Abstract
The incidence of lumbar spondylolysis is affected by sex, race, and congenital abnormalities. These differences suggest a genetic component to the etiology of spondylolysis. However, no definitive evidence has been presented regarding the inheritance of lumbar spondylolysis. We report familial cases of lumbar spondylolysis in 7- and 4-year-old brothers and their father, each of whom visited our clinic complaining of low back pain. Spondylolysis in the fifth lumbar vertebra (L5) was identified in both boys and their father from clinical, radiographic, computed tomographic, and magnetic resonance imaging examinations. Conservative treatment was provided for both boys. No bony union of any spondylolytic lesions was obtained, but they returned to sports activity without low back pain. Frequent development of spondylolysis, even at younger ages, in all male family members might indicate an underlying genetic etiology in lumbar spondylolysis, primarily in the form of autosomal dominant inheritance. However, information on patients and their parents should be considered carefully, as bony union with conservative therapy is not expected in such patients.
Keywords: Lumbar spine, spondylolysis, spondylolisthesis, hereditary pathogenesis, familiarity, familial occurrence
Introduction
Lumbar spondylolysis involves a bone defect in the pars interarticularis that connects the upper and lower zygapophyseal joints. The prevalence of spondylolysis has been estimated at 3% to 7% in the general pediatric population.1–5 Lumbar spondylolysis is the leading cause of back pain in children and adolescents, particularly among athletes,4,5 and the primary etiology is considered to be stress fractures owing to repeated mechanical stress.6,7
Lumbar spondylolysis occurs more frequently in men than in women.1–4 In addition, the incidence of lumbar spondylolysis varies not only by sex, but also by race, at 6.4% among white men, 2.8% among black men, 2.3% among white women, and 1.1% among black women.1 Furthermore, lumbar spondylolysis also frequently presents as a sign in patients with spina bifida occulta, autosomal dominant brachydactyly, and scoliosis.2,4,8 These biases in incidence owing to sex, race, and concomitant congenital anomalies suggest a genetic predisposition to lumbar spondylolysis as an etiological factor. However, studies of fetal specimens and cadavers of stillborn infants have indicated that spondylolysis does not arise during the fetal period.9,10 Therefore, definitive evidence for a genetic predisposition to lumbar spondylolysis is lacking.
In this report, we present rare cases of lumbar spondylolysis from two generations in a family, identified in 7- and 4-year-old brothers, and subsequently confirmed in their father.
Case presentation
Case 1
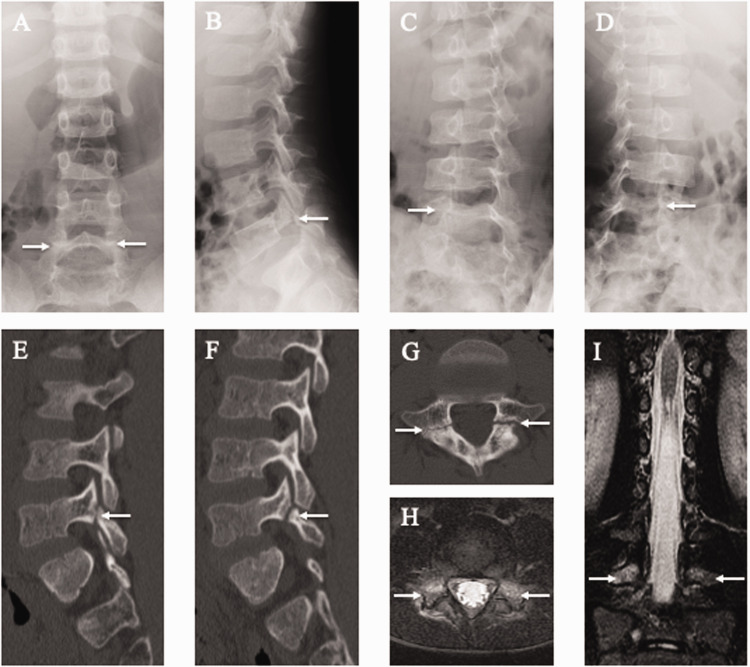
A 7-year-old first-born son visited our clinic complaining of low back pain for 6 months that had arisen while playing soccer. Plain radiography showed bilateral spondylolysis in the fifth lumbar vertebra (L5) and spina bifida below the first sacral vertebra (S1) (Figure 1a–d). Furthermore, high signal changes in bilateral pedicles were evident on short tau inversion recovery (STIR)-magnetic resonance imaging (MRI) (Figure 1e, f), indicating an adjacent pars early stress fracture. The patient stopped sports activities and wore a hard brace for 6 months, but no bony union of any spondylolytic lesions was obtained. Computed tomography (CT) multi-planar reconstruction images after 6 months of bracing demonstrated signs of terminal-stage spondylolysis (Figure 1g–i). However, he returned to playing soccer without low back pain.
