Abstract
Background
Clinically effective and safe genotyping relies on correct reference sequences, often represented by haplotypes. The 1000 Genomes Project recorded individual genotypes across 26 different populations and, using computerized genotype phasing, reported haplotype data. In contrast, we identified long reference sequences by analyzing the homozygous genomic regions in this online database, a concept that has rarely been reported since next generation sequencing data became available.
Study design and methods
Phased genotype data for a 80.6 kb region of chromosome 1 was downloaded for all 2,504 unrelated individuals of the 1000 Genome Project Phase 3 cohort. The data was centered on the ACKR1 gene and bordered by the CADM3 and FCER1A genes. Individuals with heterozygosity at a single site or with complete homozygosity allowed unambiguous assignment of an ACKR1 haplotype. A computer algorithm was developed for extracting these haplotypes from the 1000 Genome Project in an automated fashion. A manual analysis validated the data extracted by the algorithm.
Results
We confirmed 902 ACKR1 haplotypes of varying lengths, the longest at 80,584 nucleotides and shortest at 1,901 nucleotides. The combined length of haplotype sequences comprised 19,895,388 nucleotides with a median of 16,014 nucleotides. Based on our approach, all haplotypes can be considered experimentally confirmed and not affected by the known errors of computerized genotype phasing.
Conclusions
Tracts of homozygosity can provide definitive reference sequences for any gene. They are particularly useful when observed in unrelated individuals of large scale sequence databases. As a proof of principle, we explored the 1000 Genomes Project database for ACKR1 gene data and mined long haplotypes. These haplotypes are useful for high throughput analysis with next generation sequencing. Our approach is scalable, using automated bioinformatics tools, and can be applied to any gene.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12859-021-04169-6.
Introduction
Data generated by next generation sequencing (NGS) are often utilized in the emerging fields of precision and personalized medicine. This massively parallel processing chemistry can identify genetic factors that predict treatment and response to therapies. Reference nucleotide sequences are critical for analyzing NGS data, as exemplified by routine clinical diagnosis for HLA antigens [1].
Genotype phasing is the process to determine if genetic variants, often single nucleotide variations, called SNVs, belong to 2 separate chromosomes (in trans). If SNVs are located on the same chromosome (in cis), they constitute a haplotype or an allele. Genotype phasing has often been inferred using computational methods [2, 3], which are prone to certain types of error [4]. These errors are encountered in samples harboring novel variants, low frequency or rare variants, and structural variants [5]. Almost all of these errors can be precluded by laboratory based methods, such as sequencing the genomes of both parents and sibling offspring [6], physical separation of homologous chromosomes in diploid cells [7, 8], sequencing in sperm cells [9], allele specific PCR [10], single DNA molecule dilution [11] and single molecule sequencing chemistry [12, 13]. These laboratory based methods are, however, labor-intensive and time consuming, and thus infrequently applied in clinical diagnostics.
The human genome contains many regions that are known as long contiguous stretches of homozygosity (LCSH) [14,15]. Their presence in unrelated individuals across different populations is attributed to a lower average recombination rate in these regions of the human genome [14].
The human atypical chemokine receptor 1 gene (ACKR1, MIM #613,665) [16] encodes a multi-pass trans-membrane glycoprotein. It is a receptor for pro-inflammatory cytokines [17] and malaria Plasmodium parasites (P. vivax and P. knowlesi) [18]. The ACKR1 glycoprotein carries the five antigens of the Duffy blood group system (Fy) [19, 20]. Recent sequencing studies in the ACKR1 gene have identified approximately 30 haplotypes, albeit at limited lengths of 2.1 kb [21], 2.5 kb [22], 5.2 kb [23], and 5.6 kb [24], respectively. We previously applied these ACKR1 haplotypes to predict the Duffy phenotype in Neanderthal samples [21]. Later, high-coverage genome sequences of Neanderthals were established [25–27], which confirmed our prediction [21]. A recent similar comparative study, involving long genomic segments, identified a 50 kb segment in humans, which was inherited from Neanderthals and represented a genetic risk factor in SARS-CoV-2 infection [28].
The 1000 Genomes Project (1000GP) provides a comprehensive database of genotypes and haplotypes in 2,504 unrelated individuals across 26 populations worldwide [29, 30]. As a proof of principle using data from the 1000GP for the ACKR1 gene, we establish a list of 902 haplotypes, some more than 80 kb long. Our scalable approach can be applied to any gene in any population.
Materials and methods
Algorithm workflow
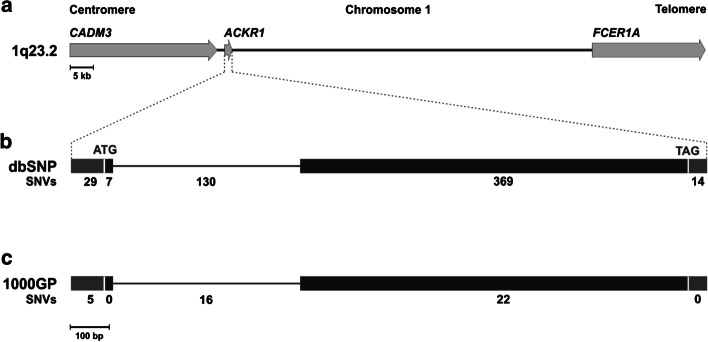
A Python algorithm was developed (Supplementary Information, File S1) to download and analyze genotype data for 80.6 kb region of chromosome 1 (between positions NC_000001.11: 159,203,314–159,283,887) flanked between 2 genes, CADM3 and FCER1A, and encompassing the ACKR1 gene (Fig. 1) for all 2,504 unrelated individuals of the final release 1000GP panel (Phase 3; GRCh38) using Bcftools [31]. The SNV data was downloaded from the dbSNP database [32]. Individual sequences with heterozygosity at a single site or with complete homozygosity were automatically extracted as an unambiguous ACKR1 haplotype that can be considered experimentally confirmed, which applied a time-proven concept [4]. The algorithm outputs three files: a sequence file containing the distinct haplotypes, a meta-data file containing information about the population in which the haplotypes are found, and a folder containing graphical representations of the population distribution of the distinct haplotypes.
