Abstract
Objective
This study examined the role of agrin in the development of cholangiocarcinoma (CCA).
Methods
Western blotting was performed to detect the expression of target genes. The correlation between agrin expression and prognosis was analyzed using the Kaplan–Meier method. Proliferation, migration, invasion, and tumorigenesis were examined in CCA cells and tissues using the Cell Counting Kit-8 assay, cell cycle analysis, transwell migration assay, and nude mouse tumorigenicity assay in vivo, respectively.
Results
Agrin expression was significantly upregulated in CCA tissues compared with that in adjacent non-tumor tissues, and agrin expression was correlated with poorer tumor characteristics such as portal vein tumor thrombus, intrahepatic metastasis, and worse survival. Forced agrin expression in CCA cells apparently promoted proliferation, colony formation, migration, invasion, and cell cycle progression, but agrin depletion had the opposite effects. Furthermore, agrin-depleted CCA cells developed fewer and smaller tumors than control cells in vivo. Mechanistic analyses indicated that agrin activated the Hippo signaling pathway and induced the translocation of YAP to the nucleus.
Conclusions
Agrin promoted CCA progression by activating the Hippo signaling pathway, suggesting its promise as a target for CCA therapy.
Keywords: Cholangiocarcinoma, agrin, YAP, metastasis, proliferation, invasion, tumorigenesis, Hippo pathway
Introduction
Cholangiocarcinoma (CCA) is the most frequent biliary adenocarcinoma, accounting for 10% to 15% of primary liver cancers, and it is associated with high malignancy potential.1 According to its origination, CCA is classified as intrahepatic or extrahepatic CCA, and the latter includes perihilar and distal CCA. Most patients with CCA are diagnosed at a median age of 65 years, but patients with primary sclerosing cholangitis commonly present with CCA at ≤40 years of age.2 The high incidence of hepatolithiasis and high prevalence of hepatitis B virus may be the main causes of CCA initiation in China.3 In recent decades, there has been increasing concern over the rising incidence and high tumor-related death rates of CCA globally. For patients with CCA, surgery is the only potentially curative option. Unfortunately, rapid preoperative tumor progression and high postoperative tumor recurrence rates significantly limit R0 resection rates and long-term tumor-free survival (TFS).4 Recently, great advances have been made in tumor management,5 and related immunotherapy has been developed, which strongly suggests that further understanding of the molecular mechanism underlying tumor progression and recurrence would be useful for improving prognosis.
Agrin is a 210-kDa basal lamina-related heparan sulfate proteoglycan that exists as either a shorter type II transmembrane protein or a secreted protein in the extracellular matrix.6 Its two parts have been revealed to have completely different functions. Specifically, the carboxy-terminal end exhibits synaptogenic activity and triggers acetylcholine receptors (AChRs) by activating the low-density lipoprotein receptor-related protein 4 (Lrp4) receptor and muscle-specific kinase (MuSK), and the amino-terminal end consists of a signal sequence necessary for the secretory pathway and an agrin domain that is required for binding with basal lamina-associated laminins.7 Most studies investigated the essential role of agrin in the formation of the vertebrate neuromuscular junction during embryogenesis. For example, Nitkin et al.8 and Jones et al.9 confirmed that ectopic agrin expression could promote the formation of postsynaptic specializations, such as the induction of endplate-specific gene transcription of the e-subunit of AChR in vivo and aggregates of several important molecules on cultured myotubes in vitro. Conversely, the inhibition of agrin resulted in few pre- and postsynaptic specializations, as well as death at birth in mice because of non-functional respiratory musculature.10 In addition, several recent studies verified that agrin is necessary for tumor initiation and progression. Sayan et al.11 demonstrated that agrin promoted cellular proliferation, migration, and invasion in hepatocellular carcinoma and activated the FAK signaling pathway through formation of the agrin–Lrp4–MuSK signaling complex. Rivera reported that agrin depletion decreased malignant potential and inhibited the phosphorylation of FAK, ERK, and cyclin D1 in oral squamous cell carcinoma cells.12 However, the relationship between agrin and CCA development has not yet been explored. Thus, the present study examined the role of agrin in regulating cell biofunction in CCA.
Materials and methods
Clinical materials and sample preparation
The present study was performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and approved by the Ethical Committee at the Second Affiliated Hospital of Wenzhou Medical University, Wenzhou University, (No. LCKY2020-110). The requirement for individual consent for this retrospective analysis was waived. All clinical samples used in the study were obtained from patients with hepatocellular carcinoma at the Second Affiliated Hospital of Wenzhou Medical University, Wenzhou University (Wenzhou, China) between 2017 and 2019.
