Abstract
Balloon pulmonary angioplasty (BPA) is an emerging treatment option for patients with chronic thromboembolic pulmonary hypertension (CTEPH) who have inoperable, segmental/subsegmental disease, or residual disease after pulmonary endarterectomy. In the past decade, advances in the techniques for BPA have led to better clinical outcomes with improvements in hemodynamics, pulmonary perfusion, exercise tolerance, functional capacity, and quality of life. We present the experience with BPA at our university, the largest CTEPH center in the world, followed by reviewing the published data regarding the efficacy and safety of BPA in patients with CTEPH. There is increasing evidence to support that the initial hemodynamic improvement is sustained for ≥3 years after the procedure. Although infrequent, complications observed with BPA are associated with pulmonary vascular injury or rarely reperfusion pulmonary edema. As the technique for percutaneous pulmonary artery revascularization has improved, the procedural risk and complications have continued to decrease. This promising technique continues to develop, and future research is required to demonstrate the long-term benefits of BPA, standardize the technique, and define a uniform institutional infrastructure for providing BPA as a part of the treatment of CTEPH.
Keywords: chronic thromboembolic pulmonary hypertension, balloon pulmonary angioplasty, pulmonary hypertension
Introduction
Balloon pulmonary angioplasty (BPA) is an emerging percutaneous treatment option for patients with chronic thromboembolic pulmonary hypertension (CTEPH). The procedure uses angioplasty techniques to widen narrowed or occluded pulmonary arteries, with the aim of disrupting organized, flow-limiting obstructions, improving pulmonary vascular blood flow, and ultimately leading to revascularization of diseased areas in the lung.1 Although pulmonary endarterectomy (PEA) surgery is the treatment of choice for eligible patients with CTEPH,2 guidelines recommend that BPA may be considered in patients who are technically inoperable, have an unfavorable surgical risk–benefit ratio, or have persistent/recurrent pulmonary hypertension (PH) after PEA.3 It is recommended that eligibility for BPA should be assessed by a multidisciplinary team and the procedure be performed in experienced, high-volume, expert CTEPH centers.2,3
The first case report of BPA in a patient with CTEPH was published in 1988, with a subsequent case series published in 2001.4,5 Since then, BPA techniques have been progressively refined, leading to improved clinical outcomes and fewer complications.6–13 In particular, data have been published from several Japanese and European centers that have been instrumental in demonstrating the efficacy and safety of BPA.9,13–17 Mizoguchi et al.15 published an observational study of 68 Japanese patients with inoperable CTEPH who underwent 255 BPA sessions resulting in a reduction in mean pulmonary arterial pressure (mPAP) from 45.4 ± 9.6 to 24.0 ± 6.4 mmHg, an improved 6-min walking distance (6MWD) from 296 ± 108 m to 368 ± 83 m, and a reduced World Health Organization functional class (WHO FC) from III to II (all P < 0.01). However, after BPA, 60% of patients developed a reperfusion injury, of which 6% of patients required mechanical ventilation, and the overall mortality rate was 1.5%.
Our university BPA experience
We introduced the BPA procedure at our university, the largest CTEPH center in the world, in early 2015. Having been introduced to the technique by colleagues in Japan, we sought to further refine and standardize the technique, equipment, and treatment strategy.18 During our university CTEPH Symposium, a BPA procedure was demonstrated live by Dr. Ehtisham Mahmud (the first author of this article).
Equipment
The equipment used for BPA procedures at our university is shown in Table 1. Target vessels are evaluated before and during BPA by selective segmental pulmonary artery angiography to assess vessel size, lesion size and characteristics, and pulmonary artery flow.18 Venous access is gained with a 9-French sheath, preferably via the femoral vein or alternatively via the internal jugular vein, and a 6-French 90 cm sheath is telescoped through the access sheath toward the target pulmonary artery segment over a 0.035-inch wire. A 6-French 110 cm guiding catheter (Judkins Right 4, Multipurpose, Extra Backup, or Hockeystick, depending on the location of the target lesion) is then inserted through the sheath to the target pulmonary artery.
Table 1.
Equipment used for BPA at our university.