Figure 1.
Results of lumbar spine radiography, computed tomography (CT), and magnetic resonance imaging (MRI) of the 7-year-old first-born son. Spondylolysis (white arrows) is observed at the fifth lumbar vertebra (L5) on posteroanterior (a), lateral (b), 45° right anterior oblique (c), and 45° left anterior oblique (d) radiographs and right parasagittal (e), left parasagittal (f), and axial CT images parallel to the L5 vertebral arch (g). Axial short tau inversion recovery (STIR)-MRI parallel to the L5 vertebral arch (h) and coronal STIR-MRI (i). Note the signal hyperintensity (white arrows) in the pedicle, indicating marrow edema. This is a sign of an early-stage stress fracture of the pars interarticularis.
Case 2
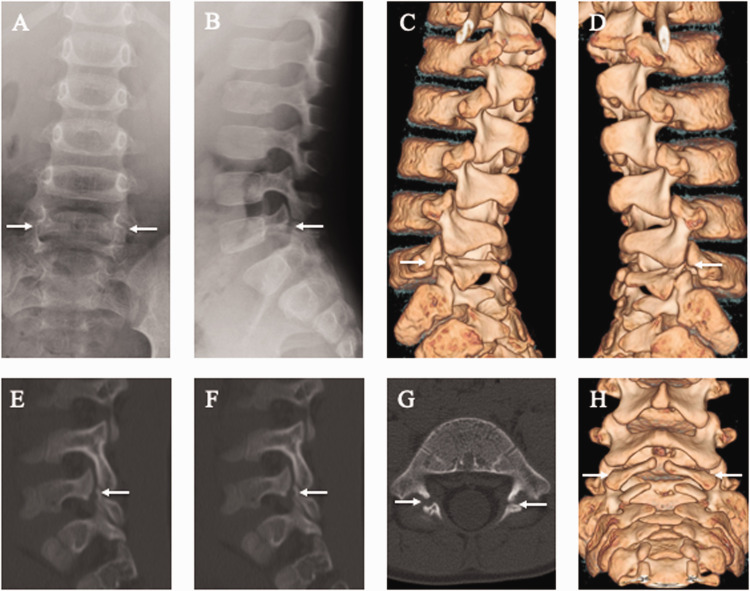
The 4-year-old second-born son with 1 year of experience playing soccer came to our hospital because of low back pain for 2 weeks. Plain radiography showed bilateral spondylolysis at L5 (Figure 2a, b). Moreover, CT multi-planar reconstruction images demonstrated signs of terminal-stage bilateral L5 spondylolysis and spina bifida below the S1 level (Figure 2c–h). Because defects at this stage have no chance of bony union under conservative treatment, treatment was aimed at achieving pain control. Symptoms were relieved after 1 month of wearing a soft brace, and he returned to playing soccer without low back pain.
Figure 2.
Lumbar spine radiographs and computed tomography (CT) images of the 4-year-old second-born son. Spondylolysis (white arrows) is evident at the fifth lumbar vertebra (L5) in posteroanterior (a) and lateral (b) radiographs and three-dimensional (3D)-CT images (c, d, h). Spondylolysis (white arrows) is apparent at L5 on right parasagittal (e), left parasagittal (f), and axial CT images parallel to the L5 vertebral arch (g).