Fig. 1.
Schematic representation of chromosome 1 region analyzed. The ACKR1 gene is bordered by the 2 genes CADM3 in centromeric and FCER1A in telomeric direction at chromosomal position 1q23.2 (a). The structure of the ACKR1 gene (b) comprises 2 exons (closed boxes) and include the coding sequence (CDS, black) and the 5’- and 3’-untranslated region (UTR, grey). The intron 1 joins the 2 exons (black line). The number of SNVs observed in the for the dbSNP (b) and 1000GP databases (c) are shown for the 5’-UTR, CDS, intron, CDS and 3’-UTR
Validation
Phased haplotype data for 80.6 kb region of chromosome 1 (between positions NC_000001.11: 159,203,314–159,283,887) was manually downloaded for all 2535 individuals of the 1000GP panel (Phase 3; GRCh37) from the 1000 Genomes browser. After removing 31 related individuals, haplotype data from 2504 unrelated individuals was imported into Microsoft Excel. Individuals with heterozygosity at a single site or with complete homozygosity in the 1,626 nucleotide-long ACKR1 gene (NG_011626.3; NC_000001.11:159,204,875–159,206,500) allowed unambiguous assignment of an ACKR1 haplotype. These unambiguous ACKR1 haplotypes were further analyzed individually using Excel spreadsheets, and their sequences were extended in both 5'- and 3'-directions until a heterozygous SNV was encountered. The region between 2 SNVs was catalogued as a haplotype and compared with the previous automated results. The manual analysis was performed and thus a validation dataset generated before the Python algorithm was developed.
Neanderthal genome
The published DNA sequence of the Neanderthal genome (Chagyrskaya, Altai, and Vindija 33.19, http://cdna.eva.mpg.de/neandertal/) [25–27] was analyzed (Integrative genomics viewer version 2.3.20) [33] and aligned to the human genome (NCBI Build GRCh38/hg38). We searched for the longest match, if any, with the haplotypes in the 1000GP.
Results
Using the 1000GP database and a Python algorithm, we extracted and catalogued long haplotypes that encompassed the ACKR1 gene and were flanked between 2 SNVs (Fig. 1). Among 2,504 individuals included in the 1000GP database, 1,520 individuals were homozygous for the 1,626 nucleotide-long ACKR1 gene or heterozygous with only 1 SNV. The ACKR1 sequences for these individuals were further analyzed both upstream and downstream of ACKR1 gene until SNVs were encountered. The extension in both directions allowed us to identify long ACKR1 haplotypes that can be considered experimentally verified. The results obtained with our computational approach were validated by a manual method, performed in a blinded fashion.
ACKR1 and SNVs
For the ACKR1 gene (Fig. 1), the dbSNP database [32] lists 549 SNVs spread over 1,626 nucleotides (Fig. 1b). We encountered, however, only 43 SNVs of the ACKR1 gene in the 1000GP database (Fig. 1c) out of the 549 known SNVs.
ACKR1 haplotypes
We identified 31 distinct haplotypes with ≥ 10 observations (Table 1). They ranged in length from 2,383 nucleotides to 17,739 nucleotides. A total of 902 haplotypes were observed, ranging in length from 1,901 nucleotides to 80,584 nucleotides, some extending into the adjacent CADM3 and FCER1A genes (Fig. 2). The combined length of haplotype sequences comprised 19,895,388 nucleotides with a median of 16,014 nucleotides (Quartile 1 – Quartile 3: 7,588 – 30,729 nucleotides; Interquartile Range: 23,141 nucleotides). The length of the haplotypes was inversely proportional to the number of observations (Fig. 3). Most of the common haplotypes (70.13%) were small (< 10 kb; Table 2) and ranged in length between 1,901 to 9,927 nucleotides. The most common ACKR1 allele observed was the Duffy-null allele (FY*02 N.01) followed by FY*A (FY*01) and FY*B (FY*02), respectively (Table 3). For each of these 3 common ACKR1 alleles, we were able to identify reference sequences longer than 80 kb (Table 3).
Table 1.