| Routine BPA equipment |
| • 9-French outer sheath, 12 cm length |
| • Swan--Ganz catheter (baseline hemodynamics) |
| • Wedge catheter (0.035″ lumen) |
| • 0.035″ J-tipped wire, exchange length |
| • 6-French Brite-tip inner sheath, 90 cm length |
| • 6-French FR4 guiding catheter, 110 cm length |
| • Stiff angled Glidewire, 150 cm length |
| • 0.014″ workhorse guidewire (BMW, Runthrough, SION blue), 180 cm length |
| • 2.0–4.0 × 15–20 mm rapid-exchange semi-compliant balloon |
| Specialized BPA equipment |
| • Navvus microcatheter (distal pressure measurement) |
| • Noncompliant, sculpting, or scoring balloons (recalcitrant lesions) |
The use of several BPA tools has been optimized at our university to improve outcomes and minimize vascular injury.18 During BPA, 0.014-inch guidewires with soft, atraumatic tips are used to cross lesions, while polymer-jacketed wires are generally avoided, to reduce the risk of perforations. Balloon inflation is performed using compliant and noncompliant balloons with a diameter of 2–5 mm. Sculpting or scoring balloons are used for recalcitrant lesions, but use of cutting balloons is avoided due to the risk of vessel injury. Measurement of pressure distal to the target lesion can characterize the hemodynamic significance of angiographic abnormalities and provide information on potential for reperfusion pulmonary edema (RPE),19 although this is not routinely performed at our university. At many centers, pressure wires are used to make this measurement, but a pressure-measuring catheter Navvus (ACIST, Eden Prairie, MN) that can be used with any commercially available wire is used more frequently at our institution. Intravascular imaging modalities, such as intravascular ultrasound and optical coherence tomography, can also be used for lesion characterization and BPA guidance. However, these technologies are rarely used for BPA at our university due to increased cost, risk of potential complications, case prolongation, and lack of evidence for improved outcomes.
Approach
Anticoagulation is provided during the procedure using heparin, administered to achieve an activated clotting time of 200–250 s. After injection of a 50/50 contrast/saline solution, single-plane pulmonary angiography is performed (biplane angiography is used for diagnostic purposes only). Typical lesions observed with angiography include fibrous webs, bands, intimal irregularities, occlusions, and pouches. Balloon sizing is largely based on angiographic assessment with very low complication rates; however, intravascular imaging modalities may be of value to accurately determine optimal balloon size and reduce the risk of BPA complications.15,20 Data from a small study (n = 9) suggest that optical coherence tomography may be superior to intravascular ultrasound in measuring luminal diameters for balloon size determination due to the improved resolution of the technique.20 However, the forceful injection of contrast required for optical coherence tomography may increase the perfusion pressure, resulting in pulmonary injury.13
To determine the initial region of treatment, angiographic and perfusion (ventilation--perfusion (V/Q) scanning or perfusion-mapped computed tomography) assessment can help to determine which lung areas are amenable to BPA or which under-perfused lung areas should be prioritized. Bilateral lower lobes can be differentially affected; therefore, perfusion defects on nuclear imaging can help guide which areas to treat initially. Typically, the patients require 2–6 BPA sessions, with one or two pulmonary lobes (2–5 segments) treated per session. The number of vessels treated in each session is limited by the total fluoroscopy time and contrast volume.18 Treatment is limited to a single lung in each session to facilitate management of complications should they occur (Fig. 1). Two sessions, treating one lung at a time, are performed during a 3- to 7-day period, and patients return within 1–3 months for subsequent treatment sessions. Patients receive moderate sedation during the procedure but must not be deeply sedated as deep breath holds are imperative for technical success of the procedure.21 In cases of severe PH (mPAP > 50 mmHg), simpler lesions are addressed initially, to reduce the mPAP sufficiently to minimize the risk of procedural complications which are associated with higher degrees of PH. Smaller-diameter balloons may also be used in such instances to limit the risk of reperfusion injury. After each procedure, patients are admitted for monitoring, typically for 24 h, and are started on an intravenous heparin infusion to provide a bridge to outpatient oral anticoagulation. Baseline and interval assessments to measure clinical response to BPA therapy include V/Q scanning, echocardiography, 6MWD, hemodynamics, and N-terminal prohormone of brain natriuretic peptide levels.
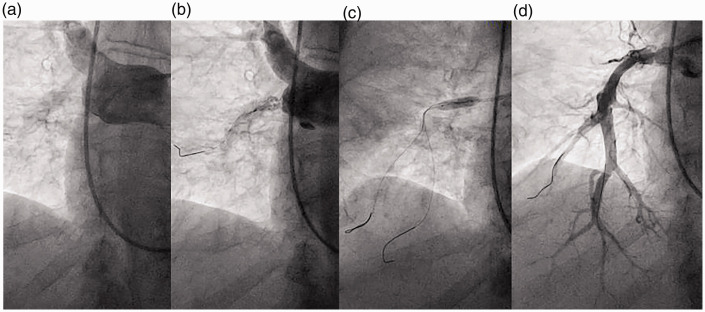
Fig. 1.
Balloon pulmonary angioplasty of right pulmonary artery pouch occlusion. A small fenestration emanating from the baseline interlobar pouch occlusion (a) is crossed using a workhorse wire and dilated using a 2-mm-diameter balloon (b). Serial dilatation using noncompliant balloons up to a 5-mm diameter (c) restores perfusion to branches of the A9 and A10 lower lobe segments (d). The patient was ineligible for pulmonary endarterectomy due to numerous prohibitive surgical comorbidities.