Case 3
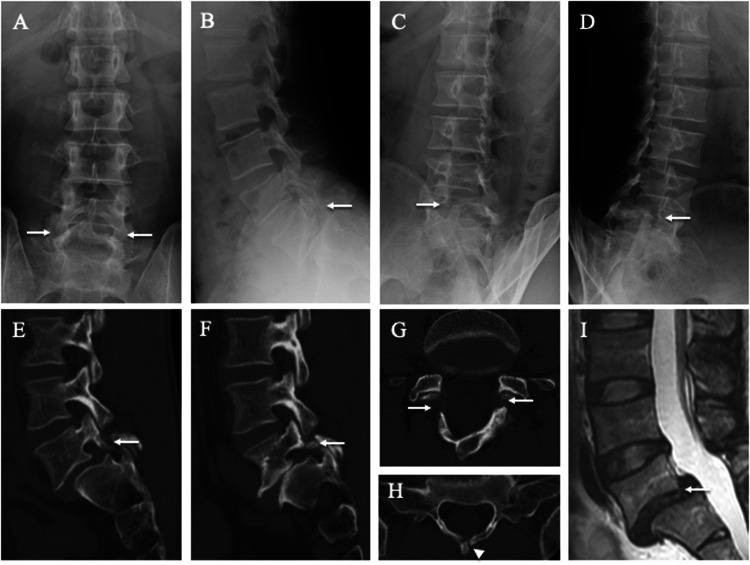
The 39-year-old father was employed in the Japan Ground Self-Defense Forces. He had a history of several acute attacks of low back pain in adulthood, but no history of low back pain during childhood or adolescence. Plain radiography, CT, and MRI showed L5 spondylolisthesis and spina bifida at S1 (Figure 3a–i). The L5 laminae were asymmetrical and fused to the spinous process bilaterally (Figure 3g). In contrast, both S1 laminae reached the midline but were not fused to the spinous process (Figure 3h). In addition, the patient’s father (the paternal grandfather of Cases 1 and 2) had a history of spinal fusion for lumbar spondylolisthesis, although no images were available.
Figure 3.
Lumbar spine radiographs, computed tomography (CT), and magnetic resonance images (MRI) of the 39-year-old father. Spondylolisthesis (white arrows) is evident at the fifth lumbar vertebra (L5) on posteroanterior (a), lateral (b), 45° right anterior oblique (c), and 45° left anterior oblique (d) radiographs and right parasagittal (e), left parasagittal (f), and axial CT images parallel to the L5 (g) and first sacral (S1) (h) vertebral arches. The white arrowhead indicates spina bifida of S1 (h). L5 vertebra slippage is apparent on MRI (i).
Case 4 (non-spondylolysis case)
The boys’ 37-year-old mother was asymptomatic. However, she was worried about spondylolysis and visited our clinic. She underwent clinical and radiological examinations, and all investigations were negative for lumbar spondylolysis and spina bifida.
Discussion
This report presents rare familial cases of lumbar spondylolysis that were identified in 7- and 4-year-old brothers as well as in their father. We recognized two essential issues in these patients. First, these familial cases suggest autosomal dominant inheritance as a primary genetic predisposition to lumbar spondylolysis. Second, achieving bony union in familial and extremely young patients with lumbar spondylolysis might be challenging with conservative treatment.
An elevated incidence of lumbar spondylolysis among the family members of affected individuals has long been debated. Previous epidemiological studies have reported that 14.9% to 22.0% of the first-degree relatives of affected patients developed lumbar spondylolysis or spondylolisthesis.11,12 In addition, another study examining the families of 23 individuals with lumbar spondylolysis in a single area of the east coast of the USA found lumbar spondylolisthesis in 31.8% of fathers, 17.4% of mothers, and 34.0% of male siblings.2 These frequencies are higher than those in the general population. However, compared with Western countries,2,11–13 there is less evidence of familial lumbar spondylolysis from Asian countries.14–17 A previous study reported L5 spondylolysis with spina bifida from the fourth lumbar vertebra (L4) to the second sacral vertebra (S2) in three brothers from the same Japanese family.16 Similar to this report, our patients also showed obvious spina bifida, indicating a possible inherent genetic weakness of the pars that might facilitate stress fractures. Despite substantial debate regarding the genetic etiology of lumbar spondylolysis, the specific genetic mode has not been fully elucidated.