Cell lines and cell transfection
Two human CCA cell lines (HUCCT-1 and CCLP-1) were purchased from the Institutes of Biological Sciences (Shanghai, China) and cultured following the manufacturer’s instructions. HUCCT-1 and CCLP-1 cells were incubated in RPMI 1640 complete medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific) and antibiotics (50 U/ml penicillin and 50 mg/ml streptomycin, both from Gibco) in a 5% CO2 incubator at 37°C. Lentivirus encoding both a short hairpin RNA (shRNA) (5′-CAGGAGAAUGUCUUCAAGATT-3′) specifically targeting human agrin and green fluorescent protein (GeneCopoeia, Rockville, MD, USA), as well as the pcDNA3.1 vector containing the full-length cDNA of agrin (GeneCopoeia), were used to infect the cells. Lentivirus encoding a scramble sequence (GeneCopoeia) was used as a control. Briefly, virus (multiplicity of infection = 20) was added into cells at logarithmic phase supplemented with 1 µL of polybrene (MedChemExpress, Monmouth Junction, NJ, USA). After incubation for 24 hours, medium including the virus was removed. An immunofluorescence microscope (Macklin, Shanghai, China) was used to observe the intensity of virus expression. Stably transfected cells were selected using puromycin (Glpbio, Montclair, CA, USA).
RNA extraction and reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
The extraction of total RNA from tissues and cells was performed using TRIZOL reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. Then, 2 µg of RNA were subjected to a reverse transcriptase reaction using a ReverTra Ace® kit (Thermo Fisher Scientific, Inc.) to synthesize cDNA. RT-qPCR was performed using SYBR Premix Ex Taq™ (Takara Bio, Inc., Kusatsu, Shiga, Japan) and the Thermal Cycler Dice Detection System (Takara Bio). The housekeeping gene β-actin was selected as an endogenous control. The 2−△△Ct method was used to quantify the results. All reactions were performed in triplicate. The following primers were used to specially amplify the agrin gene and β-actin: agrin forward, 5′-ACACCGTCCTCAACCTGAAG-3′; agrin reverse, 5′-CCAGGTTGTAGCTCAGTTGC-3′; β-actin forward, 5′-AGAGCCTCGCCTTTGCCGATCC-3′; β-actin reverse, 5′-CTGGGCCTCGTCGCCCACATA-3′.
Cell cycle analysis
Cells transfected with agrin plasmid or shRNA were subjected to serum starvation for 24 hours to induce cell cycle synchronization and then seeded at a density of 10,000 cells per 6-cm plate. Monolayer cells were harvested at approximately 70% confluency as single-cell suspensions and fixed in 90% ethanol overnight. Following washing twice with PBS, cells were incubated with 0.5 mL of DNA Prep Stain (GeneCopeia) in the dark for 30 minutes at room temperature, followed by flow cytometric analysis. The percentage of the cell population in each phase was calculated using ModFit LT software (Verity Software House, Topsham, ME, USA).
Colony formation
Colony formation assay was performed by seeding CCA cells in six-well plates. In total, 1000 cells were seeded in 2 mL of 10% FBS-containing medium, which was replaced every 3 days. After incubation for 2 weeks, the colonies were fixed with 95% methanol, stained with 0.4% crystal violet, photographed, and counted.
Cell Counting Kit-8 (CCK-8) assay
The effects of agrin on CCA cell viability was detected using the CCK-8 assay (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) assay. Briefly, CCA cells were seeded in a 96-well plate at a density of 1000 cells per well. Following culture for 24, 48, 72, 96 and 120 hours, fresh medium containing 10% CCK-8 was added to the well, and the absorbance at a wavelength of 450 nm was detected as an indicator of cell viability.
Cell migration and invasion assays
Migration and invasion assays were conducted using the transwell migration assay (24-well insert, 8.0-μm pores, Corning, Corning, NY, USA). CCA cells (1 × 104/well) were suspended in 100 μL of FBS-free culture medium and placed into the upper chamber. The upper chamber was coated with Matrigel (Corning) for the invasion assay, whereas no Matrigel was used for the migration assay. Medium containing 10% FBS was added into the bottom chamber to drive cell translocation at 500 μL per well. After 24, 48 and 72 hours, cells on the upper surface of the chamber were cleaned with cotton swabs, and those on the bottom surface were reserved and fixed in 95% methanol for 20 minutes, stained with 0.4% crystal violet, visualized, and counted using a light microscope (×100 magnification).
Wound-healing assay
CCA cells with agrin depletion or overexpression were plated in six-well plates. When monolayer CCA cells reached 80% to 90% confluence, a scratch was made using Culture-Insert (Ibidi, Gräfelfing, Germany). Following incubation for 24 and 48 hours, the migrated distances were recorded as an indicator of migration.
