is significantly associated with higher post-operative mortality after PEA,28 and as a result there is interest in using medical therapy as a bridge to PEA. Preliminary studies of pre-PEA therapy with intravenous prostacyclin or bosentan have shown that medical therapy can improve hemodynamics compared with no pre-PEA treatment, although there was no difference in post-PEA hemodynamics compared with controls.29,30 There are, however, conflicting data on the effect of pre-PEA medical therapy, and bridging therapy has not been evaluated in randomized controlled trials. For example, a retrospective analysis of data from UCSD showed only minimal benefit of pre-PEA treatment on mean pulmonary artery pressure, with no impact on post-PEA outcomes.31 Notably, pre-PEA medical therapy was associated with a significant delay in time to referral for PEA. In a pilot study, however, patients with operable CTEPH were randomized to bosentan (n = 8) or standard of care (n = 7) for 16 weeks: those who received bosentan showed significant improvements in imaging parameters of right ventricular function and remodeling compared with those who did not.32 Data from the European CTEPH registry have shown that 2% of operable patients received pre-PEA medical therapy in real-world practice, and a multivariate analysis found that use of bridging therapy was a significant independent predictor of mortality.15 The authors note, however, that bridging therapy was used in patients with the most severe hemodynamic impairment and suggest that the results may also be related to delays in carrying out PEA and possible effects of pulmonary arterial hypertension-targeted therapies on the properties of chronic thromboembolic material, increasing the difficulty of surgery. The potential for increased fragility of the vessel wall or thrombus in patients receiving medical therapy is currently a subject of active debate. The PEA Bridging Study (ClinicalTrials.gov: NCT03273257) was intended to investigate riociguat versus placebo in operable CTEPH with high PVR, but slow recruitment and limitations imposed by the COVID-19 pandemic have meant that the trial has been terminated.
Conclusions
PEA is the guideline-recommended treatment of choice for CTEPH as it has excellent long-term outcomes, and advances in surgical techniques are leading to refinement of operability definitions and improved outcomes. As a result, many previously inoperable patients with more distal disease or higher surgical risk can now be considered operable at expert centers. Despite these improvements, however, there will still be patients who are ineligible for PEA, while others develop recurrent/persistent PH after surgery. Long-term follow-up after PEA is therefore essential for all patients. In the future, multimodal therapy with PEA, BPA, and/or medical therapy is likely to be an important treatment option for many patients.
Acknowledgments
This article is based on a presentation by Professor Madani at the University of California San Diego CTEPH Symposium, November 15–16, 2019. Professor Madani reviewed each draft and approved the final draft for submission.
Footnotes
Conflict of interests: Professor Madani reports consultancy for MSD/Bayer, consultancy for Wexler Surgical, consultancy for Actelion, and Executive Board membership of the International CTEPH Association, CTEPH.com, outside the submitted work.
Funding: Medical writing assistance was provided by Richard Murphy PhD of Adelphi Communications Ltd, Bollington, UK, funded by Bayer US LLC, Whippany, New Jersey, USA.
Ethical approval: Not applicable.
Guarantor: Professor Madani is the guarantor of the accuracy of the data presented in this review.
References
- 1.Mahmud E, Madani MM, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol 2018; 71: 2468–2486. [DOI] [PubMed] [Google Scholar]
- 2.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madani MM. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovasc J 2016; 12: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 5.Madani M, Ogo T, Simonneau G. The changing landscape of chronic thromboembolic pulmonary hypertension management. Eur Respir Rev 2017; 26: 170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 7.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 8.Quadery SR, Swift AJ, Billings CG, et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J 2018; 52: 1800589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 2011; 141: 383–387. [DOI] [PubMed] [Google Scholar]
- 11.D'Armini AM, Morsolini M, Mattiucci G, et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2014; 148: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 12.Lankeit M, Krieg V, Hobohm L, et al. Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2018; 37: 250–258. [DOI] [PubMed] [Google Scholar]
- 13.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 14.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the UK national cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 16.Vuylsteke A, Sharples L, Charman G, et al. Circulatory arrest versus cerebral perfusion during pulmonary endarterectomy surgery (PEACOG): a randomised controlled trial. Lancet 2011; 378: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 17.Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 18.Taboada D, Pepke-Zaba J, Jenkins DP, et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014; 44: 1635–1645. [DOI] [PubMed] [Google Scholar]
- 19.Guth S, Wiedenroth CB, Rieth A, et al. Exercise right heart catheterisation before and after pulmonary endarterectomy in patients with chronic thromboembolic disease. Eur Respir J 2018; 52: 1800458. [DOI] [PubMed] [Google Scholar]
- 20.Olgun Yildizeli S, Kepez A, Tas S, et al. Pulmonary endarterectomy for patients with chronic thromboembolic disease. Anatol J Cardiol 2018; 19: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astashchanka A, Fernandes T, Papamatheakis DG, et al. Risk factors and outcomes for patients requiring repeat thromboendarterectomy. Am J Respir Crit Care Med 2020; 201: A6065. [Google Scholar]
- 22.Higgins JR, Kim NH, Kerr K, et al. A comparison of short term outcomes of minimally invasive versus sternotomy pulmonary thromboendarterectomy. J Heart Lung Transplant 2018; 37: S25–S26. [Google Scholar]
- 23.Madani M, Higgins J. Minimally invasive pulmonary thromboendarterectomy: a novel technique. In: ISMICS, New York, NY, USA, 29th May to 1st June 2019. https://meetings.ismics.org/abstracts/2019/C8.cgi
- 24.Wiedenroth CB, Liebetrau C, Breithecker A, et al. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2016; 35: 591–596. [DOI] [PubMed] [Google Scholar]
- 25.Yanaka K, Nakayama K, Shinke T, et al. Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc 2018; 7: e008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araszkiewicz A, Darocha S, Pietrasik A, et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2019; 278: 232–237. [DOI] [PubMed] [Google Scholar]
- 27.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 28.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. [DOI] [PubMed] [Google Scholar]
- 29.Nagaya N, Sasaki N, Ando M, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest 2003; 123: 338–343. [DOI] [PubMed] [Google Scholar]
- 30.Reesink HJ, Surie S, Kloek JJ, et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2010; 139: 85–91. [DOI] [PubMed] [Google Scholar]
- 31.Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 2009; 120: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 32.Surie S, Reesink HJ, Marcus JT, et al. Bosentan treatment is associated with improvement of right ventricular function and remodeling in chronic thromboembolic pulmonary hypertension. Clin Cardiol 2013; 36: 698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993; 103: 685–692. [DOI] [PubMed] [Google Scholar]
- 34.Gopalan D, Blanchard D, Auger WR. Diagnostic evaluation of chronic thromboembolic pulmonary hypertension. Ann Am Thorac Soc 2016; 13(Suppl 3): S222–S239. [DOI] [PubMed] [Google Scholar]
- 35.Simonneau G, Torbicki A, Dorfmüller P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160112. [DOI] [PMC free article] [PubMed] [Google Scholar]