While BPA of total occlusions has been associated with higher complications and lower success rates, successful revascularization can be achieved, as demonstrated in this manuscript (Fig. 1) and others.1,11,18 Such lesions are commonly treated by PEA, but opportunities for BPA arise in patients ineligible for PEA due to surgical comorbidities, inoperable distal disease, or patient preference. For these lesions, prudent BPA treatment strategies are used to mitigate complications. First, initial treatment of simpler nonocclusive lesions is prioritized to optimize hemodynamics in the context of severe PH. Second, atraumatic workhorse wires supported by balloon catheters, rather than jacketed or higher tip-load specialty wires that may be related to vessel perforation,11,13 are used to traverse each occlusion. Last, techniques to confirm safe wire positioning, such as lesion Dottering, serial angiography, and low-pressure initial balloon inflations, are employed. In general, BPA of total occlusions can be performed using these techniques along with a detail-oriented and cautious approach.
Patient selection for BPA
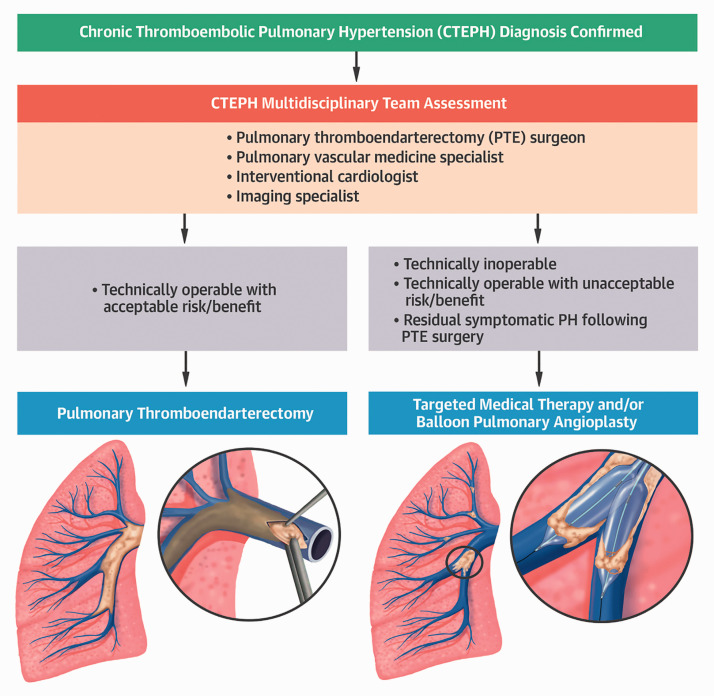
Selection of patients with CTEPH for BPA is not well standardized, and the assessment of operability depends on surgical expertise, patient comorbidities, disease distribution, severity of vascular occlusion, lesion types, hemodynamic impairment, and patient preference.1,18 A multidisciplinary expert team is needed to assess the objective data and risk–benefit profile for patients who are ineligible for PEA, technically operable for PEA but with an unacceptable risk–benefit ratio, or who have residual symptomatic PH following PEA (Fig. 2).1 The surgical classification for level of disease in patients with CTEPH is based on the location of the chronic thromboembolism.3 Patients with level III chronic thromboembolism (starting at the level of the segmental arteries) or level IV chronic thromboembolism (starting at the level of the subsegmental arteries) are best suited for BPA; however, this classification system has not been optimized for BPA as it is determined during surgery.
Fig. 2.
Suggested CTEPH treatment algorithm by the multidisciplinary of our university CTEPH team. Once the diagnosis of CTEPH is confirmed, patients are evaluated for PEA (also referred to as PTE) surgery. Targeted medical therapy and/or balloon pulmonary angioplasty (BPA) is considered in cases deemed to be inoperable, with persistent symptomatic pulmonary hypertension after PEA/PTE surgery, or with an unacceptable surgical risk/benefit ratio. Patients often undergo combined medical therapy and BPA for optimal hemodynamic and clinical results.
CTEPH: chronic thromboembolic pulmonary hypertension; PTE: pulmonary thromboendarterectomy; PH: pulmonary hypertension.