Western blotting
Total protein was extracted from CCA cells on ice using RIPA lysis buffer (Thermo Fisher Scientific) supplemented with protease and phosphatase inhibitors (Thermo Fisher Scientific). The total protein concentration was measured using the BCA assay (Pierce, Rockford, IL, USA). The electrophoretic separation of proteins was performed in a 4% to 20% gradient sodium dodecyl sulfate polyacrylamide gel (GenScript, Piscataway, NJ, USA). The protein on the gel was then transferred to 0.45-μm PVDF membranes (MilliporeSigma, Burlington, MA, USA). Following blocking with Tris-buffered saline containing 5% non-fat milk and 0.1% Tween-20 for 1 hour, the membrane was incubated with target primary antibodies overnight at 4°C. After washing three times with TBST, the membrane was incubated with the secondary antibody for 1 hour at room temperature at a dilution of 1:5000. The membrane was visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce) and analyzed using Lab Image software (Bio-Rad, Hercules, CA, USA).
Tumor xenograft experiment
A total of 1 × 106 cells with agrin depletion or overexpression suspended in 100 µL of medium, as well as control cells, were subcutaneously injected into the lateral flanks of 6-week-old male BALB/c nude mice (n = 5 per group). Tumor volume was measured weekly with a caliper according to the following equation: tumor size = (width2 × length)/2. Four weeks after the injection, all mice were sacrificed, and tumors were harvested.
Immunohistochemistry (IHC)
Tumor tissue sections (4 µm) were deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval. Next, endogenous peroxidase activity was quenched with 3% hydrogen peroxide, and non-specific binding was blocked with 5% FBS. After incubation with primary antibodies overnight at 4°C, the sections were further incubated with the corresponding HRP-conjugated secondary antibodies at room temperature for 1 hour. The target protein expression was visualized using 3,3′-diaminobenzidine and counterstaining with hematoxylin.
Cell apoptosis assay
To assay apoptosis, ICC cells with normal or depleted agrin expression were cultured in medium under conventional conditions for 24 hours. Then, a FITC-conjugated Annexin-V and 7-AAD kit (eBioscience, San Diego, CA, USA) was used according to the manufacturer’s recommendations. Cells located in quadrants Q2 and Q4 were FITC-positive and 7-AAD–negative, respectively. These cells were considered apoptotic.
Statistical analysis
All experiments were performed three times, and related data are presented as the mean ± standard deviation or frequency. The difference between experimental groups (CCA vs. normal tissues, portal vein tumor thrombus [PVTT] vs. non-PVTT, tumor size ≥ 5 cm vs. tumor size < 5 cm, multiple tumor lesions vs. single tumor lesion) was statistically analyzed using Student’s t-test. Frequency data were analyzed using Fisher’s exact test. The CA199 level exceeded 12,000 kU/L (the maximum value of the clinical test) in some patients, and thus, CA199 levels were analyzed using the chi-squared test. Statistical analysis was conducted using SPSS 19.0 software (IBM Corp., Armonk, NY, USA), and P < 0.05 indicated a statistically significant difference.
Results
Pattern and significance of agrin expression in patients with CCA
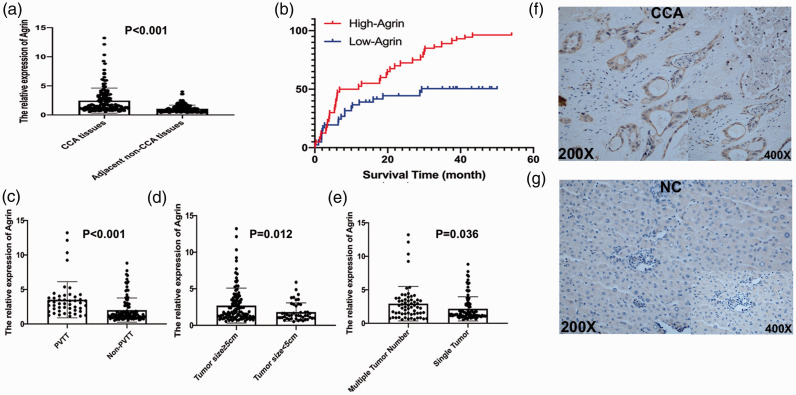
To explore the potential effects of agrin on CCA, agrin mRNA expression was detected using RT-qPCR in 162 paired CCA specimens, revealing that agrin was significantly upregulated in CCA tissues compared with its expression in adjacent non-tumor tissues (P < 0.001, Figure 1a). According to the median agrin mRNA level, the patients were divided into two groups. Differences in clinicopathological factors and demographic characteristics between the two groups are presented in Table 1. Kaplan–Meier analysis revealed that patients with high agrin expression had higher rates of CCA recurrence and poorer tumor-free survival (TFS) following surgical resection (Figure 1b). Furthermore, increased agrin expression in patients with CCA was correlated with PVTT (P < 0.001, Figure 1c), tumor size (P = 0.012, Figure 1d), and the number of tumors (P = 0.036, Figure 1e), suggesting a stimulatory role of agrin in CCA development. Moreover, IHC illustrated that agrin protein levels were substantially higher in CCA tissues than in normal tissues (Figure 1f–g). The postoperative 3-year overall survival rate was 49.5% in the low agrin expression group, versus 13.7% in the high agrin expression group. In addition, agrin was identified via Cox proportional hazards regression to be an independent risk factor for CCA (hazard ratio = 2.47; 95% confidence interval = 1.63–3.75; P < 0.001; Table 2). These results were also verified in a CCA cohort from The Cancer Genome Atlas (Figure S1).