Experimentally confirmed ACKR1 haplotypes with ≥ 10 observations in the 1000GP database*
| Haplotype | Length (nucleotides) | Observations (n) | Total | ||||
|---|---|---|---|---|---|---|---|
| Super-population† | |||||||
| AFR | AMR | EAS | SAS | EUR | |||
| 01 | 3385 | 1 | 39 | 149 | 83 | 28 | 300 |
| 02 | 3386 | 1 | 37 | 149 | 76 | 21 | 284 |
| 03 | 5168 | 161 | 1 | 0 | 0 | 0 | 162 |
| 04 | 5168 | 160 | 1 | 0 | 0 | 0 | 161 |
| 05 | 2483 | 107 | 1 | 0 | 0 | 0 | 108 |
| 06 | 2483 | 107 | 1 | 0 | 0 | 0 | 108 |
| 07 | 4871 | 0 | 6 | 42 | 0 | 1 | 49 |
| 08 | 4871 | 0 | 5 | 42 | 0 | 1 | 48 |
| 09 | 4376 | 0 | 0 | 36 | 0 | 0 | 36 |
| 10 | 4376 | 0 | 0 | 35 | 0 | 0 | 35 |
| 11 | 6276 | 27 | 0 | 0 | 0 | 0 | 27 |
| 12 | 6276 | 25 | 0 | 0 | 0 | 0 | 25 |
| 13 | 9091 | 20 | 1 | 0 | 0 | 0 | 21 |
| 14 | 17,406 | 0 | 4 | 1 | 15 | 1 | 21 |
| 15 | 14,785 | 0 | 4 | 4 | 10 | 2 | 20 |
| 16 | 2383 | 0 | 5 | 0 | 3 | 11 | 19 |
| 17 | 17,405 | 19 | 0 | 0 | 0 | 0 | 19 |
| 18 | 2383 | 0 | 5 | 0 | 3 | 11 | 19 |
| 19 | 17,739 | 16 | 1 | 0 | 0 | 0 | 17 |
| 20 | 3385 | 0 | 2 | 0 | 7 | 7 | 16 |
| 21 | 2620 | 0 | 7 | 0 | 0 | 9 | 16 |
| 22 | 2620 | 0 | 7 | 0 | 0 | 9 | 16 |
| 23 | 6310 | 0 | 0 | 15 | 0 | 0 | 15 |
| 24 | 6310 | 0 | 0 | 15 | 0 | 0 | 15 |
| 25 | 4869 | 0 | 3 | 10 | 2 | 0 | 15 |
| 26 | 2706 | 0 | 1 | 1 | 4 | 8 | 14 |
| 27 | 2706 | 0 | 1 | 1 | 4 | 8 | 14 |
| 28 | 9092 | 11 | 1 | 0 | 0 | 0 | 12 |
| 29 | 4644 | 0 | 2 | 0 | 4 | 5 | 11 |
| 30 | 4643 | 11 | 0 | 0 | 0 | 0 | 11 |
| 31 | 4644 | 0 | 2 | 0 | 4 | 4 | 10 |
Fig. 2.

ACKR1 haplotypes observed in the 1000GP. A total of 902 unique haplotypes were observed and sorted according to their length (bars). All haplotypes comprise the ACKR1 gene (shaded column), their positions in the ACKR1 gene locus (top, see Fig. 1) is indicated. The cumulative number is listed (right). Haplotypes of similar lengths are grouped together (for exact lengths see Supplementary Information, Excel files S1 and S2)
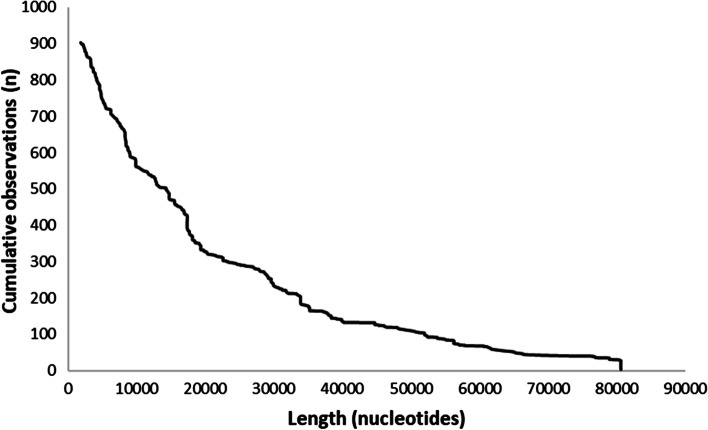
Fig. 3.
Correlation between length and observations of ACKR1 haplotypes. The length of the ACKR1 haplotypes (x-axis) observed in the 1000GP was inversely proportional to the number of observations (y-axis)
Table 2.
ACKR1 haplotypes and length distribution in the 1000GP database among 1520 individuals
| Length range (nucleotides) | ACKR1 haplotypes | |
|---|---|---|
| Observations* (n) | Frequency (%) | |
| < 10,000 | 2,132 | 70.13 |
| 10,000 – 19,999 | 468 | 15.39 |
| 20,000 – 29,999 | 128 | 4.21 |
| 30,000 – 39,999 | 132 | 4.34 |
| 40,000 – 49,999 | 34 | 1.12 |
| 50,000 – 59,999 | 52 | 1.71 |
| 60,000 – 69,999 | 26 | 0.86 |
| 70,000 – 79,999 | 16 | 0.53 |
| ≥ 80,000 | 52 | 1.71 |
| Total | 3,040 | 100 |
*Among 2,504 individuals included in the 1000GP database, 1,520 individuals (3,040 chromosomes) were homozygous for the 1,626 nucleotide-long ACKR1 gene or heterozygous with only 1 SNV
Table 3.
Length distribution of the 3 common ACKR1 alleles observed in the 1000GP
| ISBT allele | Haplotype* | Observations† | Length range | Mean ± standard deviation | Median |
|---|---|---|---|---|---|
| FY*01 | TGCCGCGCCGCGGGC | 389 | 2241—80,576 | 24,628 ± 22,298 | 16,874 |
| FY*02 | TACCGCGCCGCGGGC | 166 | 1901—80,576 | 24,851 ± 23,186 | 13,779 |
| FY*02 N.01 | CACCGCGCCGCGGGC | 344 | 1977—80,584 | 18,482 ± 15,755 | 15,125 |
| Total | 899 | 1901—80,584 | 22,098 ± 19,903 | 16,315 |
ACKR1 alleles in the Neanderthal samples
The 3 Neanderthal samples were GATA box negative (-67 T) and represented the ancestral FY*B allele (Table 4). None of the 3 Neanderthal ACKR1 sequences (Chagyrskaya, Altai, and Vindija 33.19) fully matched any of the 902 haplotypes. The 2 haplotypes closest to the Neanderthal sequences had 1 mismatch in the GATA box (Table 4).