Although congenital vertebral defects occur during the formation of the neural arches and result in complete or incomplete spina bifida, spondylolysis has never been identified in newborns.9,10 A 3.5-month-old infant with spondylolysis described by Borkow and Kleiger represents the youngest case reported to date.18 The occurrence increases gradually between 6 and 8 years of age.12,13 To the best of our knowledge, our case of a 4-year-old Japanese boy with symptomatic lumbar spondylolysis represents the youngest recorded Japanese patient.4,19
In previous reports of Japanese families with lumbar spondylolysis, as in the present report, all cases of lumbar spondylolysis were identified in male family members, with none in female family members.16,17 In one study, the pedigrees of the relatives of Finnish patients with spondylolysis were consistent with autosomal dominant inheritance and incomplete penetrance (approximately 75%).13 A family study in Iran reached similar conclusions: in X-linked recessive diseases, sons and daughters of affected fathers and non-affected carrier mothers had a 50% chance of developing the pathology.20 Generally, owing to the small size of the Y chromosome, Y-linked diseases are rare. The possibility of heredity cannot be excluded for lumbar spondylolysis in the present family, partly because the siblings were 7 and 4 years old, which is extremely young compared with the peak age of spondylolysis incidence. This report synthesized previous evidence to support the possibility of autosomal dominant inheritance among patients with lumbar spondylolysis. Our experience suggests that high demand exists for genetic studies of lumbar spondylolysis. Recent functional genomics and in vivo studies have identified autosomal dominant SLC26A2 mutations in dysplastic spondylolysis.21,22 However, no existing reports describe specific genetic variants in patients with isthmic spondylolysis, which is more common than dysplastic spondylolysis. Further studies are needed to identify specific genetic alleles predisposing patients to pars fractures.
Both the 7- and 4-year-old patients in this report failed to achieve bony union. Bony unions of defects in the lumbar spine were reportedly achieved more often during the apophyseal stage (10–17 years old) than during the cartilaginous stage (4–12 years old).23 Lumbar spondylolysis among elementary school age children suggests a congenital predisposition owing to aberrant osteogenesis during the growth period, as the abnormality has frequently been identified as a terminal-stage bone defect, sometimes asymptomatic and frequently in association with spina bifida occulta.24 The possibility of difficulty in obtaining bony union for lumbar spondylolysis in familial and younger cases is implied.
In conclusion, we presented three cases with lumbar spondylolysis in two, and potentially three, generations of a single family. These familial cases suggest the possibility of autosomal dominant inheritance as the primary genetic predisposition in lumbar spondylolysis. Clinicians should recognize the possibility of lumbar spondylolysis occurring even in younger patients (4–7 years old) when the patients complain of low back pain and have a family history of spondylolysis and spina bifida occulta. However, information on patients and their parents should be considered carefully because bony union with conservative therapy is not expected in such patients.