Figure 1.
In 162 paired specimens, agrin was significantly overexpressed in CCA tissues compared with its levels adjacent non-CCA tissues according to RT-PCR (a). Furthermore, additional overexpression of agrin was correlated with increases of mortality rates (b), PVTT (c) tumor size (d), and tumor number (e). Immunohistochemistry also revealed that agrin protein expression was significantly higher in CCA tissues than in normal tissues (f–g)
CCA, cholangiocarcinoma; PVTT, portal vein tumor thrombus.
Table 1.
Correlation of agrin expression with clinicopathological features in patients with cholangiocarcinoma.
| Variables |
Tumor agrin expression |
P a | |
|---|---|---|---|
| Low | High | ||
| Age | 0.084 | ||
| ≤50 years | 35 | 47 | |
| >50 years | 46 | 34 | |
| Sex | <0.001 | ||
| Male | 33 | 56 | |
| Female | 48 | 25 | |
| PVTT | 11 | 42 | <0.001 |
| Tumor size | <0.001 | ||
| ≤5 cm | 68 | 33 | |
| >5 cm | 13 | 48 | |
| Tumor number | 0.038 | ||
| Single | 27 | 41 | |
| Multiple | 54 | 40 | |
| CA199 > 37 kU/L | 68 | 58 | 0.088 |
| CEA > 5 ng/mL | 37 | 34 | 0.751 |
| GGT > 50 U/L | 64 | 56 | 0.209 |
| Histopathologic grade | 0.090 | ||
| Well + moderately differentiated | 31 | 20 | |
| Poorly differentiated | 50 | 61 | |
aStatistical analyses were performed using the chi-squared test.
PVTT, portal vein tumor thrombus; CEA, carcinoembryonic antigen; GGT, gamma-glutamyltransferase.
Table 2.
Agrin expression is an independent predictive factor for prognosis.
| Variables |
Cumulative recurrence |
Overall survival |
||
|---|---|---|---|---|
| HR (95% CI) | P a | HR (95% CI) | P a | |
| Age (years) | ||||
| ≥50 vs. <50 | 0.63 (0.42–0.95) | 0.028 | 0.84 (0.54–1.31) | 0.436 |
| Sex | ||||
| Male vs. female | 1.42 (0.98–2.07) | 0.065 | 1.06 (0.87–1.29) | 0.543 |
| PVTT | ||||
| Present vs. absent | 0.98 (0.68–1.41) | 0.895 | ||
| Tumor number | ||||
| Multiple vs. single | 0.84 (0.51–1.36) | 0.476 | ||
| Histopathologic grade | ||||
| Well + moderately differentiated vs. poorly differentiated | 1.66 (1.07–2.59) | 0.025 | 1.18 (0.73–1.92) | 0.491 |
| Tumor size | ||||
| >5 cm vs. ≤5 cm | 0.88 (0.59–1.33) | 0.567 | ||
| Agrin expression | ||||
| High vs. low | 2.65 (1.79–3.89) | <0.001 | 2.47 (1.63–3.75) | <0.001 |
aCox proportional hazards regression. PVTT, portal vein tumor thrombus; HR, hazard ratio; CI, confidence interval.
Agrin promotes CCA cell proliferation
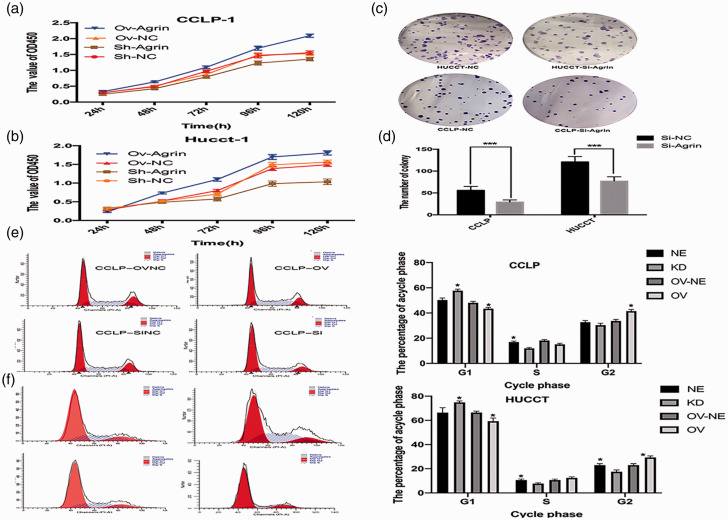
Recombinant pcDNA3.1-agrin containing the agrin gene was transfected into CCLP and HUCCT cells to induce agrin expression. After verifying the effectiveness of the agrin plasmid, CCK-8 and colony formation assays were performed to explore the effects of agrin on CCA cell proliferation. Compared with the effects of empty plasmid transfection, agrin-overexpressing CCA cells exhibited higher viability (Figure 2a–b). By contrast, agrin depletion via shRNA lentiviral vector transfection significantly suppressed CCA cell growth and colony formation (both P < 0.001, Figure 2c–d). In cell cycle analysis, agrin overexpression facilitated the G1-S/G2 cell cycle transition in CCA cells, whereas this transition was prevented by agrin depletion (Figure 2e–f).