Table 4.
ACKR1 alleles in the 1000GP and 3 Neanderthal samples
| Haplotype | Observations | Nucleotides position* | Length (base pairs) | |||||
|---|---|---|---|---|---|---|---|---|
| Species | Population† | n | c.-67 T > C | c.21 + 115 T > C | c.21 + 235 T > C | c.125G > A | ||
| NG_011626.3‡ | H. sapiens | NA | NA | T | T | T | G | 1,626 |
| HAP897 | H. sapiens | ACB | 1 | C | C | T | A | 2,032 |
| HAP899 | H. sapiens | LWK | 1 | C | C | T | A | 1,978 |
| Chagyrskaya | H. neanderthalensis | NA | 1 | T | C | T | A | NA |
| Altai | H. neanderthalensis | NA | 1 | T | C | T | A | NA |
| Vindija | H. neanderthalensis | NA | 1 | T | C | Y | A | NA |
*Nucleotide positions are shown according to the human reference sequence (NG_011626.3) and defined using the first nucleotide of the coding sequence (CDS) of the NM_002036.2 isoform as nucleotide position 1. Only variant positions with respect to the 2,032 nucleotides of the HAP897 are listed
†ACB = African Caribbeans in Barbados; LWK = Luhya in Webuye, Kenya
‡ACKR1 reference allele per ISBT [95]
NA, not applicable; Y = T or C
Discussion
In the current study, we identified 902 experimentally confirmed reference haplotypes for the ACKR1 gene, using only publicly available data from the large scale 1000GP study database. Our approach is easily scalable. It can be applied to similar databases, including the UK10K Consortium [34], the African Genome Variation Project [35] and the upcoming All of Us Research Program [36]. For proof of principle, we demonstrated the application using a Python algorithm for one gene. The approach can, however, define reference sequences for any segment of the genome, with genes or without.
We showed that reference sequences can be obtained from databases and verified without ambiguity at lengths exceeding 80 kb. Such reference sequences can be catalogued inexpensively for use in clinical diagnostics. The catalogue comprised the set of the longest unique haplotypes that can be distinguished by the gene’s nucleotide sequence. In clinical diagnostics with molecular-based assays, common and well documented (CWD) [37] reference haplotypes are routinely applied, for example in HLA typing [1]. Exact matching at the haplotype level improves survival following bone marrow transplantation [38] and reduces alloimmunization in chronically transfused patients [39–41]. A limited number of common haplotypes represented the majority in the population [42], and identifying haplotypes from databases is an economical way to obtain such reference sequences.
Apart from clinical diagnostics, long-range haplotypes are also useful to understand the influence of environment on positive selection of genes in human populations [43], for association mapping of genes that contribute to disease and other phenotypes [44], for correlating the geographical distribution of haplotypes with endemicity of disease [45], for identifying evolutionarily conserved elements and regulatory elements [46], and for improving the reliability of genotype imputation [47]. Long haplotypes identified by using SNV data from high-density oligonucleotide arrays and the International HapMap Project [48] have been shown to be population dependent and can provide important insights into human evolutionary history [49]. These studies may also identify regions of positive selection with important roles in human health and disease [50].
Next generation sequencing is increasingly used for blood group genes [51–78]. In contrast to HLA [79], most blood group genes lack well documented long reference sequences associated with them [80]. Hence, a comprehensive reference database for blood group genes will facilitate blood group genotyping by NGS. The Erythrogene database [59] contains the complete coding region sequence of many different blood group alleles obtained from the 1000GP. However, it lacks information for sequence variants in the non-coding regions, such as promoter, splice sites and long intronic regions, which can also affect the expression of antigens and helps to ascertain the allele and its coding sequence [81–84].
A large number of haplotypes were more than 50 kb long with some extending at least to 80.5 kb in length (Fig. 2). Our observations are consistent with previous reports suggesting that most of the human genome is contained in blocks of a few kb to more than 100 kb [85, 86]. However, most of the ACKR1 haplotypes in the 1000GP were small and concentrated closely around the ACKR1 gene. The number of haplotypes decreased as their length increased and extended into the intergenic regions (Fig. 3). This is explained because most of the variants in the dbSNP database resides in the intergenic regions [87].
Our 2 haplotypes HAP897 and HAP899 (Additional file 4:Table S3), observed once each in African populations, were closest to the 3 Neanderthal samples. Both haplotypes carried the GATA box mutation (c.-67C), which all Neanderthal samples lacked (c.-67T). Individuals homozygous for the GATA box mutation (c.-67C) do not express the Duffy glycoprotein on the red cell surface [81] making them resistant to invasion by the malarial parasite P. vivax [88–90]. This similarity in alleles, discrepant at nucleotide position c.-67 only, was consistent with the fact that the GATA box mutation (c.-67C) started to spread in Africa only around 30,000 years ago [91], while the 3 Neanderthals Vindija, Altai and Chagyrskaya are 50,000, 120,000 and 50,000 years old, respectively [25–27].