BPA complications
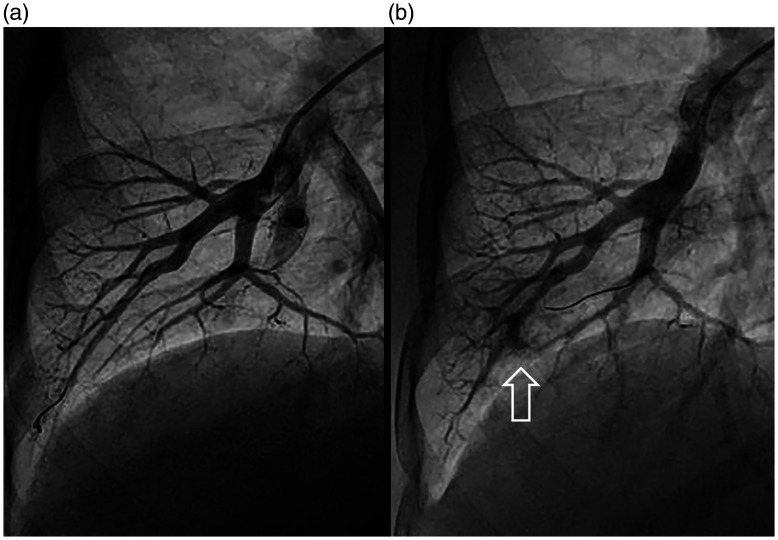
The types of complications with BPA have recently been classified as those occurring during the procedure and those occurring after the procedure.3 The complications that can arise during BPA include vascular injury (wire perforation, balloon over-dilatation, high-pressure contrast injection) with or without hemoptysis, vascular dissection, allergic reaction to contrast, and adverse reaction to conscious sedation or local anesthesia. Post-BPA complications include lung injury (radiographic opacity, with or without hemoptysis and with or without hypoxemia), acute kidney injury, and access-site complications. Of these, the most common complications are various forms of procedural pulmonary vascular injury and, rarely, RPE, a form of lung injury (Fig. 3).18
Fig. 3.
Lung injury during a BPA procedure. Baseline appearance of a right A8 segment (a) is compared beside an angiogram following guidewire insertion into the A8 medial branch, patient hemoptysis, and angiographically apparent distal vessel injury (b, arrow).
Management of hemoptysis during BPA involves immediate balloon tamponade of the injured vessel.18 Oxygenation management is also provided, including oropharyngeal suctioning and supplemental oxygen, cessation or reversal of anticoagulation, and repeat balloon tamponade as necessary. For persistent pulmonary hemorrhage, mechanical ventilation and extracorporeal membrane oxygenation may be required, as well as bailout transcatheter coil embolization, covered stent implantation, and/or gelfoam/adipose injection.
Our university BPA outcomes
At the time of our university CTEPH Symposium, analysis of the prospective BPA registry at our university (NCT03245268) revealed that 95 patients (59.4 ± 14.7 years; 34% male) had been treated at our institution since the initiation of the program. Baseline characteristics revealed that 57% were on chronic oxygen therapy, 81% had a prior history of pulmonary embolism, 16% had a history of pulmonary thromboendarterectomy, and 17% had a splenectomy. The majority of patients (84%) had distal segmental/subsegmental disease, while 17% had prohibitively high surgical risk.
The 95 patients underwent a total of 402 treatment sessions. The average number of BPA sessions per patient was 4.2, with 3.2 segments targeted per session. After BPA, several hemodynamic parameters (right atrial pressure, systolic pulmonary arterial pressure, diastolic pulmonary arterial pressure, mPAP, pulmonary artery wedge pressure, cardiac output, and pulmonary vascular resistance (PVR)) were significantly improved, as were WHO FC and 6MWD. Modest decreases in PH-targeted medical therapy were also observed. In the 39 patients with a baseline mPAP > 30 mmHg who had completed all their treatment sessions, there was a reduction in mPAP (42.7 ± 7.3 to 34.1 ± 7.9 mmHg; P < 0.01) and a reduction in mean PVR (6.1 to 3.9 Wood Units, P < 0.01). There was an improvement in WHO FC (80% Class III/IV to 35% Class III/IV, P < 0.01) and 6MWD (368 m to 421 m, P < 0.01). Analysis of our university BPA registry showed hemoptysis in 8% of procedures, lung vascular injury in 1.5% without any mortality or patient requiring intubation.
Literature review of BPA outcomes
Short-term improvements in hemodynamics, pulmonary perfusion, exercise tolerance, functional capacity, and quality of life have been demonstrated after BPA from multiple international centers.13–16,22–25 A meta-analysis of 17 observational studies that included 670 patients who received BPA (median of four BPA sessions per patient across studies) found that: mPAP decreased by 14.2 mmHg (95% confidence interval (CI): –18.9, –9.5; P < 0.00001), PVR decreased by 303.5 dyn·s/cm5 (95% CI: –377.6, –229.4; P = 0.0001), mean right atrial pressure decreased by 2.7 mmHg (95% CI: –4.1, –1.3; P = 0.02), and cardiac output increased by 0.2 L/min (95% CI: 0.0, 0.3; P < 0.00001).24 Additionally, there was a significant increase in 6MWD (+67.3 m (95% CI: 53.8, 80.8); P < 0.0001) after BPA.