Figure 2.
Compared with the findings in control cells, agrin overexpression increased cell viability but agrin depletion reversed this effect in CCLP (a) and HUCCT cells (b). Agrin suppression decreased the number of colonies (c–d). Agrin overexpression facilitated the G1-S/G2 cell cycle transition, but agrin depletion promoted cell cycle arrest (e). (f) The percentage of cells in each cycle phase.
Agrin depletion induces apoptosis in CCA cell lines
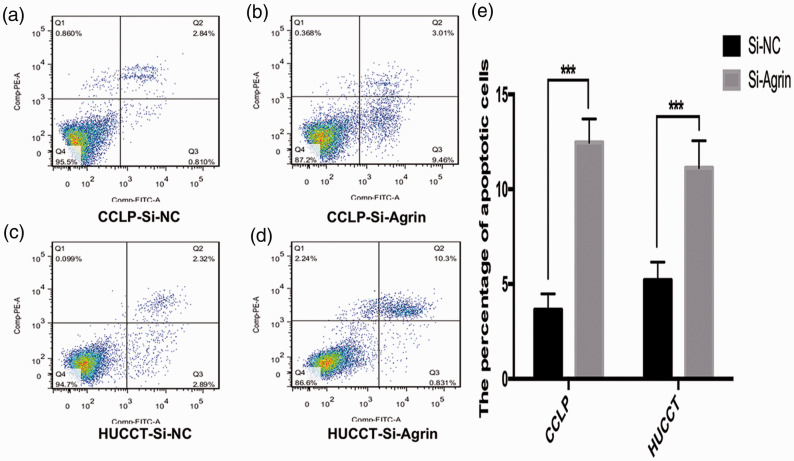
Flow cytometry was performed to clarify the role of agrin depletion on apoptosis in CCA cells. Agrin depletion induced apoptosis in HUCCT and CCLP cells, as determined by FITC/7AAD staining. Agrin-depleted CCLP cells exhibited a higher percentage of apoptotic cells (11.13 ± 0.76%) than negative control cells (5.12 ± 0.45%, P < 0.05), and the similar trend was observed in HUCCT cells (12.45 ± 0.68% vs. 3.6479 ± 0.34%, P < 0.05, Figure 3).
Figure 3.
Flow cytometry illustrated that agrin-depleted cholangiocarcinoma cells exhibited a higher percentage of apoptotic cells. (a–b) CCLP cells, 11.13 ± 0.76% vs. 5.12 ± 0.45%, P < 0.05. (c–d) HUCCT cells, 12.45 ± 0.68% vs. 3.6479 ± 0.34%. (e) The percentage of apoptotic cells.
Agrin enhances CCA metastasis in vitro
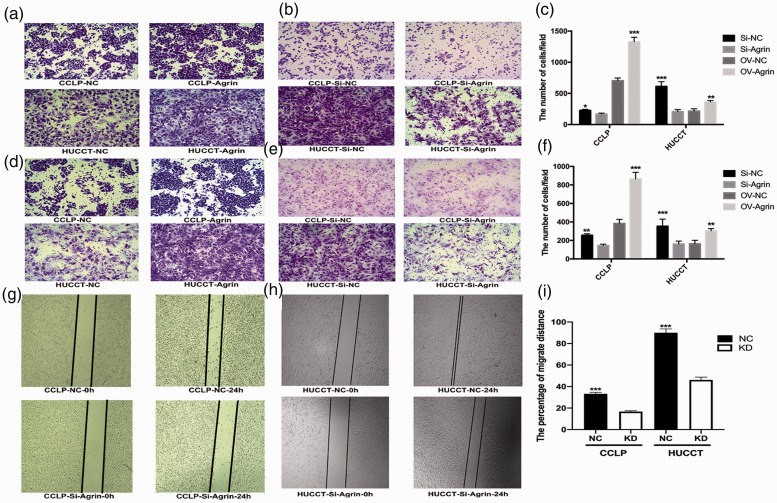
Transwell and wound-healing assays were used to evaluate the effects of agrin on the migration and invasion of CCA cells in vitro. Agrin shRNA-transfected cells were less likely to migrate to the upper chamber than control cells, and this effect was blocked by ectopic agrin expression (all P < 0.05, Figure 4a–f). Moreover, the wound-healing rate at 24 hours was significantly lower in agrin-depleted CCA cells (P < 0.001, Figure 4g–i). These results suggested that agrin enhanced CCA cell migration and invasion.