In clinical diagnostics for patients, long-range haplotypes harboring novel or rare SNVs can only be detected when the haplotype is sequenced at full-length [92]. Using Sanger sequencing, we have previously characterized the ERMAP [93], ICAM4 [94], and ACKR1 [23] blood group genes at the haplotype level and identified prevalent long-range reference alleles, a time consuming and low throughput approach. We showed in this study how long contiguous stretches of homozygosity (LCSH) can serve to generate a database of long haplotypes, as defined by full length nucleotide sequences rather than the concatenation of known SNVs. Relying on SNV data would miss patients carrying novel or rare alleles with possible clinical relevance, which are not identical to the reference sequences. Features of the 1000GP allowed us to catalogue these extended nucleotide sequences with population specific frequencies. Our approach will enable the positive identification of patients carrying these reference sequences.
We plan to extend this approach to all blood group systems recognized by the International Society of Blood Transfusion (ISBT) [95]. A tool under development will allow researchers the customized online extraction of long haplotypes from databases and genes or genomic regions of their choice. Eventually, our approach can be applied to any region of a chromosome. For now, the 902 ACKR1 alleles identified through our novel approach will be useful as templates for analyzing data from NGS, thus enhancing the reliability of clinical diagnostics.
Web Resources
1000 Genomes browser (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/) accessed on Aug 05, 2019. ISBT (https://www.isbtweb.org/fileadmin/user_upload/Table_of_blood_group_systems_v6.0_6th_August_2019.pdf). Max Planck Institute for Evolutionary Anthropology (http://cdna.eva.mpg.de/neandertal/).
Supplementary Information
Additional file 1 File S1. Python algorithm.
Additional file 2 Table S1. Populations in the 1000GP database.
Additional file 3 Table S2. Sequence data for the 902 long range ACKR1 haplotypes in the 1000GP.
Additional file 4 Table S3. Metadata file for the ACKR1 long range haplotypes in the 1000GP.
Additional file 5Table S4. Exonic SNV distribution in the 902 experimentally confirmed ACKR1 haplotypes.
Acknowledgements
Bo Lan participated in the study during his Summer Internship Program at NIH in 2019.
Author's contribution
WAF and KS conceived the study; KS designed the analysis and downloaded the data; ASF programmed the algorithm; WAF, KS, ASF and BL analyzed the data; WAF, ASF and KS wrote the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided by the National Institutes of Health (NIH). This work was supported in part by the Intramural Research Program (projects ZIC CL002128 and RASCL#727301) of the NIH Clinical Center at the National Institutes of Health. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets analyzed and generated during the current study are available as supplementary tables and at 1000 Genomes browser (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript.
Competing interests
None.
Statement of disclaimer
The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robinson J, et al. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423–431. doi: 10.1093/nar/gku1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halldórsson, B. V. et al. A survey of computational methods for determining haplotypes. In: Istrail S., Waterman M., Clark A. (eds) Computational methods for SNPs and haplotype inference. RSNPsH 2002. Lecture Notes in Computer Science. Springer, Berlin, Heidelberg. 2983, 26–47, doi.org/10.1007/1978-1003-1540-24719-24717_24713 (2004).
- 3.Al Bkhetan Z, Zobel J, Kowalczyk A, Verspoor K, Goudey B. Exploring effective approaches for haplotype block phasing. BMC Bioinform. 2019;20:540. doi: 10.1186/s12859-019-3095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark AG. Inference of haplotypes from PCR-amplified samples of diploid populations. Mol Biol Evol. 1990;7:111–122. doi: 10.1093/oxfordjournals.molbev.a040591. [DOI] [PubMed] [Google Scholar]
- 5.Glusman G, Cox HC, Roach JC. Whole-genome haplotyping approaches and genomic medicine. Genome Med. 2014;6:73. doi: 10.1186/s13073-014-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roach JC, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science. 2010;328:636–639. doi: 10.1126/science.1186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L, et al. Direct determination of molecular haplotypes by chromosome microdissection. Nat Methods. 2010;7:299–301. doi: 10.1038/nmeth.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Chen X, Wong WH. Completely phased genome sequencing through chromosome sorting. Proc Natl Acad Sci U S A. 2011;108:12–17. doi: 10.1073/pnas.1016725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkness EF, et al. Sequencing of isolated sperm cells for direct haplotyping of a human genome. Genome Res. 2013;23:826–832. doi: 10.1101/gr.144600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbeithuber, B., Heissl, A. & Tiemann-Boege, I. in Haplotyping: Methods and Protocols (eds Irene Tiemann-Boege & Andrea Betancourt) 3–22 (Springer New York, 2017).