These data include the largest multicenter registry of all patients undergoing BPA in Japan between November 2004 and March 2013 (n = 308; 1408 BPA sessions) where the mPAP decreased from 43.2 ± 11.0 to 24.3 ± 6.4 mmHg after the final BPA procedure (n = 249; P < 0.001) with a significant reduction in the use of pulmonary arterial hypertension-targeted medical therapies (P < 0.001).9 Complications after BPA occurred during 36% of sessions and 4% of patients died during follow-up. Several European studies have also been published demonstrating improved outcomes after BPA.7,8,12,16 Most recently, in a retrospective study of 154 French patients with CTEPH (1006 BPA sessions), significant improvements were observed in WHO FC, 6MWD (mean change +45 m), mPAP (–26%), and PVR (–43%) (all P < 0.001), with complications reported in 11% of sessions.17
Significant improvements in functional status after BPA have been reported in several studies16,25 including an observational study in Norway in which 73 BPA sessions were performed in 20 patients with CTEPH, leading to an improvement in WHO FC from 3.0 ± 0.5 to 2.0 ± 0.5 (P < 0.001).16 Similarly, a retrospective analysis of 24 Japanese patients with inoperable CTEPH who underwent 1–6 BPA sessions per patient showed a numerical improvement in WHO FC I/II/III/IV from 0/3/18/8% to 11/14/3/0%.25 Improvements in ventilatory parameters such as peak workload, peak oxygen consumption, pulse oximetry, ventilatory response to carbon dioxide production,23 and indices of right ventricular function26 have also been reported after BPA in patients with CTEPH. Furthermore, improvements in quality of life after BPA have been observed in 25 patients with inoperable or persistent CTEPH who underwent a total of 96 sessions.22
Studies have demonstrated that the improvements observed immediately after BPA are maintained in the long term (≥3 years of follow-up), although the data are more limited than those demonstrating short-term improvements.9,27 In a Japanese center where 649 BPA sessions were performed in 170 patients with CTEPH between 2009 and 2016, the 1-, 3-, and 5-year overall survival rates were 99% (95% CI: 95, 100), 98% (95% CI: 94, 99), and 96% (95% CI: 86, 99), respectively.27 Initial improvements in mPAP and PVR, though not cardiac index, were maintained for >3.5 years. The percentage of patients not requiring pulmonary arterial hypertension-targeted drugs increased from 9% at baseline to 72% (95% CI: 65, 78) at long-term follow-up (>3.5 years) after BPA. Freedom from home oxygen therapy also increased numerically, from 15% at baseline to 39% (95% CI: 25, 52) at long-term follow-up. Long-term improvements were also seen in a multicenter registry that assessed 308 patients who underwent 1408 procedures at seven institutions in Japan.9 Overall survival was 97% (95% CI: 94, 98) at 1 and 2 years and 95% (95% CI: 89, 97) at 3 years after the initial BPA procedure. At a mean of 425.5 ± 280.9 days after the final procedures (n = 196), initial improvements in hemodynamic parameters were maintained, with a decrease in mPAP from 43.2 ± 11.0 to 24.3 ± 6.4 mmHg after final BPA and 22.5 ± 5.4 mmHg at follow-up (both P < 0.001). Significant reductions in the concomitant use of pulmonary arterial hypertension-targeted therapy and oxygen supplementation were also observed during long-term follow-up in this study.
While technical and clinical success rates are often cited by BPA centers, the occurrence of BPA “nonresponse” is not as commonly reported or well appreciated for multiple reasons. First, there is no universal definition of BPA technical or clinical response (e.g. by procedural steps, angiography, hemodynamic measurements, walking distance, functional classification, or perfusion imaging). Second, BPA treatments are not uniform; complete treatment for different patients is achieved by a different number of treatment sessions, and each BPA session addresses a different vascular territory with variable burden of disease. Third, detectable response to each treatment can be delayed by days to weeks, and there is no universal time frame or number of sessions within which definitive treatment response must be determined. Last, the most common measures of BPA response (including right heart catheterization hemodynamics, functional classification, 6MWD, and medical regimen surveillance) may not be sensitive enough to detect response to every BPA treatment. Despite these limitations, and to avoid treatment failures, an approach to treat all vessels and lesions as completely and safely as possible is prudent.