Figure 4.
Ectopic agrin expression promoted migration and invasion (a) but agrin depletion apparently decreased migration and invasion in CCLP cells (b). (c) Mean number of cells per field. (d) Ectopic agrin expression promoted migration and invasion in HUCCT cells. (e) Agrin depletion apparently decreased migration and invasion in HUCCT cells. (f) Mean number of cells per field. The wound-healing rate over 24 hours was significantly inhibited following agrin depletion in CCLP (g) and HUCCT cells (h). (i) Percent migration distance.
Agrin increases CCA cell tumorigenesis in vivo
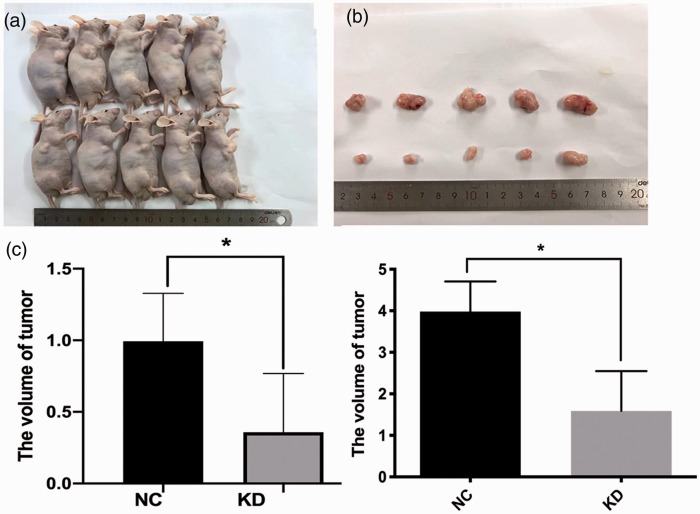
We investigated the role of agrin in tumor growth in nude mice. Agrin shRNA-transfected and negative-control CCLP cells were subcutaneously injected into nude mice (n = 5 per group). Compared with the control findings, mice injected with agrin-depleted cells displayed apparent decreases in tumor size and weight (both P < 0.05, Figure 5).
Figure 5.
Compared with the control findings, agrin-depleted CCLP cells exhibited apparently decreased tumorigenesis. (a) Tumors in mice. (b) Tumors excised from mice. (c) Tumor volume. (d) Tumor weight.
Agrin promotes YAP and TAZ expression
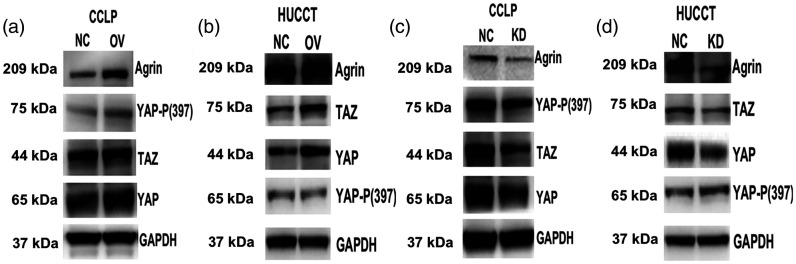
Previous data disclosed that agrin activated Lrp4/MuSK receptor-mediated signaling pathways to maintain the integrity of cellular focal adhesion,13 a critical mediator of the Hippo signaling pathway.14 We therefore analyzed the role of agrin in regulating the Hippo signaling pathway. Western blotting demonstrated that the upregulation of agrin slightly increased the phosphorylation of MOB1 in CCA cells (Figure 6a–b), whereas it promoted YAP phosphorylation in CCLP cells (Figure 6a) but not in HUCCT cells (Figure 6b). However, agrin-overexpressing CCA cells exhibited apparent increases in YAP and TAZ expression. As expected, agrin depletion noticeably inhibited the expression of YAP and TAZ in CCLP (Figure 6c) and HUCCT cells (Figure 6d). These data further confirmed the effect of agrin on promoting Hippo–YAP signaling activation.
Figure 6.
Western blotting revealed that agrin upregulation significantly increased TAZ and YAP protein expression in CCLP (a) and HUCCT cells (b). Conversely, agrin depletion inhibited TAZ and YAP expression in CCLP (c) and HUCCT cells (d).