- 11.Zheng GX, et al. Haplotyping germline and cancer genomes with high-throughput linked-read sequencing. Nat Biotechnol. 2016;34:303–311. doi: 10.1038/nbt.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhoads A, Au KF. PacBio sequencing and its applications. Genom Proteom Bioinform. 2015;13:278–289. doi: 10.1016/j.gpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain M, Olsen HE, Paten B, Akeson M. The Oxford Nanopore MinION: delivery of nanopore sequencing to the genomics community. Genome Biol. 2016;17:239. doi: 10.1186/s13059-016-1103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li LH, et al. Long contiguous stretches of homozygosity in the human genome. Hum Mutat. 2006;27:1115–1121. doi: 10.1002/humu.20399. [DOI] [PubMed] [Google Scholar]
- 15.Gibson J, Morton NE, Collins A. Extended tracts of homozygosity in outbred human populations. Hum Mol Genet. 2006;15:789–795. doi: 10.1093/hmg/ddi493. [DOI] [PubMed] [Google Scholar]
- 16.Nibbs RJB, Graham GJ. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 17.Horuk, R. The Duffy antigen receptor for chemokines DARC/ACKR1. Front Immunol 6, doi: 10.3389/fimmu.2015.00279 (2015). [DOI] [PMC free article] [PubMed]
- 18.Miller LH, Mason SJ, Dvorak JA, Mcginniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium-Knowlesi) malaria - duffy blood-group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 19.Meny GM. The Duffy blood group system: a review. Immunohematology. 2010;26:51–56. doi: 10.21307/immunohematology-2019-202. [DOI] [PubMed] [Google Scholar]
- 20.Meny GM. An update on the Duffy blood group system. Immunohematology. 2019;35:11–12. doi: 10.21307/immunohematology-2020-005. [DOI] [PubMed] [Google Scholar]
- 21.Schmid P, Ravenell KR, Sheldon SL, Flegel WA. DARC alleles and Duffy phenotypes in African Americans. Transfusion. 2012;52:1260–1267. doi: 10.1111/j.1537-2995.2011.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fichou Y, et al. Defining blood group gene reference alleles by long-read sequencing: proof of concept in the ACKR1 gene encoding the duffy antigens. Transfusion Med Hemotherapy. 2020;47:23–32. doi: 10.1159/000504584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Q, Srivastava K, Gebremedhin A, Makuria AT, Flegel WA. Long-range haplotype analysis of the malaria parasite receptor gene ACKR1 in an East-African population. Hum Genome Var. 2018;5:26. doi: 10.1038/s41439-018-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava K, et al. ACKR1 alleles at 5.6 kb in a well-characterized renewable US Food and Drug Administration (FDA) reference panel for standardization of blood group genotyping. J Mol Diagn. 2020;22:1272-1279. doi:10.1016/j.jmoldx.2020.06.014. [DOI] [PMC free article] [PubMed]
- 25.Prüfer K, et al. The complete genome sequence of a neanderthal from the Altai mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prüfer K, et al. A high-coverage Neandertal genome from Vindija Cave in Croatia. Science. 2017;358:655–658. doi: 10.1126/science.aao1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mafessoni F, et al. A high-coverage Neandertal genome from Chagyrskaya Cave. Proc Natl Acad Sci U S A. 2020;117:15132–15136. doi: 10.1073/pnas.2004944117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020 doi: 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- 29.Genomes Project, C. et al. A global reference for human genetic variation. Nature. 2015;526:68–74. 10.1038/nature15393. [DOI] [PMC free article] [PubMed]
- 30.Sudmant PH, et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics (Oxford, England) 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherry ST, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson JT, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter K, et al. The UK10K project identifies rare variants in health and disease. Nature. 2015;526:82–90. doi: 10.1038/nature14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurdasani D, et al. The African genome variation project shapes medical genetics in Africa. Nature. 2015;517:327–332. doi: 10.1038/nature13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denny JC, et al. The "All of Us" research program. N Engl J Med. 2019;381:668–676. doi: 10.1056/NEJMsr1809937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mack SJ, et al. Common and well-documented HLA alleles: 2012 update to the CWD catalogue. Tissue Antigens. 2013;81:194–203. doi: 10.1111/tan.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tay GK, et al. Matching for MHC haplotypes results in improved survival following unrelated bone marrow transplantation. Bone Marrow Transpl. 1995;15:381–385. [PubMed] [Google Scholar]
- 39.Chou ST, Liem RI, Thompson AA. Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol. 2012;159:394–404. doi: 10.1111/bjh.12061. [DOI] [PubMed] [Google Scholar]
- 40.Tournamille C, et al. Partial C antigen in sickle cell disease patients: clinical relevance and prevention of alloimmunization. Transfusion. 2010;50:13–19. doi: 10.1111/j.1537-2995.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 41.Allen ES, et al. Immunohaematological complications in patients with sickle cell disease after haemopoietic progenitor cell transplantation: a prospective, single-centre, observational study. Lancet Haematol. 2017;4:e553–e561. doi: 10.1016/s2352-3026(17)30196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slater N, et al. Power laws for heavy-tailed distributions: modeling allele and haplotype diversity for the national marrow donor program. PLoS Comput Biol. 2015 doi: 10.1371/journal.pcbi.1004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallender EJ, Lahn BT. Positive selection on the human genome. Hum Mol Genet. 2004 doi: 10.1093/hmg/ddh253. [DOI] [PubMed] [Google Scholar]