Footnotes
Ethics statement: At our institution, case reports do not require ethics review committee approval. The parents of the patients described in this paper provided written informed consent for publication.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Kinshi Kato https://orcid.org/0000-0002-1749-7274
Michiyuki Hakozaki https://orcid.org/0000-0003-1641-0795
References
- 1.Roche MB, Rowe GG. The incidence of separate neural arch and coincident bone variations; a survey of 4,200 skeletons. Anat Rec 1951; 109: 233–252. [DOI] [PubMed] [Google Scholar]
- 2.Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am 1984; 66: 699–707. [PubMed] [Google Scholar]
- 3.Beutler WJ, Fredrickson BE, Murtland A, et al. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine (Phila Pa 1976) 2003; 28: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 4.Sakai T, Sairyo K, Takao S, et al. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine (Phila Pa 1976) 2009; 34: 2346–2350. [DOI] [PubMed] [Google Scholar]
- 5.Crawford CH, 3rd, Ledonio CG, Bess RS, et al. Current evidence regarding the surgical and nonsurgical treatment of pediatric lumbar spondylolysis: a report from the Scoliosis Research Society Evidence-Based Medicine Committee. Spine Deform 2015; 3: 30–44. [DOI] [PubMed] [Google Scholar]
- 6.Chosa E, Totoribe K, Tajima N. A biomechanical study of lumbar spondylolysis based on a three-dimensional finite element method. J Orthop Res 2004; 22: 158–163. [DOI] [PubMed] [Google Scholar]
- 7.Terai T, Sairyo K, Goel VK, et al. Stress fracture as the beginning of spondylolysis occurs from the ventral aspect of pars interarticularis. A clinical and biomechanical study. J Bone Joint Surg Br 2010; 92: 1123–1127. [DOI] [PubMed] [Google Scholar]
- 8.Mays S. Spondylolysis, spondylolisthesis, and lumbo-sacral morphology in a medieval English skeletal population. Am J Phys Anthropol 2006; 131: 352–362. [DOI] [PubMed] [Google Scholar]
- 9.Rowe GG, Roche MB. The etiology of separate neural arch. J Bone Joint Surg Am 1953; 35: 102–110. [PubMed] [Google Scholar]
- 10.Kaneko F. Studies on the relation between spondylolysis and developmental anatomies of lumbosacral region. J Jpn Orthop Assoc 1977; 51: 1237–1253 (in Japanese). [Google Scholar]
- 11.Wynne-Davies R, Scott JH. Inheritance and spondylolisthesis: a radiographic family survey. J Bone Joint Surg Br 1979; 61-B: 301–305.383720 [Google Scholar]
- 12.Albanese M, Pizzutillo PD. Family study of spondylolysis and spondylolisthesis. J Pediatr Orthop 1982; 2: 496–499. [DOI] [PubMed] [Google Scholar]
- 13.Haukipuro K, Keranen N, Koivisto E, et al. Familial occurrence of lumbar spondylolysis and spondylolisthesis. Clin Genet 1978; 13: 471–476. [DOI] [PubMed] [Google Scholar]
- 14.Yano T, Miyagi S, Ikari T. Studies of familial incidence of spondylolysis. Singapore Med J 1967; 8: 203–206. [PubMed] [Google Scholar]
- 15.Young KJ, Koning W. Spondylolysis of L2 in identical twins. J Manipulative Physiol Ther 2003; 26: 196–201. [DOI] [PubMed] [Google Scholar]
- 16.Yamada A, Sairyo K, Shibuya I, et al. Lumbar spondylolysis in juveniles from the same family: a report of three cases and a review of the literature. Case Rep Orthop 2013; 2013: 272514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yurube T, Kakutani K, Okamoto K, et al. Lumbar spondylolysis: a report of four cases from two generations of a family. J Orthop Surg (Hong Kong) 2017; 25: 2309499017713917. [DOI] [PubMed] [Google Scholar]
- 18.Borkow SE, Kleiger B. Spondylolisthesis in the newborn. Clin Orthop 1971; 81: 73–76. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida T. Diagnosis and conservative therapy of lumbar spondylolysis in the growing period. J Lumbar Spine Disord 2003; 9: 15–22 (in Japanese). [Google Scholar]
- 20.Shahriaree H, Sajadi K, Rooholamini SA. A family with spondylolisthesis. J Bone Joint Surg Am 1979; 61: 1256–1258. [PubMed] [Google Scholar]
- 21.Cai T, Yang L, Cai W, et al. Dysplastic spondylolysis is caused by mutations in the diastrophic dysplasia sulfate transporter gene. Proc Natl Acad Sci U S A 2015; 112: 8064–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng C, Lin X, Liu H, et al. Phenotypic characterization of Slc26a2 mutant mice reveals a multifactorial etiology of spondylolysis. FASEB J 2020; 34: 720–734. [DOI] [PubMed] [Google Scholar]
- 23.Fujii K, Katoh S, Sairyo K, et al. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. The radiological outcome after conservative treatment. J Bone Joint Surg Br 2004; 86: 225–231. [DOI] [PubMed] [Google Scholar]
- 24.Sakai T, Goda Y, Tezuka F, et al. Characteristics of lumbar spondylolysis in elementary school age children. Eur Spine J 2016; 25: 602–606. [DOI] [PubMed] [Google Scholar]