Discussion
CCA is a malignant cancer with high incidence rates globally, but its underlying molecular mechanism has yet to be clarified. Great advances have been made in understanding CCA, but the tumor recurrence rate for CCA remains high after surgery. Therefore, the need for a potentially effective approach against CCA is urgent. Recently, multiple lines of evidence indicated that agrin plays an oncogenic role in promoting tumorigenesis.15 In this study, the function of agrin in CCA-related processes was investigated for the first time. The results demonstrated that ectopic agrin expression promoted proliferation, colony formation, migration, invasion, and tumorigenesis in CCA cells. The frequently increased expression of agrin in clinical CCA specimens and its correlation with poorer tumor characteristics and higher postoperative tumor recurrence rates were also verified. Because cytomembrane proteins are the most well-known therapeutic targets in multiple types of malignancies, the present results suggested that targeting agrin may be a promising treatment strategy for CCA.
The underlying molecular mechanism of the function of agrin was first defined in cultured myotubes. Wallace et al.16 reported the important role of agrin in promoting AChR aggregation by inducing tyrosine phosphorylation of the AChR β-subunit. Several proteins act as agrin receptors, including the receptor tyrosine kinase MuSK, which can form a primary structural scaffold for recruiting synaptic components. In addition, agrin activated MuSK with a Z-site consisting of 8, 11, or 19 amino acids to promote the organization of AChRs and other neuromuscular junction components.17 Another study demonstrated that agrin could potentially treat myasthenia gravis and other neuromuscular disorders by increasing the number of AChRs and enhancing the signal transduction of the neuromuscular junction.18 These results provided solid evidence that agrin induces the activation of the AChR signaling pathway. Recent studies revealed a close relationship between AChRs and tumor development. Following AChR agonist treatment, multiple cancer cells underwent epithelial–mesenchymal transition (EMT), and the metastatic ability of the cells was increased through the activation of M2 muscarinic receptors (M2Rs); however, blockade of M2R signaling reversed this phenomenon.19,20 EMT is necessary for cancer cells to breach the underlying basement membrane and extracellular matrix. However, Chakraborty et al. revealed that the molecular mechanism underlying the regulation of EMT by agrin does not involve AChRs. The study also demonstrated that agrin promoted invadopodia formation and activated the integrin–FAK pathway to regulate extracellular matrix degradation and drive EMT, suggesting that the biofunction of agrin is not limited to the neuromuscular junction.13
Furthermore, Dasgupta et al.21 defined the role of agrin in increasing intracellular Ca2+ concentrations after KCl or caffeine therapy to promote the development of excitation–contraction coupling and myotube maturation. Pirkmajer et al.22 revealed that neural agrin increased both the expression and activity of Na+/K+-ATPase to regulate skeletal muscle function in response to extrinsic stimuli. Moll et al.23 demonstrated that agrin binds to α-dystroglycan and promotes the stabilization of the laminin α5 chain to attenuate dystrophic symptoms. These findings suggested that the mechanism of action of agrin is far from being clarified. Membrane α-dystroglycan serves as a mechanical bridge between the cytoskeleton and extracellular matrix, and it binds dystrophin with other associated proteins, thereby forming the dystrophin–glycoprotein complex24 and promoting cardiomyocyte differentiation and regeneration.25 The genes of the dystrophin–glycoprotein complex were recently disclosed to be targets of the Hippo signaling pathway, and inhibition of the link between the dystrophin–glycoprotein complex and the musculoskeletal system reduced Hippo signaling26 and matrix rigidity.27 This evidence hinted at a close correlation between Hippo signaling and agrin. Therefore, in the present study, we investigated whether the effects of agrin on tumorigenesis depend on the Hippo signaling pathway. After upregulating agrin, increased YAP phosphorylation and decreased YAP phosphorylation were observed in CCA cells, and these changes were blocked by agrin-shRNA transfection. Consistent with these findings, Chakraborty et al.28 reported that agrin relied on the Hippo pathway to enhance oncogenic activities.
The nuclear complex YAP/TAZ combines multiple transcription factors to promote a series of oncogenic transcriptions and increase the malignant ability of tumors.14 Its function and expression are tightly regulated by two major upstream kinases (Mst1/2 and LATS1/2), which act as converging effectors of the Hippo pathway.29,30 The mechanism by which agrin regulates YAP activity is considered the relay of mechanosignaling from the extracellular matrix to intracellular YAP/TAZ because agrin is secreted and enriched in the basement membrane. Previous data verified that extracellular matrix stiffness is one of the main stimulants of the translocation of YAP to the nucleus, which facilitates the transcription of target genes.31 Chakraborty and colleagues built a model in which extracellular matrix stiffness was manipulated by enhancing collagen matrix concentrations. Using this model, this group observed agrin inhibition in cells cultured in stiff extracellular matrix resulted in reduced YAP nuclear localization and transcriptional activity, thereby weakening the local extracellular matrix and providing considerable contractile strength to the cancer cells.32 The other potential mechanism through which agrin regulates YAP activity is increasing cell spreading and cytoskeletal tension by manipulating F-actin distributional changes.33
Notably, this study had several inevitable limitations. First, this was a single-center study with a small number of samples, and additional validation with more samples is needed. In addition, the molecular mechanism is derived from a literature review and speculation, and verification via by gene microarray has not been performed. Hence, the basic contributing mechanisms require further assessment.