- 44.Gibson G, Muse SV. A primer of genome science. Sunderland, MA: Sinauer Associates; 2009. [Google Scholar]
- 45.Filosa S, et al. G6PD haplotypes spanning Xq28 from F8C to red/green color vision. Genomics. 1993;17:6–14. doi: 10.1006/geno.1993.1276. [DOI] [PubMed] [Google Scholar]
- 46.Li MJ, Yan B, Sham PC, Wang J. Exploring the function of genetic variants in the non-coding genomic regions: approaches for identifying human regulatory variants affecting gene expression. Brief Bioinform. 2015;16:393–412. doi: 10.1093/bib/bbu018. [DOI] [PubMed] [Google Scholar]
- 47.Gudbjartsson DF, et al. Large-scale whole-genome sequencing of the Icelandic population. Nat Genet. 2015;47:435–444. doi: 10.1038/ng.3247. [DOI] [PubMed] [Google Scholar]
- 48.The International HapMap Project Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 49.Gusev A, et al. The architecture of long-range haplotypes shared within and across populations. Mol Biol Evol. 2012;29:473–486. doi: 10.1093/molbev/msr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, et al. A whole genome long-range haplotype (WGLRH) test for detecting imprints of positive selection in human populations. Bioinformatics (Oxford, England) 2006;22:2122–2128. doi: 10.1093/bioinformatics/btl365. [DOI] [PubMed] [Google Scholar]
- 51.Stabentheiner S, et al. Overcoming methodical limits of standard RHD genotyping by next-generation sequencing. Vox Sang. 2011;100:381–388. doi: 10.1111/j.1423-0410.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- 52.Rieneck K, et al. Next-generation sequencing: proof of concept for antenatal prediction of the fetal Kell blood group phenotype from cell-free fetal DNA in maternal plasma. Transfusion. 2013;53:2892–2898. doi: 10.1111/trf.12172. [DOI] [PubMed] [Google Scholar]
- 53.Fichou Y, Audrézet MP, Guéguen P, Le Maréchal C, Férec C. Next-generation sequencing is a credible strategy for blood group genotyping. Br J Haematol. 2014;167:554–562. doi: 10.1111/bjh.13084. [DOI] [PubMed] [Google Scholar]
- 54.Wieckhusen C, Bugert P. 454-sequencing for the KEL, JR, and LAN blood groups. Methods Mol Biol. 2015;1310:123–133. doi:10.1007/978-1-4939-2690-9_11. [DOI] [PubMed]
- 55.Giollo M, et al. BOOGIE: predicting blood groups from high throughput sequencing data. PLoS ONE. 2015 doi: 10.1371/journal.pone.0124579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lane WJ, et al. Comprehensive red blood cell and platelet antigen prediction from whole genome sequencing: proof of principle. Transfusion. 2016;56:743–754. doi: 10.1111/trf.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lang K, et al. ABO allele-level frequency estimation based on population-scale genotyping by next generation sequencing. BMC Genomics. 2016;17:374. doi: 10.1186/s12864-016-2687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fichou Y, Mariez M, Le Maréchal C, Férec C. The experience of extended blood group genotyping by next-generation sequencing (NGS): investigation of patients with sickle-cell disease. Vox Sang. 2016;111:418–424. doi: 10.1111/vox.12432. [DOI] [PubMed] [Google Scholar]
- 59.Möller M, Jöud M, Storry JR, Olsson ML. Erythrogene: a database for in-depth analysis of the extensive variation in 36 blood group systems in the 1000 Genomes Project. Blood Adv. 2016;1:240–249. doi: 10.1182/bloodadvances.2016001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baronas J, Westhoff C, Vege S, Mah H, Aguad M. RHD zygosity determination from whole genome sequencing data. J Blood Disord Transfus. 2016;7:1–5. doi: 10.4172/2155-9864.1000365. [DOI] [Google Scholar]
- 61.Schoeman EM, et al. Evaluation of targeted exome sequencing for 28 protein-based blood group systems, including the homologous gene systems, for blood group genotyping. Transfusion. 2017;57:1078–1088. doi: 10.1111/trf.14054. [DOI] [PubMed] [Google Scholar]
- 62.Dezan MR, et al. RHD and RHCE genotyping by next-generation sequencing is an effective strategy to identify molecular variants within sickle cell disease patients. Blood Cells Mol Dis. 2017;65:8–15. doi: 10.1016/j.bcmd.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Chou ST, et al. Whole-exome sequencing for RH genotyping and alloimmunization risk in children with sickle cell anemia. Blood Adv. 2017;1:1414–1422. doi: 10.1182/bloodadvances.2017007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jakobsen MA, Dellgren C, Sheppard C, Yazer M, Sprogøe U. The use of next-generation sequencing for the determination of rare blood group genotypes. Transfus Med. 2019;29:162–168. doi: 10.1111/tme.12496. [DOI] [PubMed] [Google Scholar]
- 65.Schoeman EM, et al. Targeted exome sequencing defines novel and rare variants in complex blood group serology cases for a red blood cell reference laboratory setting. Transfusion. 2018;58:284–293. doi: 10.1111/trf.14393. [DOI] [PubMed] [Google Scholar]
- 66.Orzińska A, et al. A preliminary evaluation of next-generation sequencing as a screening tool for targeted genotyping of erythrocyte and platelet antigens in blood donors. Blood Transf. 2018;16:285–292. 10.2450/2017.0253-16. [DOI] [PMC free article] [PubMed]
- 67.Lane WJ, et al. Automated typing of red blood cell and platelet antigens: a whole-genome sequencing study. Lancet Haematol. 2018;5:e241–51. 10.1016/s2352-3026(18)30053-x. [DOI] [PMC free article] [PubMed]
- 68.Wheeler MM, et al. Genomic characterization of the RH locus detects complex and novel structural variation in multi-ethnic cohorts. Genet Med. 2019;21:477–486. doi: 10.1038/s41436-018-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu PC, et al. ABO genotyping with next-generation sequencing to resolve heterogeneity in donors with serology discrepancies. Transfusion. 2018;58:2232–2242. doi: 10.1111/trf.14654. [DOI] [PubMed] [Google Scholar]