BPA for chronic thromboembolic disease without PH
Patients who have dyspnea on exertion and chronic thromboembolic occlusions of the pulmonary vasculature, but who do not meet the hemodynamic definition of PH at rest, are described as having chronic thromboembolic disease (CTED).3 Patients with CTED demonstrate an abnormal pulmonary hypertensive response during exercise right heart catheterization, gas exchange inefficiency during cardiopulmonary exercise testing, or both.28 It is not clear whether these are different phenotypes of the condition.28 Furthermore, a proposed change to the PH definition from mPAP > 25 mmHg to >20 mmHg would lead to more patients being diagnosed with CTEPH rather than CTED.28 A prospective study of 34 patients with CTED in the UK reported that up to 56% had mPAP ≤20 mmHg.29
BPA has been performed in small numbers of patients with CTED. Preliminary data have shown improvements in functional parameters and hemodynamics after BPA in patients with symptomatic CTED,30–32 although larger prospective studies are required to further investigate these findings. An observational study in Germany assessing 10 patients with CTED judged to be inoperable for PEA reported a significant reduction in PVR (234 ± 68 to 167 ± 40 dyn·s/cm5; P = 0.004) and an improvement in WHO FC (I/II/III/IV: 0/10/90/0% to 40/50/10/0; P = 0.004).30 In a Japanese study of 15 patients with CTED who underwent BPA, 6MWD and hemodynamics were significantly improved, and the use of home oxygen therapy was reduced from 53% to 7% (P = 0.01).31 Another Japanese study of patients with CTED (n = 23) also demonstrated a reduction in mPAP (21.6 ± 2.3 to 17.1 ± 2.6 mmHg; P < 0.01) and PVR (278 ± 80 to 198 ± 63 dyn·s/cm5; P < 0.01) and an improvement in both peak oxygen consumption (14.6 ± 4.4 to 17.4 ± 4.2 mL/min/kg; P < 0.01) and WHO FC (I/II/III/IV; 0/10/12/1 to 9/12/2/0; P < 0.01) after BPA.32
It should be noted, however, that the data showing the effects of BPA in CTED are limited; there are several CTED phenotypes, and our understanding of the pathophysiology of CTED is incomplete.3,28 Careful selection of patients with CTED based on their individual risk–benefit profile is therefore needed. Importantly, there is no evidence yet to suggest that CTED can progress to CTEPH.3 CTEPH treatment guidelines should therefore not be applied to patients with CTED without sufficient safety and efficacy data specific to CTED for individual treatment modalities.
BPA complications
Pulmonary injury has been reported in 0–26% of BPA sessions with hemoptysis, a sign of several possible complications, also reported in 0–50% of sessions.6,13 Signs of vascular injury include hypoxemia, a new cough, tachycardia, increased PAP, and hemoptysis,3 as well as extravasation of contrast, and new localized and dense lung opacities on computed tomography scans (Fig. 4). In a study of pulmonary artery injuries in 540 BPA sessions performed in 143 patients with CTEPH,33 four categories of pulmonary artery injuries were identified: wire perforation (8%); pulmonary artery dissection (1%); pulmonary artery rupture, including oozing rupture after balloon over-dilation (0.6%); and high-pressure perfusion injury (0.3%). BPA-related vascular injury is predictive of lung injury after BPA, and its severity is exacerbated by a high mPAP.34 Post-procedure lung injury results from vascular injury that is much greater than reperfusion lung injury.3 Lung injury, with or without hemoptysis, can occur immediately or a few hours after BPA with varying severity.
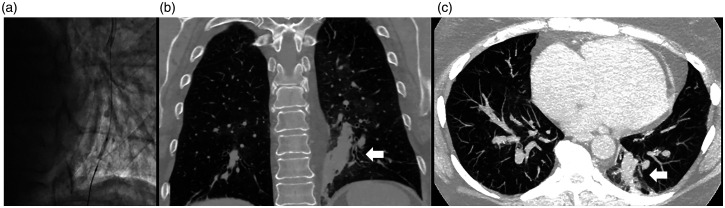
Fig. 4.
Computed tomography (CT) images after BPA. BPA of an occluded left A10 posterior basal segment is performed using a workhorse wire and 2-mm-diameter balloon low-pressure inflation without angiographic evidence of vessel injury (a). Contrast chest CT images in coronal (b) and sagittal (c) views identify the source of vessel injury (arrows) associated with patient hemoptysis during overnight observation after BPA. Occult bleeding was considered to be due to wire-induced side branch injury in the context of mPAP 50 mmHg and PVR > 8 Wood Units.
The reported incidence of RPE per BPA in the literature ranges from 0 to 61%, with RPE usually developing 24–72 h after BPA.6,13 We believe that many of the initial reports of RPE miscategorized patients as having RPE. Many of these episodes were more likely related to vascular injury. Risk factors for developing RPE include severe baseline PH (mPAP > 40 mmHg and/or PVR > 7 Wood Units) and underdeveloped bronchial arteries.13,18 The use of undersized balloons and reducing the number of vessels treated per session may help to reduce the incidence of RPE.18
The risks associated with BPA can vary depending on the level of expertise and experience of the care team. As techniques for BPA have been refined over the past 10 years, the overall risks of complications and mortality have decreased.7,13 For example, lower incidences of complications were observed in the most recent 21-month observation period compared with the initial 21-month observation period in a French study assessing 184 patients with inoperable CTEPH who underwent 1006 BPA sessions between 2014 and 2017.7 Overall complications per session reduced from 16% to 8%, lung injury from 13% to 6%, hemoptysis from 8% to 6%, and pulmonary artery perforation from 4% to 2%. The strongest predictors of lung injury were the period of BPA procedure and baseline mPAP,7 although recent data suggest that lesion morphology (specifically the presence of occlusive lesions), rather than hemodynamics, is predictive of complications.35 A study of 19 patients with CTEPH found that higher right, left, and main pulmonary artery diameter indices were significant predictors for lung injury after BPA.36 Where BPA is conducted on total occlusions, microcatheters and wires with higher tip loads and small-diameter balloons are used.13,37
Overall, periprocedural mortality rates during BPA range from 0 to 10% across several BPA studies1,18 with the latest French BPA study reporting periprocedural deaths related to severe lung injury in 2% of patients.7
Medical therapy and/or BPA
The use of medical (“bridging”) therapy before BPA, to improve hemodynamics and potentially reduce the risk of complications, or after BPA, in patients who had an inadequate response to the procedure, may be promising therapeutic options.13,38 Indeed, several studies have used pulmonary arterial hypertension-targeted drugs prior to BPA with favorable outcomes.13–15,19,39 However, robust data are lacking regarding this therapeutic hybrid approach, and prospective studies are required to understand the role of this option in the treatment pathway.