In conclusion, the present study demonstrated that agrin is an important promoter of the activation and coordination of proliferation, migration, and invasion in CCA cells, and agrin overexpression might be a prognostic marker. Additional therapeutic strategies against CCA could be developed by targeting agrin in the future.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials: The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors’ contributions: All authors contributed to the analysis and preparation of the manuscript. MH and DL conceived and design the study. MH ,BL and SJ performed the experiment and collected related data. JT and CC analyzed and interpreted the data. MH drafted the manuscript.
ORCID iD: Dan Lou https://orcid.org/0000-0002-9269-4737
Supplemental material: Supplementary material for this article is available online.
References
- 1.Siegel RL, Jemal A, Wender RC, et al. An assessment of progress in cancer control. CA Cancer J Clin 2018; 68: 329–339. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017; 11: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011; 54: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg 2012; 256: 297–305. [DOI] [PubMed] [Google Scholar]
- 5.Ma LJ, Feng FL, Dong LQ, et al. Clinical significance of PD-1/PD-Ls gene amplification and overexpression in patients with hepatocellular carcinoma. Theranostics 2018; 8: 5690–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J Cell Biol 2000; 151: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisgerber C, Bousquet R, Teillet F. [ Laparotomy with splenectomy in Hodgkin's disease. Critical study of results in 123 patients]. Nouv Presse Med 1975; 4: 1797–1800. [PubMed] [Google Scholar]
- 8.Nitkin RM, Smith MA, Magill C, et al. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol 1987; 105: 2471–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones G, Meier T, Lichtsteiner M, et al. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc Natl Acad Sci U S A 1997; 94: 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautam M, Noakes PG, Moscoso L, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 1996; 85: 525–535. [DOI] [PubMed] [Google Scholar]
- 11.Samsudin and Williams ML. Rational use of skim milk in a complete infant formula. II. Clinical study in premature infants. Am J Clin Nutr 1967; 20: 1308–1311. [DOI] [PubMed] [Google Scholar]
- 12.Rivera C, Zandonadi FS, Sanchez-Romero C, et al. Agrin has a pathological role in the progression of oral cancer. Br J Cancer 2018; 118: 1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S, Lakshmanan M, Swa HL, et al. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun 2015; 6: 6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015; 163: 811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong WC, Mei L. Agrin to YAP in Cancer and Neuromuscular Junctions. Trends Cancer 2017; 3: 247–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace BG, Qu Z, Huganir RL. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron 1991; 6: 869–878. [DOI] [PubMed] [Google Scholar]
- 17.Apel ED, Glass DJ, Moscoso LM, et al. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 1997; 18: 623–635. [DOI] [PubMed] [Google Scholar]
- 18.Ohno K, Ohkawara B, Ito M. Agrin-LRP4-MuSK signaling as a therapeutic target for myasthenia gravis and other neuromuscular disorders. Expert Opin Ther Targets 2017; 21: 949–958. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q, Gu X, Zhang C, et al. Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC). Cancer Biol Ther 2015; 16: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta P, Rizwani W, Pillai S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer 2009; 124: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandi E, Jevsek M, Mars T, et al. Neural agrin controls maturation of the excitation-contraction coupling mechanism in human myotubes developing in vitro. Am J Physiol Cell Physiol 2008; 294: C66–C73. [DOI] [PubMed] [Google Scholar]
- 22.Pirkmajer S, Chibalin AV. Na,K-ATPase regulation in skeletal muscle. Am J Physiol Endocrinol Metab 2016; 311: E1–E31. [DOI] [PubMed] [Google Scholar]
- 23.Moll J, Barzaghi P, Lin S, et al. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 2001; 413: 302–307. [DOI] [PubMed] [Google Scholar]
- 24.Henry MD, Campbell KP. Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol 1996; 8: 625–631. [DOI] [PubMed] [Google Scholar]
- 25.Bassat E, Mutlak YE, Genzelinakh A, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017; 547: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morikawa Y, Zhang M, Heallen T, et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal 2015; 8: ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yahalom-Ronen Y, Rajchman D, Sarig R, et al. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife 2015; 4: e07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty S, Njah K, Pobbati AV, et al. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep 2017; 18: 2464–2479. [DOI] [PubMed] [Google Scholar]
- 29.Moroishi T, Hayashi T, Pan WW, et al. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 2016; 167: 1525–1539.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 2008; 18: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474: 179–183. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty S, Hong W. Linking Extracellular Matrix Agrin to the Hippo Pathway in Liver Cancer and Beyond. Cancers (Basel) 2018; 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elosegui-Artola A, Andreu I, Beedle AEM, et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017; 171: 1397–1410.e14. [DOI] [PubMed] [Google Scholar]