- 70.Montemayor-Garcia C, et al. Genomic coordinates and continental distribution of 120 blood group variants reported by the 1000 Genomes Project. Transfusion. 2018;58:2693–2704. doi: 10.1111/trf.14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tounsi WA, Madgett TE, Avent ND. Complete RHD next-generation sequencing: establishment of reference RHD alleles. Blood Adv. 2018;2:2713–2723. doi: 10.1182/bloodadvances.2018017871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoeman EM, Roulis EV, Perry MA, Flower RL, Hyland CA. Comprehensive blood group antigen profile predictions for Western Desert Indigenous Australians from whole exome sequence data. Transfusion. 2019;59:768–778. doi: 10.1111/trf.15047. [DOI] [PubMed] [Google Scholar]
- 73.Orzińska A, et al. Prediction of fetal blood group and platelet antigens from maternal plasma using next-generation sequencing. Transfusion. 2019;59:1102–1107. doi: 10.1111/trf.15116. [DOI] [PubMed] [Google Scholar]
- 74.Lane WJ, et al. Automated typing of red blood cell and platelet antigens from whole exome sequences. Transfusion. 2019;59:3253–3263. doi: 10.1111/trf.15473. [DOI] [PubMed] [Google Scholar]
- 75.Halls JBL, et al. Overcoming the challenges of interpreting complex and uncommon RH alleles from whole genomes. Vox Sang. 2020 doi: 10.1111/vox.12963. [DOI] [PubMed] [Google Scholar]
- 76.Fürst D, et al. Next-generation sequencing technologies in blood group typing. Transf Med Hemother. 2020;47:4–13. 10.1159/000504765. [DOI] [PMC free article] [PubMed]
- 77.Wu PC, Pai S-C, Chen P-L. Blood group genotyping goes next generation: featuring ABO, RH and MNS. RH and MNS VOXS. 2018;13:290–297. doi: 10.1111/voxs.12426. [DOI] [Google Scholar]
- 78.Orzinska A, Guz K, Brojer E. Potential of next-generation sequencing to match blood group antigens for transfusion. Int J Clin Transfus Med. 2019;7:11–22. doi: 10.2147/IJCTM.S175142. [DOI] [Google Scholar]
- 79.Barone JC, et al. HLA-genotyping of clinical specimens using Ion Torrent-based NGS. Hum Immunol. 2015;76:903–909. doi: 10.1016/j.humimm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Reid ME. Transfusion in the age of molecular diagnostics. Hematol Am Soc Hematol Educ Program. 2009;2009:171–7. 10.1182/asheducation-2009.1.171. [DOI] [PMC free article] [PubMed]
- 81.Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10;224–8. 10.1038/ng0695-224. [DOI] [PubMed]
- 82.Lucien N, et al. Characterization of the gene encoding the human Kidd blood group/urea transporter protein. Evidence for splice site mutations in Jknull individuals. J Biol Chem. 1998;273:12973–80. 10.1074/jbc.273.21.12973. [DOI] [PubMed]
- 83.Lomas-Francis C, Reid ME. The Dombrock blood group system: a review. Immunohematology. 2010;26:71–78. doi: 10.21307/immunohematology-2019-206. [DOI] [PubMed] [Google Scholar]
- 84.Christophersen MK, et al. SMIM1 variants rs1175550 and rs143702418 independently modulate Vel blood group antigen expression. Sci Rep. 2017;7:40451. doi: 10.1038/srep40451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabriel SB, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 86.Wall JD, Pritchard JK. Haplotype blocks and linkage disequilibrium in the human genome. Nat Rev Genet. 2003;4:587–597. doi: 10.1038/nrg1123. [DOI] [PubMed] [Google Scholar]
- 87.Jin Y, Wang J, Bachtiar M, Chong SS, Lee CGL. Architecture of polymorphisms in the human genome reveals functionally important and positively selected variants in immune response and drug transporter genes. Hum Genomics. 2018;12:43. doi: 10.1186/s40246-018-0175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–304. 10.1056/nejm197608052950602. [DOI] [PubMed]
- 89.Chaudhuri A, et al. Purification and characterization of an erythrocyte membrane protein complex carrying Duffy blood group antigenicity. Possible receptor for Plasmodium vivax and Plasmodium knowlesi malaria parasite. J Biol Chem. 1989;264:13770–13774. [PubMed]
- 90.Hadley TJ, Peiper SC. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077–3091. doi: 10.1182/blood.V89.9.3077. [DOI] [PubMed] [Google Scholar]
- 91.Hamblin MT, Di Rienzo A. Detection of the signature of natural selection in humans: evidence from the Duffy blood group locus. Am J Hum Genet. 2000;66:1669–1679. doi: 10.1086/302879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suk EK, et al. A comprehensively molecular haplotype-resolved genome of a European individual. Genome Res. 2011;21:1672–1685. doi: 10.1101/gr.125047.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srivastava K, Lee E, Owens E, Rujirojindakul P, Flegel WA. Full-length nucleotide sequence of ERMAP alleles encoding Scianna (SC) antigens. Transfusion. 2016;56:3047–3054. doi: 10.1111/trf.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin Q, et al. Molecular analysis of the ICAM4 gene in an autochthonous East African population. Transfusion. 2019;59:1880–1881. doi: 10.1111/trf.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.https://www.isbtweb.org/. (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 File S1. Python algorithm.
Additional file 2 Table S1. Populations in the 1000GP database.
Additional file 3 Table S2. Sequence data for the 902 long range ACKR1 haplotypes in the 1000GP.
Additional file 4 Table S3. Metadata file for the ACKR1 long range haplotypes in the 1000GP.
Additional file 5Table S4. Exonic SNV distribution in the 902 experimentally confirmed ACKR1 haplotypes.
Data Availability Statement
The datasets analyzed and generated during the current study are available as supplementary tables and at 1000 Genomes browser (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/).