To further understand the role of BPA and medical therapy in management of CTEPH, the multicenter open-label, randomized, parallel-group riociguat versus balloon pulmonary angioplasty in non-operable chronic thrombo embolic pulmonary hypertension (RACE) study (ClinicalTrials.gov: NCT02634203) investigated the efficacy and safety of riociguat versus BPA in newly diagnosed and treatment-naive patients with CTEPH and PVR > 4 Wood Units.17 All patients had inoperable CTEPH, confirmed by multidisciplinary team assessment in the French reference center for PH. For the initial 16-week part of the study, patients were randomized to riociguat or BPA. The primary endpoint in RACE was PVR at rest at Week 26, and secondary endpoints included changes from baseline in 6MWD, WHO FC, N-terminal prohormone of brain natriuretic peptide, time to clinical worsening, and safety. Publication of the results is awaited.
Future perspectives
Although the techniques for BPA have improved over the past 10 years, a consensus-based approach toward an optimal strategy for the use of BPA in the CTEPH treatment algorithm is required. Specifically, the role of medical therapy and BPA in combination needs to be defined, and standardization of the BPA procedure and endpoints between institutions is required. There is a need for objectively adjudicated data obtained from both prospectively designed randomized controlled trials and registries. In particular, follow-up is required to determine the long-term patency and clinical success achieved with BPA. Finally, the optimal patient population who can derive the greatest benefit from BPA remains to be defined.
Conclusions
Recent advances in BPA for CTEPH are wide ranging and led by the adoption of a multidisciplinary approach toward patient evaluation and treatment. Radiation exposure to both patients and operators is decreased by using routine single-plane angiography and femoral vein access, rather than biplane angiography and neck vein access. BPA treatment strategy is now routinely enhanced by perfusion imaging guidance. Finally, the treatment technique is optimized for efficacy and safety by targeting multiple disease segments and a lower activated coagulation time therapeutic goal per treatment session.
Such advancements, along with marked improvements in BPA technique and multicenter experience, have resulted in BPA becoming a feasible treatment option for patients with CTEPH with inoperable, segmental/subsegmental disease or post-PEA residual disease. In particular, patients with segmental/subsegmental chronic thromboembolism might be well suited for a randomized clinical trial of BPA versus PEA. Additionally, further data are required to demonstrate the long-term benefits of the procedure and to guide improvements in the institutional infrastructure for providing BPA.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EM reports no relevant conflict of interest. MP reports other from The Medicines Company, other from Access Closure, outside the submitted work. LA has nothing to disclose. DP reports other from Actelion Pharmaceuticals, other from Bayer Pharmaceuticals US, Inc., outside the submitted work.
Footnotes
Author Note: This article is based on a presentation by Professor Ehtisham Mahmud at the University of California San Diego CTEPH Symposium, 15–16 November 2019, with additional material by all authors. All authors reviewed each draft and approved the final draft for submission.
Ethical approval: Not applicable.
Guarantor: EM is the guarantor of the accuracy of the data presented in this review.
Funding: Medical writing services provided by Richard Murphy, PhD, of Adelphi Communications Ltd, Macclesfield, UK, were funded by Bayer US LLC, Whippany, NJ, USA.
ORCID iD: Ehtisham Mahmud https://orcid.org/0000-0003-2493-8661
References
- 1.Mahmud E, Madani MM, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol 2018; 71: 2468–2486. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 3.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voorburg JA, Cats VM, Buis B, et al. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest 1988; 94: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 5.Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. [DOI] [PubMed] [Google Scholar]
- 6.Aoki T, Sugimura K, Tatebe S, et al. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: long-term effects and procedure-related complications. Eur Heart J 2017; 38: 3152–3159. [DOI] [PubMed] [Google Scholar]
- 7.Brenot P, Jais X, Taniguchi Y, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurzyna M, Darocha S, Pietura R, et al. Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single-centre European experience. Kardiol Pol 2017; 75: 645–654. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa A, Satoh T, Fukuda T, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes 2017; 10: e004029. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa A, Matsubara H. Balloon pulmonary angioplasty: a treatment option for inoperable patients with chronic thromboembolic pulmonary hypertension. Front Cardiovasc Med 2015; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogo T, Fukuda T, Tsuji A, et al. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur J Radiol 2017; 89: 270–276. [DOI] [PubMed] [Google Scholar]
- 12.Olsson KM, Wiedenroth CB, Kamp JC, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017; 49: 1602409. [DOI] [PubMed] [Google Scholar]
- 13.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inami T, Kataoka M, Shimura N, et al. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2014; 7: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 15.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ CardiovascInterv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 16.Andreassen AK, Ragnarsson A, Gude E, et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 17.Jais X, Brenot P, Bouvaist H, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension: results from the randomised controlled RACE study. Eur Respir J 2019; 54: RCT1885. [Google Scholar]
- 18.Mahmud E, Behnamfar O, Ang L, et al . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Interv Cardiol Clin 2018; 7: 103–117. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka M, Inami T, Kawakami T, et al. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. JACC Cardiovasc Interv 2019; 12: 1382–1388. [DOI] [PubMed] [Google Scholar]
- 20.Tatebe S, Fukumoto Y, Sugimura K, et al. Optical coherence tomography is superior to intravascular ultrasound for diagnosis of distal-type chronic thromboembolic pulmonary hypertension. Circ J 2013; 77: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 21.Ang L, McDivit Mizzell A, Daniels LB, et al. Optimal technique for performing invasive pulmonary angiography for chronic thromboembolic pulmonary disease. J Invasive Cardiol 2019; 31: E211–E219. [PubMed] [Google Scholar]
- 22.Darocha S, Pietura R, Pietrasik A, et al. Improvement in quality of life and hemodynamics in chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Circ J 2017; 81: 552–557. [DOI] [PubMed] [Google Scholar]
- 23.Fukui S, Ogo T, Goto Y, et al. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015; 180: 66–68. [DOI] [PubMed] [Google Scholar]
- 24.Khan MS, Amin E, Memon MM, et al. Meta-analysis of use of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2019; 291: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi Y, Miyagawa K, Nakayama K, et al. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention 2014; 10: 518–525. [DOI] [PubMed] [Google Scholar]
- 26.Kanar BG, Mutlu B, Atas H, et al. Improvements of right ventricular function and hemodynamics after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Echocardiography 2019; 36: 2050–2056. [DOI] [PubMed] [Google Scholar]
- 27.Inami T, Kataoka M, Yanagisawa R, et al. Long-term outcomes after percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circulation 2016; 134: 2030–2032. [DOI] [PubMed] [Google Scholar]
- 28.Heresi GA, AbuHalimeh BJ. Current Approach to Chronic Thromboembolic Disease Without Pulmonary Hypertension. In: Ford HJ, Heresi GA and Risbano MG, (eds) Pulmonary Hypertension, Respiratory Medicine. Humana, Cham, 2020. DOI: 10.1007/978-3-030-52787-7_5
- 29.Swietlik EM, Ruggiero A, Fletcher AJ, et al. Limitations of resting haemodynamics in chronic thromboembolic disease without pulmonary hypertension. Eur Respir J 2019; 53: 1801787. [DOI] [PubMed] [Google Scholar]
- 30.Wiedenroth CB, Olsson KM, Guth S, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ 2018; 8: 2045893217753122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inami T, Kataoka M, Kikuchi H, et al. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int J Cardiol 2019; 289: 116–118. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi Y, Jais X, Jevnikar M, et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2019; 38: 833–842. [DOI] [PubMed] [Google Scholar]
- 33.Inami T, Kataoka M, Shimura N, et al. Incidence, avoidance, and management of pulmonary artery injuries in percutaneous transluminal pulmonary angioplasty. Int J Cardiol 2015; 201: 35–37. [DOI] [PubMed] [Google Scholar]
- 34.Ejiri K, Ogawa A, Fujii S, et al. Vascular injury is a major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2018; 11: e005884. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda N, Kubota S, Okazaki T, et al. The predictors of complications in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Catheter Cardiovasc Interv 2019; 93: E349–E356. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto K, Nakazato K, Sakamoto N, et al. Pulmonary artery diameter predicts lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Int Heart J 2017; 58: 584–588. [DOI] [PubMed] [Google Scholar]
- 37.Jin Q, Zhao ZH, Luo Q, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: state of the art. World J Clin Cases 2020; 8: 2679–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepke-Zaba J, Ghofrani HA, Hoeper M. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160107. DOI: 10.1183/16000617.0107-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. [DOI] [PubMed] [Google Scholar]